Abstract
Cardiac hypertrophy is a physiological adaptive response by the heart to pressure overload. However, after prolonged periods, this initial adaptive response becomes maladaptive, leading to increased mortality and morbidity from heart failure. Recently, 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or statins, have been shown to inhibit cardiac hypertrophy by cholesterol-independent mechanisms. Statins block the isoprenylation and activation of members of the Rho guanosine triphosphatase (GTPase) family, such as RhoA and Rac1. Since Rac1 is a requisite component of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is a major source of reactive oxygen species (ROS) in cardiovascular cells, the ability of statins to inhibit Rac1-mediated oxidative stress makes an important contribution to their inhibitory effects on cardiac hypertrophy.
Keywords: cardiac hypertrophy, 3-hydroxyl-3-methylglutaryl coenzyme A reductase inhibitors, statins, antioxidants, small GTP-binding proteins
Introduction
Several clinical trials with 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have shown that they reduce the incidence of myocardial infarction and ischemic disease [1–3]. Although the beneficial effects of statins have been attributed to their lipid-lowering effects, subgroup analysis of the WOSCOP and CARE trials suggests that statin-treated individuals have significantly lower risks for coronary heart disease compared to age-matched placebo-treated individuals, despite comparable serum cholesterol levels [1–3]. Indeed, experimental evidence indicates that some of the cholesterol-independent or ‘pleiotropic’ effects of statins involve the improvement or restoration of endothelial function, an increase in the stability of atherosclerotic plaques, and a decrease in vascular inflammation [4–6].
Cardiac hypertrophy and small G proteins
Cardiac hypertrophy is an adaptive response of the heart to pressure overload. The molecular response to pressure overload is complex and it may include modulation of various intracellular signal pathways, such as activation of many protein kinases (mitogen activated protein kinase [MAPK] and phosphatidylinositol 3 [PI3] kinase), expression of cardiac fetal genes (atrial natriuretic factor [ANF] and myosin light chain), and increase of protein synthesis. Furthermore, pressure overload leads to the release of secretion and production of vasoactive peptides, such as angiotensin II and endothelin-1, which play pivotal roles in the induction of these hypertrophic responses [7,8]. Since cardiac myocytes convert the pressure overload into intracellular biochemical signals, the blockade of critical signaling pathways leading to cardiac hypertrophy may have therapeutic benefits. One such pathway, which involves increase in intracellular myocardial oxidative stress is mediated by small GTP-binding proteins.
Ras, Rho and Rac are members of a family of small GTP-binding proteins, which exert diverse cellular functions. They participate in cell locomotion, cytokinesis, and cytoskeletal remodeling in non-muscle cells [9,10]. In the heart, Ras, Rho and Rac are involved in the hypertrophic response [11,12]. Ras-mediated cardiac hypertrophy has been demonstrated both in vitro [13] and in vivo [14]. Transgenic mice that over-express RhoA in heart develop loss of systolic function and dilated cardiomyopathy. However, the development of cardiomyopathy is due to abnormal conduction abnormalities rather than a direct modification of myocardial architecture [15]. This effect of RhoA was unanticipated, because previously, several in vitro studies implicated RhoA in the development of hypertrophy [16]. Other studies using cultured cardio-myocytes revealed that the activation of Rac is required in phenylephrine-induced cardio-myocyte hypertrophy. In those studies, transfection of myocytes with a dominant-negative mutant of Rac1 completely inhibited the hypertrophic response to phenylephrine [11]. In addition, transgenic mice that specifically expressed activated Rac1 in the myocardium, showed severe cardiac hypertrophy and dilatation [17].
