Abstract
Here we report the crystal structure at ≈4-Å resolution of a selectively proteolyzed bovine fibrinogen. This key component in hemostasis is an elongated 340-kDa glycoprotein in the plasma that upon activation by thrombin self-assembles to form the fibrin clot. The crystals are unusual because they are made up of end-to-end bonded molecules that form flexible filaments. We have visualized the entire coiled-coil region of the molecule, which has a planar sigmoidal shape. The primary polymerization receptor pockets at the ends of the molecule face the same way throughout the end-to-end bonded filaments, and based on this conformation, we have developed an improved model of the two-stranded protofibril that is the basic building block in fibrin. Near the middle of the coiled-coil region, the plasmin-sensitive segment is a hinge about which the molecule adopts different conformations. This segment also includes the boundary between the three- and four-stranded portions of the coiled coil, indicating the location on the backbone that anchors the extended flexible Aα arm. We suggest that a flexible branch point in the molecule may help accommodate variability in the structure of the fibrin clot.
A complete understanding of blood clotting requires detailed information on the structure of the whole fibrinogen molecule and its thrombin-activated assembly into the fibrin clot. Crystallographic analysis of the molecule has been hindered, however, by its elongated, complex and flexible structure. Soluble fibrinogen (340 kDa) is a 450-Å long disulfide-linked dimer of three nonidentical polypeptide chains (termed Aα, Bβ, and γ). The N termini of the six chains from the two halves of the molecule come together to form a small globular domain (the so-called disulfide knot) in the center (see gray in Fig. 1 a and b). The C termini of each of the three chains end in globular domains, those of the Bβ and γ chains being located at the ends of the molecule (see green and red, respectively, in Fig. 1 a and b) and those of the Aα chains, the αC domains (proteolytically removed in our modified fibrinogen), generally interacting with each other adjacent to the central domain. Except for an extended flexible portion of the Aα chain immediately preceding the αC domain, the regions between the globular domains in each half-molecule are mainly α-helical and form irregular coiled coils bracketed by disulfide rings (for review, see ref 1). Until recently, single molecules of fibrinogen have been visualized in electron microscopic studies only at very low resolution (2–6) and by image processing of crystals and microcrystals that allowed a correlation of the domains (6) in the molecule with specific regions of the amino acid sequence (7–13). Fibrin assembly has been shown to occur by the thrombin cleavage of two peptides that lead to the association of fibrin monomers into two-stranded half-staggered protofibrils (14, 15); here the major contacts occur between the central and end domains on adjacent filaments and between the end domains within the same filament. The protofibrils then associate laterally to form thicker fibers. The clots, which are strengthened by covalent crosslinking of the C terminal end-to-end bonded γ domains (and at a slower rate of the αC domains), are later dissolved by plasmin primarily by proteolysis in the coiled-coil regions (16, 17).
Figure 1.
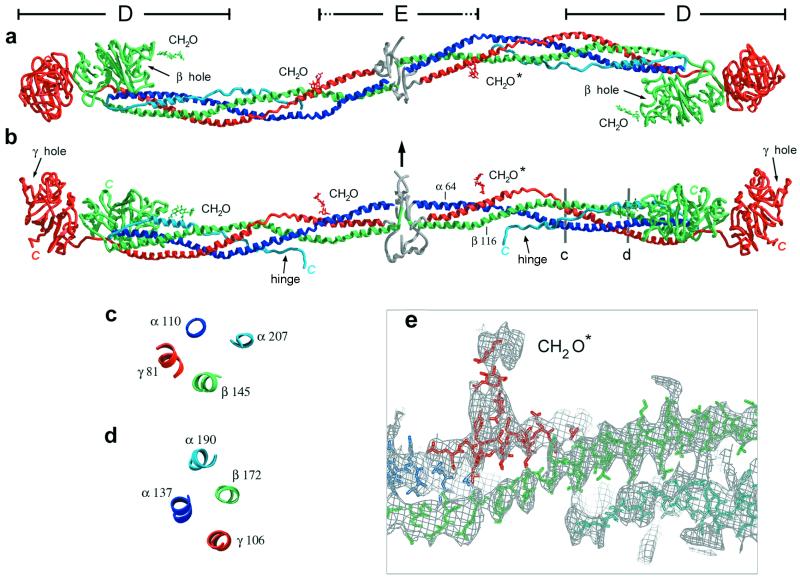
Three perpendicular views of the modified bovine fibrinogen molecule. Color scheme (for all figures) is blue for the Aα-chain, green for the Bβ-chain, and red for the γ-chain. The antiparallel portion of the Aα-chain is shown in light blue. The chains in the central N-terminal disulfide knot region cannot be traced and their general location is indicated in gray. Carbohydrates are indicated by CH2O. (Bovine residue numbers are indicated, see Methods.) (a) View showing the sigmoidal shape of the coiled-coil axis. (b) View perpendicular to that in a indicating the molecular planarity. Arrow indicates approximate 2-fold axis of the molecule, which is nearly perpendicular to the plane of the sigmoidal coiled coil. Observed C termini are indicated in italics. (c and d) Cross-sectional views of the four-stranded coiled coil at locations indicated in b. The four-stranded coiled coil is quite asymmetric and may be better described as an overlapping pair of three-stranded coiled coils that share the parallel Aα- and Bβ-chains. (The γ and antiparallel Aα-chains do not contact one another.) (e) Magnified view of b showing part of the coiled coil superimposed on an electron density map (see Methods). A polyalanine model in this segment of the antiparallel Aα-chain density (Bottom, light blue) is shown. Regular helical density is observed for the parallel chains.
