Abstract
Amino acid transformations catalyzed by a number of pyridoxal 5′-phosphate (PLP)-dependent enzymes involve abstraction of the Cα proton from an external aldimine formed between a substrate and the cofactor leading to the formation of a quinonoid intermediate. Despite the key role played by the quinonoid intermediates in the catalysis by PLP-dependent enzymes, limited accurate information is available about their structures. We trapped the quinonoid intermediates of Citrobacter freundii tyrosine phenol-lyase with l-alanine and l-methionine in the crystalline state and determined their structures at 1.9- and 1.95-Å resolution, respectively, by cryo-crystallography. The data reveal a network of protein-PLP-substrate interactions that stabilize the planar geometry of the quinonoid intermediate. In both structures the protein subunits are found in two conformations, open and closed, uncovering the mechanism by which binding of the substrate and restructuring of the active site during its closure protect the quinonoid intermediate from the solvent and bring catalytically important residues into positions suitable for the abstraction of phenol during the β-elimination of l-tyrosine. In addition, the structural data indicate a mechanism for alanine racemization involving two bases, Lys-257 and a water molecule. These two bases are connected by a hydrogen bonding system allowing internal transfer of the Cα proton.
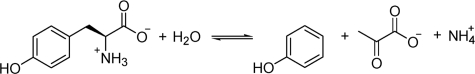
Tyrosine phenol-lyase (TPL)2 is a pyridoxal 5′-phosphate (PLP)-dependent enzyme that catalyzes at physiological conditions the reversible hydrolytic cleavage (the β-elimination reaction) of l-tyrosine to give phenol and ammonium pyruvate (Scheme 1). In vitro, this protein can catalyze a variety of reactions. In addition to the β-elimination of several β-substituted l- and d-amino acids (e.g. l-Cys, l- and d-Ser (1)), their derivatives (e.g. S-methyl-l-cysteine, β-chloroalanine (1), and S-(o-nitrophenyl)-l-cysteine (2)), and compounds structurally analogous to l-Tyr (e.g. 1-amino-2-(4-hydroxyphenyl)ethyl phosphinic acid (3)), it also catalyzes the reversal of the β-elimination reaction (4–6), the β-substitution by phenol derivatives (7–9), the racemization of l- and d-Ala (10), and the irreversible half-transamination (conversion to an α-ketoacid) of some amino acids, including l-Tyr and l-Ala (11).
SCHEME 1.
Reversible β-elimination reaction of l-tyrosine catalyzed by TPL.
The molecule of TPL is homotetrameric, both in solution and in the investigated crystal structures (12–15). It is composed of two catalytic dimers, which are held together by intertwined N-terminal arms and a hydrophobic cluster formed in the middle of the tetramer (Fig. 1). Each subunit can be divided into two conformationally invariant (rigid) regions connected via a flexible part. The large rigid region comprises the N-terminal arm, the large domain, and few small segments of the small domain, while the small rigid region is exclusively made of the remaining part of the small domain. The active site is constructed from residues belonging to the large and small rigid regions of one subunit and the large rigid region of the second subunit of the catalytic dimer. As seen in the structure of the TPL apoenzyme (15), the closed conformation is achieved by movement of the small and large rigid regions toward each other resulting in a closure of the active site.
FIGURE 1.
TPL tetramer. Ribbon diagram with different subunits shown in different colors. One catalytic dimer consists of the subunits shown in cyan and blue, while the other is formed from the subunits shown in orange and yellow. PLP and the side chains of the PLP-binding Lys-257 residues are shown as spheres. The view is along the crystallographic 2-fold axis.
The postulated mechanism of the β-elimination reaction (16) includes several key steps (Scheme 2): (a) formation of the external aldimine in the reaction of the internal aldimine with the substrate; (b) Cα proton abstraction performed by the cofactor-binding residue Lys-257 resulting in formation of the quinonoid intermediate; (c) Cγ protonation by Tyr-71 assisted by Arg-381; and, finally, (d) cleavage of the C-C bond to form phenol and the aminoacrylate intermediate. Although previous biochemical and structural data pinpointed the role of individual residues in the catalysis, the details of their interactions with the substrate and molecular interactions stabilizing their active conformation during the quinonoid intermediate formation remained obscure. This is not surprising: although the formation of a quinonoid intermediate with an amino acid is an essential step in the reaction mechanism of all PLP-dependent enzymes, this intermediate is often rather unstable preventing its analysis by crystallographic methods. Until now, structural data on the quinonoid intermediate were only available for serine hydroxymethyltransferase (17) and tryptophan synthase (18).
SCHEME 2.
Postulated reaction mechanism of β-elimination of l-tyrosine catalyzed by TPL.
Here we report crystal structures of two quinonoid intermediates of Citrobacter freundii TPL formed with amino acids l-Ala and l-Met and determined at 1.9- and 1.95-Å resolution, respectively. The first structure reveals the mechanism of the racemization of Ala. The second shows detailed interactions with the substrate side chain in the closed conformation. In addition, the data reveal the network of protein-PLP-substrate interactions that stabilize the planar geometry of the quinonoid and demonstrate the essential role of the active site closure during the physiological β-elimination of l-Tyr.
MATERIALS AND METHODS
Crystallization—TPL was produced in Escherichia coli SVS 370 cells transformed with the plasmid pTZTPL containing the TPL gene (12), and the enzyme was purified as previously described (19). Crystals of the C. freundii holo-TPL were grown at 277 K and 293 K by hanging drop vapor diffusion as described (15). The best crystals were obtained by mixing 2 μl of the protein solution (18–20 mg/ml) containing 50 mm triethanolamine (pH 8.0), 0.5 mm PLP, 1 mm dithiothreitol with an equal volume of the reservoir solution containing 50 mm triethanolamine (pH 8.0), 0.5 mm PLP, 2 mm dithiothreitol, 0.4 m KCl, and 35–38% (w/v) PEG 5000 MME. Complexes with amino acids were prepared by soaking the light yellow holo-TPL crystals for 5 min in the stabilization solution containing 40% (w/v) PEG 5000 MME, 50 mm triethanolamine buffer (pH 8.0), 0.25 m KCl, 0.2 mm PLP, 0.5 mm dithiothreitol, and either 100 mm l-Ala or 70 mm l-Met. Crystals turned dark yellow-red during soaking and were cryo-cooled by dipping into liquid nitrogen directly from the soaking solution. l-Ala and l-Met act as competitive inhibitors of TPL (20) and, when added to TPL, they predominantly form relatively stable quinonoid intermediates, both in solution (20) and in the crystal (21). Despite this, it was not possible to prepare quinonoid complexes by co-crystallization, and soaking times longer than 5 min considerably deteriorated crystal quality. Further details about preparation and stability of quinonoid intermediates are given in the supplemental data.