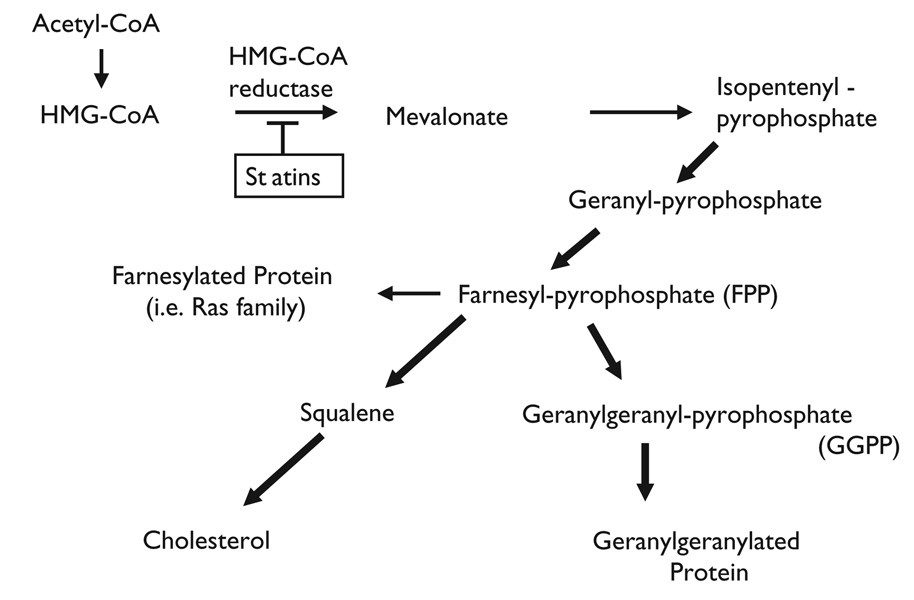
In the cholesterol biosynthetic pathway, conversion of HMG-CoA to mevalonate by HMG-CoA reductase is a rate-limiting step. Inhibition of this enzyme by statins not only leads to the reduction of cholesterol biosynthesis in the liver, but also to the reduction of the synthesis of several isoprenoid intermediates (Figure 1). These intermediates, such as farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP), serve as important lipid attachments for post-translational modification of signaling proteins, including the gamma subunit of heterotrimeric G proteins, heme-a, nuclear lamins, as well as Ras and Ras-like proteins, such as Rho and Rac [18]. Thus, protein isoprenylation allows the covalent attachment, sub-cellular localization, and intracellular trafficking of membrane-associated proteins.
Fig. 1.
Cholesterol biosynthetic pathway. HMG-CoA indicates 3-hydroxyl-3-methylglutaryl coenzyme (A) HMG-CoA reductase is a rate-limiting enzyme and this blockade (statins) leads to the reduction of other isoprenoid intermediates as well as intracellular cholesterol.
Members of Ras and Rho guanosine triphosphatase (GTPase) family are major substrates for post-translational modification by isoprenylation [18,19]. Ras translocation from the cytoplasm to the plasma membrane is dependent on farnesylation, whereas Rho translocation is dependent on geranylgeranylation [20,21]. These biochemical analyses showed that statins inhibit the development of cardiac hypertrophy through inhibition of Ras and Rho isoprenylation, leading to the accumulation of inactive Ras and Rho in the cytoplasm [22,23]. We have recently shown that Rac1 is a key mediator in the hypertrophic response. Over-expression of a dominant-negative mutant of Rac1 (N17Rac1), and to a less extent, RhoA (N19RhoA), inhibited angiotensin II-induced ANF promoter activity. Co-treatment with statins further decreased ANF promoter activity in cells transfected with N19RhoA and N17Cdc42, but not those transfected with N17Rac1. Similarly, co-treatment with GGPP reversed the inhibitory effects of statins, while GGPP could not reverse the inhibitory effect of N17Rac1 on ANF promoter activity [22].
Reduced nicotinamide adenine dinucleotide phosphate oxidase in cardiac myocyte
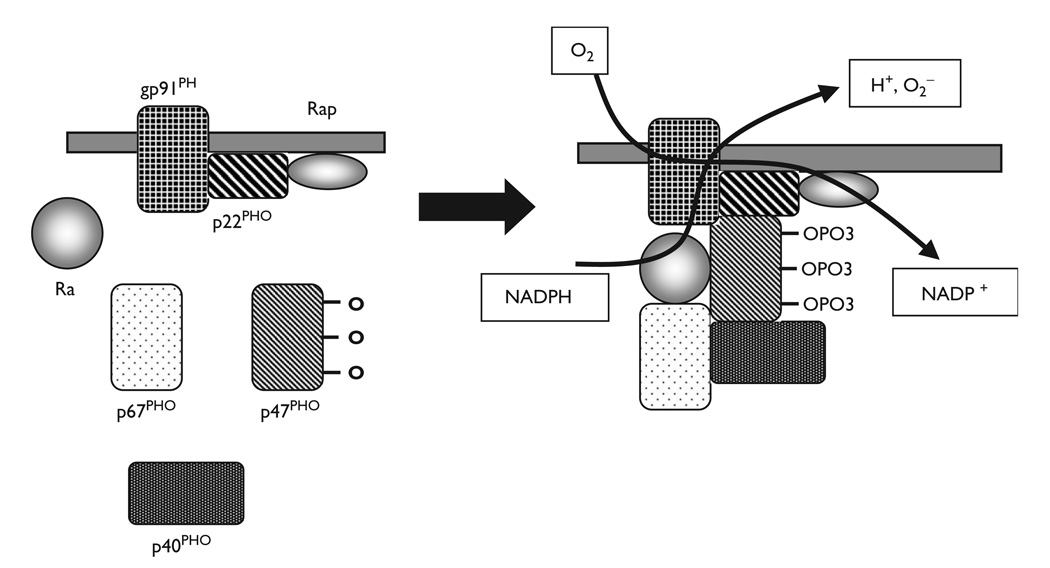
Growing evidence suggests that reactive oxygen species (ROS) may be involved in the process of cardiac hypertrophy [24,25]. Recent works strongly suggest that reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a major source of superoxide in cardiovascular cells [26]. In the resting inactive cell, three of these five components, p40PHOX(PHOX for Phagocyte Oxidase), p47PHOX and p67PHOX, exist in the cytosol, forming a complex. The other two components, p22PHOX, gp91PHOX, are bound to the membranes. Various stimuli lead to the phosphorylation of the cytosolic components and the entire cytosolic complex then migrates to the membrane (Figure 2). Importantly, not only are the core subunits required for activation, but also two low-molecular-weight guanine nucleotide-binding proteins, Rac and Rap. During activation, Rac binds guanosine triphosphate (GTP) and migrates to the membrane with the core cytosolic complex. Therefore, it has been suggested that Rac may be involved in the activation of cardiovascular NADPH oxidase.
