Abstract
Stimulation of Gq-coupled receptors activates phospholipase C and is supposed to promote both intracellular Ca2+ mobilization and protein kinase C (PKC) activation. We found that ADP-induced phosphorylation of pleckstrin, the main platelet substrate for PKC, was completely inhibited not only by an antagonist of the Gq-coupled P2Y1 receptor but also upon blockade of the Gi-coupled P2Y12 receptor. The role of Gi on PKC regulation required stimulation of phosphatidylinositol 3-kinase rather than inhibition of adenylyl cyclase. P2Y12 antagonists also inhibited pleckstrin phosphorylation, Rap1b activation, and platelet aggregation induced upon Gq stimulation by the thromboxane A2 analogue U46619. Importantly, activation of phospholipase C and intracellular Ca2+ mobilization occurred normally. Phorbol 12-myristate 13-acetate overcame the inhibitory effect of P2Y12 receptor blockade on PKC activation but not on Rap1b activation and platelet aggregation. By contrast, inhibition of diacylglycerol kinase restored both PKC and Rap1b activity and caused platelet aggregation. Stimulation of P2Y12 receptor or direct inhibition of diacylglycerol kinase potentiated the effect of membrane-permeable sn-1,2-dioctanoylglycerol on platelet aggregation and pleckstrin phosphorylation, in association with inhibition of its phosphorylation to phosphatidic acid. These results reveal a novel and unexpected role of the Gi-coupled P2Y12 receptor in the regulation of diacylglycerol-mediated events in activated platelets.
It is generally accepted that, with a very few exceptions, platelet response to soluble agonists originates from the convergence of at least two different signal transduction pathways typically initiated by the heterotrimeric G-proteins Gq and Gi. Several receptors for platelet agonists are coupled to Gq, including the P2Y1 receptor for ADP, the TPα receptor for the thromboxane A2 (TxA2),3 and the PAR1 and PAR4 receptors for thrombin (1, 2). Signaling downstream Gq involves the stimulation of members of the β subfamily of phospholipase C (PLC), which generate the second messengers inositol 1,4,5-trisphosphosphate (IP3) and diacylglycerol (DAG), responsible for Ca2+ mobilization from intracellular stores and protein kinase C (PKC) activation, respectively (3). This pathway is necessary but not sufficient to elicit a full platelet response, and the concomitant activation of a Gi-coupled receptor is absolutely mandatory (4).
To fulfill this requirement, some agonists, like ADP, directly stimulate multiple G-protein coupled receptors, one of which is associated to Gi (5, 6), whereas others, such as TxA2, rely on the auxiliary action of secondary messengers released by activated platelets to stimulate a Gi-coupled receptor (7). Despite their essential role, there are very few Gi-coupled receptors on the platelet surface, and these include the α2-adrenergic receptor for epinephrine and the P2Y12 receptor for ADP (8, 9). Therefore, ADP, which is also one of the major component of the platelet releasate, is a crucial regulator of platelet function, and its receptors have gained increasing attention as potential target for anti-thrombotic agents, including thienopyridines (9–11).
The biochemical basis for the critical role of Gi stimulation in platelet aggregation is not completely known. Inhibition of adenylyl cyclase and reduction of intracellular cAMP levels do not appear sufficient to complement Gq-dependent pathway for platelet aggregation (12). However, binding of ADP to the Gi-coupled P2Y12 receptor regulates, through the G protein βγ dimers, additional intracellular effectors, including members of the class I phosphatidylinositol 3-kinase and the small GTPase Rap1b (13–17). Activation of phosphatidylinositol 3-kinase lies upstream stimulation of Rap1b (15–18), which in turn has emerged as a critical regulator of integrin αIIbβ3 and platelet aggregation (19–21). The phosphatidylinositol 3-kinase-Rap1b pathway has been proposed to be responsible for the Gi-mediated contribution to platelet aggregation (15, 18, 22–26), but many aspects are not clearly defined.
In this work we report that whereas activation of PLC is exclusively controlled by Gq-coupled receptors, the downstream stimulation of PKC requires the concomitant activation of the Gi-coupled P2Y12 receptor. Moreover, we show that all the inhibitory effects of P2Y12 receptor antagonists on platelet function can be reversed by pharmacologic inhibition of the DAG metabolizing enzyme diacylglycerol kinase (DGK) but not by direct stimulation of PKC. These results indicate that DAG is a critical messenger for platelet aggregation regulated through Gi-dependent signaling pathways.
EXPERIMENTAL PROCEDURES
Materials—ADP, U46619, thrombin, MRS2179, 2MeSAMP, phorbol 12-myristate 13-acetate (PMA), 1,2-dioleoylglycerol, prostaglandin E1, acetylsalicylic acid, sn-1,2 dioctanoylglycerol (DiC8), and apyrase were from Sigma. [32P]Orthophosphate, myo-[2-3H]inositol (16 Ci/mmol), and [5,6,8,9,11,12,14,15-3H(N)]-arachidonic acid (189 Ci/mmol) were from GE Healthcare. Dideoxyadenosine, wortmannin, and LY294002 were from Alexis (Vinci-Biochem, Vinci, Italy). FURA-2-AM was from Calbiochem. AR-C69931MX was a generous gift from AstraZeneca (Charnwood, UK). The rabbit polyclonal antibodies against Rap1 (121) was from Santa Cruz Biotechnology (Tebu-Bio, Magenta, Italy). The rabbit polyclonal antibody against phospho(Ser) PKC substrates was from Cell Signaling Technology (Celbio, Pero, Italy).
Platelet Isolation—Human platelets were obtained from healthy volunteers in citric acid/citrate/dextrose (152 mm sodium citrate, 130 mm citric acid, 112 mm glucose). Whole blood was centrifuged at 120 × g for 10 min at room temperature. Apyrase (0.2 units/ml), prostaglandin E1 (1 μm), and acetylsalicylic acid (1 mm) were then added to the platelet-rich plasma. Platelets were recovered by centrifugation at 720 × g for 15 min, washed with 10 ml of PIPES buffer (20 mm PIPES, 136 mm NaCl, pH 6.5), and finally gently resuspended in HEPES buffer (10 mm HEPES, 137 mm NaCl, 2.9 mm KCl, 12 mm NaHCO3, pH 7.4). The cell count was typically adjusted to 0.3 × 109 platelets/ml unless otherwise stated.
