Abstract
Previous studies indicate that cerebral ischemia breaks the dynamic balance between excitatory and inhibitory inputs. The neural excitotoxicity induced by ionotropic glutamate receptors gain the upper hand during ischemia-reperfusion. In this paper, we investigate whether GluR5 (glutamate receptor 5)-containing kainate receptor activation could lead to a neuroprotective effect against ischemic brain injury and the related mechanism. The results showed that (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA), a selective GluR5 agonist, could suppress Src tyrosine phosphorylation and interactions among N-methyl-d-aspartate (NMDA) receptor subunit 2A (NR2A), postsynaptic density protein 95 (PSD-95), and Src and then decrease NMDA receptor activation through attenuating tyrosine phosphorylation of NR2A and NR2B. More importantly, ATPA had a neuroprotective effect against ischemia-reperfusion-induced neuronal cell death in vivo. However, four separate drugs were found to abolish the effects of ATPA. These were selective GluR5 antagonist NS3763; GluR5 antisense oligodeoxynucleotides; CdCl2, a broad spectrum blocker of voltage-gated calcium channels; and bicuculline, an antagonist of γ-aminobutyric acid A (GABAA) receptor. GABAA receptor agonist muscimol could attenuate Src activation and interactions among NR2A, PSD-95 and Src, resulting the suppression of NMDA receptor tyrosine phosphorylation. Moreover, patch clamp recording proved that the activated GABAA receptor could inhibit NMDA receptor-mediated whole-cell currents. Taken together, the results suggest that during ischemia-reperfusion, activated GluR5 may facilitate Ca2+-dependent GABA release from interneurons. The released GABA can activate postsynaptic GABAA receptors, which then attenuates NMDA receptor tyrosine phosphorylation through inhibiting Src activation and disassembling the signaling module NR2A-PSD-95-Src. The final result of this process is that the pyramidal neurons are rescued from hyperexcitability.
Brain functions are based on the dynamic balance between excitatory and inhibitory inputs. Cerebral ischemia breaks this balance, and the neural excitotoxicity takes over, which induces delayed neuronal cell death. Glutamate, as the primary excitatory neurotransmitter in the central nervous system, has been given widespread attention in previous studies. Ionotropic glutamate receptors, which play an important part in ischemic excitotoxicity, are divided into N-methyl-d-aspartate (NMDA),3 α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), and kainate (KA) receptors (1, 2). The NMDA receptor, as a type of ligand-gated ion channel, attracts the most attention in research of cerebral ischemia. It is composed of three types of subunits: NR1, NR2 (NR2A to -D), and NR3 (NR3A and -B) (3-5). Among these subunits, transient global ischemia increases tyrosine phosphorylation of NR2A and NR2B (6). During ischemia-reperfusion, excessive glutamate release induces the influx of Na+ and Ca2+ ions through NMDA receptors (7), and activated Src kinase can mediate tyrosine phosphorylation of NR2A and NR2B (8) and increase the activity of NMDA receptors (9). Our previous study suggested that cerebral ischemia induced autophosphorylation of Src, and then the activated Src could phosphorylate NMDA receptors in pyramidal neurons. PSD-95 (postsynaptic density protein 95) was involved in the event through forming signaling module NR2A-PSD-95-Src (10, 11).
In contrast to NMDA receptors, little is known about the role of KA receptors in ischemia-reperfusion. KA receptors are composed of five subunits: GluR5, GluR6, GluR7, KA1, and KA2 (1). A number of interesting experimental results turned our attention to the GluR5 and GluR6 subunits; GluR6-deficient mice exhibited resistance to neurotoxic effects induced by kainate (12). Knock-out of GluR6 prevented kainate-induced epileptiform bursts, whereas ablation of GluR5 led to a higher susceptibility of epileptogenic effects of kainate (13). An agonist of GluR5-containing KA receptors had antiepileptic effects (14). The results suggest that GluR6 and GluR5 may play opposing roles in brain excitability. Recently, we found that GluR6-mediated c-Jun N-terminal kinase activation is responsible for ischemic brain injury (15). We want to know whether GluR5 also plays opposing roles to GluR6 in cerebral ischemia and, if so, to identify the related mechanism. Corresponding to their distinct functions, GluR6 and GluR5 have different distribution in the hippocampus. The excitatory pyramidal cells express primarily GluR6, whereas GluR5 is expressed primarily within the inhibitory interneurons of the hippocampus (16, 17).
Compared with excitatory glutamate neurotransmission, γ-aminobutyric acid (GABA), as the primary inhibitory neurotransmitter in central nervous system, has received relatively little attention in the area of ischemic brain injury (18). GABAergic inhibitory interneurons (also called local circuit neurons) of hippocampus are the regulators of pyramidal neuron excitability (19), keeping the dynamic balance between excitatory and inhibitory inputs. Recent studies have shown that activation of GluR5-containing KA receptors facilitates GABA release from interneurons and increases tonic inhibition of pyramidal neurons (17, 20-22). In addition, GABA performs a function through acting on the GABA receptors, which can be divided into three subclasses: GABAA, GABAB, and GABAC receptors (23). Among them, GABAA receptor, which directly controls a chloride ion channel, is situated in the postsynaptic membrane. Activation of GABAA receptors enhances the influx of chloride ions, inducing hyperpolarization and attenuating cell excitability (24). In cerebral ischemia research, it was reported that GABAA receptors also take part in neuroprotection against ischemia-reperfusion (25-27).
Taken together, there is a raised possibility that activation of GluR5-containing KA receptors facilitates GABA release from interneurons. The released GABA activates postsynaptic GABAA receptors, which suppress the ischemic depolarization and then decrease the activation of NMDA receptors, performing a neuroprotective function against ischemic brain injury through enhancing inhibitory inputs and then getting excitation and inhibition back into the proper dynamic balance.
In the present study, we used (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA), a selective agonist of GluR5-containing KA receptors (28), to investigate whether activated GluR5-containing KA receptors exert neuroprotection on pyramidal neurons through suppressing Src activation and interactions among PSD-95, Src, and NR2A and then decreasing NMDA receptor-mediated excitotoxicity during ischemia-reperfusion.
EXPERIMENTAL PROCEDURES
Materials—Anti-PSD-95, anti-phosphotyrosine antibodies, alkaline phosphatase-conjugated goat anti-rabbit IgG, and goat anti-rabbit IgG were purchased from Sigma. Anti-Src, anti-NR2A, anti-NR2B, anti-phospho-Src (anti-p-Src, Tyr-416; for immunohistochemistry use) antibodies were obtained from Millipore (Billerica, MA). Anti-p-Src (Tyr-416) antibody and bicuculline were obtained from BIOMOL (Butler Pike, Plymouth Meeting, PA). Anti-actin antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). ATPA and NS3763 were purchased from TOCRIS Bioscience (Bristol, UK). 5-Bromo-4-chloro-3-indolyl-phosphate and nitro blue tetrazolium were obtained from Promega (Madison, WI). All other chemicals were got from Sigma unless indicated otherwise.
