Abstract
Under excess illumination, the Photosystem II light-harvesting antenna of higher plants has the ability to switch into an efficient photoprotective mode, allowing safe dissipation of excitation energy into heat. In this study, we show induction of the energy dissipation state, monitored by chlorophyll fluorescence quenching, in the isolated major light-harvesting complex (LHCII) incorporated into a solid gel system. Removal of detergent caused strong fluorescence quenching, which was totally reversible. Singlet-singlet annihilation and gel electrophoresis experiments suggested that the quenched complexes were in the trimeric not aggregated state. Both the formation and recovery of this quenching state were inhibited by a cross-linker, implying involvement of conformational changes. Absorption and CD measurements performed on the samples in the quenched state revealed specific alterations in the spectral bands assigned to the red forms of chlorophyll a, neoxanthin, and lutein 1 molecules. The majority of these alterations were similar to those observed during LHCII aggregation. This suggests that not the aggregation process as such but rather an intrinsic conformational transition in the complex is responsible for establishment of quenching. 77 K fluorescence measurements showed red-shifted chlorophyll a fluorescence in the 690-705 nm region, previously observed in aggregated LHCII. The fact that all spectral changes associated with the dissipative mode observed in the gel were different from those of the partially denatured complex strongly argues against the involvement of protein denaturation in the observed quenching. The implications of these findings for proposed mechanisms of energy dissipation in the Photosystem II antenna are discussed.
Photosynthetic light harvesting is a controlled process, well integrated into the light phase of photosynthesis. Under conditions of intense illumination, the photosystem II antenna undergoes a transition into a photoprotective mode to dissipate harmful excess light (1-5). This mode has been quantified by chlorophyll fluorescence measurements, enabling calculation of the non-photochemical quenching (NPQ)3 component (6, 7). The precise mechanism of NPQ is currently under intense debate. Recently, the focus of this debate has centered upon which of the pigments associated with the antenna are involved. In one model of NPQ, a zeaxanthin radical cation acts as a direct quencher of chlorophyll excited states (5). Alternatively, it has been suggested that a conformational change in light-harvesting proteins, resulting from establishment of ΔpH across the thylakoid membrane, brings about dissipative pigment interactions (2, 4, 8, 9). Specifically, an energy transfer path to a low-lying excited state of lutein 1 was revealed in experiments on isolated quenched LHCII complexes (10). In both cases, evidence of these putative quenchers in plant leaves and chloroplasts in the NPQ state has been obtained (5, 10). However, elucidation of the mechanisms involved undoubtedly will come from studies of isolated antenna components in which the fine details of structure and function can be determined.
Experiments on the main trimeric antenna complex, LHCII, showed that a highly quenched state resembling NPQ (2) could be achieved by exposure of the complex to a low detergent medium (9). These conditions inevitably caused pronounced aggregation of LHCII. An almost identical quenching state was found in the LHCII crystals (1) used for structural determination (11). Three key questions emerged from these studies. (a) Was the control of the quenching mode at the level of interactions between neighboring proteins found in these systems? (b) Was a change in protein conformation involved? (c) Which of the pigment spectral changes observed are directly associated with the quenching state?
LHCII aggregates and crystals are not suitable for many experimental approaches needed to answer these questions. Thus, it is important to develop a new methodology in which the photoprotective state can be explored. First, it is essential to test whether the aggregation of complexes is an absolute requirement of excitation energy quenching in the antenna. Second, if quenching could be induced without aggregation, it would be essential to determine whether it has the same nature as that present in aggregated LHCII. This study addresses the questions mentioned above and provides direct evidence that the mechanism of the excitation quenching in LHCII is localized at the level of monomeric LHCII, involves conformational change in the protein, and is associated with the specific alterations in configuration of pigments.
EXPERIMENTAL PROCEDURES
LHCII trimers were prepared from dark-adapted spinach leaves by an isoelectric focusing procedure as described (12). To prepare LHCIIb monomers, the LHCIIb trimers were treated with phospholipase A2 for 48 h at room temperature as reported previously (13). Immediately after treatment, samples were further purified using a seven-step exponential sucrose gradient from 0.15 to 0.87 m sucrose (14).
Oligomeric LHCII was obtained by incubation of the trimers with 50 mg of SM-2 absorbent (Bio-Rad), allowing a 10-fold decrease in the fluorescence yield. Denaturation of LHCII complexes was achieved by incubation of samples at 70 °C for 5 min.
Trimers or monomers of LHCII were introduced into a 6% polyacrylamide gel by the following procedure. Samples were diluted in a solution containing 20 mm HEPES (pH 7.8) and 0.03% n-dodecyl β-d-maltoside, mixed with a solution of acrylamide/bisacrylamide (37.5:1), and polymerized with 0.05% ammonium persulfate and 0.03% TEMED for ∼20 min using a Bio-Rad Mini-PROTEAN system. The gel thickness was 1 mm.
To induce different quenching states, strips of gel (∼1cm wide) were incubated in 20 mm HEPES (pH 7.8), allowing time for diffusion of the detergent from the gel. The fluorescence yield was measured with a Walz PAM101 fluorometer. Fluorescence quenching strength or amplitude (kd) was defined as (Fu - Fq)/Fq, where Fu and Fq are the fluorescence levels of unquenched and quenched samples, respectively.