Platelet Stimulation and Measurement of Aggregation—All the experiments were performed with 0.1–0.4-ml samples of washed platelets placed at 37 °C in an aggregometer under constant stirring in the presence of 1 mm CaCl2. Platelets were stimulated with 10 μm ADP or 1 μm U46619 for 1 min unless otherwise stated in the figure legends. Preincubation with specific inhibitors, activators, or receptor antagonists was as follows: 1 or 2.5 nm PMA for 1 min, 0.1–3.5 μm DiC8 for 1 min, 200 μm MRS2179, 1 μm AR-C69931MX or 2MeSAMP for 2 min, 1 μm R59949 for 5 min, 50 nm wortmannin, 25 μm LY294002 for 15 min, 100 μm dideoxyadenosine for 30 min. When compounds were dissolved in DMSO, an equal volume of vehicle was added to control and samples. For aggregation measurement, stimulation was prolonged up to 7 min, and light transmission was continuously monitored. All the aggregation traces reported in the figures are representative of al least three different experiments.
Measurement of Pleckstrin Phosphorylation and DiC8-PA Production Using 32P-Labeled Platelets—Platelets, at the final concentration of 109 cells/ml in PIPES buffer, were incubated with 0.1 mCi/ml 32P for 90 min at 37 °C, centrifuged at 800 × g for 15 min, and finally resuspended in HEPES buffer containing 1 mm CaCl2 and 5.5 mm glucose. Samples (0.2 ml) were preincubated with different inhibitors and then stimulated with ADP or U46619 as above indicated. The reaction was stopped by the addition 0.1 ml of SDS sample buffer 3X (37.5 mm Tris/HCl, pH 8.3, 288 mm glycine, 6% SDS, 1.5% dithiothreitol, 30% glycerol, 0.03% bromphenol blue) and by heating at 95 °C for 3 min. Identical aliquots of total platelet proteins (20 μl) were separated by SDS-PAGE on a 5–15% acrylamide gradient gel and stained with Coomassie Blue. Gels were then dried, and phosphorylation of pleckstrin was visualized upon autoradiography for about 18 h at –80 °C.
For measurement of DiC8 conversion to DiC8-PA, samples of 32P-labeled platelets (0.2 ml) were treated with 500 nm DiC8 in the presence or in the indicated concentrations of ADP plus 200 μm MRS2179, 1 μm AR-C69931MX, or 1 mm R59949 for 1 min. The reaction was stopped by the addition of 0.275 ml of chloroform and 0.55 ml of methanol. Phase partition was obtained through the addition of 0.275 ml of chloroform and 0.44 ml of 1 m HCl. Upon centrifugation at 800 × g for 10 min, the lipids recovered in the lower phase were spotted on silica gel TLC plates and eluted in ethyl acetate/iso-octane/acetic acid/water (45:20:12:6). Spots on the TLC plates were visualized by autoradiography for about 18 h at –80 °C, and DiC8-derived PA production was quantified by analysis through ImageJ software.
Evaluation of PKC-dependent Protein Phosphorylation Using an Anti-phospho(Ser) PKC Substrate Antibody—Platelet stimulation on 0.1-ml samples (see above) was stopped by the addition of 0.05 ml of SDS sample buffer 3X. Proteins were separated by SDS-PAGE on a 5–15% acrylamide gradient gel, transferred to nitrocellulose, and probed by immunoblotting using an anti-phospho(Ser) PKC substrates antibody diluted 1:1000, as previously described (24). Immunoreactive bands were visualized by enhanced chemiluminescence reaction. Quantification of pleckstrin phosphorylation was performed by densitometric analysis. All the reported figures are representative of at least three different experiments.
Measurement of Cytosolic Ca2+ Concentration—Intracellular Ca2+ concentration was measured in Fura-2-AM-loaded platelets essentially as previously described (24). For these experiments, the final platelet concentration was 2 × 108 cells/ml, and preincubation and stimulation was performed on 0.4-ml samples prewarmed at 37 °C under gentle stirring in a PerkinElmer Life Sciences LS3 spectrofluorometer in the presence of 1 mm EGTA. All determinations were repeated at least four times with platelets from different donors.
Measurement of [3H]Inositol Phosphate Production—Platelets in PIPES buffer (2 × 109 cells/ml) were labeled with 0.125 mCi/ml myo-[2-3H]inositol for 3 h at 37 °C, centrifuged, and finally resuspended in HEPES buffer containing 5.5 mm glucose, 0.5 mm RGDS, 1 mm CaCl2, and 10 mm LiCl at the concentration of 109 cells/ml. Platelet samples (0.4 ml) were incubated in the presence or absence of 1 μm AR-C69931MX and stimulated under constant stirring with 1 μm U46619. The reaction was stopped by adding 0.55 ml of chloroform and 1.1 ml of methanol. Samples were placed on ice, and phase partition was obtained by the addition of 0.55 ml of chloroform and 0.88 ml of 1 m HCl. The upper phase (2 ml), collected upon centrifugation at 800 × g for 10 min, was neutralized with 1 ml of 2 m ammonium acetate, and [3H]inositol phosphates were separated by ion exchange chromatography on 1 ml of AG 1-X resin, as previously described (27) and quantified by scintillation counting in the gel phase.
Measurement of DAG Accumulation—Platelets in PIPES buffer (109 cells/ml) were labeled with 2.5 μCi/ml [3H]arachidonic acid for 30 min at 37 °C and then resuspended in HEPES buffer containing 5.5 mm glucose, 0.5 mm RGDS, and 1 mm CaCl2. Incubation and stimulation of platelet samples (0.2 ml) as well as phase partition was performed as described for the inositol phosphates measurement; however, the lower phases (0.4 ml) were collected and dried under N2 flux. Lipids were resuspended with 20 μl of methanol:chloroform (1:1). Each sample was mixed with 1 μg of standard purified DAG and spotted on silica gel TLC plates. Elution was performed with diethyl ether:exane:acetic acid (70:30:1), and DAG spots were visualized with iodine vapors. The spots corresponding to DAG were scraped, and the radioactivity was measured by scintillation counting.
Rap1b Activation Assay—Activation of Rap1b in platelet samples (0.2 ml) was evaluated by a pulldown assay using the GST-tagged Rap binding domain of RalGDS (GST-RalGDS-RBD) essentially as previously reported (28). Quantification of Rap1b activation was performed by densitometric analysis of the immunoblots.
