Abstract
Recent studies suggest that dipyridamole (DP) may exert stroke protective effects beyond platelet inhibition. The purpose of this study is to determine whether statin and DP could enhance stroke protection through nitric oxide (NO)-dependent vascular effects. Mice were pretreated with DP (10 to 60 mg/kg, q 12 h, 3 days) alone or in combination with a statin (simvastatin; 0.1 to 20 mg/kg per day, 14 days) before transient intraluminal middle cerebral artery occlusion. Although simvastatin (1 mg/kg per day, 14 days) increased endothelial NO synthase (eNOS) activity by 25% and DP (30 mg/kg, q12 h, 3 days) increased aortic cGMP levels by 55%, neither statin nor DP alone, at these subtherapeutic doses, increased absolute cerebral blood flow (CBF) or conferred stroke protection. However, the combination of subtherapeutic doses of simvastatin and DP increased CBF by 50%, decreased stroke volume by 54%, and improved neurologic motor deficits, all of which were absent in eNOS-deficient mice. In contrast, treatment with aspirin (10 mg/kg per day, 3 days) did not augment the neuroprotective effects of DP and/or simvastatin. These findings indicate that statin and DP exert additive NO-dependent vascular effects and suggest that the combination of statin and DP has greater benefits in stroke protection than statin alone through vascular protection.
Keywords: aspirin, blood flow, cerebral ischemia, endothelium, nitric oxide synthase, stroke
Introduction
Antiplatelet agents have been the principal therapy for the secondary prevention of noncardioembolic ischemic strokes (Albers et al, 2004; Sacco et al, 2006). Treatment with aspirin (ASA) decreases the relative risk of stroke and transient ischemic attacks by 15% to 20% (Antithrombotic Trialists’ Collaboration, 2002). Because of the small magnitude of stroke reduction derived from ASA therapy, more effective antiplatelet agents or combination regimens have been tested for secondary stroke prevention. Surprisingly, dual antiplatelet therapy with ASA and clopidogrel does not confer additional stroke protection compared with ASA or clopidogrel alone, but instead increases the risk of bleeding (Bhatt et al, 2006; Diener et al, 2004). These findings suggest that further platelet inhibition beyond that of ASA or clopidogrel is not beneficial and perhaps detrimental in cerebrovascular disease. However, the addition of dipyridamole (DP) to ASA in the European Stroke Prevention Study (ESPS)-2 and European/Australian Stroke Prevention in Reversible Ischemia Trial (ESPRIT) showed an additional 20% relative and 1% absolute risk reduction of strokes and transient ischemic attacks compared with ASA alone without increased bleeding (Diener et al, 1996; Halkes et al, 2006). These findings suggest that DP may exert stroke protection by mechanism(s) beyond platelet inhibition.
Dipyridamole is a phosphodiesterase inhibitor, which increases the intracellular levels of cAMP and cGMP by preventing their conversion to AMP and GMP, respectively (Asano et al, 1977). By increasing cAMP and cGMP levels in platelets, DP reversibly inhibits platelet aggregation (Best et al, 1979). However, in addition to platelets, DP may also potentiate some of the vascular protective effects of endothelium-derived nitric oxide (NO), which increases cGMP by stimulating soluble guanylyl cyclase (Aktas et al, 2003). Endothelium-derived NO is an important regulator of cerebral blood flow (CBF), and endothelial NO synthase-deficient (eNOS−/−) mice exhibit larger stroke size after focal cerebral ischemia (Huang et al, 1996). Indeed, statins protect against stroke by increasing the expression and activity of eNOS (Endres et al, 1998). Similarly, we postulate that DP may protect against stroke by augmenting NO-dependent vascular protective effects. The purpose of this study, therefore, is to show that DP can exert stroke protective effects by mechanisms beyond platelet inhibition and that these vascular protective effects can synergize with other therapeutic agents for stroke such as ASA and statins. Accordingly, we chose the transient middle cerebral artery (MCA) occlusion model of stroke in mice, where CBF and vascular effects, rather than platelets, play a predominant role in determining stroke size. Furthermore, we showed that ASA, at a dose that has been shown to inhibit platelet aggregation in vivo, has no effect in this filament model of stroke. Thus, this study investigates potential nonplatelet effects of DP.
Materials and methods
Animals
Wild-type (WT) and eNOS−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All mice used for the experiments were 10- to 12-week-old male mice (20 g) on C57Bl/6 background. All experimental protocols were approved by the Standing Committee on Animal Care at Harvard Medical School (Boston, MA, USA).
