Abstract
To determine the roles of cholesterol and the actin cytoskeleton in apical and basolateral protein organization and sorting, we have performed comprehensive confocal fluorescence recovery after photobleaching analyses of apical and basolateral and raft- and non-raft-associated proteins, both at the plasma membrane and in the Golgi apparatus of polarized MDCK cells. We show that at both the apical and basolateral plasma membrane domains, raft-associated proteins diffuse faster than non-raft-associated proteins and that, different from the latter, they become restricted upon depletion of cholesterol. Furthermore, only transmembrane apical proteins are restricted by the actin network. This indicates that cholesterol-dependent domains exist both at the apical and basolateral membranes of polarized cells and that the actin cytoskeleton has a predominant role in the organization of transmembrane proteins independent of their association with rafts at the apical membrane. In the Golgi apparatus apical proteins appear to be segregated from the basolateral ones in a compartment that is sensitive both to cholesterol depletion and actin rearrangements. Furthermore, consistent with the role of actin rearrangements in apical protein sorting, we found that apical proteins exhibit a differential sensitivity to actin depolymerization in the Golgi of polarized and nonpolarized cells.
Raft microdomains are ordered lipid membrane domains enriched in cholesterol and sphingolipids (1). Diverse types of proteins such as glycosylphosphatidylinositol-anchored proteins (GPI-APs),2 acylated proteins and transmembrane proteins are able to segregate into these membrane domains, which have been proposed to be involved in many cellular mechanisms such as protein sorting, endocytosis, virus budding, and bacterial infection (2-5). One biochemical characteristic of the raft components is to be insoluble upon extraction in cold non-ionic detergent like Triton X-100; therefore, rafts have been assimilated to detergent-resistant membrane (DRMs). However, to date it has been very difficult to study rafts in unperturbed cells because their putative size (between 10 and 200 nm) is below the optical resolution (6). Several imaging techniques including single particle tracking, FRET, and FRAP have been developed to investigate protein and lipid organization in raft domains and their functions in membrane compartmentalization (7-13). By using FRAP, Meder et al. (30) found that raft and non-raft-associated proteins exhibit different behaviors, suggesting the existence of two environments: raft and non-raft domains at the apical surface of polarized MDCK cells.
One of our major interests is to understand the mechanism of apical sorting of GPI-APs in polarized cells. The GPI anchor was one of the first apical sorting signals to be postulated (1, 14-16); however, this hypothesis has been challenged by the finding that GPI-APs can be either apically or basolaterally sorted in polarized epithelial cells (17-20). A common characteristic of apical and basolateral GPI-APs is their association with raft microdomains. However, contrary to the basolateral ones, only apical GPI-APs are able to cluster in high molecular weight complexes in the Golgi apparatus prior to their apical sorting (17). Impairment of GPI-AP oligomerization results in mis-sorting to the basolateral surface, thus indicating that in addition to raft association, clustering of GPI-APs is required for apical sorting. The differential sorting (apical or basolateral) of raft-associated proteins can be explained by differences in the nature of the raft domain with which these proteins associate or in differences in the affinity (21).3 The role of the actin cytoskeleton in organizing and compartmentalizing the plasma membrane appears to be equally important (9, 22). To gain insights into the mechanism of apical sorting and protein compartmentalization, we analyzed the FRAP behavior of raft- and non-raft-associated proteins in apical and basolateral membranes of polarized MDCK cells, as well as in the Golgi, under conditions of raft and/or actin cytoskeleton perturbation.
Our data indicate that (i) raft domains with distinct properties exist in both apical and basolateral membranes of fully polarized MDCK cells, (ii) the actin cytoskeleton has an important role in the organization of apical transmembrane proteins independent of their association with rafts, and (iii) apical and basolateral proteins are segregated in the Golgi apparatus into two different compartments both containing cholesterol-dependent domains.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies—Cell culture reagents were purchased from Invitrogen. Phalloidin-rhodamine and latrunculin A were purchased from Invitrogen; filipin, methyl-β-cyclodextrin, water cholesterol soluble, and mevinolin were purchased from Sigma.
Cell Culture—MDCK cells were grown in Dulbecco's modified Eagle's medium containing 5% fetal bovine serum. MDCK cells were transfected with sequences encoding GFP-FR, P75-GFP, GFP-PrP, or GFP-PIT as described previously (17, 18, 23).
Depletion or Loading of Cholesterol—To deplete the cells of cholesterol, we used a previously described protocol (24, 25). Briefly MDCK cells were plated on filters, and mevinolin (10 μm) was added to the cells 24 h after plating in Dulbecco's modified Eagle's medium supplemented with 5% delipidated calf serum and mevalonate (250 μm). In supplemental Fig. S2 after 3 days on filter, MβCD (10 mm) was added to medium containing 20 mm Hepes, pH 7.5, and 0.2% bovine albumin for 30 min at 37 °C.
To load the cells with cholesterol after 3 days of polarization on filters, water-soluble cholesterol (10 mm in MβCD) was added to warm medium containing 20 mm Hepes, pH 7.5, and 0.2% bovine albumin for 30 min at 37 °C.
Water-soluble cholesterol was prepared with MβCD. The following ratio between the two chemicals determines whether the mixture will act as a cholesterol donor (ratio 1:6, cholesterol:MβCD) or acceptor (26, 27). To determine the rate of cholesterol depletion or addition, we measured cellular cholesterol levels by a colorimetric assay (cholesterol/cholesteryl ester quantification; Calbiochem) according to the manufacturer's instructions.
Perturbation of the Cytoskeleton Meshwork—To perturb the actin cytoskeleton, we incubated the polarized MDCK cells with latrunculin A (Molecular Probes) (1 μm) in Dulbecco's modified Eagle's medium containing 5% fetal bovine serum for 5 or 60 min at 37 °C before FRAP analysis or fixation for immunofluorescence.
Velocity Gradients—Velocity gradients were performed using a previously published protocol (17, 28). The cells were grown to confluency in 100-mm dishes, washed in phosphate-buffered saline containing CaCl2 and MgCl2 and lysed on ice for 30 min in 20 mm Tris, pH 7.4, 100 mm NaCl, 0.4% SDS, 0.2% Triton X-100. The lysates were scraped from dishes, sheared through a 26-gauge needle, and layered on top of a glycerol gradient (40 to 20%) after removal of nuclei by low speed centrifugation. After centrifugation at 45,000 rpm for 16 h in an ultracentrifuge (model SW 50; Beckman counter), fractions of 300 μl were harvested from the top of the gradient and trichloroacetic acid-precipitated. The proteins were revealed by Western blot using specific antibodies.
