Abstract
Siberian hamsters markedly reduce their body/lipid mass (~20–45%) in short ‘winter-like’ days (SD). Decreases in body/lipid mass associated with food deprivation or lipectomy result in increases in foraging and food hoarding. When at their SD-induced body/lipid mass nadir, food hoarding is not increased despite their decreases in body/lipid mass, but hoarding was not tested during the dynamic period of body/lipid mass loss (first 5–6 weeks of SDs). Therefore, we tested for changes in foraging/hoarding during this initial period in Siberian hamsters housed in a simulated burrow with a wheel running-based foraging system and exposed to either long ‘summer-like’ days (LD) or SDs. Two foraging effort conditions were used: 10 Revolutions/Pellet (pellet delivered after running 10 revolutions) and a Free Wheel/Free Food condition (wheel available, food pellets non-contingently available). Regardless of the foraging condition, body mass was significantly reduced across 8 weeks of SDs (~ 15%). Foraging increased after 7 weeks in SDs, but food hoarding did not increase compared to LDs. Instead food hoarding significantly decreased in SDs at Weeks 2–5 compared with Week 0 values, with the 10 Revolutions/Pellet foraging group returning to LD levels thereafter and the Free Wheel/Free Food group remaining reduced from Weeks 0–8. Collectively, we found that SDs decreased body mass, increased foraging after 7 weeks, and increased food hoarding, but only after an initial decrease and not above that seen in LDs. These data suggest that SD-induced body/lipid mass losses do not engender similar behavioral responses as seen with food deprivation or lipectomy.
Keywords: Siberian hamsters, hamsters, seasonal, appetitive behaviors, wheel running
INTRODUCTION
Obtaining sufficient energy for the maintenance of life and reproduction is essential for survival. For many animal species, especially those living in temperate climates, the presence and relative abundance of food is restricted annually and this seasonal variation has shaped seasonal cycles in animal physiology and behavior. For example, many animals exhibit seasonal cycles of breeding, adiposity, hibernation and immune responses (for review see: [1]). The most noise-free environmental cue [2] that synchronizes these physiological and behavioral cycles to the varying temperature, climate and food abundance is the daylength (for review see: [3, 4]).
For seasonal mammals, as well as some species of birds, reptiles and amphibians, the daylength is encoded into a neuroendocrine signal through the synthesis and secretion of melatonin via a multisynaptic pathway beginning with the retina and ending in the pineal gland (for review see: [4]). Melatonin is synthesized and released only at night; thus, the peak nocturnal duration of melatonin secretion faithfully codes the daylength (nightlength; for review see: [5]). For Siberian hamsters (Phodopus sungorus), long duration melatonin signals (>12 h) trigger short ‘winter-like’ day (SD) responses, whereas short duration melatonin signals (<8 h) trigger long ‘summer-like’ day (LD) responses (for review see: [5]). Thus, the nocturnal duration of melatonin release is able to transfer daylength information to many different physiological systems in photoperiodic seasonal animals and coordinate responses with annual changes in the environment (for review see: [4, 5]).
Siberian hamsters are small (< 50 g) photoperiod-responsive seasonal mammals that exhibit a broad suite of photoperiod-driven alterations in physiology and behavior [6]. Central to the work described here, Siberian hamsters decrease their body mass ~30% in SDs when food availability is lowest in nature [7], but also do so in the laboratory even when food is abundant (e.g., [8, 8–10]). In addition to the ability to cope with seasonal variation in food availability, Siberian hamsters can withstand energetic challenges that result in negative energy balance, such as food restriction/starvation, because they have ample lipid energy stores in body fat (white adipose tissue [WAT]; ~30–50 % of total body mass [11]). In the laboratory, Siberian hamsters food deprived for 56 h lose ~20–30 % of their body mass and, upon restoration of ad libitum feeding, they do not overeat [12, 13]. Instead of overeating, Siberian hamsters [12, 14] and other hamster species (e.g., Syrian [Mesocricetus auratus] [14]) increase food hoarding. Pregnant and lactating Siberian hamsters experience decreases in body/lipid mass of up to ~50 % [15, 16] and, as with food deprivation-induced decreases in body/lipid mass, they show impressive increases in food hoarding upon refeeding [16]. Because large losses of body mass in adult mammals, including Siberian hamsters, are reflected primarily as decreases in body fat, it is not surprising that direct decreases in body fat, such as occur with surgical removal of WAT (partial lipectomy), trigger increases in food hoarding [17, 18].
