Abstract
Cardiac hypertrophy is an initial physiological adaptive response by the heart to pressure overload. However, if pressure overload persists, frequently, the heart decompensates and develops ‘pathophysiological’ hypertrophy. This leads to increased mortality and morbidity and is an independent risk factor for heart failure. Because cardiac myocytes convert this pressure overload into intracellular biochemical signals, blocking this critical signaling pathway may be an important therapeutic target to prevent cardiac hypertrophy. Small GTP-binding proteins, in particular Rac1, have been suggested to play a key role in the development of cardiac hypertrophy. Recently, 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, also called statins, have been shown to inhibit cardiac hypertrophy independent of their cholesterol lowering property. Statins block the isoprenylation and activation of members of the Rho family, such as RhoA and Rac1. Rac1 also regulates NADPH oxidase, which is a major source of reactive oxygen species (ROS) in cardiovascular cells. Growing evidence suggests that ROS may be involved in the process of cardiac hypertrophy and recent research has shown that statins attenuate oxidative stress through inhibition of Rac1. Overall, these pleiotropic effects of statins will give new insights into the process of cardiac hypertrophy.
Keywords: anti-oxidants, cardiac hypertrophy, HMG-CoA reductase inhibitors, small GTP-binding proteins, statins
Introduction
Several clinical trials with 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have shown that they reduce the incidence of myocardial infarction and ischemic disease (1–3). Although the beneficial effects of statins have been attributed to their lipid-lowering effects, subgroup analysis of the WOSCOP and CARE trials suggests that statin-treated individuals have significantly lower risks for coronary heart disease compared to age-matched placebo-treated individuals, despite comparable serum cholesterol levels (1–3). Other research has found that the restoration of endothelial function by statins occurs before significant reduction in serum cholesterol levels. This is also evidence of the additional effects of statins beyond that of cholesterol reduction (4,5). Recent experimental evidence indicates that some of the cholesterol-independent or ‘pleiotropic’ effects of statins involve the improvement or restoration of endothelial function, an increase in the stability of atherosclerotic plaques, and a decrease in vascular inflammation (6–8).
Cardiac hypertrophy is an adaptive response of the heart to pressure overload. In physiological hypertrophy, such as that occurring in athletes, there is increased cardiomyocyte size and total heart weight in response to increased workload. Cardiac function is improved and contractile abnormalities are absent. In pathophysiological or excessive hypertrophy, there is a transition, which may result from a maladaptation of the coronary circulation relative to the increased cardiac myocyte size. The reduced supply of oxygen and substrates can lead to a fall in contractile function. During this process, there is increased expression of embryonic genes, such as natriuretic peptides and fetal contractile proteins, and development of cardiac fibrosis. The increased synthesis of collagen fibers, which both support the myocytes and provide a framework against which the myocytes contract, is initially adaptive and probably reversible at this stage of the process. Therefore, the onset of fibrosis, in itself, does not indicate a pathophysiological condition. However, with progressive and maladaptive hypertrophy, which is due, in part, to unrestrained positive feedback of the neurohormonal system, excessive fibrosis can eventually contribute to a fall in contractile function. From this aspect, the blockade of some critical signaling pathways, such as myocardial protein synthesis, may lead to decreased oxygen demand by cardiomyocyte at the cellular level.
The molecular response to pressure overload is complex and it may include modulation of various intracellular signal pathways, such as activation of many protein kinases (mitogen activated protein kinase (MAPK) and phosphatidyl inositol 3 (PI3) Kinase), expression of cardiac fetal genes (atrial natriuretic factor (ANF) and myosin light chain), and increase of protein synthesis. Furthermore, pressure overload leads to the release of secretion and production of vasoactive peptides, such as angiotensin II and endothelin-1, which play a pivotal role in the induction of these hypertrophic responses (9,10). Since cardiac myocytes convert the pressure overload into intracellular biochemical signals, the blockade of critical signaling pathways may be therapeutic targets to treating cardiac hypertrophy. Some signaling pathways that might be targeted are MAPK (11), calcium-dependent calcienurin/CaM kinase (12) or small GTP-binding protein, etc.