Structure Determination—Both x-ray data sets were collected at 120 K. Data for the complex with l-Ala were collected at the BW7B beamline (EMBL, Hamburg), at a wavelength of 0.862 Å using an image-plate detector (MarResearch), while the data for the complex with l-Met were collected at the BM14 beamline (ESRF, Grenoble) at a wavelength of 0.979 Å using a CCD detector (MarResearch). Data sets were processed with DENZO and SCALEPACK (22), and most other crystallographic calculations were performed using the CCP4 suite of programs (23). Initial phasing was performed by AMoRe (24) with the apoenzyme structure (PDB ID 2EZ2; (15)) as the search model. Models were built by COOT (25), water molecules were added using ARP/wARP (26), and the refinement was carried out by REFMAC (27) using the TLS option (28) with the small (residues 19–44, 346–404, 434–456) and large (residues 1–13, 45–345, 405–422) rigid regions (15) of each subunit treated as separate TLS groups. Several patches of electron density at the protein surface in both structures were interpreted as fragments of poly(ethylene glycol) monomethyl ether (29) (supplemental Fig. S1). Models of the quinonoid molecules were generated by SKETCHER; the corresponding library files used during the refinement were generated by LIBCHECK (23). The final models were validated using MolProbity (30). Crystal packing contacts were analyzed by PISA (31). Figures were generated using PyMOL (32). Data collection and refinement statistics are given in Table 1.
TABLE 1.
Crystallographic data collection and refinement statistics
| Structure | TPL-Ala | TPL-Met |
|---|---|---|
| Space group | P21212 | P21212 |
| Unit cell parameters | ||
| a (Å) | 132.9 | 132.9 |
| b (Å) | 143.7 | 143.3 |
| c (Å) | 59.6 | 59.7 |
| Resolution range (Å) | 20.0–1.89 (1.93–1.89)a | 30.0–1.95 (2.02–1.95) |
| No. of unique reflections | 89 055 (4 337) | 76 260 (6 854) |
| Rmerge (%)b | 7.0 (35.7) | 8.2 (42.5) |
| Average I/σ(I) | 12.2 (1.8) | 10.5 (2.3) |
| Data redundancyc | 3.7 (2.3) | 3.9 (3.6) |
| Completeness (%) | 97.3 (87.5) | 90.3 (82.3) |
| Wilson B-factor (Å2) | 18.9 | 27.1 |
| No. of reflections used in refinement | 87 930 (4 337) | 75 067 (7 386) |
| Rcryst (%)d | 15.8 (23.2) | 15.6 (19.2) |
| Rfree (%)d | 19.8 (30.2) | 19.2 (22.7) |
| No. of reflections used for Rfree | 1 065 (52) | 1 058 (116) |
| No. of protein residues | 912 | 912 |
| No. of water molecules | 966 | 887 |
| No. of ligands | 6 (2 K+, 2 alanine quinonoid molecules, 2 PEG fragments) | 6 (2 K+, 1 methionine quinonoid molecule, 1 PLP, 2 PEG fragments) |
| Average B-factor (Å2) | ||
| Chain A | 17.2 | 32.0 |
| Chain B | 14.3 | 29.5 |
| Water molecules | 29.6 | 41.2 |
| Overall | 17.4 | 32.0 |
| R.m.s. deviationse | ||
| Bond lengths (Å) | 0.016 (0.022) | 0.015 (0.022) |
| Bond angles (°) | 1.4 (2.0) | 1.4 (2.0) |
| Ramachandran plot (%)f | ||
| Favored | 98.1 | 97.9 |
| Outliers | 0.0 | 0.0 |
Values in parentheses are for the outer resolution shell
The value of the merging R-factor between equivalent measurements of the same reflection, Rmerge = (ΣhklΣi|<Ihkl> – Ihkl, i|)/(ΣhklΣi Ihkl, i)
The average observation of the same reflection
Crystallographic R-factors, R = (Σhkl||Fo, hkl| – |Fc, hkl||)/(Σhkl Fo, hkl|)
Target values are given in parentheses
Analyzed by MolProbity (30)
RESULTS
Crystallography—Crystals of the quinonoid intermediates with Ala and Met were obtained by soaking holo-TPL crystals in solutions containing the corresponding amino acids, followed by cryo-cooling in liquid nitrogen. Because of the significant difference in the absorption maxima of the holoenzyme and the quinonoid intermediate (∼420 and ∼500 nm, respectively (21, 33–35)), it was possible to monitor the formation of the quinonoid intermediate by a dramatic change in the color of crystals, which became dark yellow-red after 20–30 s of soaking. The crystal structures of C. freundii TPL in the complex with l-Ala (TPL-Ala) and l-Met (TPL-Met) were determined at 1.9- and 1.95-Å resolution, respectively. The electron density maps were very clear and allowed positioning of all protein residues in both structures.
Open and Closed Conformations—As in the case of the holoenzyme, the asymmetric unit contains two protein subunits, which form the catalytic dimer. Unlike holo-TPL, where both active sites are found in the open conformation (15), in the quinonoid intermediates of both TPL-Ala and TPL-Met one subunit of the catalytic dimer (denoted by A) is found in the closed conformation while the other (B) remains in the open conformation (Fig. 2). Positional adjustments between the small and large rigid regions, that accompany the closure of the active site during the quinonoid complex formation, result in formation of new crystal contacts (compared with those present in the crystals of holoenzyme). Interestingly, the observed main-chain conformations in both complexes are almost identical to those in the structure of the apo-TPL (15) with the Cα r.m.s. displacements of 0.16 Å for the closed and 0.24 Å for the open subunit of TPL-Ala (0.14 and 0.21 Å, respectively, in the case of the TPL-Met structure). The most significant difference between structures of the apoenzyme and quinonoid complexes is in the main-chain conformation of the flexible loop covering the active site in the open subunit, residues 389–393, with the maximum Cα atom displacement of 1.5 Å for the TPL-Ala and 1.2 Å for the TPL-Met complexes. The residual electron density (supplemental data) suggests that the small rigid region and the flexible parts of subunit A are discretely disordered in the TPL-Met crystal, with minor portion of subunit A found in the open conformation. Because of its low occupancy and the resulting separate unconnected peaks in the electron density maps, the open conformation of subunit A was not modeled. Such disorder was not observed for the TPL-Ala complex.