Two approaches have been taken in the crystallographic analysis of fibrinogen. One is the study of almost the whole molecule, the other of key proteolytic fragments or expressed peptides. Native fibrinogen has been difficult to crystallize. Treatment with a protease from Pseudomonas aeruginosa (18) removes part of the flexible C-terminal region of the Aα chain and some of the Bβ chain (about 15% of the molecule) (see Methods). The resulting modified molecule has high clottability and forms various microcrystals as well as crystals of flexible filaments (19, 20). Until recently, because of severe technical problems with the high solvent-content crystals, the x-ray data could not be phased. A low-resolution (≈18 Å) model showing domain substructure was derived, however, by using coordinated x-ray crystallography and cryo-electron microscopy (21) (P. Walian, personal communication).
Recently, high-resolution crystal structures have been determined for fragments of human fibrin(ogen), some of which were complexed with tetrapeptides derived from the thrombin-exposed N termini of the central region. The fragments studied include an expressed 30-kDa γ domain (22, 23), a proteolytically derived 86-kDa fragment D (24) consisting of the C-terminal β and γ globular domains and part of the coiled coil, and a fibrin degradation product containing the crosslinked D fragments (24, 25). (The structure of fragment E, the other major product of plasmin digestion, which consists of the dimeric central N-terminal portion of the molecule including the disulfide knot region between two short coiled-coil segments, has not yet been determined.) These studies have revealed two of the most important interactions that would occur at the DDE junction between three fibrin monomers in a protofibril: that of the α glycyl-prolyl-arginyl (GPR) “knob” derived from the central disulfide knot region bound in a pocket of the end γ domain (23, 24) and a slightly staggered (or “offset”) end-to-end interaction between crosslinked γ domains (24). They also have revealed the structure of the N-terminal glycyl-histidyl-arginyl (GHR) “knob” of the β chain bound in the C-terminal β domain receptor pocket (25). This interaction is implicated for the most part in slower lateral associations between protofibrils (26, 27). In addition, these studies have shown that the Aα chain reverses its direction at the C-terminal disulfide ring to form a fourth α-helical strand for at least the entire coiled coil of fragment D (24) and so are consistent with the location of the αC domain near the center of the fibrinogen molecule (3, 4, 28). Upon conversion to fibrin, this mobile domain also helps form lateral associations between protofibrils.
We now have determined the structure of a proteolytically modified bovine fibrinogen (≈285 kDa) to ≈4-Å resolution, by molecular replacement of the human fragment D structure (24) with x-ray data from cryopreserved crystals (see Methods). This procedure yielded electron density maps that reveal the paths of the individual α, β, and γ chains throughout the entire coiled coil of the molecule, including that of the E region and the connector between the D and E regions. (The density corresponding to the central disulfide knot region was, however, too weak and irregular to be accurately modeled.) This medium-resolution image of the structure is the basis for recognizing the major features reported here in Results. We then traced the chains of the coiled coil in the map by imposing essential physical constraints on this region of the molecule (see Methods). This level of detail in the structure of the coiled coil, together with the high-resolution structure of the D region, allows us to assign rather precisely the locations of key structural features in the (nearly) complete molecule. These findings reveal the relative orientation of the coiled-coil chains and the end domains in the dimeric molecule, conserved fibrin-like γ-γ interactions in the extended filament, and a critical flexible branch point at the primary plasmin-sensitive segment of the backbone. Taken together, these results have allowed us to develop an improved description for the basic protofibrillar unit in fibrin.
Methods
Crystallization.
Bovine fibrinogen used for crystallization was subjected to limited proteolysis with a lysine-specific protease from P. aeruginosa (Ps-1, Promega) (18). The resulting molecule (≈285 kDa) retains high clottability (19, 20) although sequence analysis of dissolved crystals indicates that the C-terminal third of the Aα chain (391–580) and the first 60 residues of the Bβ-chain (including the GHR sequence) have been removed. (Unless otherwise indicated, bovine residue numbering is used here. Bovine fibrinogen principally differs from human fibrinogen by insertions of three and seven residues at the N termini of the Aα and Bβ chains, respectively, except for the sequence after Aα 273 that includes multiple insertions and deletions.) The concentration of modified bovine fibrinogen typically was adjusted to ≈2.6 mg/ml in 10 mM Mes, 5 mM NaN3, 2 mM CaCl2, pH 6.2, and using the batch method and seeding at 4°C, anvil-shaped nearly 1-mm-sized crystals were obtained within 1 month from this crystallization buffer. The space group of these crystals is P21 with unit cell dimensions a = 135.1 Å, b = 98.1 Å, c = 175.0 Å, β = 92.0°, 1 molecule per asymmetric unit, and solvent content ≈70% (19–21). Essentially complete x-ray data sets to 6 Å were collected at 4°C as described (21). At 4°C, the crystals diffract beyond 6 Å primarily along the length of the molecule (in the [−3,0,1] direction).
