Abstract
Ezrin is a multidomain protein providing regulated membrane-cytoskeleton contacts that play a role in cell differentiation, adhesion, and migration. Within the cytosol of resting cells ezrin resides in an autoinhibited conformation in which the N- and C-terminal ezrin/radixin/moesin (ERM) association domains (ERMADs) interact with one another. Activation of the ezrin membrane-cytoskeleton linker function requires an opening of this interdomain association that can result from phosphatidylinositol 4,5-bisphosphate binding to the N-ERMAD and threonine 567 phosphorylation in the C-ERMAD. We have shown that ezrin can also be activated by Ca2+-dependent binding of the EF-hand protein S100P. We now provide a quantitative analysis of this interaction and map the respective binding sites to the F2 lobe in the ezrin N-ERMAD and a stretch of hydrophobic residues in the C-terminal extension of S100P. Phospholipid binding assays reveal that S100P and phosphatidylinositol 4,5-bisphosphate compete to some extent for at least partially overlapping binding sites in N-ERMAD. Using interaction-competent as well as interaction-incompetent S100P derivatives and permanently active ezrin mutants, we also show that the protein interaction and a resulting activation of ezrin promote the transendothelial migration of tumor cells. Thus, a prometastatic role of ezrin and S100P that had been proposed based on their overexpression in highly metastatic cancers is probably due to a direct interaction between the two proteins and the S100P-mediated activation of ezrin.
ERM2 (ezrin/radixin/moesin) family proteins function as membrane-cytoskeleton linkers in a number of dynamic processes ranging from the formation and maintenance of actin-rich surface structures such as microvilli to the control of cell-cell and cell-cell matrix contacts and the regulation of cell migration (for review, see Refs. 1–3). Often these processes are accompanied by the establishment of cellular polarity, one example being the front-rear asymmetry in motile cells. The latter is particularly evident in leukocyte migration that requires the regulation of ERM protein activity at the rear end of the cells for efficient migration (4, 5). In addition to providing direct physical scaffolds in the membrane skeleton, ERM proteins also function in diverse aspects of intracellular signaling, for example by directly interacting with effectors of Rho GTPase signaling (for review, see Ref. 6).
The three-modular structure of ERM proteins is ideally suited for carrying out cross-linking and signaling functions in a regulated manner. The N-terminal domain, also known as N-terminal ERM association domain (N-ERMAD) or FERM domain (for Four point one ERM), is a conserved element shared with other members of the band 4.1 superfamily of proteins. It is followed by a central α-helical domain with the propensity to form coiled-coils and a C-terminal ERMAD. Binding sites for PtdIns(4,5)P2 and several plasma membrane-resident proteins like the hyaluronate receptor CD44, and different intercellular cell adhesion molecules are located in the N-ERMAD. The C-ERMAD, on the other hand, contains an F-actin binding site, whereas the central region appears to contribute to the binding of other protein ligands like the p85 subunit of phosphatidylinositol 3-kinase in the case of ezrin (1). However, in resting, non-stimulated cells most of these binding sites are masked through intra- and intermolecular interactions between the N- and C-ERMADs, thereby keeping ERM proteins in an autoinhibited or dormant state. Specifically, binding sites for the cytoplasmic tails of plasma membrane proteins and for F-actin are inaccessible in dormant ERM proteins. Hence, inactive ERM proteins reside in the cytoplasm, and it requires cell stimulation, e.g. after exposure to growth factors, to activate the membrane-cytoskeleton cross-linking function of ERM proteins and recruit them to the membrane.
Activation of ERM proteins can occur by different means and has been studied most extensively for ezrin. Here phosphorylation at threonine 567 in the C-ERMAD, a target for protein kinase Cα acting downstream of activated plasma membrane receptors, leads to the activation of the ezrin membrane-cytoskeleton linking function (7). Ezrin also represents a substrate of different receptor tyrosine kinases and tyrosine phosphorylation, e.g. occurring after activation of the Kit and Flt3 receptors, has recently been shown to activate a metastasis-promoting function of ezrin (8). In line with a role of ezrin in tumor progression and invasion are the observations that it is required for malignant glioma cell invasion (9) and Rho/ROCK-dependent tumor cell motility in three-dimensional matrices (10). Moreover, the expression of ezrin is increased in a number of highly metastatic cancers (for review, see Refs. 11 and 12). At least partial activation of ezrin can also occur after PtdIns(4,5)P2 binding in the N-ERMAD (for review, see Refs. 1, 13, and 14).
We previously identified the Ca2+-binding protein S100P as a novel activator of ezrin. The Ca2+-regulated binding of S100P to ezrin unmasks the F-actin binding site thereby activating the ezrin molecule (15). S100P is a member of the S100 family of EF-hand Ca2+ binding proteins that function by regulating the activity of effector proteins in their Ca2+-bound state. Several S100 proteins are up-regulated in human tumor cells and have been proposed to function in metastatic processes (for review, see Ref. 16). These include S100P whose expression is markedly increased in highly metastatic non-small cell lung carcinomas (NSCLCs) correlating with an increased migration/invasion (17). Based on the apparent coregulation of the ezrin and S100P expression in metastatic tumors, we further examined the ezrin-S100P interaction and analyzed whether the S100P-mediated activation of ezrin contributes to an increased migratory phenotype of cancer cells. We show that ezrin and S100P co-localize in stimulated cells and can be co-immunoprecipitated in the presence of Ca2+. The affinity of complex formation was measured by surface plasmon resonance experiments, and structural parameters of the S100P-ezrin interaction were elucidated by mapping the respective binding sites. This led to the generation of a S100P mutant incapable of ezrin binding that served as an inert control in cell migration analyses, establishing a role of ezrin-S100P complex formation in the transendothelial migration of NSCLCs.
EXPERIMENTAL PROCEDURES
Construction of Plasmids—The pET28a+ and pEGFP-C2 constructs encoding N-terminal histidine- or GFP-tagged human WT S100P, human WT ezrin, and ezrin N-ERMAD (aa 1–323) had been described previously (15). cDNAs encoding the human S100P deletion mutants S100P 91aa and S100P 87aa and the ezrin deletion mutants ezrin 233aa, ezrin 197aa, ezrin 173aa and ezrin 82aa were generated by polymerase chain reactions using the pET28a+ plasmids containing WT S100P and WT ezrin as templates. For the recombinant expression of non-tagged proteins, the cDNAs of N-ERMAD and the S100P deletion mutants were cloned into a pET23a+ (Novagen, Madison, WI) vector modified to express the recombinant protein without T7 tag, and in the case of S100P WT, into the pKK223-3 vector (Amersham Biosciences). PCR was performed using Turbo Pfu DNA polymerase (Stratagene, La Jolla, CA) and oligonucleotide primers containing the appropriate stop codons and restriction enzyme sites. The cDNA encoding the T567D ezrin mutant protein was generated by using accordingly mutated primers in the PCR reaction. For bacterial protein expression of His- or GST-tagged derivatives, the mutant cDNAs were cloned into the pET28a+ vector (Novagen) inframe with the 6× His tag (S100P derivatives) or into the pGEX4T-1 vector (Amersham Biosciences) in-frame with the GST tag (ezrin derivatives). For migration and localization analyses, coding sequences of S100P deletion constructs and ezrin T567D were cloned into the pEGFP-C2 vector (Clontech, Mountain View, CA) to yield in-frame fusion proteins with an N-terminal EGFP.