Small G proteins and cardiac hypertrophy
Ras, Rho and Rac are members of a family of small GTP-binding proteins which exert a variety of physiological functions. It has been demonstrated that they participate in diverse cellular functions such as locomotion, cytokinesis, and cytoskeletal remodeling in non-muscle cells (13,14). The activation of Rac or Rho mediates cellular remodeling in differentiated cardiomyocytes, while myocardial architecture responds to the same signals that induce cytoskeletal reorganization in non-muscle cells (15,16). Reports on the cardiac-based effects of Ras, Rho and Rac indicate that their primary action is to modulate hypertrophic responses (17,18). Ras-mediated cardiac hypertrophy has been demonstrated both in vitro (19) and in vivo (20). Transgenic mice overexpressing RhoA exhibited symptoms consistent with the loss of systolic function and dilated cardiomyopathy. However, the development of cardiomyopathy was due to severe conduction abnormalities rather than a direct modification of myocardial architecture (21). This effect of RhoA, found in vivo, was unanticipated, because previously, several in vitro studies implicated RhoA in the development of hypertrophy (15,22). Other studies conducted with cultured cardiomyocytes revealed, that the activation of Rac is required in phenylephrine-induced cardiomyocyte hypertrophy. In those studies the transfection of myocytes with a dominant-negative Rac1 construct completely inhibited the hypertrophic response to phenylephrine (14). In addition, transgenic mice that specifically expressed activated Rac1 in the myocardium, showed severe cardiac dilation and hypertrophy (23).
Key messages
3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have been shown to inhibit cardiac hypertrophy independent of their cholesterol lowering property.
Statins block the isoprenylation and activation of small GTP-binding proteins members, such as Ras and Rho family.
Recent research has shown that statins attenuate oxidative stress through inhibition of Rac1, which has been suggested to play a key role in the development of cardiac hypertrophy
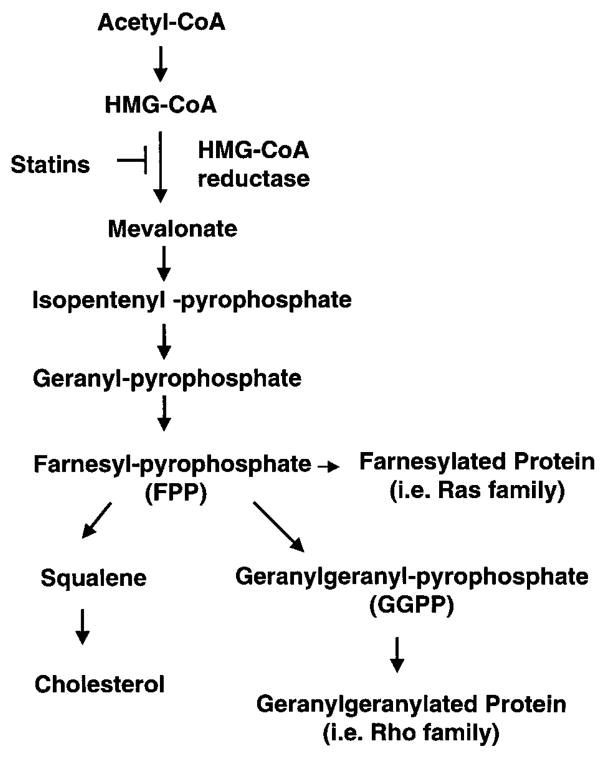
In the cholesterol biosynthetic pathway, reduction of HMG-CoA to mevalonate by the HMG-CoA reductase is a rate-limiting step. Inhibition of this enzyme by statins not only leads to the reduction of cholesterol but also the reduction of the synthesis of several isoprenoid intermediates (Fig 1). These intermediates, such as farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP), serve as important lipid attachments for posttranslational modification of a variety of proteins, including the gamma subunit of heterotrimeric G proteins, heme-a, nuclear lamins, as well as Ras and Ras-like proteins, such as Rho and Rac (24). Thus, protein isoprenylation allows the covalent attachment, subcellular localization, and intracellular trafficking of membrane-associated proteins. Importantly, members of Ras and Rho GTPase family are major substrates for posttranslational modification by prenylation (24, 25). Ras translocation from the cytoplasm to the plasma membrane is dependent on farnesylation, whereas Rho translocation is dependent on geranylgeranylation (26,27). These biochemical analyses showed that statins inhibit the development of cardiac hypertrophy through inhibition of Ras and Rho isoprenylation, leading to the accumulation of inactive Ras and Rho in the cytoplasm (28,29). We could recently elucidate that Rac1 was the key molecule in the process of cardiac hypertrophy (Fig 2). We cotransfected rat neonatal cardiac myocytes with dominant-negative mutants of Rac1, RhoA, or CDC42 and ANF promotor-luciferase reporter construct. Transfection with dominant-negative Rac1 (N17Rac1), and to a less extent, RhoA (N19RhoA), inhibited angiotensin II-induced ANF promoter activity. Cotreatment with Simvastatin further decreased ANF promoter activity in cells transfected with N19RhoA and N17Cdc42, but not those transfected with N17Rac1. Although cotreatment with GGPP reversed the inhibitory effects of Simvastatin, GGPP could not reverse the inhibitory effect of N17Rac1 on ANF promoter activity (28).