Fluorescence Microscopy—MDCK cells were grown on transwell filters for 3-4 days, washed with phosphate-buffered saline containing CaCl2 and MgCl2, fixed with 4% paraformaldehyde, and quenched with 50 mm NH4Cl. The actin cytoskeleton was revealed by using phalloidin-rhodamine (1/100). For the filipin staining, we fixed the cells for 60 min, permeabilized for 5 min with 0.075% saponin, and quenched with ammonium chloride (50 mm) before staining for 1 h with filipin (250 μg/ml) (Sigma-Aldrich). The images were acquired using a laser scanning confocal microscope (LSM 510; Carl Zeiss Microimaging, Inc.) equipped with a plan apo 63× oil immersion (NA 1.4) objective lens.
FRAP Measurements and Analysis—FRAP enabled the assessment of two parameters, the mobile fraction and the apparent diffusion coefficient. Whereas the mobile fraction corresponds to the fraction of the studied protein that is able to repopulate the bleached area, the apparent diffusion coefficient indicates the speed with which the proteins diffuse. FRAP analysis was performed on a confocal LSM 510 META from Zeiss. Using the plan apo 63× oil immersion (NA 1.4) objective lens, we monitored the fluorescence of our fused GFP protein using low intensity laser excitation (488 nm) (prebleach scans). We always kept an airy unit of 1. As a preliminary analysis to determine the mathematical model to apply for membrane proteins, different sizes of regions of interest (ROI) from 280 nm to 6.2 μm were selectively bleached (29). From those analyses it appeared that our model proteins were following a diffusion-coupled FRAP recovery (29). Furthermore, because we used a circular ROI, we analyzed our raw data using the previously described Soumpasis mathematical equation (30, 31). To compare our data with published studies, we opted for a bleaching ROI of 1.4 μm for all of our experiments. Therefore a circular region of 1.4 μm was photobleached with the same laser excitation at high intensity (decrease of the fluorescence into the ROI by 60-80%), and then the recovering of fluorescence into the bleached region over time was monitored by recording with low intensity laser (post-bleach scans) as before photobleaching. This recovery reflects the ability of unbleached fluorescent proteins around the ROI to repopulate the photobleached ROI. For each FRAP acquisition, we considered two internal controls, one that indicates over time the natural bleaching of the sample and one that gives the level of fluorescence background (29). The raw data were fitted with the Igor Pro software and an application developed in EMBL with the Soumpasis mathematical equation. FRAP recordings were obtained in CO2-independent medium (0.15 m NaCl, 0.1 m KCl, 0.1 m CaCl2, 0.1 m MgCl2, 0.2 m Hepes) at room temperature or at 37 °C.
RESULTS
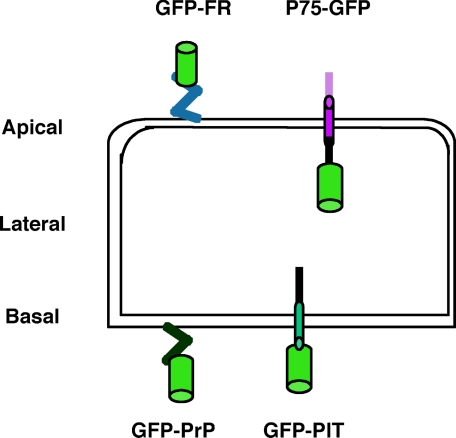
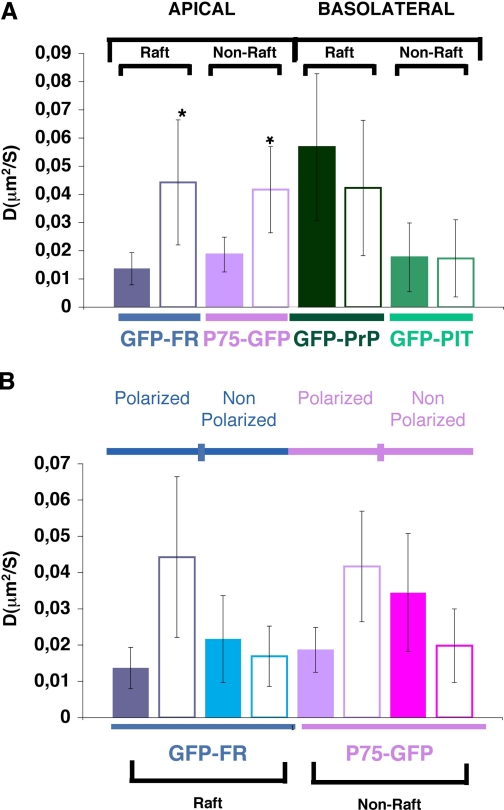
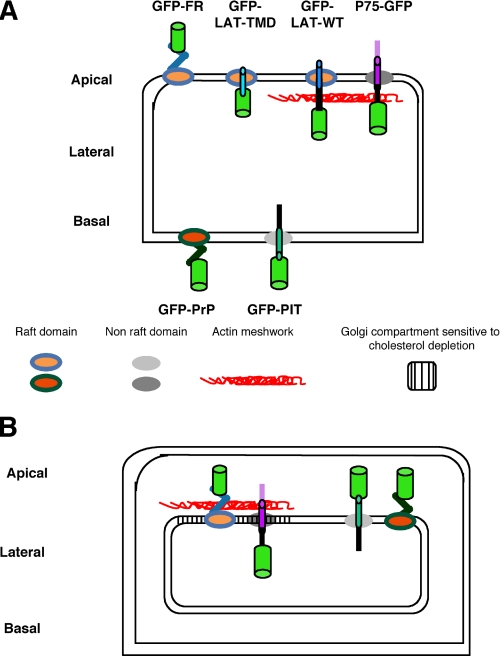
Overview of the Studied Proteins and Validation of FRAP Settings in Polarized MDCK Cells—We set up a confocal FRAP analysis and determined the mobile fraction and apparent diffusion coefficients of different proteins (see “Experimental Procedures”) at the level of the plasma membrane and the Golgi apparatus (supplemental Fig. S1) to correlate the behavior of the studied proteins with the immediate membrane environment. For raft-associated proteins, we studied GFP-FR and GFP-PrP where GFP is fused to the GPI signal attachment of either the folate receptor (GFP-FR) or the prion protein (GFP-PrP) (Fig. 1). These two chimeric proteins are excellent candidates because although both are GPI-anchored and raft-associated, they have an opposite polarity and different capacity to oligomerize.3 As non-raft proteins, we considered two transmembrane proteins fused to GFP: P75-GFP, which is apically sorted and has GFP fused to the human neurotrophin receptor (32); and GFP-PIT, which is basolaterally sorted and has GFP fused to a truncated form of the low density lipoprotein receptor (extracellular domain deleted) (23) (Fig. 1).