Data Collection at 100 K.
Crystals were soaked for 1 hr in 2 M 2-methyl-2,4-pentanediol and crystallization buffer and flash-frozen in liquid-nitrogen cooled propane. The space group and unit cell of the flash-frozen crystals are P21, a = 178.3 Å, b = 96.1 Å, c = 214.0 Å, β = 94.0°, 2 molecules per asymmetric unit, and solvent content of ≈63%. Three native data sets were collected at 100 K (two at the Brookhaven National Synchrotron Light Source, Upton, NY, and one at the Cornell High Energy Synchrotron Source, Ithaca, NY) on Image plate and charge-coupled device detectors, and integrated with denzo (29). The data were scaled by using scala (30) and reduced by using massage (N.V., unpublished work). The three data sets were sufficiently isomorphous to be merged. The relatively low completeness of the data (see Table 1) in the higher-resolution shells is caused by the high degree of anisotropic diffraction. Nevertheless, crystals at 100 K showed better diffraction in the b* direction (between layers of fibrinogen molecules) than at 4°C.
Table 1.
Data collection and refinement statistics
| Data collection | |||||
| Temperature, K | 100 | ||||
| Space group | P21 | ||||
| Unit cell | 178.3 Å | 96.1 Å | 214 Å | β = 94.0° | |
| Molecules per asymmetric unit | 2 | ||||
| Unique reflections (redundancy) | 79,465 (4.42) |
| Low-resolution limit, Å | 106 | 106 | 10.7 | 4.4 | 3.6 |
| High-resolution limit, Å | 3.4 | 10.7 | 7.6 | 4.0 | 3.4 |
| Completeness*, % | 78.5 | 99.2 | 100 | 79.1 | 46.7 |
| I/σI | 7.1 | 15.2 | 12.4 | 5.0 | 2.6 |
| Rsym,† % | 7.5 | 3.9 | 4.7 | 13.0 | 28.0 |
| Model refinement at 10.0–3.5 Å | |||
| Sigma cutoff | None | ||
| Rfactor%,‡Rfree%§ | 26.3, 37.0 | ||
| rms bond length, Å; angles ° | 0.012, 1.83 | ||
| rms dihedrals, °; improper, ° | 28.1, 1.08 |
Data are anisotropic, see Methods.
† Rsym = Σhkl Σi | Ii-〈I〉| / Σhkl ΣiI, where 〈I〉 is the mean intensity of reflection hkl.
‡ Rfactor=Σhkl | | Fobs |-| Fcalc | | / Σhkl | Fobs |, where Fcalc and Fobs are, respectively, the calculated and observed structure factor amplitudes for reflection hkl.
§ Rfree is the same as Rfactor but calculated over the 5% randomly selected fraction of the reflection data not included in the refinement.
Structure Determination.
For phasing, we positioned the human fragment D structure (24) by using amore (31) into the 100-K data (r = 0.517, correlation coefficient = 44.1 for 10- to 5-Å data before any refinement). The molecular replacement solutions yielded the expected 450-Å length between the ends of the γ domains within a molecule; they also revealed a crystal packing of symmetry-related molecules with the same offset between γ domains as observed in crosslinked D dimer from fibrin. Noncrystallographically symmetry restrained positional minimization of the fragment D structure (x-plor, ref. 32) to 3.5 Å yielded a difference map into which we could model the coiled-coil region and manually construct a mask for the entire molecule. This mask was input (using the solin option) into dm (33) to improve the connectivity of the electron density by solvent flattening. (Automated mask generation in dm excluded the coiled-coil region from the protein mask.) Generally, 10–3.5 Å maps (but note anisotropy discussed above) with 3Fo-2Fc coefficients, sigmaa weighting (34), and the dm-modified calculated phases were used for model building. Continued iterative model building of the coiled coil by using o (35), combined with positional and group B factor refinement of the residues fitted with the least uncertainty (using noncrystallographically symmetry restraints only for the fragment D region) against data modified with an anisotropic B factor, was used to arrive at our present model.
Quality of Model and Density.
At the present resolution of our diffraction data, the structures of the bovine β and γ domains are similar to those observed in human fragment D. The only significant rebuilding performed in the D region was at the N-terminal regions of its coiled coil (especially bovine β162-β167), which in fragment D are relatively disordered (25). The constraints permitting the previously unknown portion of the trimeric coiled-coil backbone (i.e., that in fragment E and the connector between fragments E and D) to be traced include: the C termini of this region treated as extensions of the fragment D coiled coil, the N termini defined by the disulfide ring, and the positions of the intervening residues restrained by the observed regular helical nature of the density, the general positioning of hydrophobic residues in the coiled-coil core, and the carbohydrate attachment site on the γ chain. The electron density was relatively well ordered in one of the half-dimers, and the coiled-coil model built into this density then was fitted into the generally less well-ordered density of the three other half-dimeric copies of the asymmetric unit. In our present model of the two molecules per asymmetric unit, the 3,073 residues that were most confidently positioned and refined include: all of the D region's β and γ domain residues (except those C-terminal peptides also not seen previously in fragment D; ref. 24), nearly all (91%) of the coiled-coil residues from one of the half-dimers, and many of the coiled-coil residues (64%, 50%, and 28%, respectively) from each of the other three half-dimers. A rough representation of part of the N-terminal disulfide knot region was constructed from the corresponding weak and irregular density. Note that the values of the resulting R factors we report (see Table 1), which appear to be high, are within the average values for proteins in this resolution range. Molecular replacement of the fragment D structure into the 4°C data (to 6-Å resolution) yielded a density map that showed the same general features observed in the 100-K maps, but at lower resolution. Coiled-coil segments derived from the 100-K map were placed into the corresponding density of the 4°C map, which was also better ordered in one of the half-dimers than in the other.