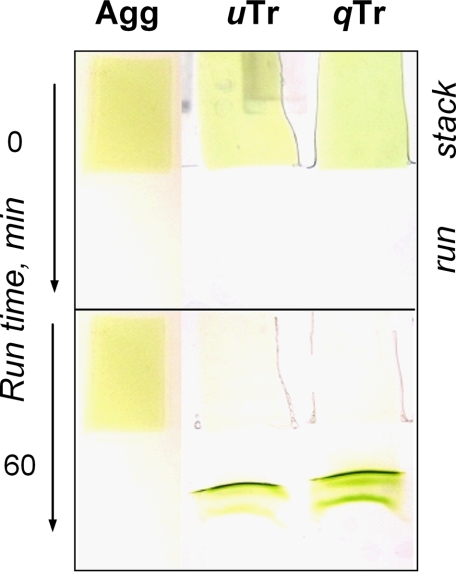
For Fig. 3, strips of LHCII gels were placed in the stacking gel area and electrophoresed for ∼60 min at 100 V in reservoir buffer containing 0.375 m Tris, 0.15 mm glycine, and 0.005% DERIPHAT (pH 7.8). The stacking gel was composed of 0.375 m Tris (pH 7.8), 0.05% ammonium persulfate, 0.03% TEMED, and 4% polyacrylamide. The running gel had the same composition except that the polyacrylamide concentration was 6%.
FIGURE 3.
Shown are the results from nondenaturing PAGE of gel fragments containing aggregate (Agg), unquenched trimer (uTr), and 10 times quenched trimer (qTr; kd = 9) (first through third lanes, respectively). Photographs of the gel were taken at t = 0 and 60 min. Aggregated LHCII was prepared prior incorporation into the gel and was 10 times quenched (kd = 9). Vertical arrows indicate the direction of phoresis. stack and run correspond to the stacking and running gels, respectively.
Absorption spectra were recorded using a Cary 500 UV-visible spectrophotometer (Varian) at 1-nm spectral resolution. Low temperature measurements were performed using an Optistat®DN LN2 cooled bath cryostat (Oxford Instruments). Samples were immersed in medium containing 70% (w/v) glycerol and 20 mm HEPES (pH 7.8).
CD spectra were recorded from 400 to 750 nm at 1-nm resolution using a J810 spectropolarimeter (Jasco). Temperature was maintained by an attached PFD425S Peltier system.
Time-correlated single-photon counting measurements were performed using a FluoTime200 picosecond fluorometer (PicoQuant). Excitation was provided at a 10-mHz repetition rate by a 650-nm laser diode. The fluorescence was detected at 680 nm with a 2-nm slit width. The pulse energy in the annihilation-free mode was <0.1 pJ. The instrument response function was in the range of 50 ps. Time-resolved emission spectra were recorded in the 665-695 nm region with 2-nm steps. FluoFit software (PicoQuant) was used for the lifetime and global analyses.
Low temperature fluorescence emission spectra were recorded using an optical multichannel analyzer-based instrument described in detail previously (9). Fluorescence was excited with 435-nm broad-band light defined by a combination of glass filters. The spectral resolution was 1 nm.
RESULTS
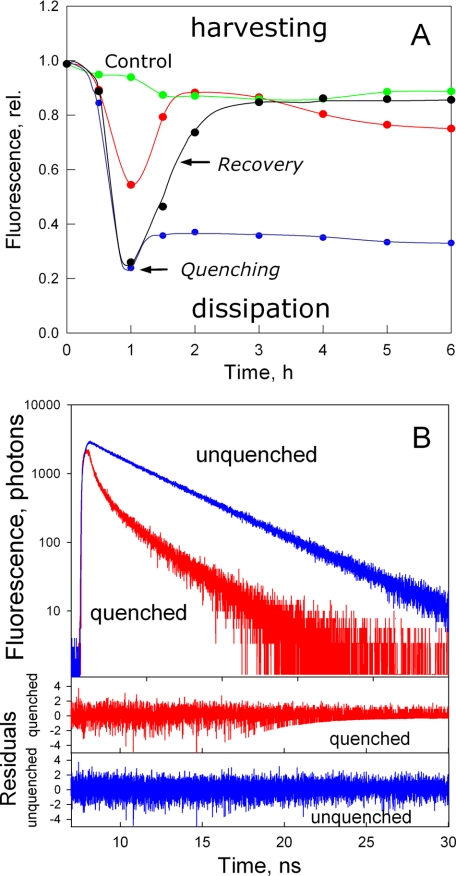
To prevent protein-protein interactions during the establishment of the photoprotective mode, trimeric LHCII complexes were incorporated into polyacrylamide gels to make LHCII gels. The proposition was that a dilute solution of protein in detergent would thereby be highly immobilized and very unlikely to form aggregates when detergent was removed. At an LHCII concentration of 10 μg/ml chlorophyll, the estimated average distance between complexes in the gel would be >100 nm. It has been previously demonstrated that the removal of detergent, acidification, and addition of dibucaine or zeaxanthin all enhance the transition of LHCII into the quenched state with varying degrees of efficiency, although in all cases the nature of the quenching state is the same (2). For study of LHCII gels, detergent removal was used as a trigger for the induction of energy dissipation (9, 10). LHCII gels were incubated at room temperature under low light conditions in medium containing different amounts of detergent (n-dodecyl β-d-maltoside) to investigate whether a quenching state could be induced. After 1 h of incubation in detergent-free buffer, the fluorescence level of the sample was decreased by ∼5-fold (Fig. 1A, black trace). Most important, the decrease in fluorescence was almost completely reversed when the gel was immersed in buffer containing detergent. A similar result was obtained if LHCII monomers were used instead of trimers (data not shown, but see below). Time-correlated single-photon counting fluorescence measurements revealed the fluorescence lifetimes of LHCII in the unquenched and quenched states in the gel (Fig. 1B and Table 1). For unquenched LHCII, the major lifetime component (86%) corresponded to a typical lifetime of the LHCII trimer (15) of ∼4.2 ns. For quenched LHCII, two dominating and much shorter lifetime components were observed, with lifetimes of 0.15 and 0.7 ns. The average fluorescence lifetimes of the unquenched and quenched LHCII gels indicated a 7-fold reduction in the fluorescence yield. This confirms that the observed fluorescence decrease in LHCII incorporated in the gel was due to a reduction in the excited state lifetime, direct proof of the formation of a highly efficient dissipative mode, as found for NPQ in vivo (16-18).