When Siberian hamsters are tested for their food hoarding after 11 weeks of SD exposure, a time after body mass/fat has reached its nadir, they do not hoard more than their LD counterparts despite these SD-induced body mass/fat losses [19]. From the above, it would be predicted that the SD-induced decreases in body and lipid mass of Siberian hamsters would markedly increase their food hoarding similarly to that occurring with food deprivation-induced losses [12, 16, 19–21] or with lipectomy [17, 18]. It may be, however, that they only do so when their body and lipid mass are most dynamically decreasing during the initial 1 to 5 or 6 weeks of SD exposure. Therefore, we asked: Does the rapid body/lipid mass loss occurring during early SD exposure trigger increase in food foraging and hoarding? This was accomplished by using our foraging/hoarding system (described below and shown and described in: [22]) that allows the measurement of food foraging, hoarding and intake. Terminal measures of WAT and testes masses, as well as serum leptin concentration also were made.
MATERIALS AND METHODS
Animals and Housing
Adult male Siberian hamsters, ~2.5–3 months old, weighing 32–50 g were obtained from our breeding colony; the colony was originally established in 1985, and has been outbred with wild caught individuals as previously described [23]. Hamsters were group housed in a LD photoperiod (16:8-h light-dark cycle [light onset at 2030]) from birth and weaned at 28 days of age. Room temperature was maintained at 21±2 °C, with relative humidity at ~50±10 %. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and were in compliance with the Public Health Service and United States Department of Agriculture guidelines.
At the start of the experiment, animals were transferred to the foraging/hoarding room and singly housed in polypropylene cages (290 × 180 × 130 mm) for two weeks to acclimate to the new light onset (0800) and single housing. The test diet (75 mg pellets, Dustless Precision Pellets (protein 18.8%, fat 5.0%, fiber 4.6%, ash 4.4%, moisture < 5.0%, carbohydrate 61.5%, caloric value: 3.68 kcal/gm; BioServ, Frenchtown, NJ) and tap water were available ad libitum throughout unless otherwise noted. Siberian hamsters were then placed into the foraging/hoarding apparatuses for one week of acclimation as previously described [22]. Briefly, two cages were connected via polyvinyl-chloride tubing (38.1 mm in diameter and ~1.5 m long), with vertical and horizontal portions to facilitate movement between the top, or ‘food cage’, and the bottom, or ‘burrow cage’. The top cage (456 × 234 × 200 mm) was equipped with a running wheel, food pellet dispenser and water bottle. The bottom cage (290 × 180 × 130 mm) contained Alpha-Dri bedding (Specialty Papers, Kalamazoo, MI) and one cotton nestlet (Ancare, Belmore, NY). The bottom cage was covered to simulate the darkness of a burrow. During the first two days of the acclimation period, the hamsters had free access to food and additionally, a pellet was dispensed for every 10 wheel revolutions. Wheel revolutions were counted using a magnetic detection system and a computer-based hardware/software package (Med Associates, Georgia, VT). On the third day, free food was removed and all animals were on a response-contingent condition for four days, where food was only obtained after 10 wheel revolutions. This 10 revolution/pellet condition was then maintained for 14 d when all three behavior measures had stabilized. At that time, animals were then assigned to one of four groups (2 foraging treatments × 2 daylength treatments). Groups were matched for average daily hoard and body mass change over the last 5 days of 10 revolutions/pellet period (baseline).
Foraging Efforts and Daylengths
Two foraging efforts used were: 1) 10 Revolutions/Pellet (10REV) and 2) Free Wheel/Free Food (FW, 300 pellets of food were available each day in a non-contingent manner with an active running wheel). The purpose of the 10REV group was to be able to measure food foraging. The purpose of the FW group was to test for non-specific alterations in locomotor activity that, without this group, would be interpreted as increases or decreases in the motivation to obtain food by the 10REV group or to be able to detect non-specific/malaise effects if FW wheel running is decreased from bona fide inhibition in the motivation to earn food. The two daylength treatments were: 1) LDs (16L:8D, light onset at 0800) and 2) SDs (8L:16D, light onset at 0700). The final number of animals per group was: a) LD/10REV (n=6), b) SD/10REV (n=10), c). LD/FW (n=7) and d). SD/FW (n=11). Animals remained in their respective treatment for 8 weeks. Body mass was recorded weekly to the nearest 0.01g and wheel running, food foraging, hoarding and intake were recorded daily within one hour of light onset.