Results
The Overall Shape of Modified Fibrinogen Is “Planar Sigmoidal.”
The shape of the fibrinogen molecule in these crystals is related to that observed earlier in projection by electron microscopy of negatively contrasted individual molecules (5), but we can now define the curvature of the coiled coils in three dimensions. The axis of the α-helical coiled-coil rod adopts a sigmoidal shape (Fig. 1a) that lies nearly in a plane (Fig. 1b). A sigmoidal shape is observed in some individual molecules (5) and in both the 4°C and 100-K crystal forms. This finding indicates that despite molecular flexibility (discussed below), the curvature appears to be an intrinsic feature of fibrinogen's coiled coil. Spanning three-quarters of the molecule's total length of 450 Å, the coiled-coil segments are crucial determinants of fibrinogen's overall architecture.
The dimeric molecule has an approximate 2-fold axis oriented nearly perpendicular to the plane containing most of the coiled-coil axis (see Fig. 1b and legend). Near the middle of the molecule, at the N termini of the two coiled coils (which are separated by only about 13 Å), the two Aα-chain α-helices of the two half-molecules are situated on the same side of the coiled coil (both “up” in Fig. 1b), whereas the two Bβ-chain α-helices (and the two γ chain α-helices) face in different directions (Fig. 1a). The symmetry axis of the molecule, as determined by the central N-terminal regions of the coiled coils, does not contain a significant translational screw component, in contrast to the “offset” nature of the end-to-end contacts (24) (see below). (The electron density of the central disulfide knot region including the α polymerization sites, poorly defined at this stage, appears for the most part as a “bulge” surrounding the first N-terminal segments of the coiled coils.) Flexibility in the coiled coil yields a somewhat more variable relationship between the half-molecules in the distal C-terminal regions. In the intact molecule, the two β domains are located roughly on opposite sides of the coiled-coil axis, and the GHR binding pockets on these domains nearly face each other. The two γ domains, located at the ends of the molecule, are essentially extensions of the coiled coils (24). The two GPR binding pockets of these domains are found on nearly the same face of the intact molecule (both “up” in Fig. 1b). This feature also is observed for the two pockets of abutting γ domains from end-to-end bonded molecules (24) and, as discussed below, is related to the overall shape of the two-stranded protofibril.
The Boundary of the Three- and Four-Stranded Portions of the Coiled Coil Lies Within a Flexible Hinge.
One of the more unexpected features reported for the human fragment D structure was the reversal in direction of the Aα chain at the C-terminal disulfide ring so that it adds a fourth, but antiparallel, strand to the coiled coil, observed at least to Aα-192 (25). In the modified bovine fibrinogen molecule, this fourth chain continues associated with the coiled coil (Fig. 1 c and d), in a partially helical fold, well past the D region (≈55 Å, ≈30 residues) and almost to the C termini of the E region. The density corresponding to the antiparallel Aα chain turns away from the main coiled coil adjacent to Aα-92, Bβ-125 (and γ60). No density is seen for a fourth strand adjacent to the E-region coiled coil (Fig. 1e).
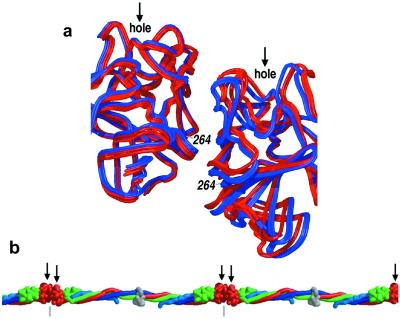
The segment of the coiled coil between the E and D regions is also the most conformationally variable part of the coiled-coil backbone in the crystals. Bending about this flexible hinge in the crystals occurs both around axes perpendicular to (Fig. 2a) and within (Fig. 2b) the plane of the coiled coil, affecting its sigmoidal shape and planarity by as much as 18°. As an illustration, such bending in the coiled coil of one of the half-molecules results in the most distal γ-domain of the intact molecule being displaced by ≈95 Å (Fig. 2b). Each of these bending modes have specific effects on the shape of the protofibril and the flexibility of the clot (discussed below).
Figure 2.