FIGURE 1.
Overview of fluorescent proteins. Two raft-associated proteins are shown: apically sorted GFP-FR containing the GPI signal attachment of the folate receptor and basolaterally sorted GFP-PrP containing the GPI signal attachment of the prion protein. Two transmembrane non-raft-associated proteins are shown: apically sorted P75-GFP containing P75 neurotrophin receptor and basolaterally sorted GFP-PIT containing PIT (truncated form of low density lipoprotein receptor deleted of its extracellular domain). All of these proteins are fused to the GFP (green cylinder) and expressed independently in MDCK cells.
All constructs were stably transfected into MDCK cells where we could compare the behavior and therefore the local environment (both at the level of the plasma membrane and in the Golgi) of (i) two differentially sorted raft-associated proteins (GFP-FR versus GFP-PrP), (ii) two apical proteins either raft- or non-raft-associated (GFP-FR or P75-GFP respectively), and (iii) two basolateral proteins either raft- or non-raft-associated (GFP-PrP or GFP-PIT, respectively).
We performed all FRAP analyses on polarized MDCK cells grown “upside-down” on small filters for three to four days to obtain fully polarized conditions as previously reported (23). Under these conditions the behavior at 25 and at 37 °C of our two model apical proteins P75-GFP and GFP-FR was similar to what was previously reported (30) (supplemental Fig. S2) and confirmed that different environments were surrounding raft- and non-raft-associated proteins. Having validated our FRAP conditions, we performed the rest of our experiments at physiological temperature (37 °C). Because, as expected, the mobile fraction of all studied proteins at this temperature was 100% (supplemental Fig. S2 and data not shown), we only measured differences in the apparent diffusion coefficient of each fusion protein.
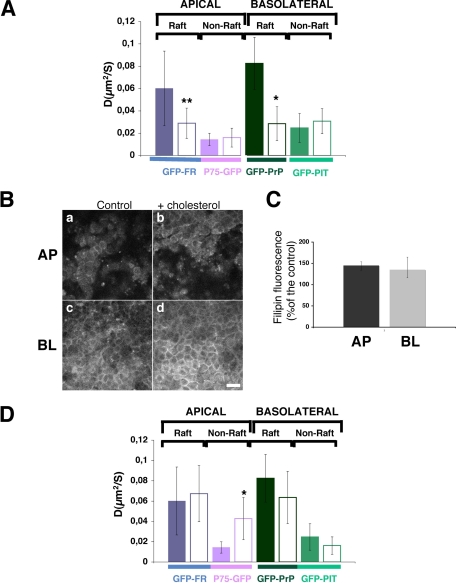
Involvement of Cholesterol in the Organization of Raft-associated Proteins at the Apical and Basolateral Plasma Membrane Domains of Polarized MDCK Cells—To understand the role of cholesterol in the organization of proteins at the apical and basolateral plasma membranes in polarized MDCK cells, we analyzed the FRAP behavior of apical and basolateral raft- and non-raft-associated proteins at steady state (referred as control) and upon modification of the cholesterol content. We found that at steady state GPI-anchored, raft-associated proteins have a significantly higher apparent diffusion coefficient compared with non-raft-associated proteins independent of their apical or basolateral localization (Fig. 2A). To investigate whether cholesterol content affects the behavior of these proteins, possibly by changing their local membrane environment, we depleted cholesterol from polarized MDCK cells using methyl-β-cyclodextrin, which sequesters cholesterol in its hydrophobic core. A short incubation of 30 min of polarized MDCK cells with MβCD (10 mm) reduced the content of cholesterol by 30% (see “Experimental Procedures”). By performing FRAP within the first 30 min after removal of the drug, we observed that the apparent diffusion coefficient of all proteins, independent of their raft association and their polarity, was significantly decreased (supplemental Fig. S3). These results are in agreement with what has been published earlier in nonpolarized COS-7 cells (33). However, because it has been shown that MβCD may induce aspecific effects not related with the depletion of cholesterol (27, 34), we decided to use an alternative approach and treated the cells with mevinolin, an inhibitor of HMG-CoA reductase, which is the rate-limiting enzyme in cholesterol synthesis (24). MDCK cells plated on filters were treated for 48 h in a delipidated medium with mevinolin (10 μm), resulting in a depletion of cholesterol of 30-40% (see “Experimental Procedures”). In contrast to the methyl-β-cyclodextrin results, upon inhibition of cholesterol synthesis only raft-associated proteins (both apical and basolateral) presented a statistically significant decrease in their apparent diffusion coefficients, whereas non-raft-associated proteins were unaffected (Fig. 2A). These results are in agreement with the fluorescence correlation spectroscopy study performed in COS-7 cells using cholesterol oxidase (35, 36). They indicate that like in nonpolarized cells, cholesterol-dependent domains exist both at the apical and basolateral membranes of polarized MDCK cells. Interestingly, we observed a difference in the statistical significance of the variation of the diffusion coefficient between apical and basolateral GPI-APs after cholesterol depletion (Fig. 2A, compare GFP-FR with GFP-PrP). Indeed, following cholesterol depletion the apparent diffusion coefficient of GFP-FR decreased with a statistical significance of p < 0.005, whereas the apparent diffusion coefficient of GFP-PrP decreased with a statistical significance of p < 0.0001. This supports the hypothesis that apical and basolateral raft domains in polarized cells have specific properties and may differ in lipid composition (37). To fully investigate the requirement for cholesterol in protein organization at the apical and basolateral membranes of polarized MDCK cells, we performed FRAP upon loading of cholesterol. To this end, we incubated polarized MDCK cells with water-soluble cholesterol for a short period of time (30 min) before proceeding to the FRAP analysis within the first 30 min after washing the cells. Measurement of total cholesterol showed an increase of 50% (see “Experimental Procedures”) also shown by the increased fluorescence signal observed by filipin staining (Fig. 2, B and C). Under these conditions, neither apical or basolateral raft-associated proteins were affected by cholesterol addition, suggesting that rafts were probably already saturated with cholesterol both at the apical and basolateral surface. In contrast, we observed a statistically significant increase of the apparent diffusion coefficient of non-raft apical protein P75-GFP upon loading of cholesterol (Fig. 2D). However, under these conditions after extraction at 4 °C in Triton X-100, P75-GFP remained soluble and did not float on sucrose density gradient (supplemental Fig. S4). This suggests that the addition of cholesterol increases the diffusional property of the apical non-raft-associated protein, but it is not enough to change the partition of a non-DRM protein to DRMs. Interestingly, the addition of cholesterol did not affect the basolateral non-raft-associated GFP-PIT, possibly because the basolateral domain is already more enriched in cholesterol compared with the apical one (compare filipin staining in Fig. 2B, panels a and c).