Drug Administration—The rats were injected intraperitoneally with bicuculline (2 mg/kg) or muscimol (Mus; 1 mg/kg) in 0.9% NaCl 30 min before ischemia. Animals were given ATPA, NS3763, or CdCl2 by means of unilateral intracerebroventricular infusion (anteroposterior, 0.8 mm; lateral, 1.5 mm; depth, 3.5 mm from the bregma). ATPA (2 nmol in 5 μl of 0.9% NaCl) was administered to the rats 20 min before ischemia. Either NS3763 (3 nmol in 5 μl of DMSO) or CdCl2 (10 nmol in 5 μl of H2O) was injected 30 min before ischemia. 10 nmol of end-phosphorothioated GluR5 antisense oligodeoxynucleotides (AS-ODNs) in 10 μl of TE buffer (10 mm Tris-HCl (pH 8.0), 1 mm EDTA) were given to the rats every 24 h for 3 days. The same dose of missense (MS) or vehicle (TE buffer) was used as a control. The sequences for GluR5 AS-ODNs were 5′-GCTTCTTAATTCATGCCGAA-3′, and sequences for MS-ODNs were 5′-GCTTCTAATTTCATGCCGAA-3′.
Animal Model of Ischemia—Adult male SD rats weighing 250-300 g were used (Shanghai Experimental Animal Center, Chinese Academy of Science). The experimental procedures were approved by local legislation for ethics of experiments on animals. Transient cerebral ischemia was induced by four-vessel occlusion, as described previously (29). Briefly, under anesthesia with chloral hydrate (300 mg/kg, intraperitoneal), vertebral arteries were electrocauterized, and common carotid arteries were exposed. On the following day, both carotid arteries were occluded with aneurysm clips to induce cerebral ischemia. After 15 min of occlusion, the aneurysm clips were removed for reperfusion. Rectal temperature was maintained at about 37 °C throughout the procedure. Rats that lost their righting reflex and whose pupils were dilated and unresponsive to light were selected for the experiments. Rats with seizures were discarded. An electroencephalography was monitored to ensure isoelectricity after carotid artery occlusion. Sham controls were performed using the same surgical procedures, except that the carotid arteries were not occluded.
Sample Preparation—Rats were decapitated immediately after 6 h of reperfusion, and then the hippocampal CA1 region was isolated and quickly frozen in liquid nitrogen. Tissues were homogenized in an ice-cold homogenization buffer containing 50 mm MOPS (pH 7.4), 100 mm KCl, 320 mm sucrose, 50 mm NaF, 0.5 mm MgCl2, 0.2 mm dithiothreitol, 1 mm EDTA, 1 mm EGTA, 1 mm Na3VO4, 20 mm sodium pyrophosphate, 20 mm β-phosphoglycerol, 1 mm p-nitrophenyl phosphate, 1 mm benzamidine, 1 mm phenylmethylsulfonyl fluoride, and 5 μg/ml each leupeptin, aprotinin, and pepstatin A. The homogenates were centrifuged at 800 × g for 10 min at 4 °C. Supernatants were collected, and protein concentration was determined by the method of Lowry et al. (30). Samples were stored at -80 °C and were thawed only once just before use.
Immunoprecipitation and Immunoblotting—Tissue homogenates (400 μg of protein) were diluted 4-fold with 50 mm HEPES buffer (pH 7.4) containing 10% glycerol, 150 mm NaCl, 1% Triton X-100, 0.5% Nonidet P40 (Nonidet P-40), and 1 mm each of EDTA, EGTA, phenylmethylsulfonyl fluoride, and Na3VO4. Samples were preincubated for 1 h with 20 μl of protein A-Sepharose CL-4B (Amersham Biosciences) at 4 °C and then centrifuged to remove protein adhered nonspecifically to protein A. The supernatants were incubated with primary antibodies for 4 h or overnight at 4 °C. Protein A (20 μl) was added to the tube, and incubation was continued for another 2 h. Samples were centrifuged at 10,000 × g for 2 min at 4 °C, and the pellets were washed three times with immunoprecipitation buffer. Bound protein was eluted by boiling at 100 °C for 5 min in SDS-PAGE loading buffer and then isolated by centrifugation. The supernatants were separated on polyacrylamide gels and then electrotransferred onto a nitrocellulose membrane (Amersham Biosciences). After blocking for 3 h in Tris-buffered saline with 0.1% Tween 20 (TBST) and 3% bovine serum albumin, membranes were incubated overnight at 4 °C with primary antibodies in TBST containing 3% bovine serum albumin. Membranes were then washed and incubated with alkaline phosphatase-conjugated secondary antibodies in TBST for 2 h and developed using nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate color substrate. The densities of the bands on the membrane were scanned and analyzed with an image analyzer (LabWorks Software; UVP, Upland, CA).
Histology and Immunohistochemistry—Rats were perfusion-fixed with 4% paraformaldehyde in 0.1 m sodium phosphate buffer (pH 7.4) under anesthesia after 5 days of brain ischemia-reperfusion. Brains were removed quickly and further fixed with the same fixation solution at 4 °C overnight. Postfixed brains were embedded in paraffin, followed by preparation of coronal sections, 5 μm thick, using a microtome (Leica RM2155; Nussloch, Germany). The paraffin-embedded brain sections were deparaffinized with xylene and rehydrated by ethanol at graded concentrations of 100-70% (v/v), followed by washing with water. The sections were stained with 0.1% (w/v) cresyl violet and examined using light microscopy. The number of surviving hippocampal CA1 pyramidal cells per 1 mm of length was counted as the neuronal density.
Immunoreactivity was determined by the avidin-biotin-peroxidase method. Briefly, sections were deparaffinized with xylene and rehydrated by ethanol at graded concentrations and distilled water. High temperature antigen retrieval was performed in 1 mm citrate buffer. To block endogenous peroxidase activity, sections were incubated for 30 min in a solution of 0.1% H2O2 in phosphate-buffered saline. After being blocked with 5% (v/v) normal goat serum in phosphate-buffered saline for 1 h at 37 °C, sections were incubated with mouse monoclonal antibody against phospho-Src (Tyr(P)-416) at 4 °C for 3 days. These sections were then incubated with biotinylated goat anti-mouse secondary antibody made up of 0.1% bovine serum albumin, 0.3% Triton X-100, and 1% normal goat serum in phosphate-buffered saline overnight and subsequently incubated with avidin-conjugated horseradish peroxidase for 1 h at 37 °C. Finally, sections were incubated with the peroxidase substrate diaminobenzidine until the desired stain intensity developed. They were then examined by light microscopy.
Cell Culture—Hippocampal neuronal cultures were prepared from 18-day-old Sprague-Dawley rat embryos as described previously (31). Briefly, hippocampi were meticulously isolated in ice-cold high glucose Dulbecco's modified Eagle's medium (Invitrogen). Hippocampal cells were dissociated by trypsinization (0.25% (w/v) trypsin and 0.02% (w/v) EDTA in Ca2+- and Mg2+-free Hanks' balanced salt solution), at 37 °C for 15 min, followed by gentle swing in plating medium (high glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 10% horse serum; Invitrogen). Cells were seeded onto poly-l-lysine (Sigma)-coated wells or coverslips at a density of 1 × 105 cells/cm2 and incubated at 37 °C in 5% CO2 atmosphere. After 24 h, cultural medium was replaced by neurobasal medium supplemented with B-27 (Invitrogen) and 0.5 mm glutamine and then half-replaced twice every week. Cultures were used after 14 days in vitro for patch clamp recording.
Patch Clamp Recording—Electrophysiological recording was performed in the conventional whole-cell patch clamp recording configuration under voltage-clamp conditions. Patch pipettes were pulled from glass capillaries with an outer diameter of 1.5 mm on a two-stage puller (PP-830; Narishige). The resistance between the recording electrode filled with pipette solution (140 mm CsCl, 10 mm tetraethylammonium, 10 mm Hepes, 5 mm EGTA, 1 mm MgCl2, 4 mm ATP, pH 7.2), and the reference electrode was 3-5 megaohms. Membrane currents were measured using a patch clamp amplifier AxonPatch 700B (Axon Instruments, Foster City, CA), filtered at 1 kHz, sampled, and analyzed using a DigiData 1322A interface and a computer with the pClamp 9.0 system (Axon Instruments). The series resistance was compensated automatically. The membrane potential was held at -60 mV throughout the experiment. All experiments were carried out at room temperature (22-25 °C).