Measurements of Food Foraging, Hoarding, and Intake
Food foraged (pellets earned) was defined as the number of pellets delivered into the top cage of the 10REV group hamsters calculated as wheel revolutions ÷ 10. Hoarded food was removed each day and was defined as the number of pellets found in the bottom cage, as well as that removed from hamsters’ check pouches. Surplus food was removed each day and defined as the food pellets that remained in the top cage that were neither ingested nor hoarded. Food intake in the 10REV group was determined by [pellets earned − (surplus food + food hoard)]. In the FW group, food intake was defined as [300 − (surplus food + food hoard)]. An electronic balance was used to weigh the food pellets and set to “parts” measurement; thus, one 75-mg food pellet was equal to 1 pellet, with fractions of a pellet calculated as well. The wheel revolutions were recorded continuously in 5 minute bins of activity and analyzed as wheel revolutions per day.
Terminal Measures
After 8 weeks of treatment, animals were lightly anesthetized with isoflurane and had ~ 400 μl of blood taken from the retro-orbital sinus. Blood was centrifuged at 4 °C for 30 minutes at 2,500 rpm; serum was then collected, stored at −20 °C before leptin analysis (mouse leptin ELISA kit, Millipore, St. Charles, MO) and was performed according to the manufacturer’s instructions. Pelage color was assessed using a four point scale 1 = summer and 4 = winter [24] and was recorded at the termination of the experiment (8 weeks). Animals were then killed by an overdose of pentobarbital sodium (300 mg/kg) and testes and epididymal-, inguinal-, and retroperitoneal WAT (EWAT, IWAT, and RWAT, respectively) were excised and weighed to the nearest 0.0001 g.
Statistical Analyses
The repeated measures data (body mass and food foraging, hoarding and intake) were transformed into percent change from initial values ([[initial value − current value]/initial value] × 100) to control for unequal initial body mass and associated measures that that occurred between groups due to the loss of animals during the study.
Statistical analyses were conducted using NCSS version 2000 (Kaysville, UT) for all measures. Body mass, wheel activity and food hoarding and intake were analyzed using three-way repeated measures analysis of variance (ANOVA; Daylength × Foraging Effort × Time; 2 × 2 × 9). Food foraging was analyzed using a two-way repeated ANOVA (Daylength, × Time; 2 × 9). The terminal measures of serum leptin concentration, pelage color, paired testes mass and fat pad mass were analyzed using two-way ANOVA (Daylength × Foraging Effort; 2 × 2). Any SD animals with paired testes mass > 250 mg were excluded from analysis, as this indicates unresponsiveness to the daylength change. Post hoc analysis was done with Tukey-Kramer Multiple-Comparison Tests when appropriate. Differences among groups were considered statistically significant if P<0.05. Exact probabilities and test values have been omitted for simplification and clarity of the presentation of the results.
RESULTS
Running Wheel Revolutions
FW running wheel activity (revolutions/day) was not affected by the photoperiod, foraging effort, time or their interaction (data not shown) indicating no non-specific effects of these treatments or their interaction on locomotor activity.
Body Mass
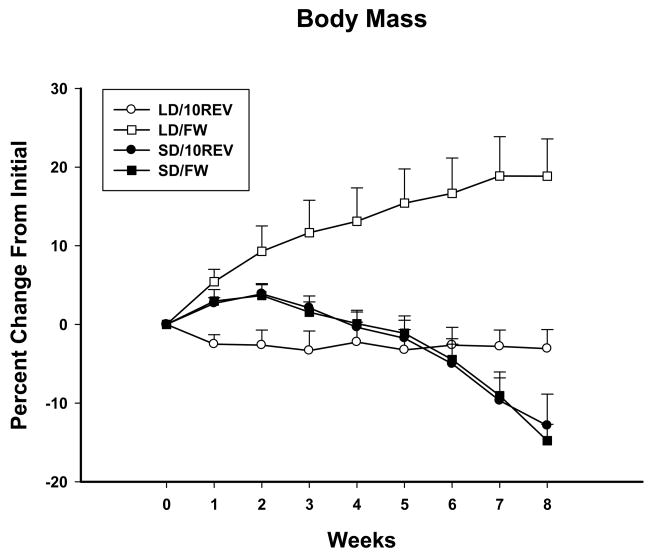
Foraging effort did not alter the SD-induced percent change in body mass for either group with significant decreases occurring from Week 7–8 in SDs (Ps<0.05; Fig. 1). In LDs, however, the FW group increased body mass after Week 2 through the end of the experiment (Ps<0.05; Fig. 1) and body mass of the 10REV group remained the same throughout the experiment. After 8 weeks of treatment, LD/FW had the greatest body mass compared with all other groups (P<0.05; Fig. 4B).