FIGURE 2.
Cholesterol plays a major role in the organization of proteins in apical and basolateral domains of polarized MDCK cells. A, apparent diffusion coefficients (D) of the four studied proteins at steady state (colored bars) and upon depletion of cholesterol synthesis by treatment with mevinolin (white bars). B, confocal pictures of filipin staining at apical pole (panels a and b) and basolateral pole (panels c and d) of polarized MDCK cells in control conditions (panels a and c) and upon loading of cholesterol (panels b and d). Bar, 10 μm. C, quantification of the filipin staining in polarized MDCK cells loaded with cholesterol. Intensity fluorescence is expressed as percentage of control cells. The experiments were performed three independent times, and the error bars are the mean ± S.D. D, apparent diffusion coefficient (D) of proteins of interest at steady state (colored bars) and upon addition of cholesterol (white bars). Fitted data are shown from at least three independent experiments (for A and D) with n > 15. The error bars are the means ± S.D. *, p < 0.0001; **, p < 0.005.
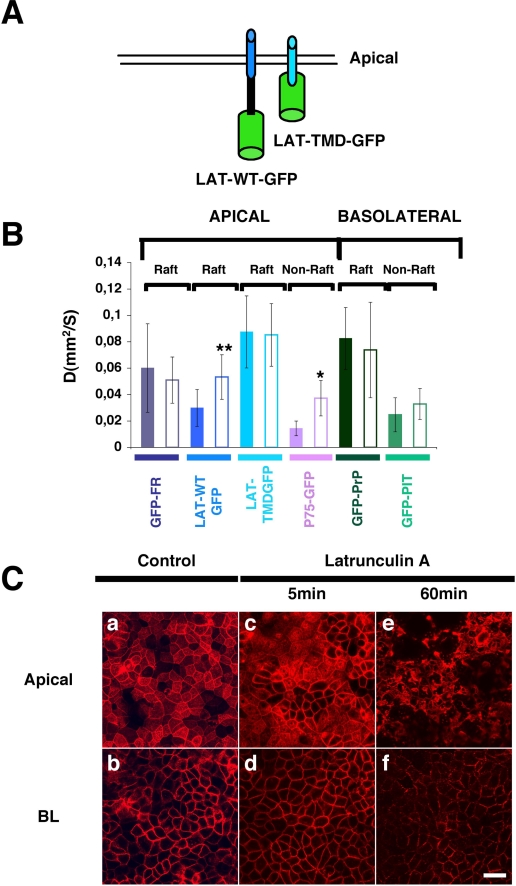
Involvement of the Actin Cytoskeleton in the Organization of Transmembrane Proteins at the Apical Membrane of Polarized MDCK Cells—It has been postulated that the plasma membrane of the fibroblast cells like normal rat kidney cells are compartmentalized and that the actin meshwork plays an essential role in the confinement of proteins. More precisely, transmembrane proteins by their interaction with actin fibers contribute to the establishment of membrane compartments in which proteins are able to diffuse. The size of these compartments ranges between 32 and 230 nm depending on the cell type (9, 22). We wanted to analyze whether the actin cytoskeleton contributed to the organization/compartmentalization of raft-associated proteins (GPI-anchored or transmembrane) at the plasma membrane of polarized MDCK cells. As apical transmembrane raft-associated protein, we considered the human LAT (linker for activation of T cells), which is the integral membrane adaptor protein linker for T cells activation. We found that two apical transmembrane raft-associated proteins (30) (Fig. 3A), LAT-WT-GFP and LAT-TMD-GFP (deleted of its cytoplasmic domain), had a significantly higher apparent diffusion coefficient than P75-GFP our model transmembrane non-raft-associated protein (p < 0.0005 and p < 0.0001, respectively) (Fig. 3B). This supports the hypothesis that the local environment provided by rafts confers a higher lateral mobility to proteins compared with proteins excluded from these lipidic domains (Fig. 2A). To define the putative role of the actin cytoskeleton in organizing raft microdomains, we perturbed the integrity of the actin meshwork by using latrunculin A, which alters the actin-monomer subunit surface and prevents polymerization (38). By staining F-actin with phalloidin-rhodamine in polarized MDCK cells grown on filters, we found that a treatment of 60 min with latrunculin A drastically affects the actin cytoskeleton (compare panels a and e or panels b and f of Fig. 3C) and the cell integrity. In contrast, after 5 min of latrunculin A treatment, the overall integrity of the actin meshwork was preserved (Fig. 3C, panels c and d). However, when we performed the FRAP analyses at this time, both apical raft- and non-raft-associated transmembrane proteins possessing a cytoplasmic domain (P75-GFP and LAT-WT-GFP) displayed a statistical significant increase in their apparent diffusion coefficients (Fig. 3B). On the contrary LAT-TMD-GFP is unaffected by latrunculin treatment. Interestingly, at steady state, LAT-TMD-GFP displays a much higher apparent diffusion coefficient than LAT-WT-GFP (p < 0.0001) (Fig. 3B), indicating that the cytoplasmic domain is necessary for mediating the restriction of LAT.
FIGURE 3.
Involvement of the actin cytoskeleton in the organization of apical pole of polarized MDCK cells. A, two apical transmembrane raft-associated proteins fused to the GFP, LAT-WT-GFP, and LAT-TMD-GFP (deleted of the cytoplasmic domain) were considered for this FRAP analysis. B, apparent diffusion coefficients of the studied proteins at steady state (colored bars) and after 5 min of latrunculin A treatment (white bars) within the first 20 min after removal of the drug because its effect is reversible (35). The experiments were performed at least three independent times, n > 15. *, p < 0.0001; **, p < 0.001. C, apical (panels a, c, and e) and basolateral (panels b, d, and f) confocal images of actin immunostained with phalloidin-rhodamine in polarized MDCK cells at steady state (panels a and b) or upon 5 min (panels c and d) or 60 min (panels e and f) treatment with latrunculin A. Bar, 10 μm.
Altogether, these results support the hypothesis that restriction of the apparent diffusion coefficient of transmembrane proteins derives from the interaction of their cytoplasmic domains with the actin meshwork independent from raft association. Interestingly, in contrast to apical proteins, basolateral proteins were unaffected by the mild latrunculin treatment (Fig. 3B).