Conformational flexibility of fibrinogen in the crystals. The diagrams show superpositions of noncrystallographically related fibrinogen molecules based on the least-squares fit of the relatively rigid coiled-coil segment: Aα104-Aα154, Bβ140-Bβ190, γ77-γ127. Among the different noncrystallographically related copies, the rms difference between the coordinates of this segment is about half that of the backbone's most flexible segment: Aα64-Aα114, Bβ100-Bβ150, γ37-γ87. (a) View, as in Fig. 1a, of one pair of molecules whose conformations differ primarily by bending within the plane of the sigmoidal coiled-coil axis. (b) View, as in Fig. 1b, of a different pair of molecules whose conformations differ primarily by bending out of the plane of the sigmoidal axis.
Structure of an Extended Filament: γ-Domain Receptor Pockets Face the Same Way.
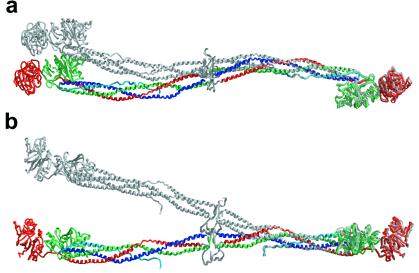
Previous low-resolution studies of various microcrystals and crystals of fibrinogen have indicated a conserved linkage between the ends of the 450-Å-long molecules that form filaments (19, 20, 36, 37). In the current x-ray structure, the specific end-to-end bonding arrangement between γ domains of symmetry-related molecules is indeed very similar (1.7-Å rms difference) to that observed in the D dimer derived from the human fibrin clot (24) (Fig. 3a). This region is distinguished by nonidentical contacts between the abutting γ domains because of a modest offset from a proper 2-fold axis. This specific relationship between the end domains is in fact conserved in both the 4°C and 100-K modified bovine fibrinogen crystal forms as well as in crystals of noncrosslinked human fragment D (24).
Figure 3.
Conserved end-to-end molecular interactions. (a) Superposition of six γ-domain dimers derived from the various crystals of modified bovine fibrinogen (red) and human fragment D and crosslinked D-dimer (blue) (24, 25) show the γ domains to be similarly “offset” from one another. This feature can be visualized by noting, for example, that γ264 of the right monomer is interacting at the edge of the γ-γ interface whereas in the left monomer it is interacting at the center of the interface. No significant difference in the offset is found among the three bovine γ-domain dimers or among the three human γ-domain dimers (pooled intra-species SD is 0.455 Å). Interspecies amino acid differences at or near the interface (e.g., γ264, which is methionine in human and serine in bovine fibrinogen) probably perturb the docking of the domains, creating a slightly less staggered offset (≈1.7-Å rms difference) in the bovine γ-dimer relative to that in the human dimer. (b) Crystal structure of an end-to-end bonded fibrinogen filament. All γ-domain receptor pockets (shown by arrows) are on the same face of the extended filament.
The structure of a single filament that might be found in fibrin thus can be directly visualized in the modified fibrinogen crystals (Fig. 3b). Both crystal forms (4°C and 100 K) are composed of end-to-end bonded fibrinogen molecules related to each other by simple unit cell translations. They reveal, for example, that all the γ-domain receptor pockets throughout the extended filament face the same way. The two-stranded protofibril found in fibrin is, however, not directly observable, because no half-staggered packing arrangement occurs in the crystals.
Discussion
Complexity of the Coiled-Coil Region.
A number of fibrinogen's properties arise from the unusual and complex design of its coiled-coil region. The heptad repeat of apolar residues (38, 39) is interrupted by so-called “stutters” (which generally lead to a modest change in the degree of supercoiling; refs. 40 and 41), and by a proline-rich kink between residues 67 and 77 of the γ chain. The unusual nature of this region in the molecule arises from the association of a fourth antiparallel strand for much, but not all, of its length (Fig. 1 a and b). This antiparallel Aα strand follows the expected left-handed supercoiled path, both in the D region and in the coiled-coil connector between the D and E regions. Note, however, that this strand is partially non-α-helical, especially beyond the fragment D region, corresponding to its low predicted coiled-coil propensity by using various sequence analysis algorithms (e.g., multicoil, ref. 42). Moreover, the resulting four-stranded coiled coil is asymmetric (Fig. 1 c and d), with the antiparallel Aα chain being positioned relatively far from the coiled coil's central axis. Taken together, these observations suggest that this fourth strand may associate only weakly with the rest of the coiled coil. This asymmetric coiled-coil structure is also consistent with the proposed evolution of fibrinogen from a homotrimeric, rather than four-stranded, precursor (43, 44).
Flexible Aα Arm.