Treatment Protocols
Dipyridamole was obtained from Boehringer Ingelheim Pharmaceuticals Inc. (Ingelheim, Germany). Aspirin and simvastatin were obtained from Merck & Co. Inc. (Rahway, NJ, USA). All other reagents unless otherwise indicated were obtained from Sigma (St Louis, MO, USA). Mice were pretreated with DP (10, 30, and 60 mg/kg, q 12 h, 3 days, orally through gavage) and simvastatin (0.1 to 20 mg/ kg per day, 14 days, intraperitoneally) or ASA (10 mg/kg per day, 3 days, intraperitoneally) before MCA occlusion. Simvastatin was activated by alkaline hydrolysis as described (Endres et al, 1998). Because of the differences in murine and human stomach pH, the absorption of DP was substantially lower in mice than that in humans. Therefore, the doses of DP were chosen on the basis of serum blood levels of DP in mice that approximate human serum levels, that is, therapeutic plasma DP level is ~1.1 to 1.3 µg/dL after 200mg oral dose.
Measurement of Plasma Dipyridamole Level
Plasma DP level was determined spectrofluorometrically under acidic and basic pH conditions to eliminate background interference as described (Oshrine et al, 2005). Briefly, 50 µL of serum was equally divided into two tubes containing either 450 µL of 15% ethanol in 50 mmol/L Gly-NaOH buffer (pH 9.8) or 450 µL of 15% ethanol in 50 mmol/L Gly-HCl buffer (pH 2.6). Measurements of fluorescence and spectra monitoring were performed on FluoroMax 3 spectrofluorometer (Jobin Yvone, Edison, NJ, USA). Fluorescence spectra were recorded in the range of wavelengths from 430 to 660nm with an excitation of 420 nm. Absorption spectra of the DP in solution buffers were recorded on a spectrophotometer Lambda 25 (Perkin-Elmer, Waltham, MA, USA). Control of pH values in solutions of studied samples was performed with Accumet AB-15 pH meter (Fisher Scientific, Pittsburgh, PA, USA) equipped with a microelectrode. Standard control solutions with different concentrations of DP were prepared in mouse plasma for calibration purposes.
Transient Middle Cerebral Artery Occlusion Model
To determine a potential nonplatelet effect of DP, we used a transient MCA occlusion model of nonthrombotic stroke in mice that is not dependent on platelet aggregation, but on endothelial function and CBF. All experiments were conducted in accordance with National Institutes of Health, Brigham and Women’s Hospital, and Massachusetts General Hospital institutional guidelines on animal experimentation. Mice were anesthetized with 2% isoflurane mixed in 70% N2O and 30% O2, and then maintained on 1.5% to 2% isoflurane in a similar gaseous mixture. Transient focal cerebral ischemia was performed using an 8-0 nylon monofilament coated with silicone, which was introduced into the internal carotid artery via the external carotid artery, and then advanced 10mm distal to the carotid bifurcation to occlude the MCA as described (Hiroi et al, 2006). Laser Doppler flowmetry of relative CBF was used to verify successful occlusion (<20% baseline value). The MCA was occluded 2 h, followed by withdrawal of filament and reperfusion for 22 h. Relative CBF returned to >95% of baseline values, indicating almost complete reperfusion without residual occlusion.
Measurement of Cerebral Infarct Size
Cerebral infarct volume was measured after reperfusion. Infarction area was measured in 2-mm-thick coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride and quantitated with MCID Elite M6 image-analysis software (Interfocus Imaging, Linton, England). Cerebral infarct volume was determined by summing up the infarcted areas. To eliminate the effects of edema, infarct size was also calculated as the ratio of ipsilateral to contralateral hemisphere.
Measurement of Cerebral Blood Flow
Absolute CBF was measured using an indicator fractionation technique as described (Laufs et al, 2000). Briefly, the right jugular vein and left femoral artery of anesthetized mice were cannulated. The mice received 1 µCi of N-isopropyl-[methyl 1,3-14C] p-iodoamphetamine from the right jugular vein as a bolus. A volume of 100 µL of arterial blood samples from the left femoral artery was collected for 20 secs (0.3 mL/min), and then the animal was decapitated and the whole brain was removed and frozen in chilled isopentane solution chilled with dry ice immediately. The frozen brains were weighed and the ischemic territory was digested with Scintigest (Fischer Scientific, Pittsburgh, PA, USA) at 50°C for 6 h. Scintillation fluid and H2O2 were added to the sample and they were shaken together for 12 h. The blood and brain samples were measured by liquid scintillation spectrometry (RackBeta 1209; Pharmacia-Wallac, Uppsala, Sweden). Cerebral blood flow was calculated as follows: CBF (mL/100 g per min) = (brain count (c.p.m.) × 0.3 (mL/min)/(blood count (c.p.m.) × brain weight (g))× 100.