FIGURE 2.
Superposition of two TPL subunits that adopt open and closed conformations. The closed conformation is created by the rotation of the small rigid region (residues 19–44, 346–404, 434–456) by about 16° around a hinge connecting it with the large rigid region (residues 1–13, 45–345, 405–422). The stereo view shows TPL-Ala subunits as Cα models superimposed using large rigid regions. The quinonoid molecules are depicted as bold ball-and-stick models. The large and small rigid regions of the closed subunit are shown in orange and pink, respectively, and the flexible parts are shown in yellow. The large and small rigid regions of the open subunit are shown in blue and green, respectively, while the flexible parts are shown in cyan.
Structure of the Quinonoid Intermediates—Previous spectroscopic studies showed that TPL forms a stable quinonoid complex in solution when incubated with l-Ala (11, 34–36). It was also suggested that a mixture of the external aldimine and the quinonoid intermediate is formed when l-Met is added to TPL in a solution (20) or in crystals (21). The main feature by which we can differentiate between the external aldimine and the quinonoid intermediates at the current resolution is the geometry around the Cα atom of the amino acid (covalently bound to the molecule of cofactor). In the external aldimine the substrate Cα atom is sp3 hybridized and has a tetrahedral geometry. This is contrary to the quinonoid intermediate in which the Cα atom is sp2 hybridized and has a planar trigonal geometry (Scheme 2). Electron density maps clearly indicate presence of the Ala quinonoid intermediate in both active sites of the catalytic dimer (Fig. 3 and supplemental Fig. S2). In contrast, in the case of the TPL-Met complex, only the active site observed in the closed conformation (subunit A) is occupied by the quinonoid intermediate (Fig. 4 and supplemental Fig. S3). The active site of the other subunit (open conformation) contains the internal aldimine with the PLP molecule covalently bound to Lys-257; its structure is essentially identical to that of the holo-TPL (15). As the external aldimine is not observed in either of the active sites, we conclude that the 425-nm peak observed previously in absorption spectra of C. freundii TPL complexed with l-Met in solution (20) and in the crystals (21) must be ascribed to the internal and not to the external aldimine.
FIGURE 3.
Enzyme interactions with the Ala quinonoid intermediate in the closed (A) and open (B) active sites. Stereo views with structures of quinonoid and two water molecules superposed with the corresponding weighted |Fo| - |Fc| electron density omit maps (green) are contoured at the 3.0σ level. Hydrogen bonds are denoted by dashed lines. Carbon atoms of residues belonging to the large rigid region are shown in orange, those from the small rigid region are shown in pink, and the residues from the neighboring subunit are shown in blue and labeled with a star. The alternate conformations of Thr-124, Thr-216, Met-288, and Phe-449 in the open conformation are shown in corresponding pale tones.
FIGURE 4.
Enzyme interactions with the Met quinonoid intermediate in the closed active site. Stereo view with the quinonoid molecule superposed with the corresponding weighted |Fo| - |Fc| electron density omit maps (green) are contoured at the 3.0σ level. Carbon atoms of residues belonging to the large rigid region are shown in orange, those from the small rigid region are shown in pink, and the residues from the neighboring subunit are shown in blue and labeled with a star. Hydrogen bonds are denoted by dashed lines.
The analysis of B-factors and difference electron density maps shows that the closed active site of TPL-Met is not fully occupied by the quinonoid intermediate. This analysis also suggests that a minor proportion of this site is occupied by the internal aldimine. Namely, the unassigned peak of 0.16 e Å-3 (3.8σ) in the σA-weighted |Fo| - |Fc| electron density map (37) is situated between Lys-257 Cε and PLP C4′, in the position where the Lys-257 Nζ would be in the internal aldimine complex (Fig. 4 and supplemental Fig. S3). However, as in the case of the open conformation of subunit A, modeling of this intermediate was precluded due to its very low occupancy (supplemental data). The data suggest that the quinonoid is bound to subunit A only when the active site is in the closed conformation, while the internal aldimine (not modeled) occurs in the minor proportion of subunits that are in the open conformation. This is further substantiated by the difference electron density observed next to the -SCH3 moiety of the Met quinonoid (Fig. 4 and supplemental Fig. S3), which could be ascribed to the conformation of Phe-123 in a minor proportion of the internal aldimine.
Protein Interactions with Quinonoid Molecules—The ε-amino group of Lys-257 is found on the re side of the quinonoid at distances of 3.8 and 3.7 Å, respectively, from the Cα atom of the quinonoid in the closed and open active site conformations of TPL-Ala (Fig. 3). The distance between these two groups is very similar (3.7 Å) in the TPL-Met complex (Fig. 4). Thus the experimental data demonstrate that Lys-257 is situated on the appropriate side of the bound substrate and, moreover, its ε-amino group is closest to the Cα atom of the quinonoid intermediate (and can come even closer by rotation around the Cδ-Cε bond). In TPL-Ala the closest entity to the Cα atom at the si side of the quinonoid is a water molecule (Wat2) found at a distance of 3.8 Å in the closed active site and 4.0 Å in the open one (Fig. 3). Wat2 is hydrogen-bonded to another water molecule (Wat3), which is situated at distances of 4.2 and 5.2 Å from the quinonoid Cα atom in the closed and open active sites, respectively. In the closed active site Wat3 is in hydrogen-bonding interactions with the Thr-124 hydroxyl and with the Arg-381 guanidinium group (Fig. 3A), but in the open active site the only protein residue to which Wat3 hydrogen bonds is Arg-381 (Fig. 3B). Wat2 is found farther from the Arg-381 guanidinium group: at distances of 4.5 and 3.5 Å in the closed and the open active sites, respectively.