FIGURE 1.
A, time course of the chlorophyll fluorescence relative yield of LHCII incorporated in polyacrylamide gel. Fluorescence was excited at 650 nm and measured in the 685-800 nm region. The arrow labeled -DM indicates an LHCII gel placed in detergent-free medium. The red trace indicates incubation of the gel containing LHCII pretreated with glutaraldehyde for 5 min before setting the gel. After 60 min, recovery of the fluorescence of the samples was achieved by placing the gel fragment in medium containing 0.03% n-dodecyl β-d-maltoside (arrow labeled +DM); the control sample (green circles) was incubated in this buffer throughout the experiment. The blue circles indicate an LHCII gel placed in recovery buffer additionally containing glutaraldehyde. rel., relative. B, time-correlated single-photon counting fluorescence measurements of unquenched (upper panel, blue trace) and quenched (red trace) LHCII in the gel. The lower panels display the difference between the three-exponential fit and the experimental data.
TABLE 1.
Fitted decay times (τ) and relative amplitudes (p) of unquenched and quenched LHCII trimers embedded in polyacrylamide gel with 95% confidence intervals
Average fluorescence lifetimes of unquenched and quenched trimers correspond to 3.73 and 0.5 ns, respectively. Quenching in LHCII trimers was achieved by incubation of the gel in detergent-free buffer for 1.5 h. CI, confidence intervals.
|
Trimer
| |||||||
|---|---|---|---|---|---|---|---|
|
Unquenched
|
Quenched
|
||||||
| τ | CI | p | CI | τ | CI | p | CI |
| ns | ns | ||||||
| 4.19 | 4.16-4.22 | 0.86 | 0.87-0.85 | 2.77 | 2.71-2.84 | 0.09 | 0.086-0.094 |
| 1.64 | 1.45-1.84 | 0.03 | 0.015-0.041 | 0.70 | 0.66-0.74 | 0.22 | 0.205-0.235 |
| 0.69 | 0.60-0.88 | 0.11 | 0.09-0.13 | 0.15 | 0.14-0.16 | 0.69 | 0.64-0.73 |
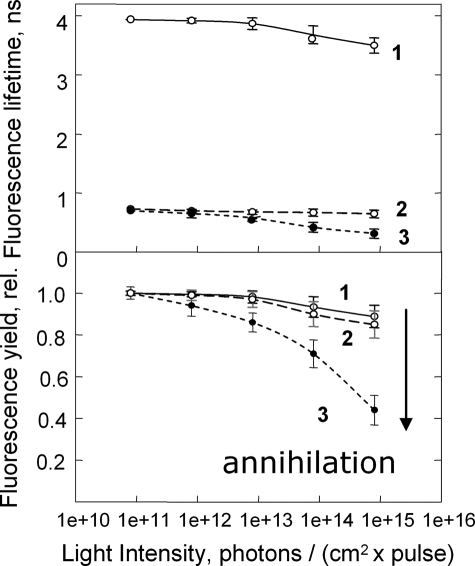
Two different approaches were used to confirm that protein aggregation had not occurred in the quenched LHCII gel. First, the presence of pigment interactions between different LHCII trimers was tested by the singlet-singlet exciton annihilation approach. Annihilation occurs when two excitons meet on one pigment molecule. The probability of the process increases with increasing light intensity and is greater the larger the number of closely interacting pigments. Thus, in LHCII aggregates, the onset of annihilation starts at much lower excitation light intensity compared with unaggregated LHCII (Fig. 2) (see also Ref. 19). This is explained by a larger pool of interacting pigments in the aggregate. In contrast, the quenched gels have almost the same light intensity dependence as the unquenched trimers (Fig. 2, lower panel), consistent with the absence of LHCII aggregates in the gel.
FIGURE 2.
Shown is the average fluorescence lifetime (upper panel) and normalized yield (lower panel) dependence upon the laser excitation light intensity probing the rate of the singlet-singlet annihilation in unquenched (trace 1) and quenched (trace 2) LHCII gels. Trace 3 represents the data for aggregated LHCII in solution. The arrow indicates the dramatic drop in the fluorescence yield of aggregated LHCII in comparison with both the unquenched and quenched trimers as the excitation photon density becomes higher. rel., relative.