Statistical Evaluation—Values were expressed as mean ± S.D. and were obtained from no fewer than five independent rats. Statistical analysis of the results was carried out by one-way analysis of variance, followed by Duncan's new multiple range method or the Newman-Keuls test. p values of <0.05 were considered significant.
RESULTS
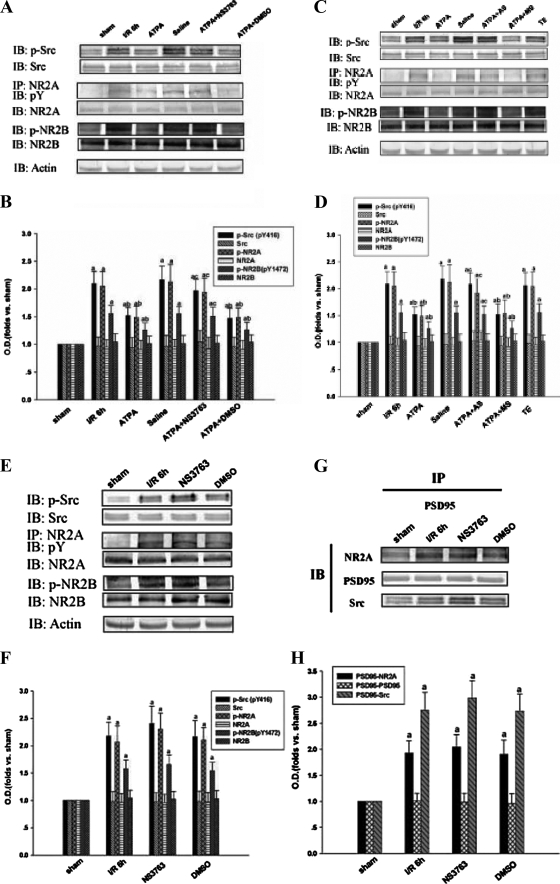
ATPA Attenuates Tyrosine Phosphorylation of Src, NR2A, and NR2B in the Hippocampal CA1 Region—Our previous study showed that tyrosine phosphorylation of NR2A and NR2B and interactions between Src and NMDA receptors reach their peak level at 6 h of reperfusion after ischemia (11, 32). In order to investigate the effects of ATPA on tyrosine phosphorylation of Src, NR2A, and NR2B, we injected ATPA to rats before ischemia and got samples at 6 h of reperfusion after ischemia. Antibody to NR2B phosphorylated on Tyr-1472 was used, because NR2B Tyr-1472 is the major phosphorylation site mediated by Src family kinases (33). Since Src is activated by autophosphorylation of tyrosine residues (Tyr-416) (34), we used antibody against Tyr(P)-416 of Src to investigate Src activation. The samples were immunoprecipitated with antibody against NR2A and then immunoblotted with antibody against phosphotyrosine. As shown in Fig. 1, A-D, for ATPA-treatment group, there was a large decline in the increased tyrosine phosphorylation of Src, NR2A, and NR2B but not for vehicle of ATPA, whereas the protein level of Src, NR2A, and NR2B did not change significantly. Among the results, two bands were detected in immunoblots with anti-p-Src or anti-Src antibody.
FIGURE 1.
ATPA attenuates tyrosine phosphorylation of Src, NR2A, and NR2B in the hippocampal CA1 region. The samples were taken at 6 h of reperfusion after 15 min of ischemia. A, C, and E, the tyrosine phosphorylation and protein levels of Src, NR2A, and NR2B were detected by immunoblotting. G, sample protein was immunoprecipitated with anti-PSD-95 antibody and then blotted with anti-NR2A, anti-PSD-95, or anti-Src antibody. B, D, F, and H, bands corresponding to the indicated protein were scanned, and the intensities were represented as -fold versus sham control. The results are given as mean ± S.D. The data were obtained from no fewer than five independent animals in each experimental group, and the results of a typical experiment were presented (n = 5). a, p < 0.05 versus sham; b, p < 0.05 versus ischemia/reperfusion 6-h treatment group; c, p < 0.05 versus ATPA treatment group. IB, immunoblotting; IP, immunoprecipitation; I/R, ischemia/reperfusion; O.D., optical density.
To further confirm that ATPA performs a function through activating GluR5, we administered selective GluR5 antagonist NS3763 or GluR5 AS-ODNs, which can suppress the expression of GluR5, to rats combined with ATPA before ischemia. As shown in Fig. 1, A-D, for the ATPA + NS3763 and ATPA + GluR5 AS-ODNs treatment groups, we found that NS3763 and GluR5 AS-ODNs could reverse the effects of ATPA on the tyrosine phosphorylation of Src and NMDA receptor, whereas GluR5 MS-ODNs and the vehicles of the above drugs had no significant effects on the phosphorylation states. However, when only NS3763 was administered before ischemia, although the tyrosine phosphorylation of Src and NMDA receptor and the interactions among Src, PSD-95, and NR2A increased slightly, the change was not statistically significant (Fig. 1, E-H). Taken together, our results suggest that activated GluR5-containing KA receptor can down-regulate ischemia-induced Src activation and then attenuate the NMDA receptor tyrosine phosphorylation.
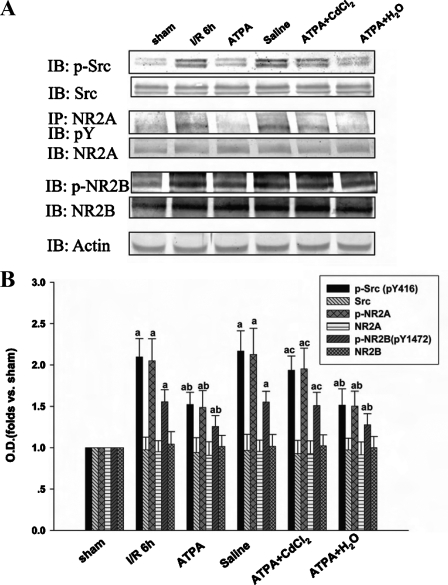
CdCl2 Suppresses the Inhibitory Effect of ATPA on Tyrosine Phosphorylation of Src, NR2A, and NR2B in the Hippocampal CA1 Region—To further examine whether the voltage-gated calcium channels play a role in the decreased phosphorylation of Src, NR2A, and NR2B in pyramidal cells of the hippocampal CA1 region induced by ATPA, we administered CdCl2, a broad spectrum blocker of voltage-gated calcium channels, to rats 30 min before ischemia. As shown in Fig. 2, for the ATPA + CdCl2 treatment group, CdCl2 could abolish the effect of ATPA on tyrosine phosphorylation of Src, NR2A, and NR2B. We also only injected CdCl2 into the rats before ischemia and then found that it had no significant effect on tyrosine phosphorylation of Src, NR2A, and NR2B (data not shown). Thus, our results suggest that the voltage-gated calcium channel is involved in the effect of activated GluR5-containing KA receptor on tyrosine phosphorylation of Src and the NMDA receptor.
FIGURE 2.