As expected, there are no water molecules at the si face of the quinonoid intermediate in TPL-Met. Instead, this space is occupied by the side chain of Met which is situated in a relatively hydrophobic environment formed by the residues Phe-36, Phe-123, Phe-448, Phe-449, and Met-379 (Fig. 4). The closest to its sulfur atom are the CZ atom of Phe-449 (at 3.7 Å) and the NH1 atom of Arg-381 (at 3.7 Å); interestingly Arg-100, a residue, which was proposed to be essential for the stabilization of the Met quinonoid intermediate (38), is found farther away with its closest atom (NH2) positioned at a distance of 4.7 Å. The hydroxyl of Tyr-71 from the adjacent subunit of the catalytic dimer is the closest protein group to the Met Cγ positioned at a distance of 3.4 Å.
In all active sites occupied by the quinonoid intermediate the phenolic hydroxyl group of Tyr-71 and the ε-amino group of Lys-257 are connected by a network of hydrogen bonding interactions passing through the hydroxyl group of Tyr-291, the water molecule Wat1, the amide group of Gln-98, the phosphate group of PLP, the guanidinium group of Arg-100 and the hydroxyl groups of Ser-51 and Ser-254 (Figs. 3 and 4). These interactions are critical for maintaining the conformation of Lys-257 and Tyr-71 side chains in the active site and define the potential route for the observed proton transfer between the Cα and Cγ atoms of the substrate (39).
Structural Rearrangements in the Active Site—All non-covalent interactions between the PLP and the active site residues observed for the internal aldimine are preserved in the quinonoid complexes. On the formation of the external aldimine the bond between Lys-257 Nζ and PLP C4′ is cleaved, and a new aldimine bond between an amino acid and PLP is formed. During this transition the pyridoxal ring has to be reoriented, as predicted (40) and later confirmed by the x-ray analysis of the aspartate aminotransferase (41). Indeed, while the PLP phosphate group in quinonoid intermediates of TPL stays in essentially the same position as in the internal aldimine, the pyridoxal ring is rotated by ∼20° around an imaginary axis passing approximately through its C3 and C5 atoms (supplemental Fig. S4). As a result, the C4′ atom of PLP is moved from Lys-257 by ∼1.1 Å compared with its position in the internal aldimine. The ε-amino group of Lys-257 in the quinonoid intermediates assumes a more relaxed, antiperiplanar conformation achieved by -98° rotation around the χ4 torsion angle, resulting in its movement toward the PLP phosphate group and formation of hydrogen bonds with PLP phosphate and the side chain hydroxyls of Ser-254 and Ser-51 (15). In addition to the salt bridge between the deprotonated hydroxyl of PLP (O3′) and the side chain of Arg-217 observed in the internal aldimine, in the quinonoid intermediates the PLP O3′ atom makes a hydrogen bond with the side chain amide of Asn-185 (Figs. 3 and 4).
The carboxylate group of each quinonoid intermediate forms several interactions with the active site residues, including a salt bridge with the guanidinium group of Arg-404 and a hydrogen bond with the hydroxyl of Thr-49 (Figs. 3 and 4). Similar interactions with Thr-49 and Arg-404 have been observed for the substrate analog 3-(4′-hydroxyphenyl)propanoate (HPPA) bound to C. freundii TPL (13) and also for a quasisubstrate N-(5′-phosphopyridoxyl)-l-tyrosine (PPT) in complex with the Erwinia herbicola TPL (14). Because the complex of TPL with HPPA resembles a Michaelis-Menten complex and the complex with PPT models the external aldimine, it appears that these interactions persist during the catalysis, from binding of the substrate to the formation of the quinonoid intermediate. The guanidinium group of Arg-217 and the amide of Asn-185 are bridging one of the substrate carboxylate oxygen atoms with the O3′ atom of PLP through hydrogen bonding and salt bridge interactions (Figs. 3 and 4). Such bridging interactions are absent in the complex of TPL with HPPA where the substrate analog is farther away and is not covalently bound to the PLP. In this complex the guanidinium group of Arg-217 is hydrogen-bonded only to the O3′ atom of PLP, while no interactions of either O3′ or the carboxylate group with Asn-185 were observed. In the complex with PPT, the side chain of Arg-217 salt bridges the O3′ atom and the carboxylate group, but Asn-185 forms a hydrogen bond only with the O3′ suggesting that the hydrogen bond with the substrate carboxylate group forms along with the quinonoid intermediate formation.
Planarity of the Quinonoid Molecules—The pyridoxal ring and the “alanine moiety,” comprising atoms N, CA, CB, C, O, and OXT, in the quinonoid molecules are almost coplanar (supplemental Figs. S2 and S3). The angle between planes of the pyridoxal ring and the “alanine moiety” (excluding oxygen atoms) is only 10° in the closed conformations of both TPL-Ala and TPL-Met complexes and 13° in the open active site of TPL-Ala complex. The carboxylate group is rotated with respect to the rest of the “alanine moiety” by 7° and 8° in the closed and open active sites of TPL-Ala, respectively. In TPL-Met this rotation amounts to 9°. Thus the interplanar angles are small and not significantly different in the different active sites. Such small angles between the planar moieties indicate that the π-electrons are highly delocalized along the system of conjugated double bonds in the quinonoid molecules.
Coordinates and structure factors of the quinonoid intermediates of TPL-Ala and TPL-Met were deposited with the Protein Data Bank with accession numbers PDB 2VLF and PDB 2VLH, respectively.