Figure 1.
Mean percent change from Week 0 body mass ± SE for Siberian hamsters housed in long daylengths while earning one food pellet per 10 wheel revolutions (○, LD/10REV, initial average body mass (g ± SEM) = 39.2 g ± 1.2), long daylengths without running wheel contingent food delivery (□, LD/FW, initial average body mass (g ± SEM) 39.6 = g ± 1.6), short daylengths while earning one food pellet per 10 wheel revolutions (●, SD/10REV, initial average body mass (g ± SEM) = 37.9 g ± 1.4), or short daylengths without running wheel contingent food delivery (■, SD/FW, initial average body mass (g ± SEM) = 44.9 g ± 1.7). * indicates within treatment group decrease from initial baseline (Week 0) for both SD housed groups, P<0.05.
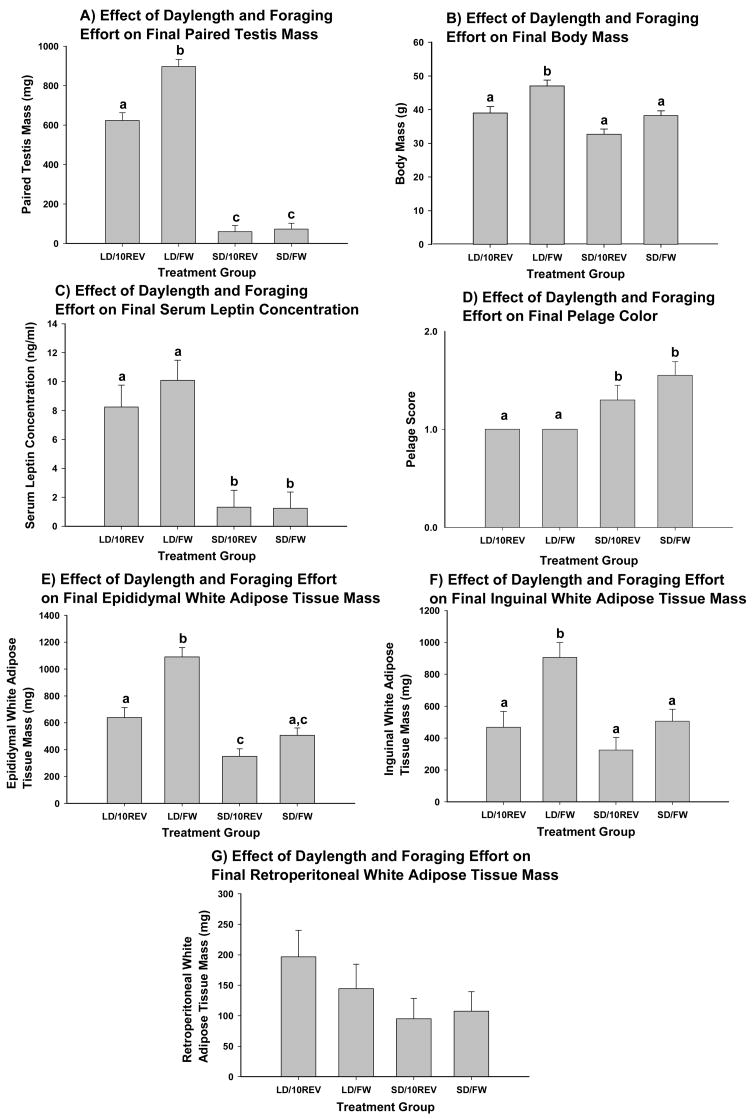
Figure 4.