CdCl2 reverses the effect of ATPA on tyrosine phosphorylation of Src, NR2A, and NR2B in hippocampal CA1 region. The samples were taken at 6 h of reperfusion after 15 min of ischemia. A, the tyrosine phosphorylation and protein level of Src, NR2A, and NR2B were detected by immunoblotting. B, corresponding bands were scanned, and the intensities were represented as -fold versus sham control. The results are given as mean ± S.D. (n = 5). a, p < 0.05 versus sham; b, p < 0.05 versus ischemia/reperfusion 6-h treatment group; c, p < 0.05 versus ATPA treatment group. IB, immunoblotting; IP, immunoprecipitation; I/R, ischemia/reperfusion; O.D., optical density.
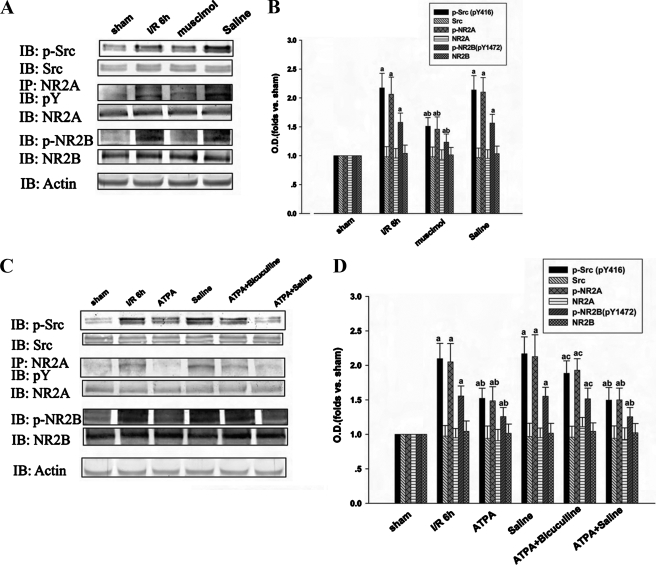
The GABAA Receptor Is Involved in the Effect of ATPA on Tyrosine Phosphorylation of Src, NR2A, and NR2B in the Hippocampal CA1 Region—It was reported that GluR5-containing KA receptors facilitate GABA release from interneurons and increase tonic inhibition of pyramidal neurons (17, 20-22). Therefore, we hypothesized that if released GABA, which was induced by activated GluR5-containing KA receptor, could affect tyrosine phosphorylation of Src, NR2A, and NR2B in postsynaptic pyramidal neurons, GABAA receptors on postsynaptic cells should be involved in the process. So we injected a GABAA receptor agonist Mus to rats before ischemia to see whether activated GABAA receptors can attenuate the tyrosine phosphorylation of Src, NR2A, and NR2B. The results confirmed the hypothesis (Fig. 3, A and B). The results suggest that the effects of activated GluR5-containing KA receptors on postsynaptic neurons may be mediated by GABAA receptors.
FIGURE 3.
GABAA receptor is involved in the effect of ATPA on tyrosine phosphorylation of Src, NR2A, and NR2B in hippocampal CA1 region. The samples were taken at 6 h of reperfusion after 15 min of ischemia. A and C, the tyrosine phosphorylation and expression level of Src, NR2A, and NR2B were detected by immunoblotting. B and D, bands were scanned, and the intensities were represented as -fold versus sham control. The results are given as mean ± S.D. (n = 5). a, p < 0.05 versus sham; b, p < 0.05 versus ischemia/reperfusion 6-h treatment group; c, p < 0.05 versus ATPA treatment group. IB, immunoblotting; IP, immunoprecipitation; I/R, ischemia/reperfusion; O.D., optical density.
To further confirm this, bicuculline, an antagonist of the GABAA receptors, combined with ATPA was administered to the rats 30 min before ischemia. The effect of ATPA on tyrosine phosphorylation of Src, NR2A, and NR2B was abolished by bicuculline (Fig. 3, C and D). Similar to the other drugs we used, single injection of bicuculline was also conducted before ischemia, and no significant change of tyrosine phosphorylation of Src, NR2A, and NR2B was found (data not shown). In summary, our results suggest that the effect of activated GluR5-containing KA receptor on tyrosine phosphorylation of Src and NMDA receptor is based on GABAA receptor activation.
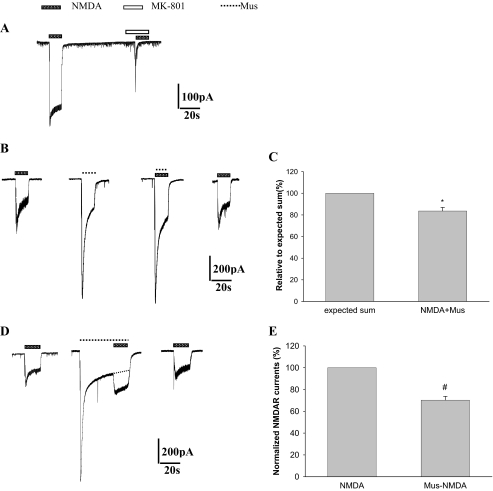
GABAA Receptor Activation Can Significantly Inhibit the NMDA Receptor-mediated Whole-cell Currents—To further investigate the role of GABAA receptors on the effects of ATPA in transient cerebral ischemia, patch clamp recording was used to detect the NMDA receptor-mediated whole-cell currents (INMDA) and the effects of GABAA receptor activator Mus on INMDA. GABAA receptor activation mediated by Mus induced an intracellular current, and co-application of Mus with NMDA evoked the current with an amplitude (83.6 ± 3.3%) that was significantly lower than the expected sum of currents evoked by Mus (IMus) and NMDA (INMDA) independently. The results suggested that there was a cross-inhibition between GABAA receptors and NMDA receptors (Fig. 4A, B and C). Moreover, GABAA receptor activator application was followed by NMDA application to further investigate the relationship between GABAA receptors and NMDA receptors (Fig. 4, D and E). Following a single application of the Mus, INMDA (66.8% ± 3.1%) became much less than the control INMDA.
FIGURE 4.
GABAA receptor activation inhibits NMDA receptor-mediated whole cell currents in cultured rat hippocampal neurons. A, NMDA-induced whole-cell currents were inhibited by NMDA receptor inhibitor MK-801. B, cross-inhibition of GABAA receptor and NMDA receptor-mediated whole-cell currents. D, effects of GABAA receptor activation on NMDA-induced whole-cell currents. The broken line indicates a single application of Mus without NMDA-induced whole-cell currents. C and E, statistic results of GABAA receptor inhibiting effects on NMDA receptor-mediated whole-cell currents. All data here are expressed as mean ± S.D. (n = 6). *, p < 0.05, compared with the group of expected sum; #, p < 0.05 compared with the group of NMDA. Concentrations were as follows: NMDA, 100 μm; MK-801, 50 μm; Mus, 15 μm.