Figure 1.
Cholesterol biosynthetic pathway. HMG-CoA indicates 3-hydroxyl-3-methylglutaryl coenzyme A. HMG-CoA reductase is a rate-limiting enzyme and this blockade (statins) leads to the reduction of other isoprenoid intermediates as well as intracellular cholesterol.
Figure 2.
Rac1 is involved in the process of cardiac hypertrophy. Effects of transfection with dominant-negative Rho mutants (N17Rac1, N19RhoA, or N17Cdc42) with and without Simvastatin (5 μM) or GGPP (10 μM) on Angiotensin(Ang) II-induced ANF promoter activity. *P<0.01 compared to transfection with vector alone (Control), †P <0.05 compared to AngII and Tx: DN-Rho.
NADPH oxidase in cardiovascular cells
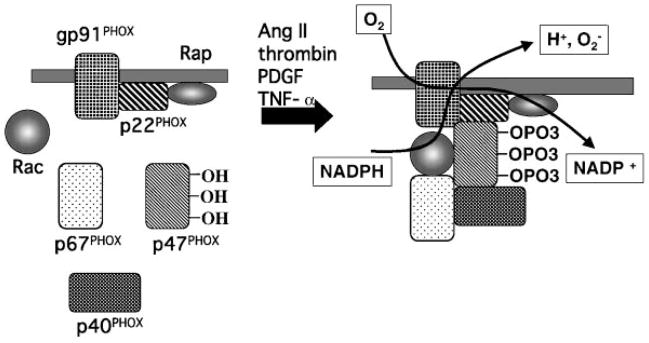
Recent work has provided strong evidence supporting the critical role of oxidative stress in the functioning of cardiovascular cells. Growing evidence suggests that reactive oxygen species (ROS) may be involved in the process of cardiac hypertrophy (30,31). It has been known for years that cardiovascular tissues can release a large amount of ROS, including superoxide, hydrogen peroxide, and nitric oxide. Recent works strongly suggest that NADPH oxidase is a major source of superoxide in cardiovascular cells (32). NADPH oxidase is a membrane-associated enzyme that catalyzes the 1-electron reduction of oxygen using NADPH or NADH as the electron donor. NADPH oxidase in leukocytes has been thoroughly studied and is found in phagocytes and B-lymphocytes. Recently 5 components have been identified in the core of the enzyme: p40PHOX (PHOX for Phagocyte Oxidase), p47PHOX, p67PHOX, p22PHOX and gp91PHOX. In the resting cell, three of these five components, p40PHOX, p47PHOX and p67PHOX, exist in the cytosol, forming a complex. The other two components, p22PHOX, gp91PHOX, are bound to the membranes. When these two groups of components are separated by their distribution in different subcellular compartments, as in the resting cell, the enzyme is inactive. Various stimuli, such as protein kinases A or C, lead to the phosphorylation of the cytosolic components and the entire cytosolic complex then migrates to the membrane (Fig 3). Not only the core subunits are required for activation, but also two low-molecular-weight guanine nucleotide-binding proteins, Rac and Rap. In the resting cell, Rac is located in the cytoplasm in a dimeric complex with Rho-GDI (Guanine nucleotide Dissociation Inhibitor) and Rap is located in membranes from which it can be copurified with the cytochrome. During activation, Rac binds guanosine triphosphate (GTP) and migrates to the membrane with the core cytosolic complex. Therefore, it has been suggested that Rac may be involved in the activation of cardiovascular NADPH oxidase.
Figure 3.
NADPH oxidase. The core enzyme comprises five components: p40PHOX (PHOX for Phagocyte Oxidase), p47PHOX, p67PHOX, p22PHOX and gp91 PHOX In the resting cell (left panel), three of these five components, p40PHOX, p47PHOX and p67PHOX, exist in the cytosol as a complex. The other two components, p22PHOX, gp91PHOX, are located in the membranes. When it was stimulated by angiotensin II, thrombin, platelet-derived growth factor (PDGF) and tumor necrosis factor-α (TNF-α) (right panel), the cytosolic component becomes heavily phosphorylayed and the entire cytosolic complex migrates to the membrane. Activation requires the participation, not only of the core subunits, but also of two low-molecular-weight guanine nucleotide-binding proteins, Rac and Rap. During activation, Rac binds guanosine triphosphate (GTP) and migrates to the membrane along with the core cytosolic complex.