Assessment of Neurologic Deficits
The neurological deficit score (NDS) was determined by two observers, who were blinded to the identity of the mice or treatment protocol. The following scoring system was used: 0, no motor deficits (normal); 1, flexion of the contralateral torso and forelimb on lifting the animal by the tail (mild); 2, circling to the contralateral side but normal posture at rest (moderate); 3, leaning to the contralateral side at rest (severe); and 4, no spontaneous movement (critical).
Measurement of Aortic Tension
Mice were killed by chloroform anesthesia followed by decapitation, and their aorta removed and immersed in physiologic solution (composition (mmol/L): NaCl 118, KCl 4.6, NaHCO3 25, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.2, glucose 10, EDTA 0.025, pH 7.4 at 37°C). The aorta was dissected, cut into 2-mm-long segments and threaded onto 40 µm stainless steel wires. Each segment was mounted in one of the four organ chambers of an isometric myograph (610M; Danish Myo Technology, Aarhus, Denmark). After mounting, each preparation was equilibrated, unstretched for 30 mins in physiologic solution, maintained at 37°C, and aerated with a gas mixture of 95% O2 and 5% CO2. The normalized passive resting force and the corresponding diameter were then determined for each preparation from its own length–pressure. Contractile responses were recorded into a computer by using a data acquisition and recording software (Myodaq and Myodata; Danish Myo Technology, Aarhus, Denmark). After normalization and 30 mins equilibration in physiologic solution, the arteries were preconstricted with 0.3 µmol/L phenylephrine. Once preconstriction reached a steady state, endothelium-dependent relaxations were studied by adding increasing concentrations of acetylcholine (ACh; 1nmol/L to 3 µmol/L). Endothelium-independent relaxations were studied by sodium nitroprusside (1 µmol/L).
Measurement of Endothelial Nitric Oxide Synthase Activity
Endothelial nitric oxide synthase activity was determined by measuring nitrite accumulation or the conversion of [3H]l-arginine into [3H]L-citrulline in the presence or absence of the competitive NOS inhibitor N(G)-nitro-l-arginine methylester (l-NAME) (1 mmol/L), as described (Laufs et al, 2000). Cells were homogenized in ice-cold PBS containing 1 mmol/L EDTA. The homogenates were centrifuged, and 5 µg of protein extracts from the supernatant were used for the eNOS assay as described. Unlabeled l-arginine was added to [3H]l-arginine (specific activity, 60 Ci/mmol) at a ratio of 3:1.
Statistical Analysis
All values are expressed as mean ± s.e.m. Blood pressure changes were examined by the paired Student’s t-test. Differences of cerebral infarction volumes between vehicle and treatment groups were determined by one-way and two-way analysis of variance test. The difference in NDS, a noncontinuous variable, was determined by the Mann– Whitney analysis and χ2 test. Values of P < 0.05 were considered statistically significant.
Results
Effects of Dipyridamole and Statin on Cerebral Blood Flow and Infarct Size
Treatment of mice with increasing concentrations of DP (10 and 30 mg/kg, q12 h, 3 days, through gavage) achieved plasma DP levels of 0.09±0.04 and 0.32±0.10 µg/mL, respectively (therapeutic plasma DP level was ~1.1 to 1.3 µg/dL). At a DP concentration of 60 mg/kg, plasma DP levels were found to be > 1.3 ±g/dL. At concentrations of 10 and 30 mg/kg, DP did not decrease cerebral infarct size (Figure 1A), increase absolute CBF in the ischemic territory, or improve NDS compared with vehicle control (data not shown) (P > 0.05 for all compared with vehicle control). At a concentration of 60 mg/kg, DP decreased cerebral infarct size by 30% and improved NDS (n=5, P < 0.05 for both compared with vehicle control).
Figure 1.
Concentration-dependent effects of (A) dipyridamole (DP; 10 to 60 mg/kg, q12 h, 3 days, gavage) or (B) simvastatin (S; 0.1 to 20 mg/kg per day, 14 days, intraperitoneally) on cerebral infarct size (stroke volume). *P<0.05 versus vehicle.
Similarly, statin (preactivated simvastatin) at concentrations of 0.1 and 1 mg/kg per day (14 days, intraperitoneally) did not affect CBF, cerebral infarct size, or neurologic deficits compared with vehicle control (n = 6 to 7, P > 0.05 for all compared with vehicle control) (Figure 1B). At concentrations of 0.1 and 1 mg/kg per day, simvastatin was subtherapeutic in terms of reducing cerebral infarct size. However, at concentrations of 10 and 20 mg/kg, statin decreased cerebral infarct size by 26% and 35%, respectively (n = 6 to 7, P < 0.05 for both compared with vehicle control) and improved NDS (n = 6 to 7, P < 0.05 for both compared with vehicle control) (data not shown).