The special role of the Aα chain also is shown by the insertion of a long flexible sequence after the coiled-coil region. Its distal C-terminal compact αC domain [≈Aα390–550 human (45)] is thus mobile in fibrin, in contrast to the fixed locations of the β and γ domains. In an intact (unmodified) fibrinogen molecule, the two αC domains appear to associate with each other to form a dimeric domain near the center of the E region (3, 4, 28). Upon release of fibrinopeptides by thrombin, the αC domains dissociate from each other and the main body of the molecule, after which they are involved in intermolecular interactions between fibrin molecules (28). These domains also are involved in the adhesion of other structures such as endothelial cells (46). The fibrinogen crystal structure indicates the location on the backbone to which the flexible Aα arm is anchored, namely the boundary between the three- and four-stranded portions of the coiled coil ≈55 Å from the E disulfide ring (Fig. 1). The portion of the Aα sequence between this coiled-coil branch point (at about residue Aα 220, but see Fig. 1 legend and Methods) and the C terminus of the Aα chain in this modified fibrinogen (at residue Aα 390, see Methods) is not seen in our electron density maps, consistent with predictions of a highly flexible region (45). From its anchor on the backbone, a flexible arm of this 170-residue length can reach any part of the fibrinogen molecule, including the center, and, if extended, its αC domain may bind to other structures more than 500 Å away.
The anchoring of the flexible Aα arm to the hinge region of the coiled coil (described above, see Fig. 2b) suggests that a “three-way” flexible junction may exist in complexes of fibrinogen with other molecules mediated by the αC domain. The elastic design of this junction may contribute to the clot's stability and to its mechanical stiffness, which is diminished when the αC domains are removed (47). This region of the molecule is also the primary target for proteolysis by plasmin.
Structure and Flexibility of the Fibrin Protofibril.
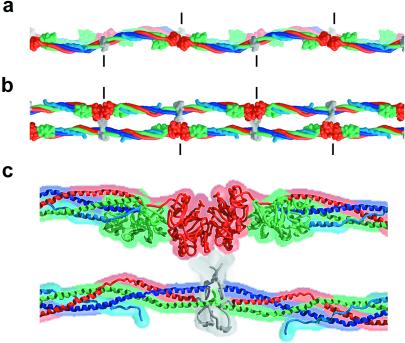
The two-stranded half-staggered protofibril is the basic structural unit formed when fibrin polymerizes. Based on the recent high-resolution structure of the fibrin D-dimer complexed with the tetrapeptide ligands derived from the E region (24, 25), we now know in detail important interactions that link three fibrin molecules from two adjacent filaments at distinct regions every 225 Å along the protofibril. The coiled coils connect these repeating DDE clusters, but they are not simply spacers. Inspection of a protofibril model based on the results reported here (see legend to Fig. 4) shows that the structural parameters (e.g., length and pitch) of the coiled coil allow an optimal orientation of the subsequent polymerization sites with respect to the adjacent filament. For example, at the E region ends of the coiled-coil α-helices (see Fig. 4 b and c), the Aα chains (dark blue) face the adjacent filament whereas the Bβ chains (green) face somewhat away from and to opposite sides of the protofibril's core. These positions are consistent with the primary role of fibrin's N-terminal α-GPR knobs, i.e., to bind within the γ-domain receptor pockets of the adjacent filament, and with the possible role of the β-GHR knobs in the slower lateral associations between protofibrils (26, 27). Similarly, the C termini of the coiled coil's antiparallel Aα chains face away from the adjacent filament, consistent with the role of the distal αC domains in forming intermolecular interactions between protofibrils in the clot (28).
Figure 4.
A model of the two-stranded protofibril of fibrin derived from a filament in the bovine fibrinogen crystal structure. (a) View as in Fig. 1a. The sigmoidal coiled-coil axes of the two filaments are in phase. (b) View as in Fig. 1b. (c) Magnified view of b, including the DDE cluster. The model was constructed so that the two filaments are half-staggered (by 225 Å), closed (the γ-domain receptor pockets face the adjacent filament), and separated by ≈60 Å (a rough estimate from figure 9 in ref. 4 and J. Weisel, personal communication). The contact area between the D and E regions is not known and is illustrated schematically here. Short vertical lines indicate the molecular boundaries. The ≈30-Å distance between the N terminus of the Aα-chain coiled coil from one filament and the γ-domain hole on the other filament (into which the GPR sequence binds) could easily be spanned by the 30 additional residues in the disulfide knot region. Similarly, the ≈100-Å distance between the N terminus of the Bβ-chain coiled coil and the β-domain receptor pocket of the adjacent filament also could be bridged by the 64 residues in this region. This long distance between the disulfide knot and the GHR sequence of the Bβ-chain also may allow binding between protofibrils.
The crystal structure of any extended fibrinogen filament, in which all the γ-domain receptor pockets face the same way, suggests that the long axes of the two filaments in a closed half-staggered protofibril would be parallel and not coil around each other. Long-pitched twisting of filaments observed in extended protofibrils and fibrin fibers (48, 49) may be accounted for by minor conformational changes in a noncrystalline environment.
The promotion of fibrin polymerization by various surfaces, fibrin's accommodation of clot retraction, as well as the twisting of fibrin fibers eventually may be understood in terms of the structural flexibility of the clot. Protofibril flexibility may arise in part from the flexible hinge at the plasmin-sensitive region of the coiled coil that is observed in the fibrinogen crystals. In the protofibril model we have put forward (Fig. 4 a and b), the planes containing the sigmoidal coiled coils of the two filaments are oriented in a “face-to-face” (rather than “edge-to-edge”) manner. The two perpendicular modes of flexibility described for the hinge (Fig. 2) would have distinct effects on the protofibril model: bending of the filaments within these planes would allow their contour lengths to remain the same (superimpose Fig. 2a on Fig. 4a), but bending out of the plane would distort the protofibril and require one filament to be stretched relative to the other (superimpose Fig. 2b on Fig. 4b). This latter mode of bending nevertheless may occur: stretching of molecules also would account for the structure of fibrin fibers, in which protofibrils at the surface and in the center are observed to undergo a similar degree of twisting with a pitch of ≈20,000 Å (48).