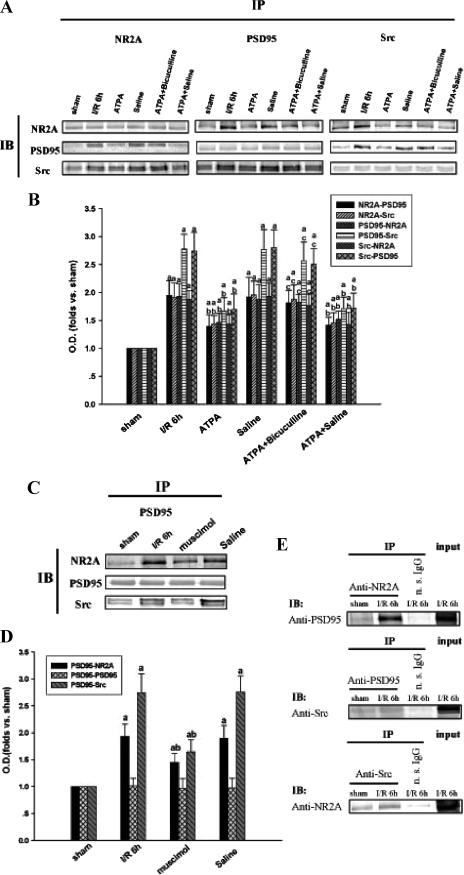
ATPA Disturbs Interactions among PSD-95, Src, and NR2A after Ischemia, and GABAA Receptors Are Involved in the Process—Our previous studies showed that the interaction between Src and NMDA receptors reaches its peak level at 6 h of reperfusion after ischemia (11), and PSD-95 is involved in the events (10). To further investigate whether the effect of ATPA on attenuating tyrosine phosphorylation of NMDA receptors and Src is related to the interactions among PSD-95, NR2A, and Src, immunoprecipitation and immunoblotting were used to examine the association among NR2A, Src, and PSD-95 after 15 min of ischemia followed by 6 h of reperfusion. Reciprocal immunoprecipitation was carried out to confirm the results. As shown in the ATPA treatment group of Fig. 5, A and B, administration of ATPA before ischemia diminished the increased interactions among NR2A, Src, and PSD-95; meanwhile, the protein levels of NR2A, Src, and PSD-95 were not altered. In addition, to confirm that the neuroprotective role of ATPA is mediated by the activation of GABAA receptors, we injected bicuculline combined with ATPA to the rats before ischemia. As shown in the ATPA + bicuculline treatment group of Fig. 5, A and B, the effect of ATPA on the interactions of NR2A, Src, and PSD-95 was inhibited by blocking GABAA receptors. Moreover, GABAA receptor agonist Mus treatment can also suppress the interactions among NR2A, Src, and PSD-95 (Fig. 5, C and D). Thus, our data suggest that the GluR5-induced disassembly of the triplicate complex NR2A-PSD-95-Src may be mediated by GABAA receptors. When immunoprecipitated with nonspecific IgG, no significant bands corresponding to PSD-95, Src, and NR2A were detected (Fig. 5E).
FIGURE 5.
ATPA disturbs interactions among PSD-95, Src, and NR2A after ischemia, and the GABAA receptor is involved in the process. The samples were taken at 6 h of reperfusion after 15 min of ischemia. A, reciprocal co-immunoprecipitation analysis of interactions among NR2A, PSD-95, and Src. Sample proteins were immunoprecipitated with anti-NR2A, anti-PSD-95, or anti-Src antibodies and then blotted with anti-NR2A, anti-PSD-95, or anti-Src antibodies. C, sample protein was immunoprecipitated with anti-PSD-95 and then blotted with anti-NR2A, anti-PSD-95, or anti-Src antibody. B and D, bands corresponding to indicated protein were scanned, and the intensities were represented as -fold versus sham control. The results are given as mean ± S.D. E, homogenates (400 μg of total protein) from sham and ischemia/reperfusion 6-h treatment groups were immunoprecipitated with anti-NR2A, anti-PSD-95, anti-Src antibody, or nonspecific IgG (n.s. IgG), and the precipitates were analyzed by immunoblotting with anti-PSD-95, anti-Src, or anti-NR2A antibody. In the input lane, 100 μg of proteins without immunoprecipitation were loaded. The data were obtained from no fewer than five independent animals in each experimental group, and the results of a typical experiment are presented. a, p < 0.05 versus sham; b, p < 0.05 versus ischemia/reperfusion 6-h treatment group; c, p < 0.05 versus ATPA treatment group. I/R, ischemia/reperfusion; IP, immunoprecipitation; IB, immunoblotting.
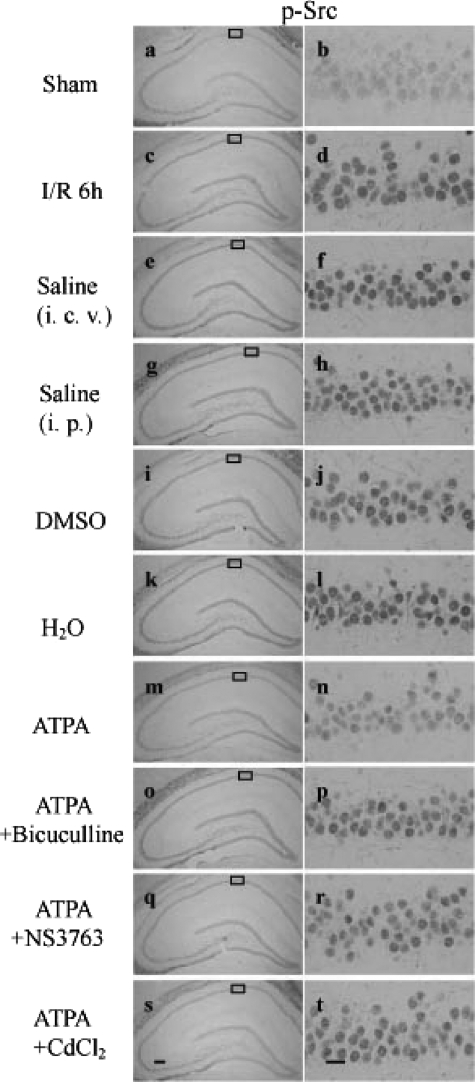
Immunohistochemistry Also Reveals the Mechanism Underlying the Effect of Activated GluR5-containing KA Receptor—Further immunohistochemical analysis also showed the neuroprotective effects of ATPA on the tyrosine phosphorylation of Src. In the sham treatment group (Fig. 6, a and b), p-Src immunoreactivity was barely detectable in the CA1 pyramidal neuron; however, the immunoreactivity significantly increased at 6 h of reperfusion (Fig. 6, c and d), and the inhibitory effect of ATPA was obvious with weak immunostaining (Fig. 6, m and n) in contrast to vehicle saline (Fig. 6, e and f). In addition, the inhibitory effect of ATPA on tyrosine phosphorylation of Src was suppressed by NS3763 (Fig. 6, q and r), CdCl2 (Fig. 6, s and t), or bicuculline (Fig. 6, o and p), whereas the vehicle of NS3763 DMSO (Fig. 6, i and j), CdCl2 H2O (Fig. 6, k and l), or bicuculline saline (Fig. 6, g and h) had no significant effect on p-Src immunoreactivity. Taken together, our results suggest that ATPA performs a neuroprotective function by acting on GluR5. The effect of activated GluR5-containing KA receptors on Src tyrosine phosphorylation is mediated by voltage-gated calcium channels and GABAA receptors.
FIGURE 6.
Immunohistochemistry reveals the mechanism underlying the neuroprotective effect of activated GluR5-containing KA receptors. Coronal sections from rats subjected to sham (a and b), 15 min of ischemia followed by 6 h of reperfusion (c and d), administration of vehicle (e-l), ATPA (m and n), ATPA plus bicuculline (o and p), ATPA plus NS3763 (q and r), or ATPA plus CdCl2 (s and t) before ischemia were immunostained with anti-p-Src (Tyr-416) antibody. Results were obtained from six independent animals in each experimental group, and the results of a typical experiment were presented. The boxed areas in the left column are shown at higher magnification in the right column. Scale bar in q, 200 μm (magnification ×40); scale bar in r, 20 μm (magnification ×400). I/R, ischemia/reperfusion; i.c.v., intracerebroventricular; i.p., intraperitoneal.