RESULTS
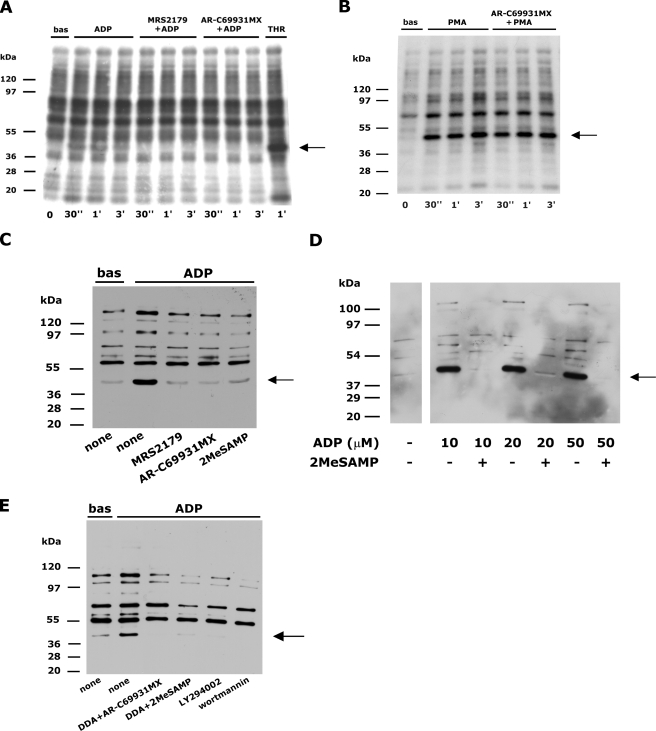
Stimulation of the Gi-coupled P2Y12 Receptor Is Necessary for ADP- or U46619-induced PKC Activation—The possible cross-talk between P2Y1 and P2Y12 receptors in the regulation of platelet PKC was evaluated by measuring the phosphorylation of the 47-kDa protein pleckstrin, the main PKC substrate, in 32P-labeled cells (29). Autoradiographic analysis allowed the detection of a clear, albeit faint, phosphorylation of pleckstrin in ADP-stimulated platelets which was rapid and transient (Fig. 1A). As expected, pleckstrin phosphorylation was totally suppressed upon blockade of the Gq-coupled P2Y1 receptor by MRS2179. Surprisingly, however, we observed that ADP-induced pleckstrin phosphorylation was also completely abrogated upon platelet incubation with AR-C69931MX, a specific antagonist of the Gi-coupled P2Y12 receptor (Fig. 1A), which is not supposed to contribute to PLC regulation. By contrast, AR-C69931MX did not alter pleckstrin phosphorylation induced by the phorbol ester PMA (Fig. 1B) or by thrombin (data not shown).
FIGURE 1.
Co-stimulation of P2Y1 and P2Y12 receptors is required for ADP-induced pleckstrin phosphorylation. A, 32P-labeled platelets were left untreated (bas) or were stimulated with 10 μm ADP in the absence of inhibitors or in the presence of the P2Y1 receptor antagonist MRS2179 (200 μm) or the P2Y12 receptor antagonist AR-C69931MX (1 μm) as indicated on the top for times ranging from 30 s to 3 min, as indicated on the bottom. As control, platelets were stimulated with 1 unit/ml thrombin (THR). Platelet proteins were separated by SDS-PAGE, and phosphorylated pleckstrin, indicated by the arrow on the right, was identified by autoradiography. B, autoradiographic analysis of pleckstrin phosphorylation in 32P-labeled platelets left untreated (bas) or stimulated with 100 nm PMA for the indicated times in the absence or in the presence of 1 μm AR-C69931MX. C, platelets were preincubated with buffer (none) or with the P2Y1 and P2Y12 receptors antagonists MRS2179 (200 μm), AR-C69931MX (1 μm), and 2MeSAMP (1 μm) as indicated on the bottom and then were left untreated (bas) or stimulated with 10 μm ADP for 1 min. PKC-directed protein phosphorylation was analyzed by immunoblotting with an anti-phospho(Ser) PKC substrates antibody. The position of the 47-kDa pleckstrin is indicated by the arrow on the right. D, platelets were incubated in the absence or presence of the P2Y12 receptor antagonist 2MeSAMP (1 μm) and then stimulated with the indicated doses of ADP for 1 min. Immunoblotting with an anti-phospho(Ser) PKC substrates antibody was used to detect phosphorylated pleckstrin, indicated by the arrow on the right. E, platelets were incubated with the P2Y12 receptor antagonists AR-C69931MX or 2MeSAMP in combination with the adenylyl cyclase inhibitor dideoxyadenosine (DDA, 100 μm) or were treated with the phosphatidylinositol 3-kinase inhibitors LY294002 (25 μm) or wortmannin (50 nm) as indicated on the bottom. Upon stimulation with 10 μm ADP, pleckstrin phosphorylation, indicated by the arrow, was analyzed by immunoblotting with the anti-phospho(Ser) PKC substrates antibody.
Because our analysis with 32P-labeled platelets revealed only a rather weak metabolic labeling of the PKC substrates, we consolidated our observations with a different, immunological approach using a phosphospecific antibody against phosphorylated PKC substrates. This anti-phospho(Ser) PKC substrates antibody detected a number of proteins in resting platelets whose reactivity was clearly increased upon stimulation with ADP (Fig. 1C). Such an increase was particularly evident for a 47-kDa band, which was identified as pleckstrin based on immunoblotting analysis performed with 32P-labeled platelets (data not shown). None of the bands depicted in Fig. 1C was detected when platelets were preincubated with the PKC inhibitor Ro31-8220 (data not shown), confirming the specificity of the antibody. Using this alternative and more sensitive approach, we confirmed and extended our observations. ADP-induced pleckstrin phosphorylation as well as phosphorylation of other unidentified PKC substrates was completely suppressed not only by MRS2179 but also by AR-C69931MX (Fig. 1C). In addition, we found that a different P2Y12 antagonist, 2MeSAMP, was as efficient as AR-C69931MX in preventing ADP-induced activation of PKC (Fig. 1C). In a dose-dependent analysis, we found that even when platelets were stimulated with doses of ADP as high as 50 μm, pleckstrin phosphorylation was completely prevented by blockade of the P2Y12 receptor (Fig. 1D). These results demonstrate that co-stimulation of both P2Y1 and P2Y12 receptors by ADP is absolutely required for activation of PKC.
In platelets, the Gi-coupled P2Y12 receptor is responsible for inhibition of adenylyl cyclase and for activation of phosphatidylinositol 3-kinase (12–16). Fig. 1E shows that when P2Y12 receptor was blocked by AR-C69931MX or 2MeSAMP, direct inhibition of adenylyl cyclase by dideoxyadenosine did not restore ADP-induced pleckstrin phosphorylation. By contrast, PKC-directed protein phosphorylation in ADP-stimulated platelets was prevented by the phosphatidylinositol 3-kinase inhibitors LY294002 and wortmannin (Fig. 1E). These results indicate that the P2Y12 receptor contributes to the regulation of PKC through the phosphatidylinositol 3-kinase-dependent pathway.