Acknowledgments
We thank A. Houdusse, M. Love, D. Himmel, K.-H. Kim, and the staffs of the Brookhaven National Laboratory and the Cornell High Energy Synchrotron Source for assistance with data collection, and J. Weisel, L. Medved', and S. Lord for critical reading of the manuscript. We also thank P. Walian for his efforts at determining the structure of modified fibrinogen by using cryo-electron crystallography and J.-F. Menetret for his excellent assistance in this project. The work has been supported by a grant to C.C. from the National Institutes of Health (AR17346), a Medical Foundation fellowship to J.H.B. supported by Fleet Bank of Massachusetts, N.A., Trustee of the Charles A. King Trust, and a grant to A.H.H.-E. from the National Institutes of Health (HL 424121).
Footnotes
Data deposition: αC coordinates have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1DEQ).
References
- 1.Doolittle R F, Everse S J, Spraggon G. FASEB J. 1996;10:1464–1470. doi: 10.1096/fasebj.10.13.8940292. [DOI] [PubMed] [Google Scholar]
- 2.Hall C E, Slayter H S. J Biophys Biochem Cytol. 1959;5:11–17. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosesson M W, Hainfeld J, Wall J, Haschemeyer R H. J Mol Biol. 1981;153:695–718. doi: 10.1016/0022-2836(81)90414-9. [DOI] [PubMed] [Google Scholar]
- 4.Erickson H P, Fowler W E. In: Molecular Biology of Fibrinogen and Fibrin. Mosesson M W, Doolittle R F, editors. New York: N.Y. Acad. Sci.; 1983. pp. 146–163. [Google Scholar]
- 5.Williams R C. In: Molecular Biology of Fibrinogen and Fibrin. Mosesson M W, Doolittle R F, editors. New York: N.Y. Acad. Sci.; 1983. pp. 180–193. [Google Scholar]
- 6.Weisel J W, Stauffacher C V, Bullitt E, Cohen C. Science. 1985;230:1388–1391. doi: 10.1126/science.4071058. [DOI] [PubMed] [Google Scholar]
- 7.Doolittle R F, Watt K W K, Cottrell A, Strong D D, Riley M. Nature (London) 1979;280:464–468. doi: 10.1038/280464a0. [DOI] [PubMed] [Google Scholar]
- 8.Henschen A, Lottspeich F, Southan C, Toepfer-Petersen E. Protides Biol Fluids Proc Colloq. 1980;28:51–56. [Google Scholar]
- 9.Chung D W, Rixon M W, Davie E W. In: Proteins in Biology and Medicine. Bradshaw R A, editor. New York: Academic; 1982. pp. 309–328. [Google Scholar]
- 10.Brown W M, Dziegielewska K M, Foreman R C, Saunders N R. Nucleic Acids Res. 1989;17:6397. doi: 10.1093/nar/17.15.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henschen A. Thromb Haemostasis. 1993;70:42–47. [PubMed] [Google Scholar]
- 12.Henschen A, Lottspeich F, Hessel B. Hoppe-Seyler's Z Physiol Chem. 1979;360:1951–1956. [PubMed] [Google Scholar]
- 13.Henschen A, McDonagh J. In: Blood Coagulation. Zwaal R F A, Hemker H C, editors. Amsterdam: Elsevier; 1986. pp. 171–241. [Google Scholar]
- 14.Stryer L, Cohen C, Langridge R. Nature (London) 1963;197:793–794. doi: 10.1038/197793a0. [DOI] [PubMed] [Google Scholar]
- 15.Weisel J W, Phillips G N, Jr, Cohen C. In: Molecular Biology of Fibrinogen and Fibrin. Mosesson M W, Doolittle R F, editors. New York: N.Y. Acad. Sci.; 1983. pp. 367–379. [Google Scholar]
- 16.Doolittle R F. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- 17.Hantgan R R, Francis C W, Marder V J. In: Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Colman R W, Hirsh J, Marder V J, Salzman E W, editors. Philadelphia: Lippincott; 1994. pp. 277–300. [Google Scholar]
- 18.Elliott B W, Jr, Cohen C. J Biol Chem. 1986;261:11259–11265. [PubMed] [Google Scholar]
- 19.Tooney N M, Cohen C. J Mol Biol. 1977;110:363–385. doi: 10.1016/s0022-2836(77)80077-6. [DOI] [PubMed] [Google Scholar]
- 20.Weisel J W, Warren S G, Cohen C. J Mol Biol. 1978;126:159–183. doi: 10.1016/0022-2836(78)90357-1. [DOI] [PubMed] [Google Scholar]
- 21.Rao S P S, Poojary M D, Elliott B W, Jr, Melanson L A, Oriel B, Cohen C. J Mol Biol. 1991;222:89–98. doi: 10.1016/0022-2836(91)90739-s. [DOI] [PubMed] [Google Scholar]