DISCUSSION
Mechanism of β-Elimination—Experimental observations presented here show and clarify the structural and mechanistic roles of several residues (Scheme 2). In particular, the structures show that Asn-185 stabilizes the quinonoid intermediate by making hydrogen bonds bridging the O3′ atom of PLP with the carboxyl of the substrate (Figs. 3 and 4), in agreement with previous propositions based on mutagenesis and kinetic studies (42). Lys-257 is ideally positioned to be the base abstracting the Cα proton with its ε-amino group being within a distance of 3.7–3.8 Å. Also, it is hydrogen-bonded to the negatively charged PLP phosphate (43) and side chain hydroxyl groups of Ser-51 and Ser-254. These interactions facilitate the Cα proton abstraction and position the ε-amino group of Lys-257 in an orientation suitable for this task. In agreement with structural observations, mutations of Ser-254 to Ala and Cys significantly reduced the enzymatic activity (44). In the structure of the Met quinonoid the Tyr-71 side chain hydroxyl is the closest group to the Cγ atom of the substrate (3.4 Å) and is thus in a suitable position for donating a proton (19). Furthermore, it is hydrogen-bonded to the positively charged side chain of Arg-100, both in TPL-Ala and TPL-Met (Figs. 3 and 4). This would facilitate the proton donation by stabilizing the negatively charged phenolate formed in that process (Scheme 2). In addition to Tyr-71, the side chain of the amino acid substrate is surrounded by Arg-100, Phe-123, Thr-124, Met-379, Arg-381, Phe-448, and Phe-449 which form a tight pocket (Fig. 4). We postulate that these residues are important for determining the specificity toward the physiological substrate. A catalytic role has been previously established only for two of these residues, Thr-124 and Phe-448 (45). Finally, we note that a hydrogen bonding network connecting the ε-amino group of Lys-257 and the phenolic hydroxyl of Tyr-71 (Figs. 3 and 4) may provide the route for the observed proton transfer between the Cα and Cγ atoms of the substrate (39). The observed 7–10% efficiency of the transfer is consistent with the number of mediating hydrogen bonds connecting the two residues.
Modeling of the physiological substrate in the active site of TPL suggests that the quinonoid intermediate with l-Tyr can be accommodated only if the active site is in the open conformation, due to unfavorable planar geometry at the Cγ atoms (15). It is likely that such a structure, like the respective Met quinonoid intermediate, is not stable in the open conformation due to the lack of interactions between the phenolic ring of the substrate and the active site residues of the small rigid region (Phe-36, Phe-448, Phe-449, Met-379, and Arg-381; Fig. 4). In the closed conformation, where such interactions are present, the phenol moiety may be accommodated only in the conformation corresponding to the keto-quinonoid intermediate, which, in accordance with the generally accepted chemical mechanism of TPL catalysis (Scheme 2), forms upon tautomerization of the phenol moiety into cyclohexadienone. Thus, the transition to the closed conformation of the active site may be considered as a driving force for the tautomerization. Our observations indicate that this process occurs through protonation of the Cγ atom by Tyr-71 assisted by the transfer of the phenolic proton to Arg-381. The following β-elimination of phenol, which is the rate-limiting stage of the whole enzymatic reaction (46), should result from the breakdown of the stereo-electronically optimally oriented bond between the Cβ and Cγ atoms. Regeneration of aromaticity in the cyclic moiety implies the appearance of the negative charge on the oxygen atom, and the return of the proton from Arg-381. The hydrophobic conditions of the closed active site should result in the phenolate anion being about 4 orders of magnitude stronger base than the guanidine (47) thus favoring such a process. Movement of the small domain toward the large one has been observed for two out of eight crystallographically independent subunits of the external aldimine complex of E. herbicola TPL with PPT (14). However, closure of the active site in these subunits is not complete. In the reported structures of quinonoid intermediates the Cζ atom of Phe-448 is closer to the ligand by 1.9 Å with respect to its position in the TPL-PPT complex.
Taken together, the structural observations indicate that the active site closure occurs after the formation of the Michaelis-Menten complex. The partial closure (as observed in the TPL-PPT complex) might occur at the stage of the external aldimine formation (Scheme 2), while the complete closure most probably occurs during or immediately after the formation of the quinonoid intermediate. Current data demonstrate that this closure protects the quinonoid molecule from the solvent and brings the catalytically important residues into appropriate positions where they can interact with the quinonoid and the emerging transitional structure. Thus the active site closure facilitates the next steps in the β-elimination mechanism, i.e. the keto-quinonoid formation and the subsequent elimination of phenol (Scheme 2), which most probably occurs in the closed active site. We suggest that after the Cβ-Cγ bond cleavage, the active site opens in order to release the products of the β-elimination reaction and to bind a new molecule of the substrate.
Mechanism of Alanine Racemization—As shown by the previous spectroscopic studies, incubation of C. freundii TPL with l-or d-Ala results in the formation of quinonoid complexes (11, 33). Therefore, the structure of C. freundii TPL-Ala may help to deduce the Ala racemization mechanism. Because the internal return of the α-hydrogen was observed in the conversion of l- to d-Ala catalyzed by TPL (48), two possible mechanisms of the Ala racemization were suggested (35). In the proposed single-base swinging door mechanism, the Ala quinonoid molecule would have to rotate by 180° in order to expose its other face to the fixed acid-base catalyst handling the proton abstraction/donation. This is unlikely because the quinonoid molecule is tightly bound in both closed and open active sites of the TPL-Ala. The principal manner of the cofactor binding is the same in all known TPL structures, so drastic reorientation of the PLP plane looks quite improbable. According to the second, two-base mechanism of Ala racemization, two different acid-base catalysts participate in the reaction (Scheme 3). Structural data on the TPL–Ala complex support the second, two-base mechanism and indicate that the ε-amino group of Lys-257 (Fig. 3) is the most probable base which abstracts the Cα proton in the l-Ala external aldimine. For the d-Ala formation, the si face of the Cα atom in the quinonoid molecule has to be protonated. The water molecules Wat2 and Wat3 are in the most suitable position for this task and, furthermore, they could be activated by the guanidinium group of Arg-381 through the hydrogen bond interactions. The observed internal return of the Cα proton could be explained by a shuttle system between Lys-257 and Wat2/Wat3 realized through a hydrogen bonding network in the closed active site. This network involves the PLP phosphate group, water molecule Wat1 and side chains of Ser-51, Ser-254, Asn-98, Arg-100, Tyr-291, and Tyr-71 (Fig. 3). We suggest that a molecule of d-Ala binds in the active site in the same manner as a molecule of l-Ala, but this means that the α-hydrogen atom is oriented in the exactly opposite direction (Scheme 3). In the case of d-Ala, the roles of the catalytic base and acid in the racemization mechanism should be mutually interchanged with those proposed for l-Ala. These could explain a higher value of Km and a lower value of kcat observed for the racemization of d-Ala in comparison to those for l-Ala (35).
SCHEME 3.
Proposed mechanism of alanine racemization catalyzed by TPL.