Similarly to the P2Y1 receptor for ADP, the platelet TxA2 receptor, TPα, is coupled to Gq and is able to stimulate PLC (1, 2). Nevertheless, platelet aggregation by TxA2 or by its stable analogue U46619 requires concomitant stimulation of the Gi-coupled P2Y12 receptor by secreted ADP (7, 22). We investigated whether the P2Y12 receptor participates in PKC regulation in U46619-activated platelets. In 32P-labeled cells, U46619 induced a stronger and more sustained phosphorylation of pleckstrin than ADP, which, however, was severally impaired when the P2Y12 receptor was blocked by AR-C69931MX (Fig. 2A). Moreover, inhibition of U46619-induced pleckstrin phosphorylation in AR-C69931MX- or 2MeSAMP-treated platelets was confirmed by the immunoblotting analysis with the anti-phospho(Ser) PKC substrates antibody (Fig. 2B). Incidentally, we noticed that the analysis of pleckstrin phosphorylation by immunoblotting with anti-phospho(Ser) PKC substrates antibody, which certainly generates stronger signals than the autoradiography approach, does not allow a reliable comparison of the strength of the response to different agonists. Although this observation has no impact on the present study, it certainly should be taken into consideration when choosing to adopt this technique. On stimulation with U46619, but not with ADP, washed platelets may form aggregates, and outside-in signaling through integrin αIIbβ3 may contribute to PLC activation and PKC stimulation. To rule out the possibility that the inhibitory effect of P2Y12 antagonists on U46619-induced pleckstrin phosphorylation was a consequence of the inhibition of aggregation, comparative analysis were performed in the absence and presence of the integrin antagonist RGDS. Fig. 2C shows that even under non-aggregating conditions U46619-induced pleckstrin phosphorylation was inhibited by AR-C69931MX. A dose-dependent analysis revealed that inhibition of pleckstrin phosphorylation by blockade of the P2Y12 receptor was still evident when platelets were stimulated with higher doses of U46619 (Fig. 2D). In all the experiments performed, we noticed that whereas inhibition of pleckstrin phosphorylation was constantly complete upon stimulation with ADP, some relevant variability was observed on stimulation with U46619 (see for instance, Figs. 2, B and C). However, an accurate quantitative analysis of several different experiments revealed that the inhibitory effects of the antagonists of the P2Y12 receptor on U46619-induced pleckstrin phosphorylation was relevant, as it accounted for about 70–80% and was statistically significant (Fig. 2E).
FIGURE 2.
Stimulation of the P2Y12 receptor by ADP is required for U46619-induced pleckstrin phosphorylation. Analysis of pleckstrin phosphorylation induced by U46619 was performed both by autoradiography with 32P-labeled platelets (panel A) and by immunoblotting with the anti-phospho(Ser) PKC substrates antibody (panels B and C). bas, untreated. Platelets were stimulated with 1μm U46619 for the indicated times (panel A) or for 1 min (panels B and C) in the absence or presence of receptor antagonists or 1 mm RGDS peptide, as indicated. In panel D platelets were stimulated with the indicated concentrations of U46619 in the absence or presence of the P2Y12 receptor antagonist AR-C69931MX (1 μm) as indicated. The arrow on the right indicates the position of the 47-kDa protein pleckstrin. Quantification of pleckstrin phosphorylation induced by U46619 was performed by densitometric analysis of seven different experiments and is reported in panel E. Pleckstrin phosphorylation induced by U46619 in the absence of inhibitors was considered as 100%. The results are the mean ± S.D. of seven different experiments (*, p < 0.01 versus control).
The P2Y12 Receptor Does Not Influence Gq-mediated Activation of Phospholipase C Induced by U46619—The effect of P2Y12 antagonists on the PKC activity could reflect a cross-talk between Gi and Gq at the level of PLC activation. Although ADP is clearly able to induce PLC activation under our experimental conditions, as revealed by measurement of PKC activation (Fig. 1) and intracellular Ca2+ mobilization (data not shown), we have been unable to reliably measure agonist-induced accumulation of inositol phosphates or DAG, probably because of the weakness of the response (data not shown), as suggested by early studies (30). Therefore, we took advantage from the evidence that the P2Y12 receptor is also implicated in PKC activation induced by U46619, which is a stronger activator of PLC. Using [3H]inositol- or [3H]arachidonic acid-labeled platelets, we found that neither accumulation of inositol phosphates nor production of DAG was affected by AR-C69931MX in U46619-treated platelets (Fig. 3A). Signals downstream of PLC branch into IP3-Ca2+ and DAG-PKC pathways. Although DAG-mediated activation of PKC was almost completely suppressed by the antagonists of P2Y12 receptor (Fig. 2), we found that, under the same conditions, IP3-mediated mobilization of intracellular Ca2+ was not affected (Fig. 3B). Altogether our results indicate that blockade of the P2Y12 receptor does not likely affect Gq-mediated PLC activation in U46619-stimulated platelets.
FIGURE 3.
Analysis of phospholipase C activation and intracellular Ca2+ mobilization. A, platelets were preincubated with buffer (black bars) or with 1 μm AR-C69931MX (white bars) and then stimulated with 1 μm U46619 for 1 min. Accumulation of DAG was measured using [3H]arachidonic acid-labeled platelets, whereas myo-[3H]inositol-labeled platelets were used to measure agonist-induced accumulation of soluble inositol phosphates (IP). Results are reported as raw measured DPM after subtraction of the radioactivity of non-stimulated samples and are the means ± S.D. of three different experiments. B, intracellular Ca2+ concentration in Fura-2-loaded platelets stimulated with 1 μm U46619 in the absence (none) or presence of apyrase or antagonists of the P2Y12 receptor as indicated. In resting platelets, the cytosolic concentration of Ca2+ was 50 ± 9 nm (n = 46). Results are the means ± S.D. of 5–6 different experiments.