Additive Effects of Dipyridamole and Statin on Cerebral Blood Flow and Infarct Size
Although there was a trend toward improvements in CBF and stroke volume in mice treated with either DP (≤ 30 mg/kg) or statin (≤ 1 mg/kg), neither was effective (n = 7 and 16, P > 0.05 for both compared with vehicle control). However, the combination of subtherapeutic concentrations of DP (30 mg/kg per day, 3 days) and simvastatin (1 mg/kg per day, 14 days) increased absolute CBF by 50%±6% (n=8, P = 0.02) and decreased cerebral infarct size by 54%±7% (n = 17, P < 0.01) (Figures 2A and 2B). This led to a substantial improvement in NDS compared with either agent alone or vehicle control (n = 6 to 7, P < 0.05 for all comparisons) (Figure 2C). Addition of a higher concentration of DP (60 mg/kg per day, 3 days) to simvastatin (1 mg/kg per day, 14 days) decreased cerebral infarct volume to 33.7 ± 6.2mm3 (P < 0.05 compared with DP 30 mg/ kg per day and simvastatin 1 mg/kg per day). These findings indicate that the combination of DP and simvastatin has additive effects on stroke protection after focal cerebral ischemia.
Figure 2.

Effects of subtherapeutic doses of simvastatin (statin, 1 mg/kg per day, 14 days) and dipyridamole (DP; 30 mg/kg, q12 h, 3 days), alone or in combination, on (A) absolute cerebral blood flow (percentage of baseline) in ischemic territory, (B) cerebral infarct size (stroke volume, mm3), and (C) frequency of NDS in WT mice. Mann–Whitney analysis and χ2 test were used to determine statistical differences in NDS. *P<0.05 versus vehicle.
Previously, we have shown that simvastatin increased CBF and decreased cerebral infarct size by upregulating eNOS (Endres et al, 1998). To determine whether statin and DP could enhance endothelium-dependent vasodilation, we measured ACh-induced relaxation of aortic strips from mice treated with vehicle, DP, or simvastatin, alone or in combination. Compared with vehicle-treated mice, the aortas from DP-treated mice exhibited similar ACh-induced vasodilation (Figure 3A). However, in statin-treated mice, ACh-induced aortic vasodilation was greater than that of the vehicle- or DP-treated mice, especially at higher ACh concentrations (0.5 to 5 µmol/L). Addition of DP to subtherapeutic dose of statin increased ACh-induced aortic vasodiation more than statin alone. These findings suggest that DP augments the endothelium-dependent response of statin. Indeed, at 20 mg/kg per day for 14 days, simvastatin increased eNOS activity by three-fold. However, in this study, using subtherapeutic dose of simvastatin at 1 mg/kg per day, alone or in combination with DP, increased aortic eNOS activity by only 25%±7% (n = 8 to 9, P < 0.05 compared with vehicle) (Figure 3B). Treatment with DP alone had no effect on aortic eNOS activity (n=8, P > 0.05 compared with vehicle). In contrast, both statin and DP increased aortic cGMP levels by 43%±5% and 55%±12%, respectively (n=6, P < 0.05 for both compared with vehicle) (Figure 3C). Surprisingly, although the combination of statin and DP increased aortic cGMP level by 49%±7% (n=6, P < 0.05 compared with vehicle), the increase in cGMP was similar to that of DP alone (P > 0.05 compared with DP or statin alone).
Figure 3.

Effects of simvastatin (statin, 1 mg/kg per day, 14 days), dipyridamole (DP, 60 mg/kg, q12 h, 3 days), alone or in combination, on (A) tension of phenylephrine (PE) preconstricted aortic strips in response to acetylcholine (ACh)-induced vasodilation, (B) aortic eNOS activity, and (C) aortic cGMP levels in WT mice. *significant difference versus control; **significant difference versus statin treatment.