In a second complementary approach, LHCII gels were subjected to electrophoresis. The gels were placed in an electrophoresis system in the stacking gel area and run for ∼60 min. LHCII migrated into the running gel area from both the quenched and unquenched gels. It is clear that highly quenched LHCII complexes moved out of the incubating gel into the running gel area along with the unquenched LHCII trimers. LHCII appeared as a mixture of LHCII monomers (Fig. 3, lower band) and trimers (upper band). An LHCII aggregate in the same gel did not migrate out of the stacking gel, as shown previously (8). Thus, the fact that quenched LHCII migrated out of the gel in the state of a trimer or monomer, not a higher oligomer, again suggested that aggregation was not involved in the establishment of the quenched mode of this complex. Rather, it points toward a conformational transition within the LHCII molecule underlying the quenching of fluorescence.
The LHCII gel allowed the exploration of one of the most controversial aspects of the quenching process, the occurrence of the protein conformational change. The protein cross-linker glutaraldehyde was used in the gel system to test this key point. In the presence of glutaraldehyde, the quenching observed after incubation in the detergent-free medium was strongly reduced (Fig. 1A, red trace); even after 6 h of incubation, the quenching constant (kd) was only 1.6, compared with the control value of 10. On the other hand, incubation of a pre-quenched LHCII gel in detergent buffer containing glutaraldehyde showed very little relaxation of quenching compared with the control (blue trace). Taken together, these data indicate that the LHCII protein undergoes conformational changes during transition both into and out of the dissipative state.
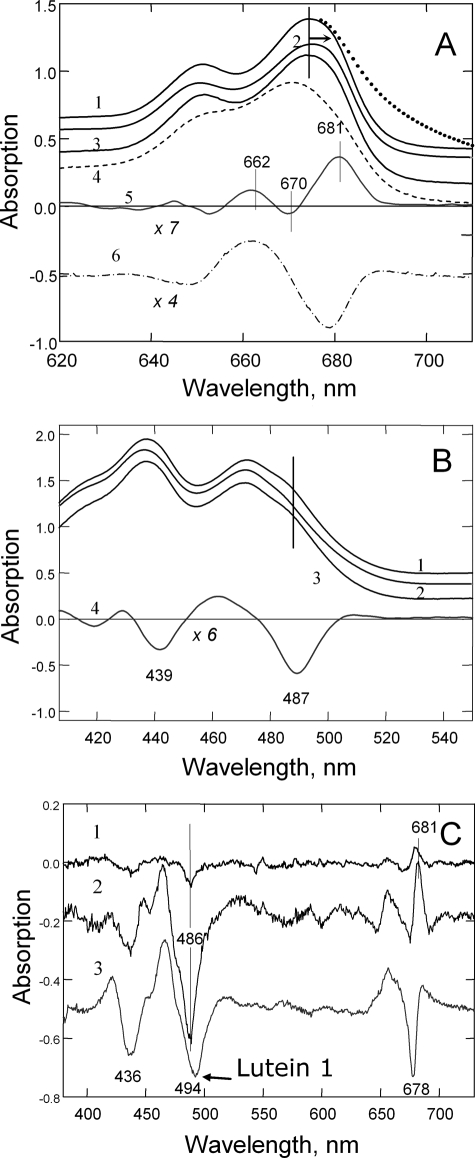
Having established that the quenching of LHCII in the gel was occurring by conformational change without protein aggregation, a range of spectroscopies were employed in an attempt to unambiguously determine the changes in pigment properties involved. Compared with that of either the unquenched or recovered gel (Fig. 4A, traces 1 and 3), the absorption spectrum of the quenched gel was characterized by a slight red shift in the chlorophyll QY region (trace 2). In contrast, in the spectrum of heat-treated denatured LHCII, the QY region of chlorophyll was strongly blue-shifted (trace 4). The difference spectra, quenched minus recovered (trace 5) and heat-treated minus control (trace 6), were clearly different, the former spectrum having the main peak at ∼681 nm, resembling that usually found for aggregation-induced quenching (20). However, as a result of light scattering from the particulate nature of the sample, the spectrum of aggregates had a pronounced long wavelength “tail” (shown by the dotted line above trace 1), which was absent in the spectrum of the quenched LHCII gel (for detailed light scattering analysis, see supplemental Figs. 1S and 2S). The difference spectrum indicated absorption changes associated with quenching in the gel (trace 5) that were more pronounced in the chlorophyll a region at 681 nm (+), 670 nm (-), and 662 nm (+) than in the chlorophyll b region at ∼650 nm. In the blue Soret region, quenching was associated with changes arising from both chlorophyll and carotenoid (Fig. 4B). A strong negative band was found from chlorophyll a at 439 nm and in the carotenoid region at 487 nm (trace 4).
FIGURE 4.
Absorption spectroscopy of LHCII complexes incorporated into polyacrylamide gel. A, room temperature absorption spectra in the red region of the unquenched (trace 1), quenched (kd = 5.0) (trace 2), and recovered (trace 3) LHCII trimers. Trace 4 is the spectrum for the heat-treated complex at 70 °C for 5 min. Traces 5 and 6 are the difference spectra for the quenched-minus-recovered and heat-treated-minus-trimeric complex, respectively. B, room temperature absorption spectra in the Soret region of the unquenched (trace 1), quenched (kd = 5.0) (trace 2), and recovered (trace 3) LHCII trimers. Trace 4 is the difference spectrum for the quenched-minus-recovered complex. C, low temperature (77 K) absorption difference spectra for quenched-minus-unquenched monomeric LHCII. Traces 1-3 correspond to kd values of 0.3, 2.5, and 10, respectively.