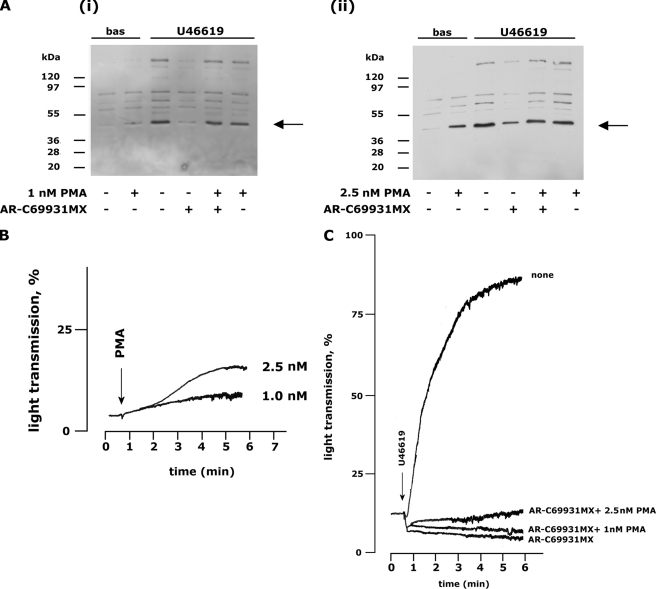
Restoration of PKC Activity Does Not Overcome the Inhibition of Platelet Aggregation Induced by P2Y12 Receptor Antagonists—It has been previously shown that both ADP- and U46619-induced platelet aggregation can be abrogated by blockade of the Gi-coupled P2Y12 receptor (5, 7). We addressed the possibility that this effect could be a consequence of the impaired PKC activation here described, and we analyzed whether restoration of PKC activity by subthreshold doses of PMA could overcome the inhibitory effect of AR-C69931MX. Using U46619-stimulated platelets like a model, we found that treatment with 1 nm PMA did not result in either pleckstrin phosphorylation or platelets aggregation, whereas 2.5 nm PMA induced a weak activation of PKC, associated to a very modest platelet aggregation (Figs. 4A and 4B). Fig. 4A shows that both 1 and 2.5 nm PMA were able to synergize with U46619 and restored normal PKC activation upon blockade of the P2Y12 receptor by AR-C69931MX. However, inhibition of U46619-induced platelet aggregation by AR-C69931MX was not overcome by either 1 or 2.5 nm PMA (Fig. 4C) despite normal PKC activation.
FIGURE 4.
Subthreshold concentrations of PMA restore agonist-induced pleckstrin phosphorylation but not platelet aggregation. A, analysis of pleckstrin phosphorylation evaluated by immunoblotting with the anti-phospho(Ser) PKC substrates antibody upon incubation with 1 nm or 2.5 nm PMA in the absence or presence of 1μm AR-C69931MX as indicated. Stimulation was with 1μm U46619 for 1 min. bas, untreated. B, analysis of platelet aggregation induced by 1 or 2.5 nm PMA. C, platelet were stimulated with 1 μm U46619 in the absence (none) or in the presence of 1 μm AR-C69931MX alone or in combination with 1 or 2.5 nm PMA as indicated on the right. The aggregation traces reported are representative of at least three different experiments.
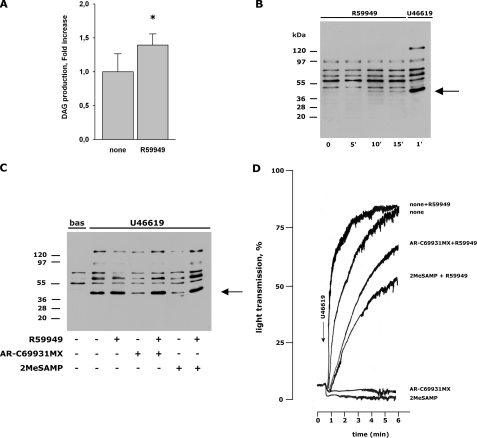
Inhibition of Diacylglycerol Kinase Bypasses the Need of P2Y12 Stimulation for Agonist-induced PKC Activation and Platelet Aggregation—The endogenous PKC activator DAG is typically metabolized and neutralized through phosphorylation by DGK, which, therefore, may indirectly regulate PKC function. To investigate whether DGK could be targeted by P2Y12, we analyzed the effect of a cell-permeable DGK inhibitor, R59949, on AR-C69931MX- and 2MeSAMP-mediated inhibition of platelet aggregation and pleckstrin phosphorylation induced by U46619. R59949 alone promoted only a small, but statistically significant increase of the levels of endogenous DAG, and caused a weak activation of PKC after 10 min (Fig. 5, A and B) but did not cause any detectable platelet aggregation (data not shown). Preincubation of platelets with R59949 for 5 min before stimulation with U46619 resulted in a very small increase of pleckstrin phosphorylation and in a modest potentiation of aggregation (Fig. 5, C and D). However, Fig. 5C shows that inhibition of PKC-directed protein phosphorylation by AR-C69931MX or 2MeSAMP in U46619-stimulated platelets was completely overcome by R59949. Similar results were also obtained when platelets were stimulated with ADP (data not shown). Interestingly, in the presence of R59949, U46619 induced evident platelet aggregation despite the inhibition of Gi signaling by AR-C69931MX or 2MeSAMP (Fig. 5D). These results indicate that the need of P2Y12 stimulation for Gq-initiated platelet aggregation can be bypassed by the inhibition of DGK rather than by activation of PKC.
FIGURE 5.
Inhibition of pleckstrin phosphorylation and platelet aggregation by P2Y12 receptor antagonists is overcome by inhibition of diacylglycerol kinase. A, accumulation of DAG in platelets treated with 1 μm R59949 for 10 min. The levels of DAG were measured using [3H]arachidonic acid-labeled platelets. The results are the mean ± S.D. of three different experiments (*, p < 0.05 versus control). B, analysis of pleckstrin phosphorylation in platelets incubated with 1 μm of the DGK inhibitor R59949 for increasing times. As control, pleckstrin phosphorylation induced by 1 μm U46619 is reported. C, platelets were incubated without or with 1 μm R59949, as indicated, left untreated (bas), or stimulated with 1 μm U46619 for 1 min in the absence or presence of the P2Y12 antagonists AR-C69931MX or 2MeSAMP (1 μm each), as indicated. Phosphorylation of pleckstrin was evaluated by immunoblotting with the anti-phospho(PKC) substrates antibody. D, platelets were treated with buffer (none) or with the P2Y12 antagonists AR-C69931MX or 2MeSAMP alone or in combination with the DGK inhibitor R59949 in a lumiaggregometer, and aggregation was initiated by the addition of 1 μm U46619 as indicated by the arrow. The figure reports aggregation traces representative of at least three different experiments.
Beside activating PKC, PLC-generated DAG regulates other intracellular effectors, including CalDAG-GEFI, a nucleotide exchange factor for the small GTPase Rap1 (20, 21), which is important for integrin αIIbβ3 activation and platelet aggregation (19). As previously reported (13), we confirmed that U46619-induced activation of Rap1b was prevented by AR-C69931MX and 2MeSAMP (Fig. 6, A and B). However, we also found that the DGK inhibitor R59949, but not the PKC activator PMA, was able to partially overcome the effect of the P2Y12 receptor blockade on agonist-induced activation of Rap1 (Fig. 6, A and B). Because the recovery of Rap1b activity was not complete in the presence of R59949, we performed an accurate quantitative analysis of immunoblots from eight different experiments. The results are reported in Fig. 6C and show that in the presence of the P2Y12 receptor antagonists, U466619-induced activation of Rap1b was restored by about 50% by R59949 and that this effect resulted statistically significant.
FIGURE 6.