- 22.Yee V C, Pratt K P, Côte H C, Trong I L, Chung D W, Davie E W, Stenkamp R E, Teller D C. Structure (London) 1997;5:125–138. doi: 10.1016/s0969-2126(97)00171-8. [DOI] [PubMed] [Google Scholar]
- 23.Pratt K P, Côte H C, Chung D W, Stenkamp R E, Davie E W. Proc Natl Acad Sci USA. 1997;94:7176–7181. doi: 10.1073/pnas.94.14.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spraggon G, Everse S J, Doolittle R F. Nature (London) 1997;389:455–462. doi: 10.1038/38947. [DOI] [PubMed] [Google Scholar]
- 25.Everse S J, Spraggon G, Veerapandian L, Riley M, Doolittle R F. Biochemistry. 1998;37:8637–8642. doi: 10.1021/bi9804129. [DOI] [PubMed] [Google Scholar]
- 26.Laurent T C, Blombäck B. Acta Chem Scand. 1958;12:1875–1877. [Google Scholar]
- 27.Hantgan R, Hermans J. J Biol Chem. 1979;254:11272–11281. [PubMed] [Google Scholar]
- 28.Veklich Y I, Gorkun O V, Medved' L V, Nieuwenhuizen W, Weisel J W. J Biol Chem. 1993;268:13577–13585. [PubMed] [Google Scholar]
- 29.Otwinowski Z, Minor W. In: Data Collection and Processing. Sawyer L, Isaacs N, Bailey S, editors. Warrington, U.K.: CLRC Daresbury Laboratory; 1993. pp. 59–62. [Google Scholar]
- 30.Evans P R. Proceedings of CCP 4 Study Weekend on Data Collection & Processing. Daresbury Laboratory, Warrington, U.K.: SERC; 1993. pp. 114–122. [Google Scholar]
- 31.Navaza J. Acta Crystrallogr A. 1994;50:157–163. [Google Scholar]
- 32.Brünger A T. x-plor: A System for X-Ray Crystallography and NMR. New Haven, CT: Yale Univ. Press; 1992. , Version 3.1. [Google Scholar]
- 33.Cowtan K D. Joint CCP 4 ESF-EACBM Newsl Protein Crystallogr. 1994;31:34–38. [Google Scholar]
- 34.Read R J. Acta Crystrallogr A. 1986;42:140–149. [Google Scholar]
- 35.Jones T A, Zou J-Y, Cowan S W, Kjeldgaard M. Acta Crystrallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 36.Weisel J W, Phillips G N, Jr, Cohen C. Nature (London) 1981;289:263–267. doi: 10.1038/289263a0. [DOI] [PubMed] [Google Scholar]
- 37.Cohen C, Weisel J W, Phillips G N, Jr, Stauffacher C V, Fillers J P, Daub E. In: Molecular Biology of Fibrinogen and Fibrin. Mosesson M W, Doolittle R F, editors. New York: N.Y. Acad. Sci.; 1983. pp. 194–213. [DOI] [PubMed] [Google Scholar]
- 38.Crick F H C. Acta Crystallogr. 1953;6:689–697. [Google Scholar]
- 39.O'Shea E K, Klemm J D, Kim P S, Alber T. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 40.Lupas A. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 41.Brown J H, Cohen C, Parry D A D. Proteins Struct Funct Genet. 1996;26:134–145. doi: 10.1002/(SICI)1097-0134(199610)26:2<134::AID-PROT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 42.Wolf E, Kim P S, Berger B. Protein Sci. 1997;6:1179–1189. doi: 10.1002/pro.5560060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henschen A, Lottspeich F. Thromb Res. 1977;11:869–880. doi: 10.1016/0049-3848(77)90115-3. [DOI] [PubMed] [Google Scholar]
- 44.Doolittle R F, Spraggon G, Everse S J. In: Plasminogen-Related Growth Factors. Bock G R, Goode J A, editors. Chichester, U.K.: Wiley; 1997. pp. 4–23. [Google Scholar]
- 45.Medved' L V, Gorkun O V, Privalov P L. FEBS Lett. 1983;160:291–295. doi: 10.1016/0014-5793(83)80985-5. [DOI] [PubMed] [Google Scholar]
- 46.Cheresh D A, Berliner S A, Vicente V, Ruggeri Z M. Cell. 1989;58:945–953. doi: 10.1016/0092-8674(89)90946-x. [DOI] [PubMed] [Google Scholar]
- 47.Collet J-P, Veklich Y, Mullin J L, Gorkun O V, Lord S T, Weisel J W. Thromb. Haemostasis. 1999. Suppl., 692 (abstr.). [Google Scholar]
- 48.Weisel J W, Nagaswami C, Makowski L. Proc Natl Acad Sci USA. 1987;84:8991–8995. doi: 10.1073/pnas.84.24.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medved' L V, Ugarova T, Veklich Y, Lukinova N, Weisel J. J Mol Biol. 1990;216:503–509. doi: 10.1016/0022-2836(90)90376-W. [DOI] [PubMed] [Google Scholar]