A two-base mechanism of Ala racemization was also proposed for alanine racemase (49–51) where the ε-amino group of the PLP-binding lysine and the hydroxyl of Tyr situated on the opposite face of the substrate Cα act as acid/base catalysts. It should be noted, however, that in the framework of the suggested racemization mechanism the quinonoid intermediates formed with l- and d-Ala should be identical in every respect except for the position of the proton abstracted from the α-position of the bound enantiomer. Thus it is reasonable to expect the existence of only one quinonoid intermediate on the racemization reaction pathway. Meanwhile, as shown by the previous rapid kinetic studies of TPL interaction with l- and d-Ala, incubation of C. freundii TPL with l-or d-Ala results in the formation of two distinct quinonoid complexes. Interconversion of the two quinonoids, proceeding through formation of a third one, is the rate-limiting step of the racemization reaction (35). It is likely that the observed slow rate of the interconversion is associated with the shuttle of the abstracted proton between the catalytic bases. This process should change charge distribution in the active site, and thus may be accompanied by considerable conformational adjustments. In this case, the chiral environment of the quinonoid formed with d-Ala could be somewhat different, and possibly even more favorable for the protonation from the si-face than that observed in the complex with l-Ala.
The Role of the Active Site Closure—Earlier biochemical studies suggested structural rearrangements in the active site upon quinonoid formation (35). The structures reported here show that such rearrangements lead to the active site closure and are accompanied by significant movement of the large and small rigid regions toward each other (∼12 Å displacement for extreme residues; Fig. 2). We note that in the crystals of holo-TPL both subunits are in the open conformation, indicating that crystal contacts do not restrict domain movement (at least not in subunit A) during the closure of the active site. This notion is further supported by the observed (but not modeled) disorder of the small rigid region and the flexible parts of subunit A in the TPL-Met structure. Interestingly, the transition from holo to quinonoid structure does not result in significant changes in the crystal unit cell dimensions (15) despite significant differences in crystal contacts of both A and B subunits. Moreover, different active site conformations (open and closed) were also found in the crystals of C. freundii apoenzyme, which have very similar unit cell parameters (15). These observations indicate that in the absence of PLP the TPL subunit can exist in both conformations and only the intersubunit interactions and interactions between two rigid regions of the same subunit determine which conformation prevails for a certain subunit of the catalytic dimer. The PLP molecule bound in the internal aldimine appears to stabilize the open conformation of the active site. This is not surprising since the first step in the enzymatic mechanism, the diffusion of the substrate molecule into the active site, must occur when it is in the open conformation. Indeed, the substrate analog HPPA, which lacks the tyrosine amino group, is bound in the open conformation of TPL (13). These observations may be explained by different interactions in the internal aldimine with respect to the other intermediates: e.g. in the internal aldimine Lys-257 is covalently bound to the PLP, while in all other intermediates Lys-257 assumes more relaxed conformation making hydrogen bonds with the PLP phosphate, Ser-254 and Ser-51 (Figs. 3 and 4); also, the orientation of the pyridoxal ring in the internal aldimine has direct influence on conformations of Phe-123, Asp-214, Thr-216, and other neighboring residues, which in turn cause slight, but presumably important, conformational changes farther from the active site and thus affecting the intersubunit and intrasubunit (between the small and large rigid regions) interactions. We conclude that whether or not the active sites of TPL in the crystalline state stay in the open conformation, depends on the presence or absence of abovementioned protein-PLP interactions.
In the TPL-Met (as well as in the TPL-Ala) crystal the entrance into the open active site is oriented toward a channel filled by solvent and it is not hindered by neighboring protein molecules, so binding of the substrate can occur. The Met quinonoid intermediate in the closed active site is stabilized by interactions of the Met side chain with Phe-36, Phe-448, Phe-449, Met-379, and Arg-381 (Fig. 4). All of these residues belong to the small rigid region and if the active site were open, they would be too far away to interact with the Met quinonoid intermediate. It is possible that binding of the substrate with a bulky side chain (such as methionine) requires conformational adjustments of the small and large rigid regions. We cannot exclude that such conformational adjustments are restricted by crystal contacts in the case of subunit B thus preventing methionine binding in this subunit. However, observation of the two different states in the crystal structure (quinonoid and internal aldimine) correlates with solution absorption spectra (20). The presence of both internal aldimine and quinonoid intermediate in the case of TPL-Met crystals may indicate some communication (cooperativity) between the two TPL active sites of the catalytic dimer. This is possible because each active site is composed of the residues from both subunits of the same catalytic dimer. It is possible that when one active site is closed and occupied by the quinonoid intermediate, the other active site stays in a form that prevents methionine binding.
Internal Return and Isotope Exchange of the Cα Hydrogen in TPL Reactions—It was mentioned above that racemization of Ala, catalyzed by TPL, is characterized by the internal return of the Cα hydrogen (48). Internal return of the Cα hydrogen to the leaving phenol group was also observed in the β-elimination reaction with the natural substrate, l-Tyr (39). The present structural data show that in both cases the internal return may occur through the system of hydrogen bonds connecting the active site residues and water molecules. The observed extents of the internal return during alanine racemization (3.5%) and β-elimination reaction (7–10%) are consistent with the number of mediating hydrogen bonds connecting the Nζ of Lys-257 with the water molecules Wat2 and Wat3 (alanine racemization, Fig. 3) and with Tyr-71 (β-elimination, Fig. 4). We note that in the closed conformation these residues and water molecules are isolated from the bulk solvent, while in the open conformation some of them are solvent accessible and thus prone to the hydrogen isotope exchange with the solvent. The rates of deprotonation-reprotonation processes (leading to the isotopic exchange) are higher than those for racemization or β-elimination reactions (35, 52). If the isotopic exchange occurred only in the open conformation, the extents of the internal return would be significantly lower than the observed ones (39, 48).