Effects of Dipyridamole and Statin in eNOS−/− Mice
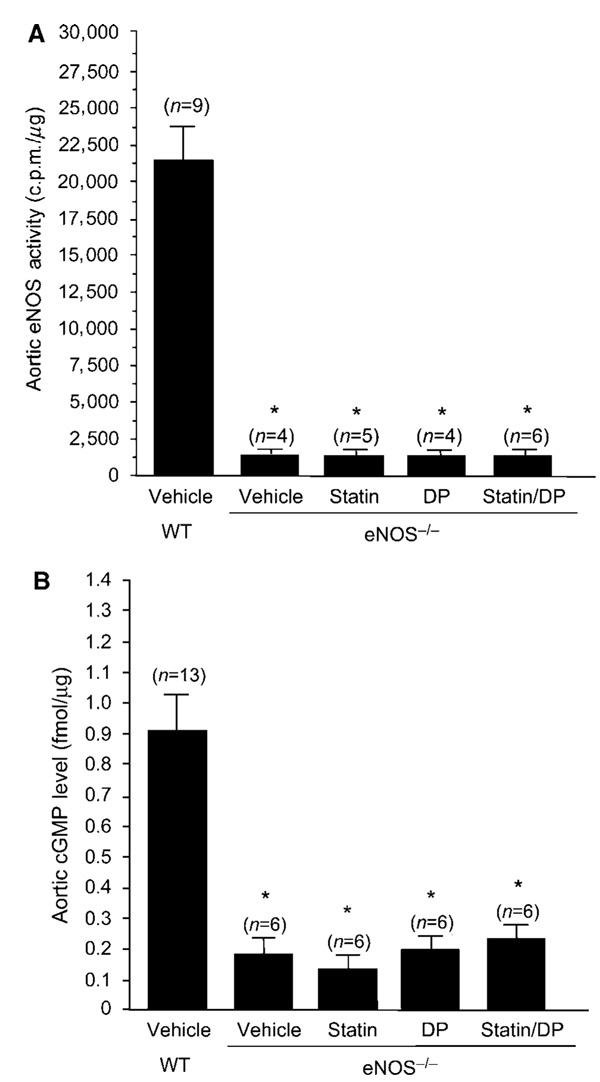
After transient MCA occlusion, cerebral infarct size was comparable between WT and eNOS−/− mice (84±9 versus 78±8mm3, n = 8 to 9, P > 0.05). In contrast, in the permanent MCA occlusion model, which results in a larger stroke size and higher mortality, eNOS−/− mice exhibited a 15% to 20% increase in cerebral infarct size compared with WT mice (Huang et al, 1996). Therefore, we elected to use the transient model of focal cerebral ischemia in our study, as the stroke sizes are similar between WT and eNOS−/− mice and also all mice survived MCA occlusion and could be analyzed. Treatment of eNOS−/− mice with either simvastatin or DP, alone or in combination, did not affect absolute CBF in the ischemic territory, cerebral infarct size, or neurologic deficits (P > 0.05 for all groups compared with vehicle control) (Figures 4A to 4C). Indeed, aortic eNOS activity and cGMP level in the eNOS−/− mice were less than 10% and 20% of WT controls, respectively (Figures 5A and 5B). Treatment with DP or statin, alone or in combination, had no effect on aortic eNOS activity and cGMP level. These findings indicate that the combination of DP and statin exert their neuroprotective effects predominantly via an eNOS-dependent mechanism, as all of the benefits of DP and statin on stroke protection were absent in eNOS−/− mice.
Figure 4.

Effects of simvastatin (statin, 1 mg/kg per day, 14 days), dipyridamole (DP, 30 mg/kg, q12 h, 3 days), alone or in combination, on (A) absolute cerebral blood flow (percentage of baseline) in ischemic territory, (B) cerebral infarct size (stroke volume, mm3), and (C) frequency of NDS in eNOS−/− mice. Mann–Whitney analysis and χ2 test were used to determine statistical differences in NDS.
Figure 5.
Effects of simvastatin (statin, 1 mg/kg per day, 14 days), dipyridamole (DP, 30 mg/kg, q12 h, 3 days), alone or in combination, on aortic (A) eNOS activity and (B) cGMP levels in eNOS−/− mice. *P<0.01 versus vehicle-WT.
Effect of Aspirin on Cerebral Infarct Size
To confirm that the transient MCA occlusion model used in this study is not dependent on thrombosis, we pretreated mice with ASA (10 mg/kg per day, 3 days, intraperitoneally). Previous study has shown that at a concentration of 10 mg/kg per day, ASA was effective in preventing platelet aggregation and thrombosis in vivo (Meng and Seuter, 1977; Rosenblum and El-Sabban, 1977). Treatment with ASA alone did not have any effect on cerebral infarct size or neurologic deficits (P =NS (nonsignificant) for both, n = 7 to 8) (Figures 6A and 6B). Indeed, after withdrawal of the filament and reperfusion, CBF in the ischemic territory returned to >90% of baseline values for vehicle and all treatment groups, suggesting little or no obstruction after 2 h of occlusion (data not shown).
Figure 6.

Effects of aspirin (ASA, 10 mg/kg per day, 3 days), dipyridamole (DP, 30 and 60 mg/kg, q12 h, 3 days), statin (simvastatin, 1 mg/kg per day, 14 days), alone or in combination, on (A) cerebral infarct size (stroke volume, mm3) and (B) frequency of NDS in WT mice. Mann–Whitney analysis and χ2 test were used to determine statistical differences in NDS. *significant difference versus vehicle; **significant difference versus ASA/DP60 treatment.