Inhibition of U46619-induced Rap1b activation by the P2Y12 receptor antagonists is reversed by R59949 but not by PMA. Rap1b activation induced by stimulation of platelets with 1 μm U46619 for 1 min was analyzed by the pulldown assay with GST-RalGDS-RBD followed by immunoblotting with anti Rap1 antibody. In panel A platelets were preincubated with either 1 μm AR-C69931MX or 1 μm 2MeSAMP, and the effect of 1 μm R59949 was analyzed. TOT, total. In panel B some samples were incubated with AR-C69931MX, and the effect of 1 nm PMA is reported. The upper panels show the active form of Rap1b (GTP-Rap1b), whereas the lower panels report the level of total Rap1b present in the platelet lysates. The levels of Rap1b activation were quantified by densitomeric analysis of the immunoblots, and the results are summarized in panel C. The amount of active Rap1b in U46619-stimulated platelets in the absence of any inhibitor was taken as 100%. Data are the mean ± S.D. of eight different experiments. The recovery of Rap1b activity caused by inhibition of DGK with R59949, albeit partial, was found statistically significant.
Finally we investigated the interplay between Gi-dependent signaling and DAG-mediated effects on platelet activation using the cell-permeable DAG analogue, DiC8. A weak platelet aggregation was observed upon treatment of platelets with 3.5 μm but not with 2 μm DiC8 (Fig. 7A). Concomitant stimulation of P2Y12 receptor through the addition of ADP in the presence of MRS2179 significantly potentiated the effect of DiC8 on platelet aggregation (Fig. 7A). DiC8-induced PKC activation was dose-dependent and occurred in a range of concentrations much lower than those required for aggregation (Fig. 7B) We found that the faint pleckstrin phosphorylation detectable at 100 nm DiC8 was clearly potentiated by the DGK inhibitor R59949 (Fig. 7Bi). TLC analysis of the lipids extracted from 32P-labeled platelets revealed that exogenous DiC8 was actively phosphorylated to DiC8-PA by a DGK activity in intact cells and that this process was inhibited upon platelet incubation with R59949 (Fig. 7C). Similarly to R59949, we found that stimulation of the P2Y12 receptor by ADP in the presence of MRS2179 resulted in a potentiation of pleckstrin phosphorylation induced by exogenous DiC8 at all the doses analyzed (Fig. 7Bii). Concomitantly, an evident inhibition of DiC8 conversion to DiC8-PA in intact platelets was observed upon stimulation with ADP. This effect of ADP in 32P-labeled cells was dose-dependent and was prevented by AR-C69931MX but not by MRS2179, indicating that it was mediated by activation of the P2Y12 receptor (Fig. 7C). These results support the hypothesis that Gi-dependent signaling potentiates the effect of endogenous DAG by limiting its conversion to PA.
FIGURE 7.
Potentiation of DiC8-mediated platelet activation by stimulation of P2Y12 receptor. A, platelet aggregation was measured upon addition of 2 μm (i) or 3.5 μm (ii) DiC8 in the presence of buffer (none) or 10 μm ADP and 200 μm MRS2179, as indicated on the right. The reported traces are representative of at least three different experiments producing comparable results. B, platelets were stimulated for 1 min with 0–500 nm DiC8, as indicated at the bottom,in the absence or presence of 1 μm R59949 (i) or 10 μm ADP and 200 μm MRS2179 (ii). PKC-directed protein phosphorylation was analyzed by immunoblotting with an anti-phospho(Ser) PKC substrates antibody. The position of the 47-kDa pleckstrin is indicated by the arrow on the right. C, 32P-labeled platelets were treated with 500 nm DiC8 or with an equivalent volume of DMSO, as indicated on the top of the panel (i), and then left untreated (none) or stimulated with the indicated doses of ADP for 1 min in the absence or in the presence of 200 μm MRS2179, 1 μm AR-C69931MX, or 1 μm R59949, as indicated. Lipids were extracted, separated by TLC, and visualized by autoradiography. A representative image is reported in panel i. Accumulation of DiC8-derived PA was quantified by densitometric analysis of the autoradiographies, and the results are reported in panel (ii). The production of DiC8-PA in the presence of ADP or ADP plus antagonists is reported as percentage of that observed in samples treated with DiC8 alone. Results are the means ± S.D. of four different experiments. *, p < 0.01 versus non-stimulated platelets.
DISCUSSION
In this work we have documented a novel interplay between Gq and Gi stimulation in human platelets as we have shown that activation of the Gi-coupled P2Y12 receptor is essential for complete PKC activation in response to stimulation of Gq-coupled receptors by ADP or U46619. The cross-talk between Gq and Gi is most likely to occur at the level of DAG metabolism, which plays a more crucial role than PKC itself in the regulation of platelet aggregation.
Gq-coupled receptors are able to stimulate PLCβ isoforms, which hydrolyze phosphatidylinositol 4,5-bisphosphate, and produce two intracellular messengers, IP3 and DAG, which mediate the release of Ca2+ from internal stores and the activation of PKC, respectively (3). According to this paradigm, we have found that blockade of ADP binding to the Gq-coupled P2Y1 receptor abolished PKC-mediated protein phosphorylation. Unexpectedly, however, we have found that antagonists of the Gi-coupled P2Y12 receptor totally prevented PKC activation as well. Therefore, stimulation of the Gq is necessary but not sufficient for ADP to induce PKC activation, and the concomitant stimulation of Gi is also required. Importantly, Gi-mediated regulation of PKC is not limited to ADP-stimulated platelets but is a common event when Gq is stimulated. It has been previously shown that stimulation of the Gq-coupled TPα receptor by the TxA2 analogue U46619 activates PLCβ but is not sufficient to trigger platelet aggregation unless activation of aGi-dependent pathway by binding of secreted ADP to the P2Y12 receptor occurs (2, 7, 22). In this context we have found that, similarly to what observed in ADP-stimulated platelets, the P2Y12 antagonists AR-C69931MX and 2MeSAMP prevented PKC-mediated protein phosphorylation induced by U46619. Therefore, even in this alternative experimental model, PKC activation is regulated by concomitant signals through Gq- and Gi-coupled receptors. Direct measurement of inositol phosphate accumulation DAG production in U46619-stimulated platelets indicates that although Gi regulates PKC activation, PLC is probably not affected by blockade of the P2Y12 receptor. Moreover, we also found that U46619-induced, IP3-dependent Ca2+ mobilization from intracellular stores was not affected by ADP scavengers or antagonists (Fig. 3B and Ref. 11) Previous works reported that ADP-induced Ca2+ mobilization is reduced by blockade of the P2Y12 receptor, suggesting a cross-talk between Gq and Gi at the level of PLC (31, 32). Using ADP as platelet agonist, we observed a comparable partial inhibition of Ca2+ release by AR-C69931MX (data not shown), but importantly, under the same conditions, pleckstrin phosphorylation was completely suppressed, indicating that Gi signaling exerts an additional specific control on the DAG-PKC pathway. Moreover, it is of note that most of our study was performed with U46619-strimulated platelets under conditions in which PLC activation and Ca2+ mobilization were found to occur normally.