TPL also catalyzes the stereospecific isotope exchange of the Cα hydrogen of several l-amino acids, which occurs with the retention of the amino acid (S)-configuration. Kinetic data showed that the Cα hydrogen isotope exchange for l-Phe proceeds via a quinonoid intermediate (53). In contrast, in the case of l-Met, an alternative mechanism of isotope exchange, not involving the quinonoid intermediate, must exist. This is because the quinonoid intermediate with l-Met is a dead-end complex as indicated by the comparison of the reaction rates of the Cα proton abstraction from the external aldimine and reprotonation of the quinonoid with the rate of the Cα hydrogen isotope exchange (53). According to this alternative mechanism, the Cα hydrogen isotope exchange for l-Met occurs by a concerted mechanism (Scheme 4) involving a six-membered transition state, which includes the ε-amino group of Lys-257 and a water molecule (D2O) hydrogen-bonded to it (53). Analysis of the structure of the closed active site of TPL-Met shows that the active site does indeed contain a water molecule (Wat1 on Fig. 4), which could perform this task. Although this water molecule is not hydrogen-bonded to the ε-amino group of Lys-257 in the structure of the quinonoid intermediate, such interaction may be favored in the external aldimine.
SCHEME 4.
Concerted mechanism of Cα hydrogen isotope exchange in the external aldimine of methionine.
Supplementary Material
Acknowledgments
We thank Robert Byrne for critically reading the manuscript and Martin Walsh for help during data collection at the BM14 beamline (ESRF, Grenoble), which is supported by the UK, BBSRC, EPSRC, and MRC Research Councils.
The atomic coordinates and structure factors (codes 2VLF and 2VLH) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (Grant 119-1193079-1084 (to D. M. and D. M.-Č.)), by the Russian Foundation for Basic Research (Grants 08-04-00117 (to T. V. D.) and 07-04-01138 (to N. G. F.)), by the Fogarty Foundation (Grant R03 TW006045-01-2A (to T. V. D.)), and by the Wellcome Trust (Fellowship 081916, to A. A. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and data.
Footnotes
The abbreviations used are: TPL, tyrosine phenol-lyase, EC 4.1.99.2; HPPA, 3-(4′-hydroxyphenyl)propanoate; MME, monomethyl ether; PEG, poly-(ethyleneglycol); PLP, pyridoxal 5′-phosphate; PPT, N-(5′-phosphopyridoxyl)-l-tyrosine; r.m.s., root-mean-square; TPL-Ala, complex of tyrosine phenol-lyase and quinonoid intermediate formed with alanine; TPL-Met, complex of tyrosine phenol-lyase and quinonoid intermediate formed with methionine.
References
- 1.Kumagai, H., Yamada, H., Matsui, H., Ohkishi, H., and Ogata, K. (1970) J. Biol. Chem. 245 1767-1772 [PubMed] [Google Scholar]
- 2.Phillips, R. S. (1987) Arch. Biochem. Biophys. 256 302-310 [DOI] [PubMed] [Google Scholar]
- 3.Faleev, N. G., Zhukov, Y. N., Khurs, E. N., Gogoleva, O. I., Barbolina, M. V., Bazhulina, N. P., Belikov, V. M., Demidkina, T. V., and Khomutov, R. M. (2000) Eur. J. Biochem. 267 6897-6902 [DOI] [PubMed] [Google Scholar]
- 4.Enei, H., Nakazawa, H., Matsui, H., Okumura, S., and Yamada, H. (1972) FEBS Lett. 21 39-41 [DOI] [PubMed] [Google Scholar]
- 5.Yamada, H., and Kumagai, H. (1975) Adv. Appl. Microbiol. 19 249-288 [DOI] [PubMed] [Google Scholar]
- 6.Yamada, H., Kumagai, H., Kashima, N., Torii, H., Enei, H., and Okumura, S. (1972) Biochem. Biophys. Res. Commun. 46 370-374 [DOI] [PubMed] [Google Scholar]
- 7.Kumagai, H., Matsui, H., Ohgishi, H., Ogata, K., Yamada, H., Ueno, T., and Fukami, H. (1969) Biochem. Biophys. Res. Commun. 34 266-270 [DOI] [PubMed] [Google Scholar]
- 8.Ueno, T., Fukami, H., Ohkishi, H., Kumagai, H., and Yamada, H. (1970) Biochim. Biophys. Acta 206 476-479 [DOI] [PubMed] [Google Scholar]
- 9.Phillips, R. S., Ravichandran, K., and Von Tersch, R. L. (1989) Enzyme Microb. Technol. 11 80-83 [Google Scholar]
- 10.Kumagai, H., Kashima, N., and Yamada, H. (1970) Biochem. Biophys. Res. Commun. 39 796-801 [DOI] [PubMed] [Google Scholar]
- 11.Demidkina, T. V., Myagkikh, I. V., and Azhayev, A. V. (1987) Eur. J. Biochem. 170 311-316 [DOI] [PubMed] [Google Scholar]
- 12.Antson, A. A., Demidkina, T. V., Gollnick, P., Dauter, Z., Von Tersch, R. L., Long, J., Berezhnoy, S. N., Phillips, R. S., Harutyunyan, E. H., and Wilson, K. S. (1993) Biochemistry 32 4195-4206 [DOI] [PubMed] [Google Scholar]
- 13.Sundararaju, B., Antson, A. A., Phillips, R. S., Demidkina, T. V., Barbolina, M. V., Gollnick, P., Dodson, G. G., and Wilson, K. S. (1997) Biochemistry 36 6502-6510 [DOI] [PubMed] [Google Scholar]
- 14.Pletnev, S. V., Antson, A. A., Sinitsyna, N. I., Dauter, Z., Isupov, M. N., Hurs, E. N., Faleev, N. G., Wilson, K. S., Dodson, G., Demidkina, T. V., and Arutyunyan, E. G. (1997) Crystallogr. Rep. 42 809-819 [Google Scholar]
- 15.Milić, D., Matković-Čalogović, D., Demidkina, T. V., Kulikova, V. V., Sinitzina, N. I., and Antson, A. A. (2006) Biochemistry 45 7544-7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips, R. S., Demidkina, T. V., and Faleev, N. G. (2003) Biochim. Biophys. Acta 1647 167-172 [DOI] [PubMed] [Google Scholar]