Addition of subtherapeutic dose of DP (30 mg/kg, q12 h, 3 days) to ASA (10 mg/kg per day, 3 days) had no effect on stroke size or neurologic deficits, suggesting that ASA, in contrast to statins, has no direct vascular protective effects and therefore cannot enhance the subtherapeutic dose of DP (Figure 6). The combination of a higher dose of DP (60 mg/kg, q12 h, 3 days) with ASA (10 mg/kg per day, 3 days) increased absolute CBF in the ischemic territory by 29%, decreased stroke size by 27%, and improved neurologic deficits (n = 7 to 10, P < 0.05 for all groups) (Figures 6A and 6B). However, all of these benefits were similar to DP (60 mg/kg, q12 h, 3 days) alone (P > 0.05 for all compared with the combination of DP and ASA) and absent in eNOS−/− mice (n = 4 to 6, P > 0.05 compared with vehicle, data not shown). Similarly, the addition of ASA to subtherapeutic dose of statin (i.e., simvastatin 1 mg/kg per day, 14 days) did not affect cerebral infarct volume or neurologic deficits compared with vehicle or statin alone. However, the addition of statin to ASA and DP (60 mg/kg, q12 h, 3 days) further decreased cerebral infarct size and improved neurologic deficits compared with ASA and DP. However, cerebral infarct size in mice treated with the combination of ASA, statin, and DP was similar to the cerebral infarct size of mice treated with the combination of statin and DP (30.5±7.1 versus 33.7±6.2mm3, P > 0.05). These findings indicate that ASA alone offers little or no neuroprotective effects in this nonplatelet-dependent model of transient MCA occlusion and suggest that statin and DP protects against cerebral ischemia by a NO-dependent mechanism.
Discussion
We have shown that DP can exert neuroprotective effects in a filament model of ischemic stroke suggesting additional benefits beyond platelet inhibition. Indeed, ASA, at a dose that has been shown to inhibit platelet aggregation and thrombosis in vivo (Meng and Seuter, 1977; Rosenblum and El-Sabban, 1977), provided no additive neuroprotective effects in this model, either in combination with DP alone or DP and statin. Indeed, the addition of subtherapeutic dose of DP or statin to ASA did not decrease cerebral infarct size compared with ASA alone. Furthermore, the combination of a higher DP dose with ASA confers similar stroke protection to that of the higher DP dose alone. These findings suggest that ASA has little or no direct vascular protective effects in this intraluminal filament model of ischemic stroke. Nevertheless, we cannot completely exclude the additional antithrombotic effects of DP in this model. Further studies using a clot embolic stroke model are required to address the role of platelet inhibition by DP and ASA.
The combination of subtherapeutic doses of statin and DP increased CBF and decreased cerebral infarct size. We have previously shown that statins at higher concentrations have ‘pleiotropic’ or direct vascular effects by upregulating eNOS expression and activity (Endres et al, 1998; Laufs et al, 1998). In agreement with NO-dependent mechanism, we found that the aortas of mice treated with statin and DP exhibited greater ACh-depedent relaxation than with statin or DP alone. Similar vascular protective effects of DP and lower dose of statin have been observed in an ischemia–reperfusion injury model (Ye et al, 2007). Indeed, the combination of statin and DP had no effect on CBF and cerebral infarct size in eNOS−/− mice. These findings indicate that DP exerts neuroprotective effects through NO-dependent mechanisms and suggest that the neuroprotection was due, in part, to vascular protection. These results may explain why the addition of DP to ASA decreases the risk for secondary strokes without incurring excess bleeding compared with ASA alone and suggest a role of statin–DP combination in the therapy for ischemic strokes.
Antiplatelet therapy has been the principal treatment for nonembolic ischemic strokes (Albers et al, 2004; Sacco et al, 2006). Interestingly, ASA at doses as low as 35 to 50mg per day achieved comparable risk reductions in stroke and transient ischemic attacks as doses of 1,000 to 1,500 mg per day, and with lesser gastrointestinal hemorrhages. However, the relatively small magnitude of stroke reduction derived from ASA has spurred the search for more effective antiplatelet agents or regimens. Surprisingly, the Management of Atherothrombosis with Clopidogrel in High-risk Patients (MATCH) and Clopidogrel for High Atherothrombosis Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) studies indicate that dual antiplatelet therapy of ASA and clopidogrel do not confer additional stroke protection compared with ASA or clopidogrel alone, but instead increases the risk of bleeding (Bhatt et al, 2006; Diener et al, 2004). These findings suggest that further platelet inhibition beyond that of ASA or clopidogrel alone is not beneficial and perhaps detrimental in cerebrovascular disease. In contrast, addition of DP to ASA in the European Stroke Prevention Study (ESPS)-2 and European/Australian Stroke Prevention in Reversible Ischemia Trial (ESPRIT) decreased the relative risk of stroke by approximately 20% compared with ASA alone, without incurring excess bleeding (Diener et al, 1996; Halkes et al, 2006). These findings suggest that DP exerts stroke protective effects possibly beyond platelet inhibition. Indeed, clinical trials with vascular protective agents, such as statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers, have all been shown to reduce the risk of secondary strokes despite having no direct effects on platelet inhibition (Amarenco et al, 2006; Dahlof et al, 2002; Heart Outcomes Prevention Evaluation Study Investigators, 2000; Sacco and Liao, 2005).