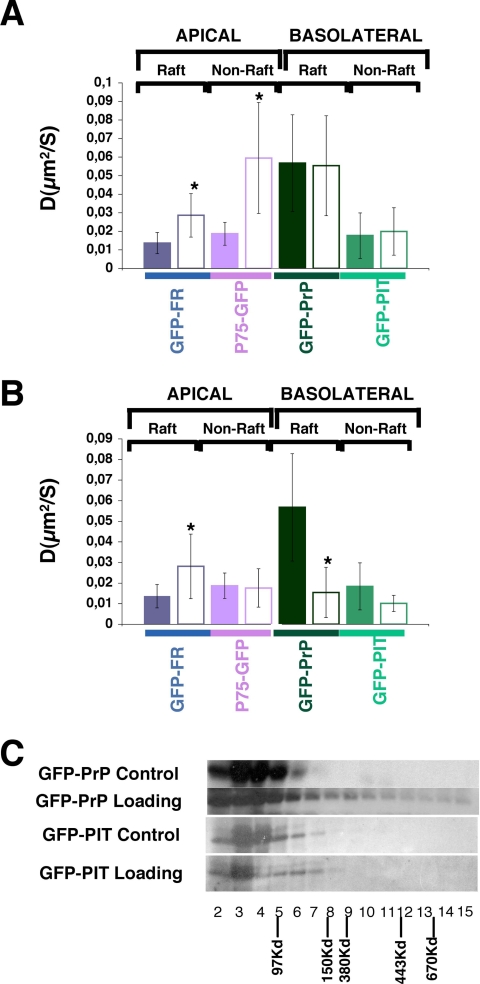
Involvement of Cholesterol and Actin in the Compartmentalization of Apical and Basolateral Raft- and Non-raft-associated Proteins at the Level of the Golgi Apparatus—Secreted proteins travel through the Golgi apparatus where they are packed in distinct vesicles before being delivered to the cell surface (32, 39). To investigate whether raft- and non-raft-associated proteins were presorted at the level of the Golgi, we analyzed their behavior in this organelle by FRAP. These experiments were done at 37 °C and at steady state without performing any temperature block to enrich proteins in the Golgi to reproduce physiological conditions as close as possible. Although we bleached through all the samples in the selected Golgi area, we monitored the fluorescence recovery in a single confocal plane where we could detect the fluorescence from the Golgi marker furin (not shown) (40, 41). We found that in the Golgi, GFP-PrP (raft-associated, basolateral) presents a much higher apparent diffusion coefficient compared with GFP-PIT (basolateral, transmembrane, non-raft-associated). Interestingly, its apparent diffusion coefficient is also higher compared with the two apical proteins, GFP-FR and P75-GFP, independently of their raft association (Fig. 4A). This is consistent with the fact that raft-associated GFP-PrP is monomeric, whereas GFP-FR forms high molecular complexes (17, 42). Furthermore we observed that upon cholesterol depletion only apical proteins display a statistically significant increase in their apparent diffusion coefficients. On the contrary, basolateral proteins (both raft and non-raft-associated) were unaffected by mevinolin treatment (Fig. 4A). The high sensitivity of apical proteins to depletion of cholesterol, independent of their raft association, suggests that they occupy a different environment from the basolateral proteins in the Golgi apparatus (Fig. 4A). To further examine the cholesterol dependence of proteins in the Golgi, we loaded cells with cholesterol and analyzed the apparent diffusion coefficients of our model proteins in the Golgi. Interestingly, the addition of cholesterol induced a significant increase of the apparent diffusion coefficient of the apical raft-associated GPI-AP (GFP-FR) (Fig. 4B). In contrast, upon loading of cholesterol the apparent diffusion coefficient of GFP-PrP (basolateral GPI-APs raft-associated protein) decreased significantly and became similar to the apparent diffusion coefficient of an apically sorted GPI-AP raft-associated proteins (Fig. 4B). This is consistent with the observation that upon cholesterol addition GFP-PrP is able to oligomerize and is apically sorted (Fig. 4C).3 Interestingly, the addition of cholesterol does not have any effect on non-raft-associated proteins (neither apical, P75-GFP, nor basolateral, GFP-PIT) (data not shown and Fig. 4C). These data indicate that the protein must already be in a raft environment to be sensitive to the action of cholesterol addition and therefore that raft- and non-raft-associated proteins are in a different environment in the Golgi apparatus.
FIGURE 4.
Diffusional mobilities of raft- and non-raft-associated proteins at the level of the Golgi compartment in polarized MDCK cells. A, apparent diffusion coefficients of our studied proteins at steady state (colored bars) and upon depletion of cholesterol by inhibition of HMG-CoA reductase (white bars). B, apparent diffusion coefficients of our studied proteins at steady state (colored bars) and upon loading of cholesterol (white bars). These experiments have been performed at least three independent times, n > 15. The error bars are the means ± S.D. with *, p < 0.0001. C, analysis by velocity gradient of oligomerization status of basolaterally sorted proteins (GFP-PrP and GFP-PIT) at steady state or upon loading of cholesterol.
To further characterize the compartment of apical and basolateral proteins in the Golgi apparatus, we decided to analyze the involvement of the actin cytoskeleton on the organization of raft- and non-raft-associated proteins at the level of Golgi membranes (Fig. 5A). We found that both apical proteins, independent of their raft association, display a significant increase in their apparent diffusion coefficients upon the addition of latrunculin A (5 min) (Fig. 5A). These results contrast with those reported very recently by Lazaro-Dieguez et al. (43) in nonpolarized cells. These authors performed inverse FRAP in COS-1 cells at the level of the Golgi complex and considered the behavior of the same raft- and non-raft-associated apical proteins that we analyzed here in polarized MDCK cells (43). In this study the exit of P75-GFP from the Golgi was inhibited after treatment of COS-1 cells with latrunculin, whereas the exit of GFP-FR was unaffected by the same treatment; thus, the authors concluded that only non-raft-associated proteins were dependent on the actin cytoskeleton in the Golgi apparatus. The difference between these reported data and ours could be the result of the difference in Golgi organization between polarized and nonpolarized cells. Importantly, the Golgi apparatus appears to have a different morphology in polarized and nonpolarized MDCK cells (data not shown). To verify this hypothesis we analyzed the FRAP behavior of apical proteins (raft- and non-raft-associated) upon perturbation of the actin cytoskeleton in MDCK cells grown on coverslips under nonpolarized conditions. We found that in nonpolarized MDCK cells latrunculin A decreases the apparent diffusion coefficient of P75-GFP in the Golgi membranes, whereas the raft-associated protein, GFP-FR, is unaffected (Fig. 5B). Thus, our results in nonpolarized MDCK cells are consistent with the data reported in COS-1 cells on the same model proteins (43), suggesting that there is a different membrane organization in the Golgi apparatus between nonpolarized and polarized cells. Interestingly, in contrast to the behavior of apical proteins, both populations of basolateral proteins (raft- and non-raft-associated) are unaffected by latrunculin addition.