Fig. 2.
NADPH oxidase. The core enzyme comprises five components: p40PHOX (PHOX for Phagocyte Oxidase), p47PHOX, p67PHOX, p22PHOX and gp91PHOX. In the resting cell (left panel), three of these five components, p40PHOX, p47PHOX and p67PHOX, exist in the cytosol as a complex. The other two components, p22PHOX, gp91PHOX, are located in the membranes. When it was stimulated, the cytosolic component becomes heavily phosphorylated and the entire cytosolic complex migrates to the membrane. Activation requires the participation, not only of the core subunits, but also of two low-molecular-weight guanine nucleotide-binding proteins, Rac and Rap. During activation, Rac binds guanosine triphosphate (GTP) and migrates to the membrane along with the core cytosolic complex.
One of the most important attributes of cardiovascular oxidase is its responsiveness to local metabolic changes, hemodynamic forces, and hormones, such as the potent vasoconstrictor angiotensin II. Angiotensin II increases the activity of vascular oxidase through NADPH oxidase activation [26,27]. Thrombin, platelet-derived growth factor (PDGF) and tumor necrosis factor-á (TNF-á) also stimulate NADPH oxidase-dependent superoxide production in vascular smooth muscle cells (SMCs) [28–30]. Recent analysis of the gp91PHOX deficient mice demonstrated that angiotensin II treatment increased cardiac hypertrophy and collagen content in wild-type but not gp91PHOX deficient mice [31]. This evidence further supports the hypothesis that oxidative stress, in particular NADPH oxidase, plays a crucial role in cardiac hypertrophy. For these reasons, it is likely that statins would inhibit cardiac hypertrophy through an antioxidant mechanism involving inhibition of Rac1 geranylgeranylation. Indeed, statins inhibit angiotensin II-induced oxidative stress and cardiac hypertrophy in rodents [22]. Perhaps, this is the mechanism by which statins inhibit cardiac hypertrophy in humans with hypercholesterolemia [32].
In summary, strong experimental evidence indicate that statins can prevent cardiac hypertrophy. This effect is mediated by Rac 1 and includes a reduction in oxidative stress. These results suggest a novel pharmacological approach to treating cardiac hypertrophy.
Acknowledgements
This work described in this article was supported by NIH (HL-52233, HL-48743, to Dr. Liao) and the American Heart Association Bugher Foundation Award (to Dr. Liao) and the Banyu Fellowship Awards in cardiovascular medicine that are sponsored by Banyu Pharmaceutical Co., Ltd., and The Merck Company Foundation (to Dr. Nakagami).
Footnotes
Conflicts of interest: none
References
- 1. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. This large randomized study demonstrates that the relative risk reduction conferred by statin treatment in individuals with high cholesterol levels.