Dipyridamole has been shown to inhibit platelet aggregation by preventing the re-uptake of adenosine (Gresele et al, 1986). Adenosine could also exert vascular effects such as vasodilation and this may also lead to neuroprotection (Gamboa et al, 2005). Indeed, the effects of DP on resistance vessels are due predominantly to potentiation of adenosine mechanisms (Gamboa et al, 2005). Furthermore, DP can increase prostacyclin (PGI2) synthesis in vascular endothelial cells (Blass et al, 1980), which could also result in vasodilation and enhanced blood flow. However, the absence of DP on stroke protection in eNOS−/− mice indicates that, at least in the murine model of transient MCA occlusion, the effect of DP on adenosine levels does not contribute significantly to stroke protection. By increasing intracellular levels of cGMP, DP could augment many of the downstream signaling pathways of NO. Loss of endothelial-derived NO activity leading to reduction in intracellular cGMP levels contributes to impaired vascular responses (Liao et al, 1991), enhanced platelet aggregation (Radomski et al, 1992), and vascular smooth muscle proliferation (Garg and Hassid, 1989). Inhibition of endothelial NO production by the eNOS inhibitor Nω-mono-methyl-l-arginine (l-NMA) causes vasoconstriction (Kurose et al, 1993), and vascular inflammation by promoting endothelial-leukocyte adhesion (De Caterina et al, 1995). Indeed, lower vascular cGMP levels in mutant mice lacking eNOS are associated with systemic and pulmonary hypertension (Huang et al, 1995; Steudel et al, 1997), greater propensity for intimal smooth muscle proliferation in response to vascular cuff injury (Moroi et al, 1998), and larger stroke sizes in response to cerebral ischemia (Huang et al, 1996). Thus, in this study, by inhibiting cGMP phosphodiesterase, DP may potentiate the down-stream effects of statins on eNOS upregulation.
Interestingly, the addition of DP to statin did not increase intracellular cGMP levels to a greater extent than either DP or statin alone. These findings suggest that elevation of cGMP may not be the only downstream mechanism responsible for beneficial effects of NO. Indeed, eNOS activity and cGMP levels, with and without statins and/or DP, were less than 10% and 20% of vehicle control, respectively. Nevertheless, another phosphodiesterase inhibitor, cilostazol, reduces the incidence of secondary stroke by 42% compared with placebo; the greatest risk reduction occurred among patients enrolled after lacunar stroke (Gotoh et al, 2000; Matsumoto, 2005). Similar to DP, the clinical benefits of cilostazol were not associated with adverse bleeding events relative to placebo. Interestingly, cilostazol has been shown to increase eNOS activity (Hashimoto et al, 2006). These findings suggest that phosphodiesterase inhibition in the vascular wall in addition to their antiplatelet effects may protect and decrease the severity of ischemic strokes. It remains to be determined whether these agents are similarly neuroprotective when given after MCA occlusion.
Acknowledgments
We thank Eun Jung Shin for technical assistance and Wolfgang Eisert for critical review of the paper.
This work was supported by grants from the National Institutes of Health (HL052233 to JKL, NS055104 to CW and NS010828 to JKL and MAM), Korean Science and Engineering Fellowship (KOSEF to H-HK), and sponsored research grants from Boehringer Ingelheim Pharmaceuticals Inc. (to JKL) and ZEMA Inc. (to H-HK).
Footnotes
Disclosures
JKL serves as a consultant for AstraZeneca, Merck, Pfizer, Boehringer Ingelheim Pharmaceuticals Inc., and ZEMA Inc. JKL is on the speaker’s bureau of AstraZeneca, Merck, Pfizer, and Boehringer Ingelheim Pharmaceuticals Inc.