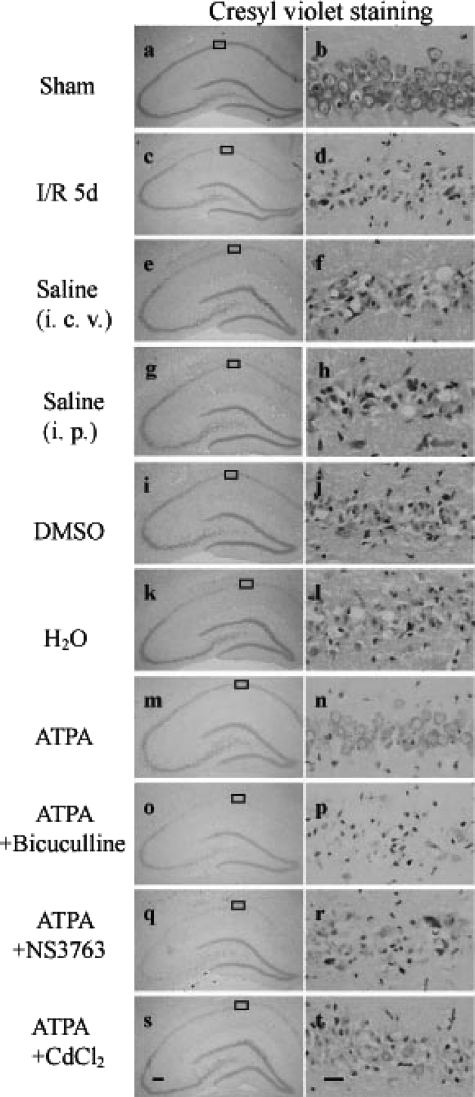
Neuroprotective Effect of ATPA against Ischemic Brain Injury in Vivo—It is well known that cerebral ischemia induces delayed neuronal death in pyramidal neurons of the hippocampal CA1 region. To investigate whether ATPA has a protective role against ischemia-reperfusion-induced neuronal cell death, cresyl violet staining was performed to examine the survival of pyramidal neurons of the hippocampal CA1 region. Rats were injected with ATPA, bicuculline, NS3763, or CdCl2 before ischemia. Results indicated that normal pyramidal cells in the sham treatment group (Fig. 7, a and b) showed round and pale stained nuclei, whereas dead cells in the ischemia-reperfusion and vehicle treatment group showed pyknotic nuclei (Fig. 7, c-l). ATPA treatment group (Fig. 7, m and n) showed a significant decrease in neuronal degeneration. However, the protective effect of ATPA disappeared when the rats were treated with bicuculline, NS3763, or CdCl2, respectively, in addition to ATPA (Fig. 7, o-t). The surviving pyramidal cells counted within a 1-mm length of the CA1 region were 205 ± 24, 19 ± 7, 20 ± 6, 21 ± 6, 22 ± 7, 18 ± 6, 113 ± 14, 24 ± 7, 27 ± 8, and 23 ± 7, corresponding to Fig. 7, b, d, f, h, j, l, n, p, r, and t, respectively. Taken together, our results suggest that activation of GluR5-containing KA receptors has a neuroprotective effect against ischemia-reperfusion-induced neuronal cell death in vivo, and furthermore voltage-gated calcium channels and GABAA receptors are involved in the event.
FIGURE 7.
Neuroprotective effect of ATPA against ischemic brain injury in vivo. Cresyl violet staining was performed on sections from the hippocampus of rats subjected to sham (a and b), 15 min of ischemia followed by 5 days of reperfusion (c and d), administration of vehicle (e-l), ATPA (m and n), ATPA plus bicuculline (o and p), ATPA plus NS3763 (q and r), or ATPA plus CdCl2 (s and t) before ischemia. Results were obtained from six independent animals in each experimental group, and the results of a typical experiment are presented here. The boxed areas in the left column are shown at higher magnification in the right column. Scale bar in q, 200 μm (magnification ×40); scale bar in r, 20 μm (magnification ×400). I/R, ischemia/reperfusion; i.c.v., intracerebroventricular.
DISCUSSION
In the present study, we report for the first time that activation of GluR5-containing KA receptors has a neuroprotective effect against ischemic brain injury by suppressing Src activation and interactions among NR2A, PSD-95, and Src and then decreasing NMDA receptor activation by attenuating NMDA receptor tyrosine phosphorylation, which are Ca2+-dependent and GABAA receptor-mediated.
In virtue of in situ hybridization, it was found that KA receptor subunit GluR6 is expressed primarily within the excitatory pyramidal cells of the hippocampus, whereas the inhibitory interneurons express primarily GluR5 (16, 17). The different distribution of these KA receptor subunits may mean that they have distinct functions in central nervous system. In epilepsy research, GluR6-deficient mice exhibited resistance to neurotoxic effects induced by kainate (12). Knock-out of GluR6 prevented kainate-induced epileptiform bursts, whereas ablation of GluR5 led to a higher susceptibility of epileptogenic effects of kainate (13), and an agonist of GluR5-containing KA receptors had antiepileptic effects (14). According to above, we infer that GluR6 and GluR5 may play opposing roles in excitability of the brain. In previous articles concerning cerebral ischemia, we found that GluR6-mediated c-Jun N-terminal kinase activation is responsible for ischemic brain injury (15). Based on this, we can hypothesize that GluR5 activation may have a neuroprotective effect against ischemic brain injury by playing opposing roles to GluR6 during ischemia-reperfusion. Our present study confirmed the hypothesis. The results show that activated GluR5-containing KA receptors have a neuroprotective effect against ischemic brain injury in vivo (Fig. 7) and can suppress the tyrosine phosphorylation of Src, NR2A, and NR2B (Fig. 1, A-D). However, when we administered a GluR5 antagonist NS3763, instead of ATPA, to rats, the tyrosine phosphorylation of Src and NMDA receptors and the interactions among PSD-95, NR2A, and Src increased slightly over the vehicle control groups, but the changes were not statistically significant (Fig. 1, E-H). This may be due to the fact that GluR5 becomes much more activated than normal when ATPA binds to it. Thus, the inhibitive effect of the GluR5 antagonist on activated GluR5 is more marked than on inactivated GluR5 at a low level of activity. An additional factor may be the limited quantity of total protein of Src in pyramidal cells. Since ischemia-reperfusion induces Src activation, if nearly all Tyr-416 of Src is phosphorylated during ischemia-reperfusion, the antagonist NS3763 treatment will not further make more Src activated. Then the increase of NMDA receptor tyrosine phosphorylation and the interactions will subsequently be affected.