FIGURE 5.
Contribution of the actin cytoskeleton in the organization of membranes in the Golgi complex in polarized and nonpolarized MDCK cells. A, apparent diffusion coefficients of our studied proteins at steady state (white boxes) and upon 5 min latruncuclin A treatment (colored bars) in Golgi membranes of polarized MDCK cells. B, apparent diffusion coefficients of apical raft-associated protein (GFP-FR) and non-raft-associated protein (P75-GFP) at steady state (colored bars) and upon 5 min (white bars) treatment with latrunculin A in Golgi membranes in polarized and nonpolarized MDCK cells. The experiments were performed at least three independent times, n > 15. *, p < 0.0001; **, p < 0.05.
DISCUSSION
Previously FRAP analysis has been used to study the involvement of rafts in the segregation and organization of proteins at the plasma membrane of nonpolarized cells (33) and at the apical plasma membrane of polarized MDCK cells grown in fully polarized conditions (30). Here we wanted to study the role of (i) cholesterol as a major raft component and of (ii) the actin cytoskeleton in the membrane organization and compartmentalization of apical and basolateral raft- and non-raft-associated proteins at the level of the apical and basolateral plasma membrane domains and at the level of the Golgi apparatus in polarized MDCK cells. Indeed both protein trafficking and Golgi membranes appear to be very different between nonpolarized and polarized cells (23, 32), highlighting the importance of analyzing membrane environments in fully polarized epithelial cells. Additionally, for the first time we investigated the putative role of cholesterol and the actin meshwork in protein organization both at the apical and basolateral domains and also at the level of the Golgi complex in correlation with protein sorting. To accomplish these studies, we set up a confocal FRAP analysis of MDCK cells grown on filters under polarized conditions (23). We found that both apical and basolateral raft-associated proteins display higher apparent diffusion coefficients compared with non-raft-associated proteins, supporting a role for rafts in protein organization both at the apical and basolateral domains of polarized cells. To sustain this hypothesis, we then analyzed the role of cholesterol by either depleting or increasing the membrane cholesterol content. Interestingly, we found that treatment with MβCD induced a significant decrease in the apparent diffusion coefficient of both raft- and non-raft-associated proteins (supplemental Fig. S3). These results are in agreement with the finding that methyl-β-cyclodextrin has other effects, unrelated to cholesterol depletion, such as global inhibition of the lateral mobility of plasma membrane proteins, irrespective of their putative association with lipid ordered domains (34, 44). However, when we lowered the cellular content of cholesterol by inhibiting its synthesis, we found an effect only on raft-associated proteins (Fig. 2A). Thus, our results support the existence of a cholesterol-dependent organization of proteins at the apical and basolateral plasma membrane of polarized cells. Interestingly, following cholesterol depletion, the apparent diffusion coefficient of basolateral GFP-PrP decreased with a higher statistical significance of compared with apical GFP-FR (Fig. 2A). These results could be interpreted in two ways: either mevinolin treatment was biased toward the cholesterol reduction from the basolateral membrane as opposed to the apical membrane, or the overall cholesterol content is higher in the basolateral membrane compared with that of the apical membrane. This latter possibility is supported both by filipin staining (Fig. 2B) and by a previous report showing that there is three times more cholesterol efflux from the basolateral than from the apical membrane in polarized MDCK cells (45). To fully investigate the requirement for cholesterol in the organization of apical and basolateral proteins in the plasma membrane, we performed the converse of these experiments and loaded cells with cholesterol (Fig. 2, B and C). FRAP analysis under these conditions revealed that only P75-GFP, the apical non-raft-associated protein, increased its apparent diffusion coefficient (Fig. 2D). These results reinforce the idea that cholesterol fluidifies membranes and confers higher diffusional properties to proteins (46). This also supports the hypothesis that the overall cholesterol content of the basolateral pole of the cell is higher than that of the apical pole, thus explaining the absence of effect observed for the non-raft-associated protein basolaterally expressed (GFP-PIT) upon cholesterol addition. Interestingly, the addition of cholesterol was not sufficient to change the partitioning of the protein from non DRMs to DRMs (supplemental Fig. S4), indicating that the proteins need to have an intrinsic ability to partition with DRMs and that changes in the cholesterol content of the plasma membrane will not alter this property. Besides cholesterol, the actin cytoskeleton has also been shown to play a role in the organization of plasma membrane proteins in fibroblasts. In particular the picket fence model proposed by the Kusumi laboratory (7-9, 11, 22) suggests that transmembrane proteins, independent of their raft association, would bind to the actin meshwork, creating small compartments into which proteins diffuse according to their local environments. By using a short treatment with latrunculin A, we demonstrated that in polarized cells, apical transmembrane proteins presented a higher diffusional property when the actin cytoskeleton was perturbed independent of their raft association (Fig. 3B). Therefore, these data indicate that apical transmembrane proteins are able to bind to the actin cytoskeleton and might play a “picket” role at the apical domain of polarized MDCK cells. Similar to fibroblasts, this role is independent of their association with rafts. In contrast, all basolateral proteins (independent of their raft association or the presence of a transmembrane domain) were insensitive to latrunculin treatment (Fig. 3B). Although in these conditions we did not reveal a role for the actin cytoskeleton in the organization of basolateral proteins in the plasma membrane, we cannot rule out a different organization (and therefore different latrunculin sensitivity) of the basolateral cytoskeleton. Similarly, at the apical pole, the GPI-AP GFP-FR did not show any modification of its apparent diffusion coefficient upon latrunculin A treatment (Fig. 3B). Although these observations are in agreement with the fluorescence correlation spectroscopy studies showing no effect of latrunculin treatment on GPI-AP in COS-7 cells (35), we cannot exclude a different sensitivity to the perturbation of the actin cytoskeleton between apical transmembrane and GPI-AP proteins.