The LHCII gels could be cooled to 77 K with liquid nitrogen, not only allowing the quenching process to be stopped at particular level but also allowing higher resolution spectroscopy to be performed. In this experiment, LHCII monomers were used. Absorption spectra were obtained from samples with kd values of 0.3, 2.5, and 10, and quenched-minus-recovered difference spectra were calculated (Fig. 4C). All major changes shown in Fig. 4, A and B, were again found: 438-439 nm (-), 486-487 nm (-), 650-652 nm (-), 658-662 nm (+), 670 nm (-), and 681 nm (+) bands. The spectral structure was dependent upon the amount of quenching; at the lowest level of quenching, the 681 nm feature emerged, indicative of the changes in the terminal emitter locus (trace 1). This band grew in intensity as the level of quenching increased, along with the negative 436 and 486 nm bands (trace 2); and at the highest level of quenching, the negative band at 436 nm further increased, whereas the 681 nm feature partially collapsed, and a negative band at 678 nm appeared (trace 3). The appearance of the negative red-shifted band suggested a further alteration in the terminal emitter locus of chlorophyll a during establishment of a highly quenched LHCII conformation. The sample with the highest extent of quenching also displayed a shift and broadening of the negative carotenoid band at ∼494-495 nm. Thus, these experiments have clearly established that the quenched LHCII state is associated with modification of the electronic states of pigments.
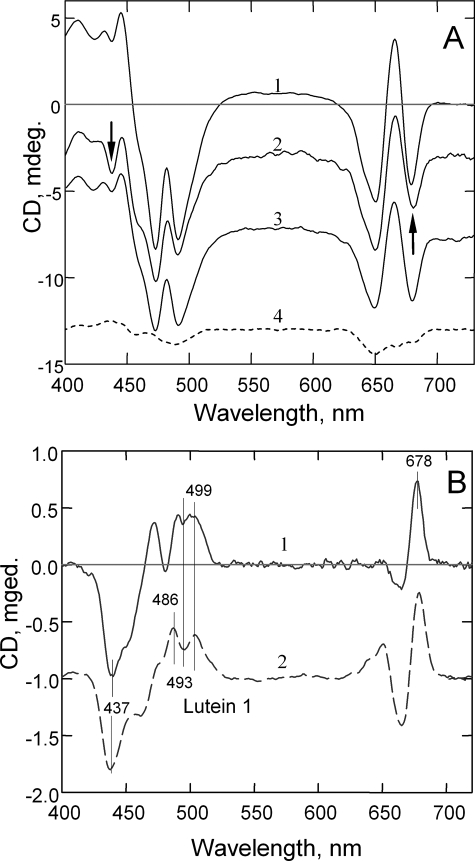
The nature of the altered pigment states was further explored by CD spectroscopy. Fig. 5A shows CD spectra for unquenched, quenched, and recovered LHCII gel samples (traces 1-3, respectively). The arrows at ∼440 and ∼680 nm indicate the most affected regions in the spectrum of quenched LHCII. On the other hand, thermal denaturation of LHCII led to a strong decrease in the whole CD spectrum and elimination of its excitonic features (trace 4) (21, 22). The difference spectrum associated with quenching in the gel (Fig. 5B, trace 1) displayed a strong (-) band at 437 nm and a group of bands in the carotenoid region: 486 nm (+), 493 nm (-), and 503 nm (+). Similar changes were found in the aggregation-related difference CD spectra (trace 2) (21). Both difference spectra showed similarity also in the red region, with the main positive maximum at 678 nm and a negative band at 665 nm (chlorophyll a).
FIGURE 5.
A, CD spectroscopy of LHCII incorporated into gel. Shown are room temperature CD spectra of unquenched (trace 1), quenched (trace 2), recovered (trace 3), and partially unfolded (70 °C, 5 min) (trace 4) trimers. The arrows indicate the most affected regions in the spectrum of the quenched sample. B, difference CD spectra for quenched-minus-recovered LHCII in the gel (trace 1) and aggregated-minus-trimeric LHCII (trace 2). mdeg., millidegrees.
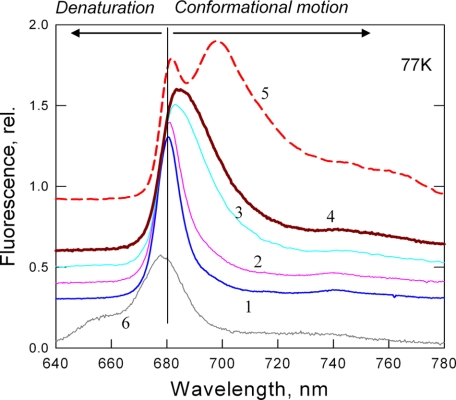
The quenched state of LHCII in aggregates and crystals has been previously associated with an unusual 77 K long wavelength fluorescence band at ∼700 nm (1, 9). Fig. 6 shows the 77 K fluorescence spectra for LHCII gels in a range of quenching magnitudes (traces 1-4), which are completely different from those recorded with heat-induced denaturation (trace 6). There was a gradual broadening of the 681 nm band and a shift toward the 700 nm region with the increase in quenching strength, indicating that the red shift is not a result of interactions between neighboring complexes. Interestingly, there was much less emission at 700 nm in the spectrum of the quenched LHCII gel in comparison with those of highly quenched aggregates. Thus, the red shift of the main band at 680 nm and the appearance of the 700 nm band may arise from two different processes. Whereas the former was related to the extent of quenching, the latter appeared to be an attribute of interacting LHCII units in the aggregate.