Cell Culture and Generation of Stable Cell Lines—Human microvascular endothelial cells (HMEC-1) were cultured in MCDB 131 medium (Invitrogen) supplemented with 10% FCS gold (PAA, Linz, Austria), 20 mm l-glutamine, 50 μg/ml gentamycin, 10 ng/ml epidermal growth factor, and 10 μg/ml hydrocortisone. SK-BR-3 cells (human mammary gland) were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 0.1 mm nonessential amino acids, 1 mm sodium pyruvate, 2 mm l-glutamine, and antibiotics. The human lung squamous carcinoma cell line HTB-58 was cultured in Eagle's modified essential medium supplemented with 0.1 mm nonessential amino acids, 1 mm sodium pyruvate, 2 mm l-glutamine, 10% heat-inactivated fetal bovine serum, and antibiotics. For the generation of stable cell lines expressing GFP-tagged WT S100P, S100P deletion mutants, ezrin T567D, or GFP alone, HTB-58 cells were transfected with the appropriate pEGFP fusion constructs and selected using the antibiotic G418 (750 μg/ml). To eliminate clone-specific effects mixed cultures of selected cell lines were used in all experiments. The expression of the GFP constructs was verified by Western blotting employing anti-EGFP antibodies as well as by fluorescence-activated cell sorting.
Immunofluorescence Staining—To induce cell stimulation accompanied by an intracellular Ca2+ transient, SK-BR-3 cells grown on coverslips were transferred to Dulbecco's modified Eagle's medium without fetal calf serum and cultured for 16 h. 10% heat-inactivated fetal bovine serum was then added, and cells were incubated for 30 min. Subsequently, cells were fixed with 3% formaldehyde in PBS for 10 min at room temperature. After quenching in 50 mm NH4Cl for 7 min, cells were permeabilized with 0.2% Triton X-100 in PBS for 2 min and incubated in PBS supplemented with 2% bovine serum albumin for 30 min. Cells were then first incubated with an anti-ezrin polyclonal antibody (Upstate Biotechnology, Inc., Lake Placid, NW) for 30 min and washed 3 times with 2% bovine serum albumin, PBS followed by incubation with an anti-S100P mouse monoclonal antibody (generated by injecting mice with purified His-tagged human S100P) for 30 min. After washing three times with PBS, primary antibodies were stained with Cy2-conjugated goat anti-rabbit and Red-X-conjugated donkey anti-mouse secondary antibodies (Dianova, Hamburg, Germany). Cells were washed 3 times with PBS, once with distilled H2O, mounted in Mowiol, and analyzed using a confocal laser scanning microscope (LSM 510; Zeiss, Jena, Germany).
Transient Transfection and Co-immunoprecipitation—Transient transfection of SK-BR-3 cells with the GFP-WT S100P expression construct was carried out by electroporation (10 μg of DNA, 200 V, 1050 microfarads) of trypsinized cells in 4-mm cuvettes. After transfection cells were seeded on 100-mm cell culture dishes and 6 h after transfection transferred to Dulbecco's modified Eagle's medium without fetal calf serum and cultured for 16 h. Cells were then lysed in 1.1 ml of lysis buffer (1% Triton X-100, 30 mm Hepes, pH 7.2, 140 mm NaCl, 1 mm dithiothreitol, 2 mm MgCl2, 1 mm EDTA, 1 mm EGTA, complete protease inhibitor mixture (Roche Applied Science)). The lysate was centrifuged for 10 min at 20,000 × g, and the resulting post-nuclear supernatant (PNS) was dialyzed against buffer D (30 mm Hepes, pH 7.2, 300 mm NaCl, 2 mm MgCl2, 10 mm β-mercaptoethanol, 1 mm PMSF, complete protease-inhibitor mixture (Roche Applied Science)). After dialysis the PNS was adjusted to 0.5 mm CaCl2, centrifuged for 20 min at 100,000 × g, and incubated overnight with 10 μg of anti-ezrin polyclonal rabbit antibodies coupled to 100 μl Dynabeads® M-280 sheep anti-rabbit (Invitrogen). As a control, an equal amount of PNS was incubated in parallel with nonspecific anti-mouse IgG polyclonal rabbit antibodies (DAKO, Glostrup, Denmark) coupled to 100 μl of Dynabeads® M-280 sheep anti-rabbit. Beads were washed 3 times with 0.1% bovine serum albumin, 0.5 mm CaCl2 in PBS and then treated for 5 min at 95 °C with an equal volume of 4× SDS sample buffer. Proteins released from the beads were analyzed by SDS-PAGE and immunoblotting.
Recombinant Expression and Purification of S100P and Ezrin—pET28a+ constructs encoding N-terminal His-tagged WT and mutant S100P as well as pGEX4T-1 constructs encoding WT ezrin and ezrin deletion mutants with an N-terminal GST tag were used to transform Escherichia coli (strain BL21 (DE3)pLysS). Transformed bacteria were grown to an A600 of 0.6, and recombinant protein expression was then induced by adding isopropyl 1-thio-β-d-galactopyranoside. After 3 h of incubation, cells were harvested by centrifugation (5000 × g, 10 min) and resuspended in lysis buffer (40 mm Hepes, pH 7.4, 300 mm NaCl, 20 mm imidazole, pH 7.4, 1 mm EDTA, 10 mm β-mercaptoethanol, 1.5 mm PMSF). Subsequently, cells were lysed by repeated freeze/thaw cycles (3 times) and sonication, and the lysates were cleared by centrifugation for 1 h at 100,000 × g.
For preparation of WT and mutant S100P, the remaining supernatant was adjusted to a Ca2+ concentration of 5 mm and applied to a phenyl-Sepharose (GE Healthcare) column equilibrated in lysis buffer containing 0.5 mm Ca2+ instead of EGTA. After extensive washing with the same buffer, bound S100P proteins were eluted with lysis buffer containing 1 mm EGTA. S100P-containing fractions were pooled, dialyzed against 20 mm imidazole, pH 7.4, 300 mm NaCl, 10 mm β-mercaptoethanol, 1.5 mm PMSF, and applied to a Ni-NTA-agarose (Qiagen, Hilden, Germany) column equilibrated in the same buffer. S100P proteins were eluted with 250 mm imidazole, pH 7.4, 300 mm NaCl, 10 mm β-mercaptoethanol, and 1 mm PMSF.
In the case of GST-tagged WT ezrin and ezrin deletion mutants the cleared bacterial lysates were applied to a glutathione-Sepharose 4B (Amersham Biosciences) column equilibrated in PBS containing 1.5 mm PMSF. After extensive washing with the same buffer, bound ezrin derivatives were eluted with 100 mm glutathione, 50 mm Tris-HCl, pH 8.0.
For preparation of non-tagged WT S100P and S100P deletion mutants, the cleared bacterial lysates were applied to a phenyl-Sepharose column and purified by developing the column as described above for the His-tagged S100P proteins. The non-tagged N-ERMAD protein was purified as described (18).