One of the most important attributes of cardiovascular oxidase is its responsiveness to local metabolic changes, hemodynamic forces, and hormones, such as the potent vasoconstrictor angiotensin II. Angiotensin II increases the activity of vascular oxidase through NADPH oxidase activation (32, 33). Thrombin, platelet-derived growth factor (PDGF) and tumor necrosis factor-α (TNF-α) also stimulate NADPH oxidase-dependent superoxide production in vascular smooth muscle cells (SMCs) (34–36). Recent analysis of the gp91PHOX deficient mice demonstrated that angiotensin II treatment increased cardiac hypertrophy and collagen content in wild-type but not gp91PHOX deficient mice (37). This evidence further supports the hypothesis that oxidative stress, in particular NADPH oxidase, plays a crucial role in cardiac hypertrophy.
The mechanism of statins in cardiac hypertrophy
From these recent growing evidences, we speculated that statins would inhibit cardiac hypertrophy through an antioxidant mechanism and focused on the inhibition of Rac1. Indeed, statins could inhibit angiotensin II-induced oxidative stress in neonatal rat cardiac myocytes (28). Moreover, in both angiotensin II-induced and pressure overload model by aortic banding, statins attenuated oxidative stress and cardiac hypertrophy in vivo (28).
Recent studies also showed that statins could inhibit cardiac hypertrophy and fibrosis in rabbit b-myosin heavy chain-Q403 mutant model due to the inhibition of Rho protein (38), and Ras signaling in rats with ascending aorta banding (39). In terms of renin-angiotensin system, it has been demonstrated that statins decrease angiotensin II type 1 receptor expression (40) and angiotensin-converting enzyme activity (41) in cardiomyocytes. These effects could partially contribute to the inhibition of cardiac hypertrophy.
Statins also increase vascular nitric oxide (NO) production (26), which potentially decreases blood pressure and attenuates the process of cardiac hypertrophy. Importantly, NO can directly contribute the inhibition of cardiac hypertrophy via cGMP pathway (42). NO can react with superoxide and form peroxynitrite, which is a more harmful radical than superoxide. Thus, one of possible mechanisms in the process of cardiac hypertrophy is the imbalance of NO and superoxide, resulting in the degradation of NO before it can reach the cardiac myocyte. There are now several lines of evidence linking excess vascular oxidative stress to the impairment of NO action in patients with diabetes or hypertension (43, 44).
In summary, there is strong evidence of statins in preventing cardiac hypertrophy. This effect is mediated by Rac 1 and includes a reduction in oxidative stress. These experimental results may suggest a novel pharmacological approach to treating cardiac hypertrophy.
Acknowledgments
The work described in this article was supported by NIH (HL-52233, HL-48743, to Dr. Liao) and the American Heart Association Bugher Foundation Award (to Dr. Liao) and the Banyu Fellowship Awards in cardiovascular medicine which are sponsored by Banyu Pharmaceutical Co., Ltd. and The Merck Company Foundation (to Dr. Nakagami).
References
- 1.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 2.Massy ZA, Keane WF, Kasiske BL. Inhibition of the mevalonate pathway: benefits beyond cholesterol reduction? Lancet. 1996;347:102–3. doi: 10.1016/s0140-6736(96)90217-2. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 4.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–93. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 5.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–31. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 6.Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–9. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 7.Fukumoto Y, Libby P, Rabkin E, Hill CC, Enomoto M, Hirouchi Y, et al. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 2001;103:993–9. doi: 10.1161/01.cir.103.7.993. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Mizuno T, et al. Angiotensin II partly mediates mechanical stress-induced cardiac hypertrophy. Circ Res. 1995;77:258–65. doi: 10.1161/01.res.77.2.258. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, et al. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–8. doi: 10.1074/jbc.271.6.3221. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie-Brown J, Fuller SJ, Bogoyevitch MA, Cowley S, Sugden PH. The mitogen-activated protein kinase kinase MEK1 stimulates a pattern of gene expression typical of the hypertrophic phenotype in rat ventricular cardiomyocytes. J Biol Chem. 1995;270:28092–6. doi: 10.1074/jbc.270.47.28092. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y, Hiroi Y, Uozumi H, Takimoto E, Toko H, Zhu W, et al. Calcineurin plays a critical role in the development of pressure overload-induced cardiac hypertrophy. Circulation. 2001;104:97–101. doi: 10.1161/01.cir.104.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 14.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 15.Pracyk JB, Tanaka K, Hegland DD, Kim KS, Sethi R, Rovira II, et al. A requirement for the rac1 GTPase in the signal transduction pathway leading to cardiac myocyte hypertrophy. J Clin Invest. 1998;102:929–37. doi: 10.1172/JCI2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH. The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of Rho kinase. J Biol Chem. 1998;273:7725–30. doi: 10.1074/jbc.273.13.7725. [DOI] [PubMed] [Google Scholar]