We have also addressed the fundamental question as to the functional implication of PKC regulation through P2Y12 receptor on platelet aggregation using the membrane-permeable PKC activator PMA. Subthreshold doses of PMA were actually able to restore normal PKC activity in U46619 (and ADP)-stimulated platelets despite P2Y12 receptor inhibition. However, under these conditions platelets were still unable to aggregate. Therefore, blockade of P2Y12 receptor still prevents U46619-induced aggregation even if PKC is normally activated. This indicates that although PKC activity is regulated by Gi, this is not the element of Gi-dependent pathway critical for platelet aggregation. A different effector similarly targeted by P2Y12 receptor must be responsible for the independent but concomitant regulation of both PKC and platelet aggregation. We provide here evidence that this target may be the PKC upstream regulator DAG. DAG is generated by activated PLC but is subsequently phosphorylated to PA by the enzyme DGK, which therefore turns off the effects of DAG as second messenger (33). In this work we document that pharmacological inhibition of DGK restores the agonist-induced phosphorylation of PKC substrates prevented by antagonists of the P2Y12 receptor and, in parallel, allows platelets to undergo aggregation. Importantly, even AR-C69931MX-promoted inhibition of Rap1 activation is overcome by pharmacologic inhibition of DGK. This finding is consistent with the notion that the main nucleotide exchange factor responsible for the activation of Rap1 expressed in platelets is the CAlDAG-GEFI, which is directly activated by DAG independently of PKC and is required for platelet aggregation (20, 21). Our results demonstrate that the need for Gi stimulation to trigger full platelet response can be bypassed by direct inhibition of DGK with R59949. Moreover, we have also directly demonstrated that stimulation of Gi hampers the conversion of exogenous DAG to PA, thus extending and potentiating its effects on platelets. It is, therefore, possible that whereas DAG is generated by PLC activated through the Gq-dependent pathway, its effective accumulation requires inhibition of DGK through Gi.
Although DGK family enzymes have been known for a long time, their biology has recently gained renewed interest (33, 34). At least 10 different DGK isoforms have been identified which in many cell types are essential for proliferative and migratory signaling (34, 35). In platelets, early works had reported that inhibitors of DGK are able to potentiate agonist-induced secretion and aggregation, but the mechanism of this action has never been investigated in detail (36–38). Unfortunately, despite all the efforts, we have been unable to demonstrate a direct regulation of endogenous DGK by Gi in agonist-stimulated platelets. Platelets express multiple DGK isoforms (33, 39), but neither specific antibodies of acceptable quality nor selective inhibitors for the different isoforms are commercially available. We have tried to measure the enzymatic activity of immunoprecipitated DGKα, one of the R59946-sensitive DGK isoforms expressed in platelets, but we have been unable to reveal any difference upon cell stimulation (data not shown). Similarly, we have been unable to detect any stimulation-dependent difference in the total DGK activity measured either in whole cell lysates, in membranes, or in cytosolic fractions (data not shown). However, it must be considered that all these measurements provide a value of the global amount of these metabolites in the cell, as it results from the comprehensive action of all the expressed isoforms, and they are neither indicative of the contribution of single isoforms nor informative as to the possibly different subcellular distribution of DAG. An increasing number of evidence in nucleated cells has indicated that DKG isoforms undergo complex but crucial spatial regulation (33–35). This may also be relevant in platelets, and the P2Y12-mediated inhibition of a particular DGK isoform may transiently occur in specific subcellular microdomains, allowing a local increase of DAG sufficient to efficiently trigger cellular responses. Nevertheless, we cannot rule out the possibility that Gi-dependent pathways target intracellular effectors different from DGK, producing effects equivalent to those produced by DKG inhibition and, thus, reverted by R59949. This possibility as well as the possible involvement and contribution of selected isoforms of DGK is a challenging task that certainly deserves further investigation but is pending on the future availability of suitable pharmacologic and genetic tools.
In conclusion, our results shed new light into the signaling pathway activated downstream of the P2Y12 receptor for ADP and required for integration of the Gq-dependent signals for platelet aggregation, reveal a novel role for Gi in the regulation of PKC activity, and point to the importance of DAG-metabolizing enzymes such as DGK in the regulation of platelet activation.
This work was supported by grants from the Ministero dell'Istruzione, Università e Ricerca Scientifica (MIUR, PRIN 2006), from the University of Pavia, and from the Consorzio Interuniversitario Biotecnologie. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TxA2, thromboxane A2; DiC8, sn-1,2 dioctanoylglycerol; PLC, phospholipase C; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphosphate; PKC, protein kinase C; DGK, diacylglycerol kinase; PMA, phorbol 12-myristate 13-acetate; PIPES, 1,4-piperazinediethanesulfonic acid; PA, phosphatidic acid.