Real Time Binding Measured by Surface Plasmon Resonance (SPR)—SPR experiments were performed with a Biacore 3000 system (GE Healthcare). Purified N-ERMAD, S100P, or the mutants indicated were immobilized on a sensor surface, and the respective binding partners (analytes) were passed over the chip in a mobile aqueous phase. Sensor Chip CM5 was obtained from GE Healthcare. The HBS-P, 0.5 mm CaCl2 flow buffer consisted of 10 mm HEPES, pH 7.4, 150 mm NaCl, 0.005% (v/v) Tween 20, 0.5 mm CaCl2 and was filtered through 0.22-μm filters (Millipore) and degassed before use. Immobilization on the sensor chips employed amine coupling according to manufacturer's instructions. Briefly, using a flow rate of 5 μl/min, the chip surface was activated with an 8-min injection of a freshly prepared 1:1 mixture of 0.2 m N-ethyl-N-(3-diethylaminopropyl) carbodiimide and 0.05 m N-hydroxysuccinimide solution followed by injection of the protein to be coupled, typically at a concentration of 30 μg/ml in acetate buffer (pH 5.5 for N-ERMAD and 4.5 for S100P immobilization). When the desired level of immobilization was achieved, unreacted N-hydroxy-succinimide-ester groups were blocked with a 7-min injection of 1 m ethanolamine hydrochloride. Sensograms (response units versus time) were recorded at 25 °C with a typical flow rate of 40 μl/min and injection times of 3 min. Controls for the contribution of the change in bulk refractive index were performed in parallel with blank flow cells that were activated with N-ethyl-N-(3-diethylaminopropyl) carbodiimide/N-hydroxy-succinimide and deactivated with ethanolamine in the absence of protein to be coupled and subtracted from all binding sensograms. Blank injections of the running buffer were also performed and subtracted from all the kinetic measurements.
To analyze the binding of WT S100P and S100P 87aa to the immobilized N-ERMAD, different concentrations of WT S100P and S100P 87aa in the flow buffer (concentration range between 0.006 and 3.5 μm) were injected into the flow cells. Each injection was repeated at least 2-fold for reproducibility. Regeneration of the surface was performed using two short pulses of HBS-P, 3 mm EGTA buffer and glycine/HCl buffer, pH 2.5.
Approximate equilibrium dissociation constants (KD) were obtained by measuring the equilibrium resonance (Req) units at several ligand concentrations at equilibrium. The affinity of the interaction, i.e. the equilibrium dissociation constant (KD), was determined from the level of binding at equilibrium as a function of the sample concentrations by BIAevalution version 4.1 software (Biacore, Inc.). The steady state binding level is related to the concentration according to the Scatchard equation: Req/C = KARmax - ReqKA, where Rmax is the resonance signal at saturation, C is the concentration of free analyte, and KA is the equilibrium association constant.
Column-based Binding Assays—The interaction between the different S100P and ezrin derivatives was also analyzed using affinity columns containing one of the binding partners in an immobilized form. To prepare affinity columns, GST-tagged proteins (N-ERMAD, ezrin 233aa, ezrin 197aa, ezrin 173aa, and ezrin 82aa) were dialyzed against PBS containing 1.5 mm PMSF and rebound to glutathione-Sepharose 4B. The material was transferred to 5 ml of polypropylene columns (Pierce) and washed with PBS containing 0.5 mm CaCl2 to remove unbound protein and adjust the fluid phase to an elevated Ca2+ concentration. His-tagged S100P derivatives were then added in the fluid phase, the columns were washed extensively with PBS containing 0.5 mm CaCl2 to remove all non-specifically bound protein, and Ca2+-dependently bound proteins were eluted with PBS containing 1 mm EGTA. Columns were then stripped with 100 mm glutathione, 50 mm Tris-HCl, pH 8.0.
For the reciprocal experiments, His-tagged proteins (purified WT or mutant S100P) were dialyzed against buffer A (30 mm Hepes, pH 7.4, 25 mm imidazole, pH 7.4, 150 mm NaCl, 1 mm MgCl2, 0.5 mm CaCl2, 10 mm β-mercaptoethanol, 1.5 mm PMSF) and rebound to Ni-NTA-agarose. 500 μg of purified His-tagged protein were mixed with 250 μl of a 50% Ni-NTA agarose slurry equilibrated in buffer A and incubated for at least 2 h at 4 °C with gentle agitation. The mixtures were then transferred to the 5-ml polypropylene columns and washed with buffer A to remove unbound protein. Subsequently, purified GST-tagged proteins (N-ERMAD, ezrin 233aa, ezrin 197aa, ezrin 173aa, ezrin 82aa) were added to the column in the fluid phase. After washing with 10 column volumes of buffer A, Ca2+-dependent bound proteins were eluted with 5 column volumes of buffer B (same as buffer A but containing 1 mm EGTA instead of 0.5 mm CaCl2). The columns were stripped of His-tagged proteins by elution with 250 mm imidazole, pH 7.4. Samples of each elution and washing step were analyzed by 15% SDS-PAGE and subsequent immunoblotting with the respective antibodies.
Chemical Cross-linking—For chemical cross-linking experiments, 4 μg of the different S100P derivatives were dialyzed against buffer C (0.2 m Hepes, pH 7.2, 10 mm β-mercaptoethanol). Ca2+ was added to a final concentration of 0.5 mm, and the samples were incubated with the homobifunctional cross-linker BS3 (Sulfo-DSS) (final concentration 5 mm) at 22 °C for 45 min. Reactions were terminated by the addition of 2% SDS (w/v) and 2% β-mercaptoethanol, and samples were analyzed by 15% SDS-PAGE.
Liposome Co-sedimentation Assays—Small unilamellar liposomes containing 97% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC, Avanti Polar Lipids, Alabaster, AL) and 3% PtdIns(4,5)P2 (Avanti Polar Lipids) were prepared by sonication in buffer L (20 mm Tris-HCl, pH 7.4, 50 mm KCl, 1 mm dithiothreitol, 1 mm NaN3, 50 μm CaCl2). Typically this yielded vesicles in the size range of 15–50 nm that could be pelleted by centrifugation for 20 min at 100,000 × g. For liposome co-sedimentation assays 100 μg of the POPC/PtdIns(4,5)P2 liposomes were mixed with 20 μg of N-ERMAD and 20 μg of the S100P derivative indicated, and the mixture was adjusted to a total volume of 200 μl with buffer L. After incubation at room temperature for 30 min, the liposomes were pelleted by high speed centrifugation (20 min 100,000 × g) and washed 2 times in buffer L. The resulting supernatants (Sn1, unbound fraction; Sn2, Sn3, wash fractions) and the remaining pellets were subjected to 15% SDS-PAGE. Controls were carried out in buffer L without Ca2+ but containing 0.1 mm EDTA and 0.1 mm EGTA. They showed the same amount of N-ERMAD binding that was, however, not affected by the addition of S100P. Additional control experiments employed PtdIns(4,5)P2-free liposomes that did not show N-ERMAD binding. Furthermore, controls revealed that the different S100P derivatives were not capable of binding to the POPC- or POPC/PtdIns(4,5)P2-containing liposomes.