Next we analyzed the apparent diffusion coefficients of our different apical and basolateral proteins at the level of the Golgi. We found that at steady state, the basolateral GPI-AP (GFP-PrP) presented a much higher apparent diffusion coefficient compared with apical proteins independent of their mode of anchorage and of their raft association (Fig. 4A). We also found that upon cholesterol depletion only apical proteins both raft- and non-raft-associated (Fig. 4A) showed a statistically significant increase in their apparent diffusion coefficients. Thus, these data suggest that in the Golgi apical and basolateral proteins are segregated in different compartments with different sensitivities to cholesterol (Fig. 6). They might also indicate that apically sorted proteins share a common environment in Golgi membranes. This hypothesis is supported by the studies of Jacob and collaborators who reported that apical proteins bud from the Golgi compartment in a common carrier prior to their segregation into distinct vesicles carrying raft-associated and non-raft-associated proteins respectively (39, 47-50). Our data are also consistent with the fact that the apparent diffusion coefficient of a protein correlates with its oligomeric state (42). In particular, we found that upon cholesterol depletion, the apical GPI-AP GFP-FR increases its apparent diffusion coefficient, and this is consistent with its reduced capacity to form high molecular weight complexes under these conditions (23). Conversely the increase of apparent diffusion coefficient of GFP-FR upon cholesterol loading can be explained in two ways: either the addition of cholesterol impairs the new formation of high molecular weight complexes, or the local membrane environment becomes more fluid. Most interestingly, we found that upon loading of cholesterol GFP-PrP increases its ability to form high molecular weight complexes (and therefore is sorted apically)3 and also reported here that its apparent diffusion coefficient is reduced (Fig. 4B). These combined data can be explained in two ways: (i) upon the addition of cholesterol a basolateral raft-associated protein shifts to a different raft environment within the Golgi that allows its oligomerization and apical sorting or (ii) cholesterol per se is able to stabilize the protein within the raft, which in turn leads to its oligomerization and apical sorting.
FIGURE 6.
Model of raft- and non-raft-associated proteins organization at the level of the polarized plasma membrane (A) and of the Golgi apparatus (B) in polarized MDCK cells. A, raft-associated proteins (GFP-FR, GFP-LAT-TMD, and GFP-LAT-WT) are surrounded by orange lipids, whereas non-raft-associated P75-GFP is represented surrounded by a different membrane environment in dark gray. At the basolateral membrane GFP-PrP raft-associated is also surrounded by a dark orange membrane environment, whereas the non-raft-associated protein GFP-PIT is surrounded by a light gray membrane environment. Raft domains appear different between the apical and the basolateral membrane (dark orange/orange) because they have a different sensitivity to cholesterol depletion. Non-raft domains are also distinct (dark gray and light gray) because only the apical non-raft domain is modified upon cholesterol loading. Only transmembrane proteins with a cytosolic domain and independently of their raft association seem to be connected to the actin meshwork. B, in the Golgi, apical and basolateral proteins already appear to be in a different environment, which does not show the same sensitivity to cholesterol depletion and actin rearrangement. In both of these environments raft- and non-raft-associated proteins are found (orange/dark orange for raft-associated proteins and gray/dark gray for non-raft-associated proteins).
Interestingly, cholesterol affects the ability of proteins to oligomerize, and therefore the sorting only of GPI-APs and not of non-raft-associated transmembrane proteins is affected (Fig. 4B). Therefore, this strongly suggests that the specific membrane environment (e.g. rafts) is able to promote/inhibit protein-protein interactions between proteins embedded within this environment. Emblematic is the case of p75-GFP, which upon cholesterol loading increases its apparent diffusion coefficient but does not become DRM-associated and does not form oligomers.
In conclusion, our data show that at the plasma membrane of polarized MDCK cells in both apical and basolateral domains, GPI-APs show a cholesterol-dependent behavior different from apical and basolateral non-raft-associated proteins; the actin cytoskeleton appears to play a role in the confinement/organization of transmembrane proteins at the level of the apical plasma membrane independent of their raft association (Fig. 6A). Furthermore, we show here that apical and basolateral proteins do not share a common environment but appear to be already segregated in the Golgi into two different compartments that display different sensitivities toward cholesterol depletion and actin perturbation (Fig. 6B). This represents the first comprehensive study of protein organization by confocal FRAP at the plasma membrane and in the Golgi of fully polarized MDCK cells in correlation with their respective sorting. Our work highlights clear differences between the various classes of proteins and between polarized and nonpolarized cells that should be taken into account in further studies.
Supplementary Material
Acknowledgments
We thank Dr. Karine Gousset and Dr. Gianni Guizzunti for critical reading of the manuscript and Pascal Roux from the Plate-Forme d'Imagerie Dynamique of Pasteur Institute for helpful discussion and technical assistance.
This work was supported by Agence Nationale de la Recherche Grant 05-BLAN 296-01 and Ministero dell' Università e della Ricerca Scientifica e Tecnologica Grant PRIN 2006. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S4.
Footnotes
The abbreviations used are: GPI, glycosylphosphatidylinositol; GPI-AP, GPI-anchored protein; DRM, detergent-resistant membrane; FR, Folate receptor; GFP, green fluorescent protein; FRAP, fluorescence recovery after photobleaching; MDCK, Madin-Darby canine kidney; ROI, regions of interest; MβCD, methyl-β-cyclodextrin.