GABAergic inhibitory interneurons of the hippocampus are the regulators of pyramidal neuron excitability (19). As described previously, interneurons in the hippocampal CA1 region express high levels of GluR5-containing KA receptors, whereas in hippocampal pyramidal cells expression of GluR5 is barely detectable (16, 17). However, as shown in our results, Src tyrosine phosphorylation (Fig. 6) and the protective role of ATPA against ischemia-reperfusion in vivo (Fig. 7) are performed in the pyramidal cells of the hippocampus. So, how does activation of GluR5-containing KA receptors on interneurons affect postsynaptic pyramidal cells? Recently, it was reported that GluR5-containing KA receptors can facilitate GABA release and increase tonic inhibition of pyramidal neurons (17, 20-22). Based on this information, we thought that the neuroprotective role of activated GluR5-containing KA receptors reflected the relationship between presynaptic and postsynaptic neurons. Therefore, we wanted to demonstrate the hypothesis about the effect of released GABA from interneurons on the pyramidal neurons by investigating the change in the postsynaptic cells induced by activating GluR5 on interneurons. Our data indicated that bicuculline, a selective GABAA receptor antagonist, could abolish the postsynaptic events induced by activating GluR5, such as suppressing Src activation and interactions among NR2A, PSD-95, and Src then decreasing NMDA receptor activation through attenuating NMDA receptor tyrosine phosphorylation. These results suggest that activated GluR5-containing KA receptors may facilitate GABA release from interneurons. However, previous studies reported different results, which suggest that activation of KA receptors on GABAergic presynaptic terminals leads to a decrease of GABA release (28, 35), and in cerebral ischemia research, corresponding results were also reported (36). Thus, two opposite hypotheses, “overinhibition” and “disinhibition,” were presented (21). Some experiments set out to investigate the inconsistency and then showed that low concentrations of GluR5-containing KA receptor agonists facilitate, whereas high concentrations suppress, GABAergic transmission in interneuron-to-pyramidal cell synapses (37). The results of the other group in our laboratory indicated that the appropriate concentration of ATPA (1-2.5 nmol) ensures that neuroprotection is performed on postsynaptic pyramidal neurons,4 which provides a basis for the dose of ATPA used in our present study. The possible mechanism underlying the concentration-dependent effect is as follows. Since KA receptor subunits can be divided into two groups on the basis of their affinity for [3H]kainate (the low affinity subunits (GluR5-GluR7) and the high affinity subunits (KA1 and KA2)) (38), a GluR5/KA2 and GluR5/GluR6 subunit combination could mediate the facilitation and inhibition of GABAergic transmission, respectively (37).
The exact intracellular processes induced by activated KA receptors remain to be determined. The influx of calcium ions into a GABAergic terminal causes release of the neurotransmitter GABA into the synaptic cleft. Previous studies showed that facilitation of GABAergic transmission by activating KA receptors did not require activation of the voltage-gated calcium channels but could be mediated by calcium influx through Ca2+-permeable GluR5-containing KA receptors (37). However, there was also a report that blockage of calcium influx by Cd2+ inhibited the KA receptor-mediated increase in GABA release (22). In the present study, our data suggest that ischemia-reperfusion GABA release, which is due to activating GluR5-containing KA receptors, requires activation of voltage-gated calcium channels, but we could not rule out the possibility of the involvement of GluR5-containing KA receptors in the entry of calcium ions.
Activated GABAA receptors play a neuroprotective role in ischemia (25-27). Our data show that activation of GABAA receptors and GluR5-containing KA receptors could lead to similar results. Moreover, bicuculline, an antagonist of GABAA receptors, could suppress the change induced by GluR5 activation in postsynaptic neurons. Thus, our results suggest that activated GluR5-containing KA receptors may facilitate GABA release from interneurons. Released GABA can activate postsynaptic GABAA receptors. Then the influx of Cl- through GABAA receptors can counter postsynaptic membrane depolarization and limit calcium entry (39). Moreover, according to the results of patch clamp recording in our present study, we directly proved that GABAA receptor activation could significantly inhibit the NMDA receptor-mediated whole-cell currents. In summary, ATPA-induced GABA release could inhibit NMDA receptors and attenuate the increased NMDA receptor tyrosine phosphorylation by suppressing Src activation and interactions among PSD-95, Src, and NR2A.
Excitotoxicity is the main mechanism of ischemic brain injury. NMDA receptors are involved in this process. Cerebral ischemia causes excessive glutamate release, which induces the influx of excessive Na+ and Ca2+ ions through NMDA receptors (7) and increases tyrosine phosphorylation of NR2A and NR2B (6). Src kinase can increase the activity of NMDA receptors (9) and also bind to NMDA receptors with its Src homology 2 domain (40). It can mediate tyrosine phosphorylation of NR2A and NR2B and the increased tyrosine phosphorylation, resulting in the potentiation of the NMDA ion channel (8, 41, 42). As a result, more Ca2+ ions enter postsynaptic pyramidal cells through NMDA receptors, which further leads to Src kinase activation. The positive feedback mechanisms induce delayed neuronal death. Our previous study showed that not only the tyrosine phosphorylation of NR2A and NR2B but also the association of NMDA receptors and Src reach their peak level at 6 h of reperfusion after ischemia (11, 32), and activated Src induces enhanced NMDA receptor tyrosine phosphorylation by means of the signaling module NR2A-PSD-95-Src during ischemia-reperfusion (10). In this study, our data suggest that activated GluR5-containing KA receptors could suppress Src activation and interactions among NR2A, PSD-95, and Src and then attenuate NMDA receptor-mediated excitotoxicity by attenuating NMDA receptor tyrosine phosphorylation.
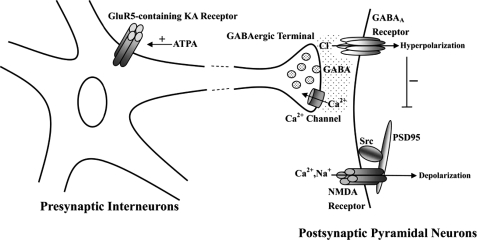
In conclusion, as depicted in Fig. 8, activated GluR5-containg KA receptors play a neuroprotective role against ischemic brain injury through an interesting pathway. Brain functions are based on the dynamic balance between excitatory and inhibitory inputs. Cerebral ischemia upsets the balance, and the neural excitotoxicity gains the upper hand. The process underlying the neuroprotective effect of GluR5 activation renders us a possible delicate regulative mechanism, which may exist not only in the pathological conditions of exitotoxicity, such as stroke, but also in normal physiological conditions; the excitatory transmitter glutamate can regulate the balance between excitatory and inhibitory synaptic inputs by activating GluR5-containing KA receptors. Since activated GluR5-containing KA receptors have a neuroprotective effect against ischemic brain injury in vivo, the signaling pathway suggests a potential new therapy for ischemia.
FIGURE 8.
Scheme summarizing the proposed mechanisms underlying neuroprotection of GluR5-containing kainate receptor activation against ischemic brain injury. Ischemia-reperfusion induces entry of excessive Na+ and Ca2+ ions through NMDA receptors. Activated Src phosphorylates NMDA receptors, which is mediated by PSD-95. The results enhance the NMDA receptor function and further promote the influx of Ca2+. The positive feedback mechanism induces the delayed neuronal death. Our present results suggest that activation of GluR5-containing KA receptors induces GABA release from the synaptic terminal of interneurons, which is Ca2+-dependent. Released GABA activates postsynaptic GABAA receptors. Then the influx of Cl- ions through the GABAA receptors can counter postsynaptic membrane depolarization and limit calcium entry and then suppress Src activation. Then the interactions among Src, PSD-95, and NR2A, decrease the activity of NMDA channels.
This work was supported by Great Research Project of the National Natural Science Foundation of China Grant 90608015, Natural Science foundation of Jiangsu Province, China, Grant BK2007546, and the President Special Grant of Xuzhou Medical College 07KJZ08 and Natural Science Foundation of the Jiangsu Higher Education Institutions of China Grants 06KJB 180116 and 07KJA18030. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations and trivial name used are: NMDA, N-methyl-d-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate; AS-ODNs, antisense oligodeoxynucleotides; ATPA, (RS)-2-Amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid; GABA, γ-aminobutyric acid; KA receptor, kainate receptor; Mus, muscimol; p-Src, phospho-Src; NS3763, 4,6-bis(benzoylamino)-1,3-benzenedicarboxylic acid.