Lipid Plate Binding Assay—Phosphoinositide binding was also analyzed by a lipid-plate assay employing immobilized PtdIns(4,5)P2 (19). Therefore, wells of 96-well microtiter plates (F96 Maxisorp, Nunc, Wiesbaden, Germany) were coated with 100 ng of PtdIns(4,5)P2, air-dried, and then blocked for 1 h with 0.5% bovine serum albumin in PBS. Purified, His-tagged N-ERMAD in the absence or presence of the S100P derivatives indicated (800 ng/well and protein) was incubated in the PtdIns(4,5)P2-coated wells for 1 h at room temperature in PBS containing 50 μm CaCl2. Subsequently, the wells were washed 3 times with PBS containing 0.05% Tween, and the amount of lipid-bound N-ERMAD was determined by a colorimetric reaction using monoclonal anti-Penta-His antibodies (Qiagen), peroxidase-coupled secondary antibodies, and 3.3′, 5.5′-tetramethyl benzidine substrate (Perbio, Bonn, Germany). Control reactions using the same conditions but PBS without Ca2+ yielded no effect of any of the S100P derivatives on the binding of N-ERMAD to immobilized PtdIns(4,5)P2.
For statistical evaluation, the amount of N-ERMAD bound to the immobilized PtdIns(4,5)P2 in the absence of Ca2+ was set to 100%. The values obtained for binding in the presence of Ca2+, i.e. under conditions where N-ERMAD-S100P complex formation occurs, were then calculated in relation to these 100% with lower values, indicating inhibition of lipid binding. Data were obtained in triplicates of at least four independent experiments, and statistical significance was calculated by unpaired Student's t test.
Transendothelial Migration Analysis—For transendothelial migration experiments, 2.2 × 105 HMEC-1 were seeded into the upper chamber of a Transwell device (Costar, Bodenheim, Germany) and cultivated for 48 h on the microporous (8 μm) filter support to generate a confluent monolayer with a defined transendothelial electrical resistance (20). 5 × 105 HTB-58 cells expressing EGFP alone or the different EGFP-S100P or EGFP-ezrin mutants were then added to the upper chamber, and their transendothelial migration was quantified by counting the number of cells that had migrated into the lower chamber within a time period of 16 h. Flow cytometry of the GFP-positive cells employed a FACSCalibur (BD Biosciences).
RESULTS
Characterization of the Ezrin-S100P Interaction—To verify that the ezrin-S100P interaction identified previously (15) also occurs in vivo, we first carried out co-localization analyses employing SK-BR-3 human mammary epithelial cells. These cells were chosen because they can be induced by serum stimulation to form abundant ezrin-positive surface protrusions and microvilli. Fig. 1A shows that S100P is also found in these surface structures showing a high degree of co-localization with ezrin. The same co-localization is seen for ectopically expressed GFP-S100P (not shown). We then performed co-immunoprecipitation experiments to confirm that the co-localization observed reflects the existence of an intracellular ezrin-S100P complex. In these experiments we used SK-BR-3 cells expressing GFP-S100P, as our monoclonal anti-S100P antibody, although working properly in immunofluorescence analyses, only shows very limited reactivity in immunoblotting. Using precipitating anti-ezrin antibodies, a co-precipitation of GFP-S100P detected with anti-GFP antibodies is clearly evident (Fig. 1B), strongly suggesting that the two proteins interact in vivo in stimulated cells.
FIGURE 1.
Co-localization and co-immunoprecipitation of ezrin and S100P in SK-BR-3 cells. A, ezrin and S100P co-localize to plasma membrane protrusions of stimulated SK-BR-3 cells. Serum-starved SK-BR-3 cells were stimulated with fetal calf serum for 30 min and then fixed with paraformaldehyde, permeabilized, and stained with rabbit anti-ezrin polyclonal and mouse anti-S100P monoclonal antibodies followed by appropriate fluorescently labeled secondary antibodies. The lower panels show a higher magnification of the area indicated highlighting the co-localization of ezrin and S100P to microvillar membrane protrusions. Bars, 10 μm. B, co-immunoprecipitation. A PNS from SK-BR-3 cells expressing GFP-S100P was subjected to immunoprecipitation with an anti-ezrin polyclonal antibody (IP) or with an unspecific polyclonal antibody as control (C). The starting material (PNS) and the precipitated proteins were probed by immunoblotting with either anti-ezrin polyclonal (upper panel) or anti-GFP monoclonal antibodies (lower panel).
Quantitative information about the affinity of the ezrin-S100P interaction was obtained by surface plasmon resonance approaches using the Biacore 3000 system. Because we had previously shown that the S100P binding site is located in the N-ERMAD of ezrin (15), we employed recombinantly expressed and purified N-ERMAD that was immobilized on the CM5 sensor chip. Titration experiments revealed that the binding of S100P occurs specifically and with an approximate KD of 0.3 μm as determined using a solid state affinity (Scatchard) approach (Fig. 2).
FIGURE 2.
Interaction of S100P with immobilized N-ERMAD analyzed by SPR. A, ∼200 response units (RU) of N-ERMAD were immobilized on the CM5 sensor chip using the amine coupling method as described under “Experimental Procedures.” S100P at concentrations of 0.006–3.5 μm was injected and allowed to react with the sensor chip surface at a flow rate of 40 μl/min and an injection time of 3 min. The average binding level at the end of injection (equilibrium) was used for calculation of the steady state affinity. B, steady state affinity determined from the level of binding at equilibrium (Req) as a function of the sample concentration. Calculation was carried out using the BIAevaluation software version 4.1, as described under “Experimental Procedures.” The Scatchard plot of the binding experiment is shown in inset C. An approximate dissociation constant of 3e-7 M was calculated for the binding of WT S100P to N-ERMAD.
Mapping of the Ezrin-S100P Interaction Site—To map the S100P binding site in N-ERMAD more precisely, we generated a series of C-terminal-truncated N-ERMAD derivatives (Fig. 3A). The mutants as well as full-length N-ERMAD were expressed as N-terminal GST-tagged derivatives, purified (Fig. 3B) and rebound to glutathione-Sepharose columns for solid phase binding assays. The different affinity matrices were then probed with purified S100P that was added to the respective column in the fluid phase. Binding reactions were carried out in the presence or absence (not shown) of Ca2+, and after extensive washing, S100P that had bound Ca2+ dependently to the N-ERMAD derivatives was eluted with an EGTA-containing buffer before stripping all proteins off the column with a glutathione-containing buffer. Fig. 4A shows that columns containing the N-ERMAD deletion derivatives ezrin 233aa, ezrin 197aa, and ezrin 173aa retain WT S100P in a Ca2+ dependent manner, whereas no S100P bound to the immobilized deletion mutant ezrin 82aa. The same results, i.e. S100P binding capability of all N-ERMAD deletions except ezrin 82aa, were obtained when the purified and soluble N-ERMAD derivatives were allowed to interact with immobilized S100P (not shown). No interaction was observed in either experiment in the absence of Ca2+ (not shown).
FIGURE 3.
N-ERMAD and S100P mutant derivatives. A, schematic representation of the different C-terminal deletion constructs. Amino acids predicted to play an important role in dimerization or target protein binding of S100P are highlighted in bold in the sequence given. B, Western blot analysis of the mutant proteins purified after recombinant expression. Lane 1, ezrin 82aa; lane 2, ezrin 173aa; lane 3, ezrin 197aa; lane 4, ezrin 233aa; lane 5, S100P 87aa; lane 6, S100P 91aa. The ezrin derivatives were detected with an anti-GST and S100P proteins with an anti-Penta-His antibody.
FIGURE 4.