Terminal measures: A) mean paired testis mass, mg ± SE, B) mean body mass, g ± SE, C) mean serum leptin concentration, ng/ml ± SE, D) average pelage score ± SE, E) mean EWAT mass, mg ± SE, F) mean IWAT mass, mg ± SE, and G) mean RWAT mass, mg ± SE. Shared letters indicate group similarity, p<0.05.
Food Foraging
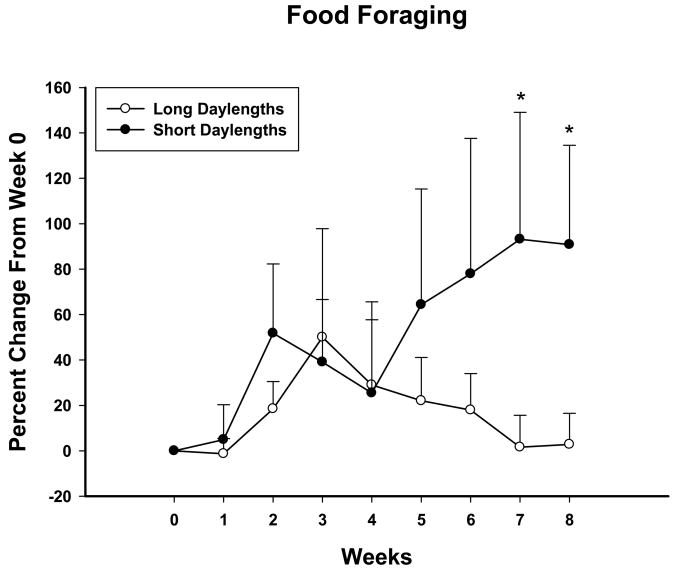
SD-housed animals had a greater percent increase in foraged food than did the LD-housed animals at Weeks 7 and 8 and was significantly increased over baseline at Weeks 5–8 (Ps<0.05; Fig. 2).
Figure 2.
Mean percent change from Week 0 daily foraging (pellets earned) ± SE for Siberian hamsters housed in long (○, initial daily average pellets earned (pellets ± SEM) = 212.0 pellets ± 28.9) or short (●, initial daily average pellets earned (pellets ± SEM) = 227.2 ± 41.5) daylengths for eight weeks (10REV groups only). * indicates significant difference between groups and an increase from baseline (Week 0), P<0.05.
Food Hoarding
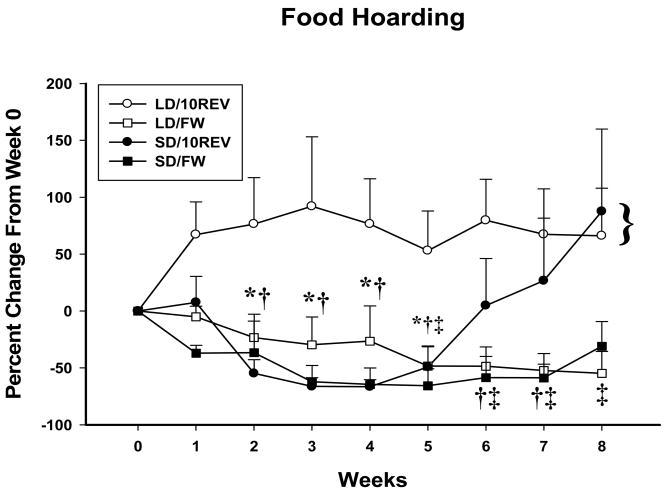
SD exposure significantly increased the percent decrease in food hoard size irrespective of foraging treatment compared with the LD/10REV treatment at Weeks 1–5 (Ps<0.05; Fig. 3). In addition, hoarding was significantly reduced at Weeks 2–5 in both SD groups compared with their respective Week 0 (baseline) values (Ps<0.05; Fig. 3). At Week 6,SD/10REV food hoarding returned to baseline values and at Week 8 was significantly increased over baseline (P<0.05; Fig. 3), but not to a greater extent than the LD/10REV. SD/FW food hoarding returned to baseline hoarding values at Week 8 (P<0.05; Fig 3). The percent change in food hoarding was significantly decreased in LD/FW animals from baseline values at Weeks 2–8 (P<0.05; Fig. 3).
Figure 3.