FIGURE 6.
Low temperature (77 K) fluorescence spectroscopy of LHCII in gels. Traces 1-4 are spectra corresponding to various levels of quenching with kd values of 0, 0.2, 2, and 9, respectively. Trace 5 is the spectrum of aggregated LHCII with kd = 9. Trace 6 is the spectrum of heat-treated trimers (70 °C, 5 min). The arrows indicate the characteristic blue shift of the unfolding/denaturation state (left arrow) and the red shifts for conformational transition (right arrow). The vertical line indicates the 680 nm fluorescence band of unquenched trimers. rel., relative.
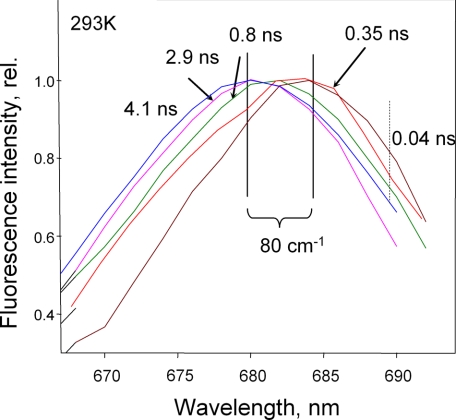
Time-resolved fluorescence spectroscopy was performed to determine whether the transition from the unquenched to the quenched state occurred by switching a population of LHCII complexes into a dissipative state with a characteristic short lifetime. Fig. 7 shows a series of spectra around the maximum of LHCII fluorescence at 680 nm. The major component of 4.1 ns of trimeric LHCII in the unquenched state had a maximum at ∼680 nm. Ten times quenched LHCII in the gel displayed a multicomponent picture. It possessed 2.9-, 0.8-, 0.35-, and 0.04-ns components. It was interesting to note that the time-resolved spectra of these components revealed the tendency for red shift for the shorter lifetimes. Hence, the strongest shift in the spectrum of unquenched trimers (∼80 cm-1) was observed for the fastest component (0.04 ns). The spectrum revealed a pronounced shoulder in the 688-695 nm region (indicated in Fig. 7 by the dotted vertical line) Therefore, these data have clearly revealed the presence of not one but several quenching states/conformations in LHCII.
FIGURE 7.
Time-resolved fluorescence spectra of various fluorescence components of LHCII trimers. 4.1 ns is the major component of the unquenched trimer (blue line). 2.9 ns, 0.8 ns, 0.35 ns, and 0.04 ns are different components of quenched LHCII in the gel with kd = 9. The relative (rel.) amplitudes of these components were 0.02, 0.08, 0.5, and 0.4, respectively. The solid vertical lines define the spectral shift between the longest and shortest components of 80 cm-1. The dotted vertical line highlights the presence of a longer wavelength fluorescence shoulder in the spectrum of the 0.04-ns component.
DISCUSSION
The results obtained in this study provide important insights into the role of protein conformational changes and aggregation in the formation of excitation quenchers in LHCII. It has been previously argued that aggregation per se could give rise to artifactual quenching and that no conformational change was occurring (23). The finding that fluorescence quenching in LHCII can be obtained without protein aggregation strongly argues against such a proposal and indicates that the cause is indeed a change in protein conformation within each monomeric LHCII unit, as originally hypothesized (4). This conclusion also explains the early reports of the lack of tight correlation between the level of fluorescence quenching and the extent of aggregation in isolated LHCII (24).
Recently, it was demonstrated that the same conformational change in LHCII, which is manifested as a twist in the Raman configuration of the bound neoxanthin molecule, is detected in the NPQ state in vivo in leaves and chloroplasts (10). We suggest therefore that aggregation of LHCII is preceded by this conformational change explored here in detail. In this sense, aggregation in vivo may be thought of as a means of simply minimizing the energy of the quenched state and pulling the equilibrium in its favor.
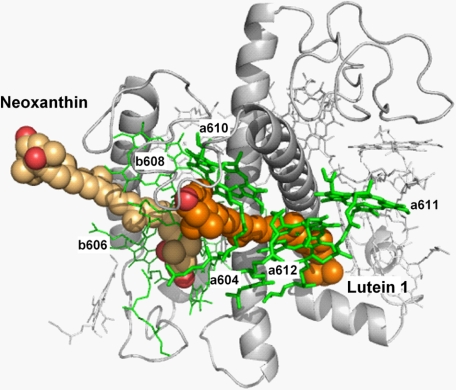
The experimental approach used in this study allows the unambiguous interpretation of the changes in pigment configuration accompanying quenching for the first time. Two pigment domains within the complex appear to cooperatively interact in this conformational switch (Fig. 8). The neoxanthin domain contains chlorophylls a604, b606, b607, and b608, which display quenching-related changes in absorption and alterations in the CD signal structure. This domain has been previously highlighted an indicator of the conformational change not just in vitro but also in vivo (1, 4, 10). The second domain, the putative quenching site, contains the terminal emitter group of pigments (chlorophylls a610, a611, and a612), which give rise to the red-shifted chlorophyll a at 681 nm paralleled by changes in the Soret band at ∼438 nm. The absorption change peaking at 494 nm and the CD transitions at 492 (-) and 499 (+) correspond to lutein 1, the latter implying involvement of excitonic interactions between lutein and its neighbors. It has been recently established that the quenching in aggregates of LHCII arises from the formation of an energy transfer pathway to a low-lying excited state of lutein 1 (10).