Direct interaction between WT S100P and different N-ERMAD derivatives. A, affinity chromatography analysis. The purified GST-tagged ezrin deletion mutants ezrin 233aa, ezrin 197aa, ezrin 173aa, and ezrin 82aa were bound to glutathione-Sepharose columns, and purified WT S100P was added in the fluid phase. Binding reactions were carried out in the presence of Ca2+ (see “Experimental Procedures”), and flow-through fractions were collected (FT). After extensive washing with a Ca2+-containing buffer, the columns were developed with an EGTA-containing buffer to elute Ca2+ dependently bound proteins (E). Finally, all bound proteins were stripped with a glutathione-containing buffer (S). Equivalent amounts of all fractions were subjected to SDS-PAGE and analyzed by Western blotting. S100P was detected using an anti-Penta-His antibody and the N-ERMAD derivates using an anti-GST antibody. Note the binding of WT S100P to all ezrin derivatives except ezrin 82aa. Minor variations in the EGTA elution profiles, e.g. between the experiments using ezrin 197aa and ezrin 173aa, are due to the column and fraction sizes differing slightly from experiment to experiment. B, SPR analysis of the interaction of N-ERMAD and ezrin 82aa with immobilized S100P. ∼200 RU of S100P were amine-coupled to the CM5 sensor chip. N-ERMAD (upper sensogram) and ezrin 82aa (lower sensogram) were injected at a flow rate of 30 μl/min and injection times of 3 min. C, interaction of S100P with amine-coupled N-ERMAD (upper curve) and amine-coupled ezrin 82aa mutant (lower curve) analyzed by SPR. ∼200 RU of N-ERMAD and ezrin 82aa were immobilized on the CM5 sensor chip, and 3.5 μm WT S100P were injected at a flow rate of 40 μl/min and injection times of 3 min. All sensograms are corrected using a blank flow cell as reference. RU, response units.
The critical ezrin 82aa mutant was also compared with full-length N-ERMAD using Biacore experiments employing WT-S100P immobilized on the sensor chip. In contrast to the high affinity interaction seen for N-ERMAD, no appreciable binding was detected for ezrin 82aa (Fig. 4B). Similarly, when full-length N-ERMAD or ezrin 82aa were immobilized on the sensor chip to approximately the same level, binding of WT S100P was only observed for the full-length protein (Fig. 4C). These analyses identify residues 82–173 as being of prime importance for the interaction with Ca2+-activated S100P. The region in question overlaps with the F2 lobe of the crystallized N-terminal FERM domain of ezrin (21) that most likely undergoes structural alterations during activation of dormant ezrin. Parts of the F2 lobe overlapping with the S100P binding site have been shown to be important for PtdIns(4,5)P2 binding, suggesting possible competitions (see below).
We next attempted to identify the site for N-ERMAD interaction in S100P. Mutagenesis and crystallographic analyses of different S100-target peptide complexes, including those of S100A10 and A11 with their annexin A2 and A1 target peptides, had revealed that several residues form a nonlinear, conformational interaction pocket in the biologically active dimer of the S100 protein. Although residues in the N-terminal sequence preceding the first EF-hand and in the linker between the two EF-hands contribute to the interaction, a crucial part of the binding site involves a short patch of conserved hydrophobic residues in the C-terminal region (22–25). Sequence comparisons of different S100 proteins reveal that residues Tyr-88 and Phe-89 of S100P are likely candidates to form this hydrophobic patch (not shown). To elucidate their contribution to N-ERMAD binding, we generated C-terminal-truncated S100P derivatives either containing (S100P 91aa) or missing (S100P 87aa) the hydrophobic patch (Fig. 3A). Both truncation mutants could be expressed and purified as soluble proteins (Fig. 3B) and showed biochemical properties indistinguishable from WT S100P. In particular, the Ca2+-dependent interaction with hydrophobic matrices like phenyl-Sepharose was unaffected by the respective truncations (not shown). However, N-ERMAD binding is specifically and significantly perturbed in one of the S100P mutants. Although S100P 91aa bound to immobilized N-ERMAD in a Ca2+ dependent manner, showing a behavior similar to that of WT S100P, the S100P 87aa derivative is markedly compromised in its capability of interacting with N-ERMAD (Fig. 5A). Again, Biacore experiments were employed to substantiate these findings. As compared with the high affinity binding of WT S100P (KD of 0.3 μm, see above), no significant interaction of S100P 87aa with N-ERMAD immobilized on the sensor chip was observed. Even using high concentrations of S100P 87aa in the titration experiments did not reveal any binding sufficient for calculating a KD (Fig. 5C).
FIGURE 5.
Direct interaction between N-ERMAD and C-terminal-truncated S100P. A, affinity chromatography analysis. Purified GST-tagged N-ERMAD was immobilized on glutathione-Sepharose beads, and purified WT S100P, S100P 91aa, and S100P 87aa were added in the fluid phase. Binding reactions were carried out in the presence of Ca2+, and after extensive washing with a Ca2+-containing buffer, the columns were developed with an EGTA-containing buffer to elute Ca2+ dependently bound proteins (E). Subsequently, all bound proteins were stripped with a glutathione-containing buffer (S). Equivalent amounts of all fractions were analyzed by Western blotting using anti-Penta-His (S100P) and anti-GST antibodies (N-ERMAD). B and C, SPR analysis of the interaction of WT S100P (B) and S100P 87aa (C) with amine-coupled N-ERMAD. WT S100P and S100P 87aa (0.006–3.5 μm) were injected into a flow cell containing the immobilized N-ERMAD at 40 μl/min flow rate and injection time of 3 min. Note the complete loss of binding in the case of S100P 87aa. RU, response units.
Because certain hydrophobic residues in the C-terminal extensions of S100 proteins have been implicated in providing dimer contacts and because dimer formation is required for specific target protein binding, we next analyzed dimer formation of the S100P mutants using a chemical cross-linking approach. As depicted in Fig. 6, incubation of WT S100P and both S100P deletion mutants in the presence of the cross-linker BS3 results in the formation of covalently linked dimers with apparent molecular masses of ∼25 kDa. A less pronounced band of ∼25 kDa also appears in the non-cross-linked specimen and most likely resembles the product of intermolecular interactions resistant to SDS. Such products are also observed for other S100 proteins in the absence of any cross-linking agent (not shown). Thus, the chemical cross-linking experiments reveal that the C-terminal extension of S100P (residues 88–95) is dispensable for dimerization. This conclusion is in line with the S100P crystal structure which shows no significant contribution of residues 88–95 to formation of the hydrophobic dimer interface (26).
FIGURE 6.
Chemical cross-linking of WT and mutant S100P. WT S100P and the S100P truncation mutants S100P 91aa and S100P 87aa were adjusted to 0.5 mm Ca2+ and incubated with the homobifunctional cross-linker BS3 (C). In control experiments, samples were incubated without cross-linker (-). Products of the reactions were subjected to 15% SDS-PAGE and visualized by silver staining. For representation of this figure, the original gel was cut between lanes 1 and 2 and lanes 4 and 5 to remove lanes not relevant for the experiment.