Mean percent change from Week 0 daily food hoard ± SE, for Siberian hamsters housed in long daylengths while earning one food pellet per 10 wheel revolutions (○, LD/10REV, initial daily average pellets hoarded (pellets ± SEM) = 97.6 pellets ± 17.5), long daylengths without running wheel contingent food delivery (□, LD/FW, initial daily average pellets hoarded (pellets ± SEM) = 85.4 pellets ± 19.1), short daylengths while earning one food pellet per 10 wheel revolutions (●, SD/10REV, initial daily average pellets hoarded (pellets ± SEM) = 73.9 pellets ± 17.4), or short daylengths without running wheel contingent food delivery (■, SD/FW, initial daily average pellets hoarded (pellets ± SEM) = 86.7 pellets ± 17.4). * indicates significant decrease from initial baseline (Week 0) for SD/10REV, † indicates significant decrease from initial baseline (Week 0) for SD/FW, and ‡ indicates significant decrease from initial baseline (Week 0) for LD/FW. Bracket indicates between group similarity within time point, P<0.05.
Food Intake
The percent change in food intake was not significantly changed by SD exposure, regardless of the foraging treatment, at any point across the experiment, although the SD/FW approached a significant decrease in Week 7 and 8 (P=0.07; data not shown). This finding is consistent with the delay in decreases in food intake that ultimately becomes significant 10–12 weeks after SD exposure [11].
Terminal Measures
Testes Mass
All SD-housed animals had regressed gonads (<250mg), which is somewhat unusual as in this species as ~ 30 % of animals typically are non-responsive to SD exposure [25]. LD/FW hamsters had significantly larger testes than all other groups (Ps<0.05; Fig. 4A).
Serum Leptin Concentrations
Regardless of the foraging treatment, the serum leptin concentration was significantly less in SD- than in LD-housed hamsters (Ps<0.05; Fig. 4C), as expected [26].
Pelage Color
Irrespective of foraging treatment, SD-housed hamsters had a significantly greater shift to the white winter pelage than their LD-housed counterparts after 8 weeks of SD exposure (Ps<0.05; Fig. 4D).
WAT Pad Masses
EWAT mass was significantly smaller in SD within each foraging treatment (10REV and FW; P<0.05; Fig. 4E). IWAT mass was decreased in the SD and the LD/10REV groups compared to the LD/FW animals (P<0.05; Fig. 4F). RWAT was unaffected by daylength, foraging effort or their interaction.
DISCUSSION
Despite the lack of increases in hoarding by SD-acclimated Siberian hamsters tested at their body/lipid mass nadir (11–13 weeks of SD exposure; [19]), we predicted that foraging and hoarding would increase above that seen in LD-housed animals during the dynamic period of SD-induced body and lipid mass loss (~0–6 wk SD exposure [27]) that was included in the 8 wks of SD exposure tested here. This prediction was based on the marked increases in these behaviors that occur during the dynamic decreases in body and lipid mass associated with food deprivation [18, 19, 21], pregnancy [28] and lipectomy [17, 18]. Indeed, food hoarding was increased beginning ~5–6 weeks after SD exposure, when body mass decreased most rapidly compared with their LD-exposed counterparts for the SD/10REV hamsters. These increases, however, followed unexpected initial (~2–5 wk) decreased food hoarding and were not above that of LD/10REV Siberian hamsters. The increased food hoarding also was associated with decreased body, IWAT, and EWAT masses (but not RWAT mass) as well as decreased serum leptin concentrations at the end of the experiment (8 weeks post SD exposure).
In SDs, foraging for food (SD/10REV group) was increased compared with LDs at Weeks 7 and 8, and this increase coincided with the onset of a significant SD-induced body mass loss. This finding is similar to the foraging increases found with refeeding after food deprivation (e.g., [21]). Not surprisingly, LD/10REV hamsters did not alter their foraging for food, as their body mass did not change significantly from initial values.