- 17.Wang SM, Tsai YJ, Jiang MJ, Tseng YZ. Studies on the function of rho A protein in cardiac myofibrillogenesis. J Cell Biochem. 1997;66:43–53. [PubMed] [Google Scholar]
- 18.Thorburn J, Xu S, Thorburn A. MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. EMBO J. 1997;16:1888–900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller SJ, Gillespie-Brown J, Sugden PH. Oncogenic src, raf, and ras stimulate a hypertrophic pattern of gene expression and increase cell size in neonatal rat ventricular myocytes. J Biol Chem. 1998;273:18146–52. doi: 10.1074/jbc.273.29.18146. [DOI] [PubMed] [Google Scholar]
- 20.Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270:23173–8. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 21.Sah VP, Minamisawa S, Tam SP, Wu TH, Dorn GW, Ross J, Jr, et al. Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J Clin Invest. 1999;103:1627–34. doi: 10.1172/JCI6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sah VP, Hoshijima M, Chien KR, Brown JH. Rho is required for Galphaq and alpha1-adrenergic receptor signaling in cardiomyocytes. Dissociation of Ras and Rho pathways. J Biol Chem. 1996;271:31185–90. doi: 10.1074/jbc.271.49.31185. [DOI] [PubMed] [Google Scholar]
- 23.Sussman MA, Welch S, Walker A, Klevitsky R, Hewett TE, Price RL, et al. Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J Clin Invest. 2000;105:875–86. doi: 10.1172/JCI8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 25.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 26.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–35. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 27.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–71. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 28.Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108:1429–37. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dechend R, Fiebeler A, Park JK, Muller DN, Theuer J, Mervaala E, et al. Amelioration of angiotensin II-induced cardiac injury by a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor. Circulation. 2001;104:576–81. doi: 10.1161/hc3001.092039. [DOI] [PubMed] [Google Scholar]
- 30.Bell JP, Mosfer SI, Lang D, Donaldson F, Lewis MJ, Vitamin C. quinapril abrogate LVH and endothelial dysfunction in aortic-banded guinea pigs. Am J Physiol Heart Circ Physiol. 2001;281:H1704–10. doi: 10.1152/ajpheart.2001.281.4.H1704. [DOI] [PubMed] [Google Scholar]
- 31.Date MO, Morita T, Yamashita N, Nishida K, Yamaguchi O, Higuchi Y, et al. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J Am Coll Cardiol. 2002;39:907–12. doi: 10.1016/s0735-1097(01)01826-5. [DOI] [PubMed] [Google Scholar]
- 32.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 33.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–95. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 34.Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, et al. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J Biol Chem. 1999;274:19814–22. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- 35.De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J. 1998;329:653–7. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-kappaB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation. 1997;96:2361–7. doi: 10.1161/01.cir.96.7.2361. [DOI] [PubMed] [Google Scholar]
- 37.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91phox-containing NADPH oxidase in Angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–6. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 38.Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, et al. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation. 2001;104:317–24. doi: 10.1161/hc2801.094031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Indolfi C, Lorenzo ED, Perrino C, Stingone AM, Curcio A, Torella D, et al. Hydroxymethylglutaryl Coenzyme A reductase inhibitor simvastatin prevents cardiac hypertrophy induced by pressure overload and inhibits p21ras activation. Circulation. 2002;106:2118–2124. doi: 10.1161/01.cir.0000034047.70205.97. [DOI] [PubMed] [Google Scholar]
- 40.Wassmann S, Laufs U, Baumer AT, Muller K, Konkol C, Sauer H, et al. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59:646–54. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 41.Luo JD, Zhang WW, Zhang GP, Guan JX, Chen X. Simvastatin inhibits cardiac hypertrophy and angiotensin-converting enzyme activity in rats with aortic stenosis. Clin Exp Pharmacol Physiol. 1999;26:903–8. doi: 10.1046/j.1440-1681.1999.03165.x. [DOI] [PubMed] [Google Scholar]
- 42.Wollert KC, Fiedler B, Gambaryan S, Smolenski A, Heineke J, Butt E, et al. Gene transfer of cGMP-dependent protein kinase I enhances the antihypertrophic effects of nitric oxide in cardiomyocytes. Hypertension. 2002;39:87–92. doi: 10.1161/hy1201.097292. [DOI] [PubMed] [Google Scholar]
- 43.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22–8. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wever RM, Luscher TF, Cosentino F, Rabelink TJ. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation. 1998;97:108–12. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]