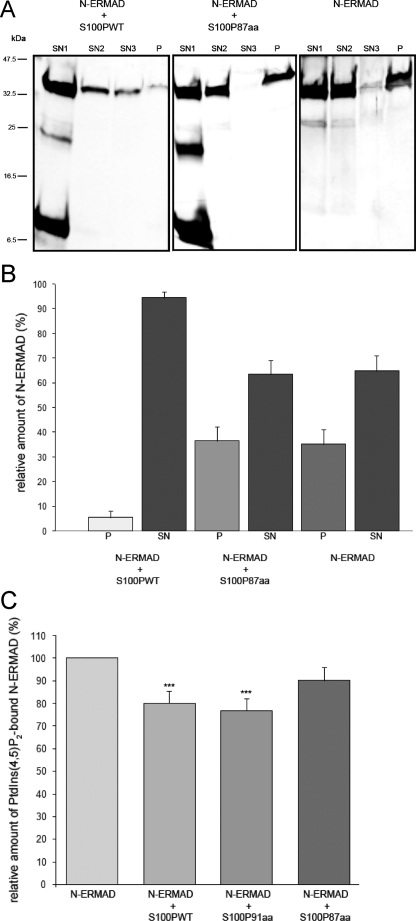
S100P Competes with PtdIns(4,5)P2 for Ezrin Binding—As indicated above, the S100P binding site maps to a region in ezrin (aa 82–173) previously shown to participate in PtdIns(4,5)P2 binding. By employing internal deletions and C-terminal truncations of the ezrin N-terminal domain Barret et al. (27) identified amino acids 12–115 and 233–310 as being important for PtdIns(4,5)P2 binding. Because of this partial overlap, we next sought to determine the influence of S100P on the binding of N-ERMAD to PtdIns(4,5)P2. Therefore, we first employed a liposome co-sedimentation assay previously used to reveal the N-ERMAD-PtdIns(4,5)P2 interaction (27). POPC liposomes containing 3% PtdIns(4,5)P2 were incubated with a mixture of N-ERMAD and the S100P derivatives indicated. After pelleting and washing of the liposomes, associated proteins were identified by SDS-PAGE, and the relative amount of lipid-bound N-ERMAD was determined densitometrically. Fig. 7A shows that in contrast to the positive control (assay containing N-ERMAD alone) the inclusion of WT S100P results in a significant decrease in the amount of N-ERMAD pelleted together with the PtdIns(4,5)P2-containing liposomes (see also densitometric quantification of several independent experiments in Fig. 7B). In contrast, the C-terminal truncation mutant S100P 87aa, shown above to be incapable of binding N-ERMAD, does not affect the amount of N-ERMAD recovered in the PtdIns(4,5)P2 liposome pellet (Fig. 7, A and B). Control experiments revealed that the effect of S100P on the POPC/PtdIns(4,5)P2 liposome binding of N-ERMAD most likely requires the Ca2+ conformation of S100P and is due to the direct N-ERMAD-S100P interaction as (a) no inhibitory effect of S100P on the PtdIns(4,5)P2 binding of N-ERMAD was observed in the absence of Ca2+, and (b) the different S100P derivatives were not capable of interacting with the liposomes themselves (not shown).
FIGURE 7.
S100P interferes with the PtdIns(4,5)P2 binding of ezrin. A and B, liposome co-sedimentation assays. A, POPC vesicles containing 3% PtdIns(4,5)P2 were incubated with N-ERMAD in the absence or presence of WT S100P and S100P 87aa, respectively. Liposomes were pelleted by high speed centrifugation and then subjected to two successive washing steps. The resulting supernatants (Sn1, Sn2, and Sn3) and final pellets (P) were analyzed by SDS-PAGE and silver staining. B, statistical evaluation of three independent liposome pelleting assays (SN, Sn1, Sn2 and Sn3 combined; P, pellet). Note that WT S100P interferes to some extent with the binding of N-ERMAD to PtdIns(4,5)P2-containing liposomes, i.e. the amount of N-ERMAD in the liposome pellet fraction is markedly decreased in the presence of WT S100P. This inhibition is not seen with S100P 87aa. C, lipid plate assay revealing the binding of N-ERMAD to immobilized PtdIns(4,5)P2 in the absence or presence of S100P derivatives. After PtdIns(4,5)P2 immobilization, lipid binding of N-ERMAD was assessed as described under “Experimental Procedures” in the presence or absence of WT S100P, S100P 91aa, and S100P 87aa. Statistical significance in all analyses was calculated using unpaired student's t test (N-ERMAD-WT S100P, p = 0.0003, n = 4; N-ERMAD-S100P 91aa, p = 0.0001, n = 4). Note the reduction in PtdIns(4,5)P2-bound N-ERMAD in the presence of WT S100P and S100P 91aa that is not seen with the S100P 87aa mutant. The inhibitory effect of S100P on the PtdIns(4,5)P2 binding of N-ERMAD is less pronounced in the lipid plate as compared with the liposome co-sedimentation assay, most likely due the higher density and local concentration of PtdIns(4,5)P2 present on the lipid plate.
To confirm the inhibitory effect of S100P on ezrin PtdIns(4,5)P2 binding, we carried out an independent set of lipid binding experiments. These approaches employed lipid plate binding assays with immobilized PtdIns(4,5)P2 that had been used previously to measure the PtdIns(4,5)P2 binding of annexin A2 and the pleckstrin homology domain of phospholipase C-δ1 (19). In line with the liposome co-sedimentation analyses, these experiments also revealed that WT S100P and S100P 91aa but not S100P 87aa interfered to some extent with the PtdIns(4,5)P2 binding of N-ERMAD. The inhibitory effect was again strictly Ca2+-dependent but was less pronounced as compared with that seen in the liposome binding experiments, possibly due to the high density of PtdIns(4,5)P2 present on the lipid plates. Together the lipid binding experiments indicate that only S100P derivatives capable of interacting with N-ERMAD interfere with efficient PtdIns(4,5)P2 binding of N-ERMAD, suggesting a partial competition of S100P with PtdIns(4,5)P2 for structurally overlapping binding motifs in ezrin.
Relevance of the S100P-Ezrin Interaction for Tumor Cell Migration—S100P has been implicated in the metastatic cell migration in NSCLC and together with S100A2 is up-regulated in metastatic versus non-metastatic tumors from NSCLC patients (17). Ezrin is also up-regulated in certain metastatic cancers (12), and its activation by phosphorylation appears to be involved in certain migratory events (7, 9, 10). Because our previous analysis had revealed that, at least in vitro, ezrin can also be activated by S100P binding (15), we analyzed whether the S100P-ezrin interaction could be relevant to tumor cell migration. Therefore, we recorded the spontaneous transendothelial migration of NSCLC HTB-58 cells expressing certain S100P and ezrin mutants. HTB-58 lines stably expressing EGFP (control) or EGFP fusion constructs of WT S100P, S100P 91aa, S100P 87aa, or ezrin T567D were generated and maintained as mixed cultures of different selected clones to eliminate clone-specific effects. Expression of the respective constructs was verified by fluorescence-activated cell sorting analysis and fluorescence microscopy (not shown) as well as Western blotting (Fig. 8A). All cells showed strong and comparable signals of the ectopically expressed GFP or GFP fusion proteins. For all clones, migration through an endothelial cell monolayer was analyzed in a two-compartment (Transwell) system. When compared with EGFP-expressing control cells, expression of the permanent active ezrin mutant T567D leads to a significant increase in spontaneous transendothelial migration, indicating that activation of ezrin is involved in NSCLC migration across endothelial monolayers (Fig. 8B). Likewise, we detected a consistent and significant increase in spontaneous transendothelial migration of cell cultures stably overexpressing S100P. Similarly, expression of the C-terminal-truncated S100P 91aa mutant, which still contains the ezrin binding site, resulted in an increase in spontaneous migration by ∼40% over EGFP-overexpressing HTB-58 cells (Fig. 8B). In contrast, expression of the S100P 87aa deletion mutant, which still forms a dimer but is incapable of binding ezrin, did not increase the extent of spontaneous transendothelial migration. Thus, only S100P derivatives capable of interacting with and thereby activating ezrin induce an increase in the spontaneous migration of NSCLC HTB-58 cells, in line with a role of complex formation in the regulation of this type of cell migration.