S. Paladino, S. Lebreton, S. Tivodar, V. Campana, R. Tempre, and C. Zurzolo, submitted for publication.
References
- 1.Simons, K., and Ikonen, E. (1997) Nature 387 569-572 [DOI] [PubMed] [Google Scholar]
- 2.Cherukuri, A., Dykstra, M., and Pierce, S. K. (2001) Immunity 14 657-660 [DOI] [PubMed] [Google Scholar]
- 3.Leser, G. P., and Lamb, R. A. (2005) Virology 342 215-227 [DOI] [PubMed] [Google Scholar]
- 4.Manes, S., del Real, G., Lacalle, R. A., Lucas, P., Gomez-Mouton, C., Sanchez-Palomino, S., Delgado, R., Alcami, J., Mira, E., and Martinez, A. C. (2000) EMBO Rep. 1 190-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen, D. H., and Taub, D. D. (2004) Mol. Interv. 4 318-320 [DOI] [PubMed] [Google Scholar]
- 6.Pike, L. J. (2006) J. Lipid Res. 47 1597-1598 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki, K. G., Fujiwara, T. K., Edidin, M., and Kusumi, A. (2007) J. Cell Biol. 177 731-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki, K. G., Fujiwara, T. K., Sanematsu, F., Iino, R., Edidin, M., and Kusumi, A. (2007) J. Cell Biol. 177 717-730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morone, N., Fujiwara, T., Murase, K., Kasai, R. S., Ike, H., Yuasa, S., Usukura, J., and Kusumi, A. (2006) J. Cell Biol. 174 851-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirchev, R., and Golan, D. E. (2001) Blood Cells Mol. Dis. 27 143-147 [DOI] [PubMed] [Google Scholar]
- 11.Kusumi, A., Koyama-Honda, I., and Suzuki, K. (2004) Traffic 5 213-230 [DOI] [PubMed] [Google Scholar]
- 12.Kenworthy, A. K., and Edidin, M. (1998) J. Cell Biol. 142 69-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenworthy, A. K., and Edidin, M. (1999) Methods Mol. Biol. 116 37-49 [DOI] [PubMed] [Google Scholar]
- 14.Lisanti, M. P., Caras, I. W., Davitz, M. A., and Rodriguez-Boulan, E. (1989) J. Cell Biol. 109 2145-2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown, D. A., Crise, B., and Rose, J. K. (1989) Science 245 1499-1501 [DOI] [PubMed] [Google Scholar]
- 16.Lisanti, M. P., Sargiacomo, M., Graeve, L., Saltiel, A. R., and Rodriguez-Boulan, E. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 9557-9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paladino, S., Sarnataro, D., Pillich, R., Tivodar, S., Nitsch, L., and Zurzolo, C. (2004) J. Cell Biol. 167 699-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zurzolo, C., Lisanti, M. P., Caras, I. W., Nitsch, L., and Rodriguez-Boulan, E. (1993) J. Cell Biol. 121 1031-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipardi, C., Nitsch, L., and Zurzolo, C. (1999) Biochimie (Paris) 81 347-353 [DOI] [PubMed] [Google Scholar]
- 20.Benting, J. H., Rietveld, A. G., and Simons, K. (1999) J. Cell Biol. 146 313-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brugger, B., Graham, C., Leibrecht, I., Mombelli, E., Jen, A., Wieland, F., and Morris, R. (2004) J. Biol. Chem. 279 7530-7536 [DOI] [PubMed] [Google Scholar]
- 22.Murase, K., Fujiwara, T., Umemura, Y., Suzuki, K., Iino, R., Yamashita, H., Saito, M., Murakoshi, H., Ritchie, K., and Kusumi, A. (2004) Biophys. J. 86 4075-4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paladino, S., Pocard, T., Catino, M. A., and Zurzolo, C. (2006) J. Cell Biol. 172 1023-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller, P., and Simons, K. (1998) J. Cell Biol. 140 1357-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipardi, C., Nitsch, L., and Zurzolo, C. (2000) Mol. Biol. Cell 11 531-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christian, A. E., Haynes, M. P., Phillips, M. C., and Rothblat, G. H. (1997) J. Lipid Res. 38 2264-2272 [PubMed] [Google Scholar]
- 27.Zidovetzki, R., and Levitan, I. (2007) Biochim. Biophys. Acta 1768 1311-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheiffele, P., Roth, M. G., and Simons, K. (1997) EMBO J. 16 5501-5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprague, B. L., and McNally, J. G. (2005) Trends Cell Biol. 15 84-91 [DOI] [PubMed] [Google Scholar]
- 30.Meder, D., Moreno, M. J., Verkade, P., Vaz, W. L., and Simons, K. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 329-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soumpasis. (1983) Biophys. J. 41 95-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreitzer, G., Schmoranzer, J., Low, S. H., Li, X., Gan, Y., Weimbs, T., Simon, S. M., and Rodriguez-Boulan, E. (2003) Nat. Cell Biol. 5 126-136 [DOI] [PubMed] [Google Scholar]
- 33.Kenworthy, A. K., Nichols, B. J., Remmert, C. L., Hendrix, G. M., Kumar, M., Zimmerberg, J., and Lippincott-Schwartz, J. (2004) J. Cell Biol. 165 735-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shvartsman, D. E., Gutman, O., Tietz, A., and Henis, Y. I. (2006) Traffic 7 917-926 [DOI] [PubMed] [Google Scholar]
- 35.Lenne, P. F., Wawrezinieck, L., Conchonaud, F., Wurtz, O., Boned, A., Guo, X. J., Rigneault, H., He, H. T., and Marguet, D. (2006) EMBO J. 25 3245-3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marguet, D., Lenne, P. F., Rigneault, H., and He, H. T. (2006) EMBO J. 25 3446-3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tivodar, S., Paladino, S., Pillich, R., Prinetti, A., Chigorno, V., van Meer, G., Sonnino, S., and Zurzolo, C. (2006) FEBS Lett. 580 5705-5712 [DOI] [PubMed] [Google Scholar]
- 38.Morton, W. M., Ayscough, K. R., and McLaughlin, P. J. (2000) Nat. Cell Biol. 2 376-378 [DOI] [PubMed] [Google Scholar]
- 39.Jacob, R., Heine, M., Alfalah, M., and Naim, H. Y. (2003) Curr. Biol. 13 607-612 [DOI] [PubMed] [Google Scholar]
- 40.Liu, G., Thomas, L., Warren, R. A., Enns, C. A., Cunningham, C. C., Hartwig, J. H., and Thomas, G. (1997) J. Cell Biol. 139 1719-1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polishchuk, R., Di Pentima, A., and Lippincott-Schwartz, J. (2004) Nat. Cell Biol. 6 297-307 [DOI] [PubMed] [Google Scholar]
- 42.Lippincott-Schwartz, J., Snapp, E., and Kenworthy, A. (2001) Nat. Rev. Mol. Cell. Biol. 2 444-456 [DOI] [PubMed] [Google Scholar]
- 43.Lazaro-Dieguez, F., Colonna, C., Cortegano, M., Calvo, M., Martinez, S. E., and Egea, G. (2007) FEBS Lett. 581 3875-3881 [DOI] [PubMed] [Google Scholar]
- 44.Hancock, J. F. (2006) Nat. Rev. Mol. Cell. Biol. 7 456-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Remaley, A. T., Farsi, B. D., Shirali, A. C., Hoeg, J. M., and Brewer, H. B., Jr. (1998) J. Lipid Res. 39 1231-1238 [PubMed] [Google Scholar]
- 46.van Meer, G. (2005) EMBO J. 24 3159-3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delacour, D., and Jacob, R. (2006) Cell Mol Life Sci. 63 2491-2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacob, R., and Naim, H. Y. (2001) Curr. Biol. 11 1444-1450 [DOI] [PubMed] [Google Scholar]
- 49.Delacour, D., Greb, C., Koch, A., Salomonsson, E., Leffler, H., Le Bivic, A., and Jacob, R. (2007) Traffic 8 379-388 [DOI] [PubMed] [Google Scholar]
- 50.Delacour, D., Cramm-Behrens, C. I., Drobecq, H., Le Bivic, A., Naim, H. Y., and Jacob, R. (2006) Curr. Biol. 16 408-414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.