References
- Aktas B, Utz A, Hoenig-Liedl P, Walter U, Geiger J. Dipyridamole enhances NO/cGMP-mediated vasodilator-stimulated phosphoprotein phosphorylation and signaling in human platelets: in vitro and in vivo/ex vivo studies. Stroke. 2003;34:764–769. doi: 10.1161/01.STR.0000056527.34434.59. [DOI] [PubMed] [Google Scholar]
- Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:483S–512S. doi: 10.1378/chest.126.3_suppl.483S. [DOI] [PubMed] [Google Scholar]
- Amarenco P, Bogousslavsky J, Callahan A, III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Ochiai Y, Hidaka H. Selective inhibition of separated forms of human platelet cyclic nucleotide phosphodiesterase by platelet aggregation inhibitors. Mol Pharmacol. 1977;13:400–406. [PubMed] [Google Scholar]
- Best LC, McGuire MB, Jones PB, Holland TK, Martin TJ, Preston FE, Segal DS, Russell RG. Mode of action of dipyridamole on human platelets. Thromb Res. 1979;16:367–379. doi: 10.1016/0049-3848(79)90084-7. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- Blass KE, Block HU, Forster W, Ponicke K. Dipyridamole: a potent stimulator of prostacyclin (PGI2) biosynthesis. Br J Pharmacol. 1980;68:71–73. doi: 10.1111/j.1476-5381.1980.tb10700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3- hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa A, Abraham R, Diedrich A, Shibao C, Paranjape SY, Farley G, Biaggioni I. Role of adenosine and nitric oxide on the mechanisms of action of dipyridamole. Stroke. 2005;36:2170–2175. doi: 10.1161/01.STR.0000179044.37760.9d. [DOI] [PubMed] [Google Scholar]
- Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, Shinohara Y, Itoh E, Matsuda T, Sawada T, Yamaguchi T, Nishimaru K, Ohashi Y. Cilostazol stroke prevention study: a placebo-controlled doubleblind trial for secondary prevention of cerebral ischemia. J Stroke Cerebrovasc Dis. 2000;9:147–157. doi: 10.1053/jscd.2000.7216. [DOI] [PubMed] [Google Scholar]
- Gresele P, Arnout J, Deckmyn H, Vermylen J. Mechanism of the antiplatelet action of dipyridamole in whole blood: modulation of adenosine concentration and activity. Thromb Haemost. 1986;55:12–18. [PubMed] [Google Scholar]
- Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Miyakoda G, Hirose Y, Mori T. Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis. 2006;189:350–357. doi: 10.1016/j.atherosclerosis.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Cheng SY, Liao JK. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA. 2006;103:14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-l-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, Granger DN. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993;73:164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Chui DS, Yang SX, Simoncini T, Yamada M, Rabkin E, Allen PG, Huang PL, Bohm M, Schoen FJ, Moskowitz MA, Liao JK. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- Liao JK, Bettmann MA, Sandor T, Tucker JI, Coleman SM, Creager MA. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res. 1991;68:1027–1034. doi: 10.1161/01.res.68.4.1027. [DOI] [PubMed] [Google Scholar]
- Matsumoto M. Cilostazol in secondary prevention of stroke: impact of the Cilostazol Stroke Prevention Study. Atheroscler Suppl. 2005;6:33–40. doi: 10.1016/j.atherosclerosissup.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Meng K, Seuter F. Effect of acetylsalicylic acid on experimentally induced arterial thrombosis in rats. Naunyn Schmiedebergs Arch Pharmacol. 1977;301:115–119. doi: 10.1007/BF00501425. [DOI] [PubMed] [Google Scholar]
- Moroi M, Zhang L, Yasuda T, Virmani R, Gold HK, Fishman MC, Huang PL. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshrine B, Malinin A, Pokov A, Dragan A, Hanley D, Serebruany V. Criticality of pH for accurate fluorometric measurements of dipyridamole levels in biological fluids. Methods Find Exp Clin Pharmacol. 2005;27:95–100. doi: 10.1358/mf.2005.27.2.876284. [DOI] [PubMed] [Google Scholar]
- Radomski MW, Rees DD, Dutra A, Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992;107:745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum WI, El-Sabban F. Platelet aggregation in the cerebral microcirculation: effect of aspirin and other agents. Circ Res. 1977;40:320–328. doi: 10.1161/01.res.40.3.320. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–e449. [PubMed] [Google Scholar]
- Sacco RL, Liao JK. Drug insight: statins and stroke. Nat Clin Pract Cardiovasc Med. 2005;2:576–584. doi: 10.1038/ncpcardio0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- Ye Y, Lin Y, Perez-Polo R, Huang MH, Hughes MG, McAdoo DJ, Manickavasagam S, Uretsky BF, Birnbaum Y. Enhanced cardioprotection against ischemia– reperfusion injury with a dipyridamole and low-dose atorvastatin combination. Am J Physiol Heart Circ Physiol. 2007;293:H813–H818. doi: 10.1152/ajpheart.00210.2007. [DOI] [PubMed] [Google Scholar]