FIGURE 8.
Transendothelial migration of HTB-58 cell lines stably expressing different ezrin and S100P derivatives. A, stable expression of GFP fusion proteins of WT S100P, S100P 91aa, S100P 87aa, and ezrin T567D in HTB-58 cells was verified by Western blotting of total cell lysates with a monoclonal anti-GFP antibody. Probing of the same blot with anti-vimentin antibodies served to control for equal loading. B, statistical evaluation of transendothelial migration assays employing the different cell lines. The migration of GFP-positive HTB-58 cells through a monolayer of HMEC-1 grown on microporous filter support was measured as described under “Experimental Procedures.” Note that cells expressing WT S100P, S100P 91aa, or the permanently active ezrin mutant T567D show increased migration as compared with control cells expressing GFP alone. In contrast, expression of S100P 87aa caused no significant effect on the transendothelial migration of HTB-58 cells.
DISCUSSION
Using direct binding assays we show here that Ca2+ bound S100P interacts with a sequence motif in the N-ERMAD of ezrin that overlaps partially with the PtdIns(4,5)P2 binding site. The latter had been demonstrated previously to involve residues 12–115 and 233–310 (27), whereas a crucial determinant for S100P binding is located between residues 82 and 173 (Fig. 4). Thus, the first part of the PtdIns(4,5)P2 binding site could participate in S100P binding in line with the partial competition between S100P and PtdIns(4,5)P2 observed in the direct binding assays (Fig. 7). The residues required for S100P binding map to lobe F2 in ezrin's FERM domain (21). Interestingly, residues 135–150 and 155–180 within this lobe undergo structural changes upon ezrin activation. In dormant ERM proteins residues within these regions directly contact hydrophobic counterparts in the C-terminal autoinhibitory helix A that in turn contributes to complete folding of the hydrophobic core of the FERM domain in so-called keystone interactions (21, 28). It appears likely that the hydrophobic side chains of S100P identified here to crucially contribute to N-ERMAD binding (Tyr-88, Phe-89) can replace the C-ERMAD helix thereby releasing the autoinhibition and contributing as keystone interaction partners to N-ERMAD folding. It is worth noting that similar keystone interactions appear to dictate the interaction of another S100 protein, S100A10, with its binding partner annexin A2. Here, hydrophobic residues in the C-terminal helix of the S100 protein contact hydrophobic side chains in an amphiphatic N-terminal helix of annexin A2 (25) thereby stabilizing helix formation (29). Thus, the activation of ezrin induced by S100P binding most likely results from a replacement by S100P of the C-ERMAD. How this relates structurally to the PtdIns(4,5)P2-mediated activation of ezrin that also results in the unmasking of the C-terminal F-actin binding site (30) remains to be determined. Nonetheless, within the context of intracellular ezrin activation, it appears that S100P and PtdIns(4,5)P2 are alternative activators that are probably used in different signaling pathways, the first one strictly depending on intracellular Ca2+ rise.
Our interaction analysis led to the identification of an S100P mutant derivative that had lost the ezrin binding site albeit showing the major biochemical features of wild-type S100P, i.e. Ca2+ binding and dimerization. Thus, this S100P 87aa mutant is an important tool in assessing specific intracellular functions of S100P in the context of its ezrin binding. We have used the S100P 87aa mutant as a negative control in the tumor cell migration assays and, thus, were able to conclude that the stimulatory effect of S100P on transendothelial tumor cell migration is most likely due to its ability to activate ezrin by direct interaction. Although S100P derivatives capable of binding ezrin (WT and 91aa) promote an increased transmigration, S100P 87aa fails to do so. S100P expression has been correlated in several cases with tumor progression, and the extent of transcriptional up-regulation is particularly pronounced in highly metastatic tumors (17, 31, 32). Likewise, ezrin expression has been linked to tumor progression and tumor cell migration, most noticeably in highly metastatic tumors (33, 34). In several cases these expression changes have been linked to a functional activation of ezrin, correlating with ezrin phosphorylation and the involvement of a Rho/ROCK-dependent signaling pathway (for reviews, see Refs. 6 and 12). A tumor migration promoting role of ezrin can also be deduced from RNA interference studies showing that down-regulation of the protein significantly reduces the spontaneous migration of carcinoma cells (35). Here we show that expression of the phospho-mimicking T567D mutant of ezrin increases the migratory potential of NSCLCs, in particular their transendothelial passage. Although this directly links ezrin activation to NSCLC invasion, it also enabled us to use this cell model to correlate the S100P-dependent activation of ezrin to tumor cell migration. Altogether, these findings, thus, are in line with various expression studies showing an up-regulation of both proteins in highly migratory tumor cells.
Other S100 proteins have also been implicated in tumor progression and the development of metastases. Most notably, S100A2 is up-regulated in NSCLC, gastric cancer, and lymphoma and S100A4 is elevated in breast cancer patients with its expression correlating with an aggressive metastatic phenotype (for reviews, see Refs. 36–38). Several models have been proposed to account for such tumor promoting activities of S100 proteins. These include intracellular roles for S100A4 as a direct regulator of p53 and the non-muscle myosin IIa and extracellular roles for the same protein that when given to tumor cells can induce the up-regulation of matrix metalloproteinases by yet unknown mechanisms (for reviews, see Refs. 37 and 38). Our studies correlate the pro-migratory effect of S100P overexpression to its binding to and activation of ezrin, thus a clear intracellular function. Future experiments have to reveal where in the migrating NSCLCs ezrin activation occurs and whether other metastasis-associated S100 proteins also function by regulating events in the cortical cytoskeleton.
Acknowledgments
We thank Dr. E. Liebau (Department of Molecular Physiology, University of Muenster) for access to the Biacore 3000 system and Dr. U. Bierfreund for suggestions concerning the SPR experiments.
This work was supported by a Deutsche Forschungsgemeinschaft Grant Ge 514/5-2). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ERM, ezrin/radixin/moesin; ERMAD, ERM association domain; PtdIns(4,5)P2, phosphatidylinositol 4,5-bisphosphate; WT, wild type; aa, amino acids; GST, glutathione S-transferase; EGFP, enhanced green fluorescent protein; PBS, phosphate-buffered saline; PMSF, phenylmethylsulfonyl fluoride; NSCLC, non-small cell lung carcinomas; HMEC, human microvascular endothelial cells; PNS, post-nuclear supernatant Ni-NTA, nickel-nitrilotriacetic acid; SPR, surface plasmon resonance; POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine.