References
- 1.Woulfe, D. S. (2005) J. Thromb. Haemost. 3 2193–2200 [DOI] [PubMed] [Google Scholar]
- 2.Offermanns, S., Toombs, C. F., Hu, Y. H., and Simon, M. I. (1997) Nature 389 183–186 [DOI] [PubMed] [Google Scholar]
- 3.Rhee, S. G. (2001) Annu. Rev. Biochem. 70 281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorsam, R. T., and Kunapuli, S. P. (2004) J. Clin. Investig. 113 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin, J., and Kunapuli, S. P. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 8070–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckly, A., Gendrault, J.-L., Hechler, B., Cazenave, J.-P., and Gachet, C. (2001) Thromb. Haemostasis 85 694–701 [PubMed] [Google Scholar]
- 7.Paul, B. Z., Jin, J., and Kunapuli, S. P. (1999) J. Biol. Chem. 274 29108–29114 [DOI] [PubMed] [Google Scholar]
- 8.Yang, J., Wu, J., Kowalska, M. A., Dalvi, A., Prevost, N., O'Brien, P. J., Manning, D., Poncz, M., Lucki, I., Blendy, J. A., and Brass, L. F. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 9984–9989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollopeter, G., Jantzen, H. M., Vincent, D., Li, G., England, L., Ramakrishnan, V., Yang, R.-B., Nurden, P., Nurden, A., Julius, D., and Conley, P. B. (2001) Nature 409 202–207 [DOI] [PubMed] [Google Scholar]
- 10.Foster, C. J., Prosser, D. M., Agans, J. M., Zhai, Y., Smith, M. D., Lachwicz, J. E., Zhang, F. L., Gustafson, E., Monsma, F. J., Wiekowski, M. T., Abbondanzo, S. J., Cook, D. N., Bayne, M. L., Lira, S. A., and Chintala, M. S. (2001) J. Clin. Investig. 107 1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gachet, C. (2008) Thromb. Haemostasis 99 466–472 [DOI] [PubMed] [Google Scholar]
- 12.Daniel, J. L., Dangelmaier, C., Jin, J., Kim, Y. B., and Kunapuli, S. P. (1999) Thromb. Haemostasis 82 1322–1326 [PubMed] [Google Scholar]
- 13.Lova, P., Paganini, S., Sinigaglia, F., Balduini, C., and Torti, M. (2002) J. Biol. Chem. 277 12009–12015 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch, E., Bosco, O., Tropel, P., Laffargue, M., Calvez, R., Altruda, F., Wymann, M., and Montrucchio, G. (2001) FASEB J. 15 2019–2021 [DOI] [PubMed] [Google Scholar]
- 15.Jackson, S. P., Schoenwaelder, S. M., Goncalves, I., Nesbitt, W. S., Yap, C. L., Wright, C. E., Kenche, V., Anderson, K. E., Dopheide, S. M., Yuan, Y., Sturgeon, S. A., Prabaharan, H., Thompson, P. E., Smith, G. D., Shepherd, P. R., Daniele, N., Kulkarni, S., Abbott, B., Saylik, D., Jones, C., Lu, L., Giuliano, S., Hughan, S. C., Angus, J. A., Robertson, A. D., and Salem, H. H. (2005) Nat. Med. 11 507–514 [DOI] [PubMed] [Google Scholar]
- 16.Woulfe, D., Jiang, H., Mortensen, R., Yang, J., and Brass, L. F. (2002) J. Biol. Chem. 277 23382–23390 [DOI] [PubMed] [Google Scholar]
- 17.Cosemans, J. M., Munnix, I. C., Wetzker, R., Heller, R., Jackson, S. P., and Heemskerk, J. W. (2006) Blood 108 3045–3052 [DOI] [PubMed] [Google Scholar]
- 18.Lova, P., Paganini, S., Hirsch, E., Barberis, L., Wymann, M., Sinigaglia, F., Balduini, C., and Torti, M. (2003) J. Biol. Chem. 278 131–138 [DOI] [PubMed] [Google Scholar]
- 19.Bertoni, A., Tadokoro, S., Eto, K., Pampori, N., Parise, L., White, G. C., and Shattil, S. J. (2002) J. Biol. Chem. 277 25715–25721 [DOI] [PubMed] [Google Scholar]
- 20.Crittenden, J. R., Bergmeier, W., Zhang, Y., Piffath, C. L., Liang, Y., Wagner, D. A., Housman, D. E., and Graybiel, A. M. (2004) Nat. Med. 10 982–986 [DOI] [PubMed] [Google Scholar]
- 21.Bernardi, B., Guidetti, G. F., Campus, F., Crittenden, J. R., Graybiel, A. M., Balduini, C., and Torti, M. (2006) Blood 107 2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dangelmaier, C., Jin, J., Smith, J. B., and Kunapuli, S. P. (2001) Thromb. Haemostasis 85 341–348 [PubMed] [Google Scholar]
- 23.Kauffenstein, G., Bergmeier, W., Eckly, A., Ohlmann, P., Leon, C., Cazenave, J. P., Nieswandt, B., and Gachet, C. (2001) FEBS Lett. 505 281–290 [DOI] [PubMed] [Google Scholar]
- 24.Campus, F., Lova, P., Bertoni, A., Sinigaglia, F., Balduini, C., and Torti, M. (2005) J. Biol. Chem. 280 24386–24395 [DOI] [PubMed] [Google Scholar]
- 25.Larson, M. K., Chen, H., Kahn, M. L., Taylor, A. M., Fabre, J.-E., Mortensen, R. M., Conley, P., and Parise, L. V. (2003) Blood 101 1409–1415 [DOI] [PubMed] [Google Scholar]
- 26.Nieswandt, B., Bergmeier, W., Eckly, A., Schulte, V., Ohlmann, P., Cazenave, J.-P., Zirngibl, H., Offermanns, S., and Gachet, C. (2001) Blood 97 3829–3835 [DOI] [PubMed] [Google Scholar]
- 27.Torti, M., Balduini, C., Ramaschi, G., and Sinigaglia, F. (1992) Cell Biochem. Funct. 10 53–59 [DOI] [PubMed] [Google Scholar]
- 28.Lova, P., Campus, F., Lombardi, R., Cattaneo, M., Sinigaglia, F., Balduini, C., and Torti, M. (2004) J. Biol. Chem. 279 25299–25306 [DOI] [PubMed] [Google Scholar]
- 29.Tyers, M., Rachubinski, R. A., Stewart, M. I., Varrichio, A. M., Shorr, R. G. L., Haslam, R. J., and Harley, C. B. (1988) Nature 333 470–473 [DOI] [PubMed] [Google Scholar]
- 30.Vickers, J. D., Kinlough-Rathbone, R. L., Packham, M. A., and Mustard, J. F. (1990) Eur. J. Biochem. 193 521–528 [DOI] [PubMed] [Google Scholar]
- 31.Sage, S. O., Yamoah, E. H., and Heemskerk, J. W. (2000) Cell Calcium 28 119–126 [DOI] [PubMed] [Google Scholar]
- 32.Hardy, A. R., Jones, M. L., Mundell, S. J., and Poole, A. W. (2004) Blood 104 1745–1752 [DOI] [PubMed] [Google Scholar]
- 33.Luo, B., Regier, D. S., Prescott, S. M., and Topham, M. K. (2004) Cell. Signal. 16 983–989 [DOI] [PubMed] [Google Scholar]
- 34.Merida, I., Avina-Flores, A., and Merino, E. (2008) Biochem. J. 409 1–18 [DOI] [PubMed] [Google Scholar]
- 35.Baldanzi, G., Cupriti, S., Chianale, F., Gnocchi, V. F., Rainero, E., Porporato, P. E., Filigheddu, N., van Blitterswijk, W. J., Parolini, O., Bussolino, F., Sinigaglia, F., and Graziani, A. (2008) Oncogene 27 942–956 [DOI] [PubMed] [Google Scholar]
- 36.Bishop, W. R., and Bell, R. M. (1986) J. Biol. Chem. 261 12513–12519 [PubMed] [Google Scholar]
- 37.Packham, M. A., Livne, A. A., Ruben, D. H., and Rand, M. L. (1993) Biochem. J. 209 849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunn, D. L., and Watson, S. P. (1987) Biochem. J. 243 809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yada, Y., Ozeki, T., Kanoh, H., and Nozawa, Y. (1990) J. Biol. Chem. 265 19237–19243 [PubMed] [Google Scholar]