FIGURE 8.
Neoxanthin and lutein 1 domains in the structure of LHCII. Neoxanthin is a chlorophyll b-enriched domain interacting with the lutein 1 domain. The latter carries terminal emitter chlorophylls (a610, a611, and a612) where the energy dissipation takes place.
Fluorescence lifetime measurements revealed several life-time components associated with the quenched state, suggesting the existence of a number of different states of the complex with a varying energy-quenching strength. A similar conclusion was drawn from investigation of fluorescence lifetimes of LHCII incorporated into liposomes (27). Furthermore, the time-resolved fluorescence spectrum measurements revealed the appearance of a number of red-shifted components with short fluorescence lifetimes. This red shift could reflect a stepwise promotion of pigment-pigment interactions between lutein 1 and chlorophylls a610, a611, and a612 in the terminal emitter domain. Hence, the non-photochemical quenching switch in LHCII appears to have a gradual nature as opposed to an on-off switch. Detailed fluorescence lifetime measurements and time-resolved fluorescence spectra for leaves and chloroplasts with a wide range of NPQ levels are required to explore whether this switch enables all these states in vivo or if only one or two particular states are allowed, as proposed previously (18). It is interesting to note that, in all cases, the presence of zeaxanthin bound at either peripheral or internal sites in any LHCII protein is insufficient to induce significant fluorescence quenching in vitro (25-27). Therefore, there is a need for a protein conformational change in LHCII, as demonstrated recently to fully activate NPQ in vivo (10). The establishment of the existence of a conformational switch enabling highly efficient energy dissipation within the Photosystem II antenna is a clear advance in comparison with the other models of photoprotection in Photosystem II. Recently published work on the role of zeaxanthin cation in NPQ does not provide any evidence for the required conformational change and provides neither a connection to a significant energy dissipation process (28) nor an explanation of the zeaxanthin-independent NPQ (29). We have shown that the quenching is an internal event within one LHCII unit free from interactions with others.
In conclusion, the discovery that fluorescence quenching can be induced in LHCII immobilized in a solid state gel has allowed important advances to be made in our understanding of the photoprotective antenna switch, demonstrating that it is a cooperative protein and pigment conformational change. It was shown that quenching does not require interactions between complexes, but is a process that involves structural alterations within individual LHCII subunits. Because the LHCII gel is devoid of optical artifacts, for the first time, clear information was obtained about the changes in pigment states in the quenching mode, proving the involvement of neoxanthin and lutein 1 domains.
In vivo, during the formation of NPQ, through the dynamics of these domains, this switch is controlled and amplified by other factors. These are (a) protein-protein interactions between neighboring antenna complexes in the grana macrostructure (4, 30), sometimes under stress conditions, forming large aggregates in which deeply quenched states may be stabilized (31, 32); (b) zeaxanthin, critical for the full expression of NPQ in vivo, allosterically favoring the quenched conformation of LHCII (4, 33); and (c) the ΔpH via the action of the PsbS protein acting as the main physiological trigger of the switch (34).
Supplementary Material
Acknowledgments
We thank Professor Herbert van Amerongen (Wageningen University) for advice regarding the singlet-singlet annihilation experiments.
This work was supported by grants (to A. V. R. and P. H.) from the Biotechnology and Biological Sciences Research Council and The Royal Society and by the INTRO2 Marie Curie Research Training Network of the European Union. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1S and 2S.
Footnotes
The abbreviations used are: NPQ, non-photochemical quenching; LHCII, light-harvesting antenna complex of Photosystem II; TEMED, N,N,N′,N′-tetramethylethylenediamine.