References
- 1.Bretscher, A., Edwards, K., and Fehon, R. G. (2002) Nat. Rev. Mol. Cell Biol. 3 586-599 [DOI] [PubMed] [Google Scholar]
- 2.Fievet, B., Louvard, D., and Arpin, M. (2007) Biochim. Biophys. Acta 1773 653-660 [DOI] [PubMed] [Google Scholar]
- 3.Hughes, S. C., and Fehon, R. G. (2007) Curr. Opin. Cell Biol. 19 51-56 [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga-Ohara, N., Takahashi, A., Uchiyama, T., and Sasada, M. (2002) Exp. Cell Res. 278 112-122 [DOI] [PubMed] [Google Scholar]
- 5.Lee, J. H., Katakai, T., Hara, T., Gonda, H., Sugai, M., and Shimizu, A. (2004) J. Cell Biol. 167 327-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivetic, A., and Ridley, A. J. (2004) Immunology 112 165-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng, T., Parsons, M., Hughes, W. E., Monypenny, J., Zicha, D., Gautreau, A., Arpin, M., Gschmeissner, S., Verveer, P. J., Bastiaens, P. I., Parker, P. J. (2001) EMBO J. 20 2723-2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monni, R., Haddaoui, L., Naba, A., Gallais, I., Arpin, M., Mayeux, P., and Moreau-Gachelin, F. (2008) Blood 111 3163-3172 [DOI] [PubMed] [Google Scholar]
- 9.Wick, W., Grimmel, C., Wild-Bode, C., Platten, M., Arpin, M., and Weller, M. (2001) J. Neurosci. 21 3360-3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahai, E., and Marshall, C. J. (2003) Nat. Cell Biol. 5 711-719 [DOI] [PubMed] [Google Scholar]
- 11.McClatchey, A. I. (2003) Nat. Rev. Cancer 3 877-883 [DOI] [PubMed] [Google Scholar]
- 12.Curto, M., and McClatchey, A. I. (2004) Cancer Cell 5 113-114 [DOI] [PubMed] [Google Scholar]
- 13.Bretscher, A., Chambers, D., Nguyen, R., and Reczek, D. (2000) Annu. Rev. Cell Dev. Biol. 16 113-143 [DOI] [PubMed] [Google Scholar]
- 14.Mangeat, P., Roy, C., and Martin, M. (1999) Trends Cell Biol. 9 187-192 [DOI] [PubMed] [Google Scholar]
- 15.Koltzscher, M., Neumann, C., Konig, S., and Gerke, V. (2003) Mol. Biol. Cell 14 2372-2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donato, R. (2001) Int. J. Biochem. Cell Biol. 33 637-668 [DOI] [PubMed] [Google Scholar]
- 17.Diederichs, S., Bulk, E., Steffen, B., Ji, P., Tickenbrock, L., Lang, K., Zänker, K. S., Metzger, R., Schneider, P. M., Gerke, V., Thomas, M., Berdel, W. E., Serve, H., Müller-Tidow, C. (2004) Cancer Res. 64 5564-5569 [DOI] [PubMed] [Google Scholar]
- 18.Smith, W. J., and Cerione, R. A. (2002) Acta Crystallogr. D Biol. Crystallogr. 58 1359-1361 [DOI] [PubMed] [Google Scholar]
- 19.Rescher, U., Ruhe, D., Ludwig, C., Zobiack, N., and Gerke, V. (2004) J. Cell Sci. 117 3473-3480 [DOI] [PubMed] [Google Scholar]
- 20.Kielbassa-Schnepp, K., Strey, A., Janning, A., Missiaen, L., Nilius, B., and Gerke, V. (2001) Cell Calcium 30 29-40 [DOI] [PubMed] [Google Scholar]
- 21.Smith, W. J., Nassar, N., Bretscher, A., Cerione, R. A., and Karplus, P. A. (2003) J. Biol. Chem. 278 4949-4956 [DOI] [PubMed] [Google Scholar]
- 22.Kube, E., Becker, T., Weber, K., and Gerke, V. (1992) J. Biol. Chem. 267 14175-14182 [PubMed] [Google Scholar]
- 23.Seemann, J., Weber, K., and Gerke, V. (1996) Biochem. J. 319 123-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rety, S., Osterloh, D., Arie, J. P., Tabaries, S., Seeman, J., Russo-Marie, F., Gerke, V., and Lewit-Bentley, A. (2000) Structure 8 175-184 [DOI] [PubMed] [Google Scholar]
- 25.Rety, S., Sopkova, J., Renouard, M., Osterloh, D., Gerke, V., Tabaries, S., Russo-Marie, F., and Lewit-Bentley, A. (1999) Nat. Struct. Biol. 6 89-95 [DOI] [PubMed] [Google Scholar]
- 26.Zhang, H., Wang, G., Ding, Y., Wang, Z., Barraclough, R., Rudland, P. S., Fernig, D. G., and Rao, Z. (2003) J. Mol. Biol. 325 785-794 [DOI] [PubMed] [Google Scholar]
- 27.Barret, C., Roy, C., Montcourrier, P., Mangeat, P., and Niggli, V. (2000) J. Cell Biol. 151 1067-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson, M. A., Reczek, D., Bretscher, A., and Karplus, P. A. (2000) Cell 101 259-270 [DOI] [PubMed] [Google Scholar]
- 29.Johnsson, N., Marriott, G., and Weber, K. (1988) EMBO J. 7 2435-2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janke, M., Herrig, A., Austermann, J., Gerke, V., Steinem, C., and Janshoff, A. (2008) Biochemistry 47 3762-3769 [DOI] [PubMed] [Google Scholar]
- 31.Guerreiro Da Silva, I. D., Hu, Y. F., Russo, I. H., Ao, X., Salicioni, A. M., Yang, X., and Russo, J. (2000) Int. J. Oncol. 16 231-240 [PubMed] [Google Scholar]
- 32.Wang, G., Platt-Higgins, A., Carroll, J., de Silva Rudland, S., Winstanley, J., Barraclough, R., and Rudland, P. S. (2006) Cancer Res. 66 1199-1207 [DOI] [PubMed] [Google Scholar]
- 33.Khanna, C., Wan, X., Bose, S., Cassaday, R., Olomu, O., Mendoza, A., Yeung, C., Gorlick, R., Hewitt, S. M., and Helman, L. J. (2004) Nat. Med. 10 182-186 [DOI] [PubMed] [Google Scholar]
- 34.Yu, Y., Khan, J., Khanna, C., Helman, L., Meltzer, P. S., and Merlino, G. (2004) Nat. Med. 10 175-181 [DOI] [PubMed] [Google Scholar]
- 35.Rossy, J., Gutjahr, M. C., Blaser, N., Schlicht, D., and Niggli, V. (2007) Exp. Cell Res. 313 1106-1120 [DOI] [PubMed] [Google Scholar]
- 36.Marenholz, I., Heizmann, C. W., and Fritz, G. (2004) Biochem. Biophys. Res. Commun. 322 1111-1122 [DOI] [PubMed] [Google Scholar]
- 37.Helfman, D. M., Kim, E. J., Lukanidin, E., and Grigorian, M. (2005) Br. J. Cancer 92 1955-1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garrett, S. C., Varney, K. M., Weber, D. J., and Bresnick, A. R. (2006) J. Biol. Chem. 281 677-680 [DOI] [PubMed] [Google Scholar]