Q. Lv, Y. Liu, J. Xu, and G. -Y. Zhang, unpublished data.
References
- 1.Hollmann, M., and Heinemann, S. (1994) Annu. Rev. Neurosci. 17 31-108 [DOI] [PubMed] [Google Scholar]
- 2.Seeburg, P. H. (1993) Trends Neurosci. 16 359-365 [DOI] [PubMed] [Google Scholar]
- 3.Andersson, O., Stenqvist, A., Attersand, A., and von Euler, G. (2001) Genomics 78 178-184 [DOI] [PubMed] [Google Scholar]
- 4.Chatterton, J. E., Awobuluyi, M., Premkumar, L. S., Takahashi, H., Talantova, M., Shin, Y., Cui, J., Tu, S., Sevarino, K. A., Nakanishi, N., Tong, G., Lipton, S. A., and Zhang, D. (2002) Nature 415 793-798 [DOI] [PubMed] [Google Scholar]
- 5.Laube, B., Kuhse, J., and Betz, H. (1998) J. Neurosci. 18 2954-2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takagi, N., Shinno, K., Teves, L., Bissoon, N., Wallace, M. C., and Gurd, J. W. (1997) J. Neurochem. 69 1060-1065 [DOI] [PubMed] [Google Scholar]
- 7.Lee, J. M., Zipfel, G. J., and Choi, D. W. (1999) Nature 399 (suppl) A7-A14 [DOI] [PubMed] [Google Scholar]
- 8.Ali, D. W., and Salter, M. W. (2001) Curr. Opin. Neurobiol. 11 336-342 [DOI] [PubMed] [Google Scholar]
- 9.Yu, X. M., Askalan, R., Keil, G. J., II, and Salter, M. W. (1997) Science 275 674-678 [DOI] [PubMed] [Google Scholar]
- 10.Hou, X. Y., Zhang, G. Y., and Zong, Y. Y. (2003) Neurosci. Lett. 343 125-128 [DOI] [PubMed] [Google Scholar]
- 11.Liu, Y., Zhang, G., Gao, C., and Hou, X. (2001) Brain Res. 909 51-58 [DOI] [PubMed] [Google Scholar]
- 12.Mulle, C., Sailer, A., Perez-Otano, I., Dickinson-Anson, H., Castillo, P. E., Bureau, I., Maron, C., Gage, F. H., Mann, J. R., Bettler, B., and Heinemann, S. F. (1998) Nature 392 601-605 [DOI] [PubMed] [Google Scholar]
- 13.Fisahn, A., Contractor, A., Traub, R. D., Buhl, E. H., Heinemann, S. F., and McBain, C. J. (2004) J. Neurosci. 24 9658-9668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalilov, I., Hirsch, J., Cossart, R., and Ben-Ari, Y. (2002) J. Neurophysiol. 88 523-527 [DOI] [PubMed] [Google Scholar]
- 15.Pei, D. S., Wang, X. T., Liu, Y., Sun, Y. F., Guan, Q. H., Wang, W., Yan, J. Z., Zong, Y. Y., Xu, T. L., and Zhang, G. Y. (2006) Brain 129 465-479 [DOI] [PubMed] [Google Scholar]
- 16.Bahn, S., Volk, B., and Wisden, W. (1994) J. Neurosci. 14 5525-5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bureau, I., Bischoff, S., Heinemann, S. F., and Mulle, C. (1999) J. Neurosci. 19 653-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz-Bloom, R. D., and Sah, R. (2001) J. Neurochem. 77 353-371 [DOI] [PubMed] [Google Scholar]
- 19.Freund, T. F., and Buzsaki, G. (1996) Hippocampus 6 347-470 [DOI] [PubMed] [Google Scholar]
- 20.Christensen, J. K., Paternain, A. V., Selak, S., Ahring, P. K., and Lerma, J. (2004) J. Neurosci. 24 8986-8993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cossart, R., Esclapez, M., Hirsch, J. C., Bernard, C., and Ben-Ari, Y. (1998) Nat. Neurosci. 1 470-478 [DOI] [PubMed] [Google Scholar]
- 22.Jiang, L., Xu, J., Nedergaard, M., and Kang, J. (2001) Neuron 30 503-513 [DOI] [PubMed] [Google Scholar]
- 23.Bormann, J. (2000) Trends Pharmacol. Sci. 21 16-19 [DOI] [PubMed] [Google Scholar]
- 24.Oja, S. S., Korpi, E. R., and Saransaari, P. (1990) Neurochem. Res. 15 797-804 [DOI] [PubMed] [Google Scholar]
- 25.Lyden, P. D., and Hedges, B. (1992) Stroke 23 1463-1469; Discussion 1469-1470 [DOI] [PubMed] [Google Scholar]
- 26.Lyden, P. D., and Lonzo, L. (1994) Stroke 25 189-196 [DOI] [PubMed] [Google Scholar]
- 27.Shuaib, A., Mazagri, R., and Ijaz, S. (1993) Exp. Neurol. 123 284-288 [DOI] [PubMed] [Google Scholar]
- 28.Clarke, V. R., Ballyk, B. A., Hoo, K. H., Mandelzys, A., Pellizzari, A., Bath, C. P., Thomas, J., Sharpe, E. F., Davies, C. H., Ornstein, P. L., Schoepp, D. D., Kamboj, R. K., Collingridge, G. L., Lodge, D., and Bleakman, D. (1997) Nature 389 599-603 [DOI] [PubMed] [Google Scholar]
- 29.Pulsinelli, W. A., and Brierley, J. B. (1979) Stroke 10 267-272 [DOI] [PubMed] [Google Scholar]
- 30.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem. 193 265-275 [PubMed] [Google Scholar]
- 31.Jiang, Q., Gu, Z., Zhang, G., and Jing, G. (2000) Brain Res. 857 71-77 [DOI] [PubMed] [Google Scholar]
- 32.Pei, L., Li, Y., Zhang, G. Y., Cui, Z. C., and Zhu, Z. M. (2000) Acta Pharmacol. Sin. 21 695-700 [PubMed] [Google Scholar]
- 33.Nakazawa, T., Komai, S., Tezuka, T., Hisatsune, C., Umemori, H., Semba, K., Mishina, M., Manabe, T., and Yamamoto, T. (2001) J. Biol. Chem. 276 693-699 [DOI] [PubMed] [Google Scholar]
- 34.Cooper, J. A., and MacAuley, A. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 4232-4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Moreno, A., Herreras, O., and Lerma, J. (1997) Neuron 19 893-901 [DOI] [PubMed] [Google Scholar]
- 36.O'Neill, M. J., Bogaert, L., Hicks, C. A., Bond, A., Ward, M. A., Ebinger, G., Ornstein, P. L., Michotte, Y., and Lodge, D. (2000) Neuropharmacology 39 1575-1588 [DOI] [PubMed] [Google Scholar]
- 37.Braga, M. F., Aroniadou-Anderjaska, V., Xie, J., and Li, H. (2003) J. Neurosci. 23 442-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chittajallu, R., Braithwaite, S. P., Clarke, V. R., and Henley, J. M. (1999) Trends Pharmacol. Sci. 20 26-35 [DOI] [PubMed] [Google Scholar]
- 39.Lee, J. M., Grabb, M. C., Zipfel, G. J., and Choi, D. W. (2000) J. Clin. Invest. 106 723-731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takagi, N., Cheung, H. H., Bissoon, N., Teves, L., Wallace, M. C., and Gurd, J. W. (1999) J. Cereb. Blood Flow Metab. 19 880-888 [DOI] [PubMed] [Google Scholar]
- 41.Wang, Y. T., and Salter, M. W. (1994) Nature 369 233-235 [DOI] [PubMed] [Google Scholar]
- 42.Wang, Y. T., Yu, X. M., and Salter, M. W. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 1721-1725 [DOI] [PMC free article] [PubMed] [Google Scholar]