- 17.Szebenyi, D. M. E., Liu, X., Kriksunov, I. A., Stover, P. J., and Thiel, D. J. (2000) Biochemistry 39 13313-13323 [DOI] [PubMed] [Google Scholar]
- 18.Barends, T. R. M., Domratcheva, T., Kulik, V., Blumenstein, L., Niks, D., Dunn, M. F., and Schlichting, I. (2008) ChemBioChem 9 1024-1028 [DOI] [PubMed] [Google Scholar]
- 19.Chen, H. Y., Demidkina, T. V., and Phillips, R. S. (1995) Biochemistry 34 12276-12283 [DOI] [PubMed] [Google Scholar]
- 20.Faleev, N. G., Ruvinov, S. B., Demidkina, T. V., Myagkikh, I. V., Gololobov, M. Y., Bakhmutov, V. I., and Belikov, V. M. (1988) Eur. J. Biochem. 177 395-401 [DOI] [PubMed] [Google Scholar]
- 21.Phillips, R. S., Demidkina, T. V., Zakomirdina, L. N., Bruno, S., Ronda, L., and Mozzarelli, A. (2002) J. Biol. Chem. 277 21592-21597 [DOI] [PubMed] [Google Scholar]
- 22.Otwinowski, Z., and Minor, W. (1997) Methods Enzymol. 276 307-326 [DOI] [PubMed] [Google Scholar]
- 23.Collaborative Computational Project, Number 4. (1994) Acta Crystallogr. Sect. D. 50 760-76315299374 [Google Scholar]
- 24.Navaza, J. (1994) Acta Crystallogr. Sect. A. 50 157-163 [Google Scholar]
- 25.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. Sect. D. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 26.Perrakis, A., Morris, R., and Lamzin, V. S. (1999) Nat. Struct. Biol. 6 458-463 [DOI] [PubMed] [Google Scholar]
- 27.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. Sect. D. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 28.Winn, M. D., Isupov, M. N., and Murshudov, G. N. (2001) Acta Crystallogr. Sect. D. 57 122-133 [DOI] [PubMed] [Google Scholar]
- 29.Hašek, J. (2006) Z. Kristallogr. Suppl. 23 613-618 [Google Scholar]
- 30.Davis, I. W., Leaver-Fay, A., Chen, V. B., Block, J. N., Kapral, G. J., Wang, X., Murray, L. W., Arendall, W. B., III, Snoeyink, J., Richardson, J. S., and Richardson, D. C. (2007) Nucleic Acids Res. 35 W375-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krissinel, E., and Henrick, K. (2007) J. Mol. Biol. 372 774-797 [DOI] [PubMed] [Google Scholar]
- 32.DeLano, W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific, Palo Alto, CA
- 33.Demidkina, T. V., and Myagkikh, I. V. (1989) Biochimie (Paris) 71 565-571 [DOI] [PubMed] [Google Scholar]
- 34.Muro, T., Nakatani, H., Hiromi, K., Kumagai, H., and Yamada, H. (1978) J. Biochem. (Tokyo) 84 633-640 [DOI] [PubMed] [Google Scholar]
- 35.Chen, H., and Phillips, R. S. (1993) Biochemistry 32 11591-11599 [DOI] [PubMed] [Google Scholar]
- 36.Kumagai, H., Yamada, H., Matsui, H., Ohkishi, H., and Ogata, K. (1970) J. Biol. Chem. 245 1773-1777 [PubMed] [Google Scholar]
- 37.Read, R. (1986) Acta Crystallogr. Sect. A. 42 140-149 [Google Scholar]
- 38.Mouratou, B., Kasper, P., Gehring, H., and Christen, P. (1999) J. Biol. Chem. 274 1320-1325 [DOI] [PubMed] [Google Scholar]
- 39.Faleev, N. G., Lyubarev, A. E., Martinkova, N. S., and Belikov, V. M. (1983) Enzyme Microb. Technol. 5 219-224 [Google Scholar]
- 40.Ivanov, V. I., and Karpeisky, M. Y. (1969) Adv. Enzymol. Relat. Areas Mol. Biol. 32 21-53 [DOI] [PubMed] [Google Scholar]
- 41.Kirsch, J. F., Eichele, G., Ford, G. C., Vincent, M. G., Jansonius, J. N., Gehring, H., and Christen, P. (1984) J. Mol. Biol. 174 497-525 [DOI] [PubMed] [Google Scholar]
- 42.Barbolina, M. V., Phillips, R. S., Gollnick, P. D., Faleev, N. G., and Demidkina, T. V. (2000) Protein Eng. 13 207-215 [DOI] [PubMed] [Google Scholar]
- 43.Schnackerz, K. D., Keller, J., Phillips, R. S., and Toney, M. D. (2006) Biochim. Biophys. Acta 1764 230-238 [DOI] [PubMed] [Google Scholar]
- 44.Papisova, A. I., Bazhulina, N. P., Faleev, N. G., and Demidkina, T. V. (2003) Dokl. Biochem. Biophys. 391 225-228 [DOI] [PubMed] [Google Scholar]
- 45.Demidkina, T. V., Barbolina, M. V., Faleev, N. G., Sundararaju, B., Gollnick, P. D., and Phillips, R. S. (2002) Biochem. J. 363 745-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faleev, N. G., Spirina, S. N., Demidkina, T. V., and Phillips, R. S. (1996) J. Chem. Soc. Perkin Trans. 2 1996 2001-2004 [Google Scholar]
- 47.Faleev, N. G. (2003) in Protein Structures. Kaleidoscope of Structural Properties and Structures (Uversky, V. N., ed), Research Signpost, Kerala, India
- 48.Palcic, M. M., Shen, S. J., Schleicher, E., Kumagai, H., Sawada, S., Yamada, H., and Floss, H. G. (1987) Z. Naturforsch. 42 307-318 [DOI] [PubMed] [Google Scholar]
- 49.Stamper, C. G. F., Morollo, A. A., and Ringe, D. (1998) Biochemistry 37 10438-10445 [DOI] [PubMed] [Google Scholar]
- 50.Sun, S., and Toney, M. D. (1999) Biochemistry 38 4058-4065 [DOI] [PubMed] [Google Scholar]
- 51.Watanabe, A., Yoshimura, T., Mikami, B., and Esaki, N. (1999) J. Biochem. (Tokyo) 126 781-786 [DOI] [PubMed] [Google Scholar]
- 52.Chen, H., Gollnick, P., and Phillips, R. S. (1995) Eur. J. Biochem. 229 540-549 [PubMed] [Google Scholar]
- 53.Faleev, N. G., Demidkina, T. V., Tsvetikova, M. A., Phillips, R. S., and Yamskov, I. A. (2004) Eur. J. Biochem. 271 4565-4571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.