References
- 1.Pascal, A. A., Liu, Z., Broess, K., van Oort, B., van Amerongen H., Wang, C., Horton, P., Robert, B., Chang, W., and Ruban, A. (2005) Nature 436 134-137 [DOI] [PubMed] [Google Scholar]
- 2.Horton, P., Ruban, A. V., and Walters, R. G. (1996) Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 655-684 [DOI] [PubMed] [Google Scholar]
- 3.Holt, N. E., Fleming, G. R., and Niyogi, K. K. (2004) Biochemistry 43 8281-8289 [DOI] [PubMed] [Google Scholar]
- 4.Horton, P., Wentworth, M., and Ruban, A. (2005) FEBS Lett. 579 4201-4206 [DOI] [PubMed] [Google Scholar]
- 5.Holt, N. E., Zigmantas, D., Valkunas, L., Li, X.-P., Niyogi, K. K., and Fleming, G. R. (2005) Science 307 433-436 [DOI] [PubMed] [Google Scholar]
- 6.Quick, W. P., and Horton, P. (1984) Proc. R. Soc. Lond. B Biol. Sci. 226 237-247 [Google Scholar]
- 7.Schreiber, U. (1986) Photosynth. Res. 9 261-272 [DOI] [PubMed] [Google Scholar]
- 8.Ruban, A. V., Rees, D., Pascal, A. A., and Horton, P. (1992) Biochim. Biophys. Acta 1102 39-44 [Google Scholar]
- 9.Ruban, A. V., and Horton, P. (1992) Biochim. Biophys. Acta 1102 30-38 [Google Scholar]
- 10.Ruban, A. V., Berera, R., Ilioaia, C., van Stokkum, I. H. M., Kennis, J. T. M., Pascal, A. A., van Amerongen, H., Robert, B., Horton, P., and van Grondelle, R. (2007) Nature 450 575-578 [DOI] [PubMed] [Google Scholar]
- 11.Liu, Z. F., Yan, H. C., Wang, K. B., Kuang, T. Y., Zhang, J. P., Gui, L. L., An, X. M., and Chang W. R. (2004) Nature 428 287-292 [DOI] [PubMed] [Google Scholar]
- 12.Ruban, A. V., Young, A., Pascal, A., and Horton, P. (1994) Plant Physiol. 104 227-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruban, A. V., Pesaresi, P., Wacker, U., Irrgang, K.-D. J., Bassi, R., and Horton, P. (1998) Biochemistry 37 11586-11591 [DOI] [PubMed] [Google Scholar]
- 14.Ruban, A. V., Lee, P. J., Wentworth, M., Young, A. J., and Horton, P. (1999) J. Biol. Chem. 274 10458-10465 [DOI] [PubMed] [Google Scholar]
- 15.den Hollander, W. T. F., Bakker, J. G. C., and van Grondelle, R. (1993) Biochim. Biophys. Acta 725 492-507 [Google Scholar]
- 16.Gilmore, A. M., Hazlett, T. L., and Govindjee (1995) Proc. Natl. Acad. Sci. U. S. A. 92 2273-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner, B., Goss, R., Richter, M., Wild, A., and Holzwarth, A. R. (1996) J. Photochem. Photobiol. B Biol. 36 339-350 [Google Scholar]
- 18.Gilmore, A. M., Shinkarev, V. P., Hazlett, V. P., and Govindjee (1998) Biochemistry 37 13582-13593 [DOI] [PubMed] [Google Scholar]
- 19.Barzda, V., Gulbinas, V., Kananavicius, R., Cervinskas, V., van Amerongen, H., van Grondelle, R., and Valkunas, L. (2001) Biophys. J. 80 2409-2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruban, A. V., Calkoen, K., Kwa, S. L. S., van Grondelle, R., Horton, P., and Dekker, J. P. (1997) Biochim. Biophys. Acta 1321 61-70 [Google Scholar]
- 21.Kwa, S. L. S., Groeneveld, G. F., Dekker, J. P., van Grondelle, R., van Amerongen, H., Lin, S., and Struve, W. S. (1992) Biochim. Biophys. Acta 1101 143-146 [Google Scholar]
- 22.Ide, J. P., Klug, D. R., Kuhlbrandt, W., Giorgi, L. B., and Porter, G. (1987) Biochim. Biophys. Acta 893 349-364 [Google Scholar]
- 23.Standfuss, J., Terwisscha van Scheltinga, A. C., Lamborghini, M., and Kuhlbrandt, W. (2005) EMBO J. 24 919-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchhoff, H., Hinz, H. J., and Rösgen, J. (2003) Biochim. Biophys. Acta 1606 105-116 [DOI] [PubMed] [Google Scholar]
- 25.Moya, I., Silvestri, M., Vallon, O., Cinque, G., and Bassi, R. (2001) Biochemistry 40 12552-12561 [DOI] [PubMed] [Google Scholar]
- 26.Amarie, S., Standfuss, J., Barros, T., Kulbrandt, W., Dreuw, A., and Wachtveitl, J. (2007) J. Phys. Chem. B 111 3481-3487 [DOI] [PubMed] [Google Scholar]
- 27.Crimi, M., Dorra, D., Bosinger, C. S., Giuffra, E., Holzwarth, A. R., and Bassi, R. (2001) Eur. J. Biochem. 268 260-267 [DOI] [PubMed] [Google Scholar]
- 28.Avenson, T. J., Ahn, T. K., Zigmantas, D., Niyogi, K. K., Li, Z., Ballotarri, M., Bassi, R., and Fleming, G. R. (2007) J. Biol. Chem. 283 3550-3558 [DOI] [PubMed] [Google Scholar]
- 29.Ahn, T. K., Avenson, T. J., Ballottari, M., Cheng, Y. C., Niyogi, K. K., Bassi, R., and Fleming, G. R. (2008) Science 320 794-797 [DOI] [PubMed] [Google Scholar]
- 30.Dekker, J. P., and Boekema, E. J. (2005) Biochim. Biophys. Acta 1706 12-39 [DOI] [PubMed] [Google Scholar]
- 31.Tang, Y., Wen, X., Lu, Q., Yang, Z., Cheng, Z., and Lu, C. (2007) Plant Physiol. 143 629-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busch, F., Huner, N. P., and Ensminger, I. (2007) Plant Physiol. 143 1242-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horton, P., Ruban, A. V., and Wentworth, M. (2000) Philos. Trans. R. Soc. Lond. B Biol. Sci. 355 1361-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, X.-P., Gilmore, A. M., Cafarri, S., Bassi, R., Golan, T., Kramer, D., and Niyogi, K. (2004) J. Biol. Chem. 279 22866-22874 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.