- 2.Massy ZA, Keane WF, Kasiske BL. Inhibition of the mevalonate pathway: benefits beyond cholesterol reduction? Lancet. 1996;347:102–103. doi: 10.1016/s0140-6736(96)90217-2. [DOI] [PubMed] [Google Scholar]
- 3. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. This large randomized study demonstrates that the relative risk reduction conferred by statin treatment in individuals with average cholesterol levels.
- 4.Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 5.Fukumoto Y, Libby P, Rabkin E, Hill CC, Enomoto M, Hirouchi Y, et al. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 2001;103:993–999. doi: 10.1161/01.cir.103.7.993. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, et al. Angiotensin II partly mediates mechanical stress-induced cardiac hypertrophy. Circ Res. 1995;77:258–265. doi: 10.1161/01.res.77.2.258. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, et al. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–3228. doi: 10.1074/jbc.271.6.3221. [DOI] [PubMed] [Google Scholar]
- 9.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 10.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 11.Wang SM, Tsai YJ, Jiang MJ, Tseng YZ. Studies on the function of RhoA protein in cardiac myofibrillogenesis. J Cell Biochem. 1997;66:43–53. [PubMed] [Google Scholar]
- 12.Thorburn J, Xu S, Thorburn A. MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. Embo J. 1997;16:1888–1900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller SJ, Gillespie-Brown J, Sugden PH. Oncogenic src, raf, and ras stimulate a hypertrophic pattern of gene expression and increase cell size in neonatal rat ventricular myocytes. J Biol Chem. 1998;273:18146–18152. doi: 10.1074/jbc.273.29.18146. [DOI] [PubMed] [Google Scholar]
- 14.Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 15.Sah VP, Minamisawa S, Tam SP, Wu TH, Dorn GW, Ross J, Jr, et al. Cardiac-specific over-expression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J Clin Invest. 1999;103:1627–1634. doi: 10.1172/JCI6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sah VP, Hoshijima M, Chien KR, Brown JH. Rho is required for Galphaq and alpha1-adrenergic receptor signaling in cardiomyocytes. Dissociation of Ras and Rho pathways. J Biol Chem. 1996;271:31185–31190. doi: 10.1074/jbc.271.49.31185. [DOI] [PubMed] [Google Scholar]
- 17.Sussman MA, Welch S, Walker A, Klevitsky R, Hewett TE, Price RL, et al. Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J Clin Invest. 2000;105:875–886. doi: 10.1172/JCI8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 19.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 20.Laufs U, La Fata V, Plutzky J, Liao JK. Up-regulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 21. Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. This study demonstrates an important principle of cholesterol-independent effects of statin treatment in human endothelial cells showing that inhibition of isoprenylation of the small G protein RhoA by statin treatment leads to increased eNOS mRNA stability.
- 22. Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108:1429–1437. doi: 10.1172/JCI13350. This is the first study to show that statins can prevent the development of cardiac hypertrophy through a non-lipid, antioxidant mechanism.
- 23.Dechend R, Fiebeler A, Park JK, Muller DN, Theuer J, Mervaala E, et al. Amelioration of angiotensin II-induced cardiac injury by a 3-hydroxy-3- methylglutaryl coenzyme a reductase inhibitor. Circulation. 2001;104:576–581. doi: 10.1161/hc3001.092039. [DOI] [PubMed] [Google Scholar]
- 24.Bell JP, Mosfer SI, Lang D, Donaldson F, Lewis MJ. Vitamin C and quinapril abrogate LVH and endothelial dysfunction in aortic-banded guinea pigs. Am J Physiol Heart Circ Physiol. 2001;281:H1704–H1710. doi: 10.1152/ajpheart.2001.281.4.H1704. [DOI] [PubMed] [Google Scholar]
- 25.Date MO, Morita T, Yamashita N, Nishida K, Yamaguchi O, Higuchi Y, et al. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in an in vivo murine pressure-overload model. J Am Coll Cardiol. 2002;39:907–912. doi: 10.1016/s0735-1097(01)01826-5. [DOI] [PubMed] [Google Scholar]
- 26.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 27.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 28.Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, et al. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J Biol Chem. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- 29.De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J. 1998;329:653–657. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-κB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation. 1997;96:2361–2367. doi: 10.1161/01.cir.96.7.2361. [DOI] [PubMed] [Google Scholar]
- 31.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 32.Lee TM, Chou TF, Tsai CH. Association of pravastatin and left ventricular mass in hypercholesterolemic patients: role of 8-iso-prostaglandin F2alpha formation. J Cardiovasc Pharmacol. 2002;40:868–874. doi: 10.1097/00005344-200212000-00007. [DOI] [PubMed] [Google Scholar]