Others have reported that the responses to SDs are blocked or attenuated with access to running wheels in this species, including the normal SD-induced decreases in testes and body mass [29, 30]. That is, running wheel access (but not the requirement to wheel run to earn food) increases body mass regardless of the daylength [28, 29]. In the present study, only the LD/FW group significantly increased their body mass, whereas LD/10REV did not change their body mass and SDs decreased body mass loss after ~7 weeks regardless of the foraging requirement. The reason for the discrepant results between the present study and those previously reporting wheel running-triggered increases in body mass is unclear. The lack of body mass gain with running wheel access, whether required for food delivery or voluntary, is not unique to this study [21, 22]. Briefly, we previously varied the foraging effort (the number of wheel revolutions required to earn a pellet) and body mass was not increased at any foraging effort level, despite the use of a running wheel [21, 22]. In addition, in another previous experiment, we triggered SD-like responses in LD-housed hamsters by injecting melatonin 3 h before lights-out thereby extending the endogenous nocturnal melatonin signal to be SD-like [30]. Melatonin injections decreased body mass to the same extent whether the animals had access to running wheels or were sedentary [30]. Therefore, the conditions under which wheel running increases body mass in Siberian hamsters are unclear.
None of the Siberian hamsters housed in SDs were SD non-responsive, similar to a previous study that showed a decrease in non-responsiveness when SD-housed Siberian hamsters had access to a running wheel [31]. Thus, SDs triggered the typical reductions in body mass, as reflected in decreased WAT masses for some (EWAT and IWAT), but not all (RWAT) fat depots, and decreased testes mass (gonadal regression), as well as progression toward a white winter pelage [5]. The mechanism underlying the universal responsiveness to SD exposure in Siberian hamsters given access to a running wheel is unknown, but may to act through the thalamic intergeniculate leaflet [32].
We are puzzled by the initial decrease in food hoard size during the first 5 weeks of SD exposure in both foraging effort groups and the LD/FW group, a decrease not mirrored in food intake nor foraging (SD/10REV). Thus, of the food earned for the SD/10REV hamsters, less was hoarded and food intake was not significantly changed resulting in more surplus pellets (pellets not eaten or hoarded, data not shown). Both of the FW groups also exhibited an initial decrease in food hoarding similar to the SD/10REV group and this decrease continued in the LD/FW animals, with a slight rebound to initial levels of hoarding in the SD/FW hamsters. It should be noted that the decreased food hoarding in both FW groups was not due to an increased food intake that might reduce food brought back to the burrow cage.
The factors underlying the initial SD-induced decreased food hoarding and subsequent increased food hoarding after ~ 6 wk of SDs are not known. It seems unlikely that either effect is due to marked changes in orexigenic or anorexigenic peptides that have been demonstrated to affect foraging and food hoarding by Siberian hamsters housed in this foraging/hoarding apparatus [33–37]. That is, gene expression for neuropeptide Y, agouti-related protein (melanocortin receptor inverse agonist), ghrelin, α-melanocyte-stimulating hormone (melanocortin receptor agonist) and several other neurochemicals do not change seasonally in Siberian hamster brain (for review see: [38]), but all of those substances affect food hoarding in Siberian hamsters [33–37].
At the end of the eight week exposure to LDs or SDs, serum leptin concentrations were significantly lower in SD-housed versus LD-housed hamsters. Because this was a single-point determination, however, no correlations can be made or conclusions drawn for the changes in serum leptin concentrations and the changing patterns of food hoarding or intake. The SD-induced, terminally decreased serum leptin concentrations are not surprising as leptin varies seasonally in these animals in apparent accordance with their seasonal changes in body fat (e.g., [26]). SDs alter components of the leptin signaling pathway within the arcuate nucleus in this species, leading to increased leptin sensitivity in SDs [39–41] that likely is due to the rapid SD-induced down regulation of suppressor of cytokine signaling 3 (SOCS3; for review see: [42]). Down regulation of SOCS3, in turn, inhibits Janus-activating kinase2 and signal transducers and activator of transcription (for review see: [43]). This rapid SD-induced down regulation of SOCS3 and subsequent increase in leptin sensitivity occurs at a time when leptin concentrations are still elevated in Siberian hamsters [38]. Thus, it is possible that the initial SD-induced decreased food hoarding seen here is due to transient increased sensitivity to leptin that occurs while leptin titers are still elevated. The subsequent gradual increased food hoarding could, in turn, be due to the decreases in serum leptin that occurs with continued SD exposure. Such a scenario infers an inhibitory effect of leptin on this food hoarding. Indeed, we have recently shown that exogenous leptin given peripherally or centrally decreases food hoarding by Siberian hamsters housed in the foraging/hoarding apparatus in LDs [44] and a similar inhibitory effect of peripheral leptin on food hoarding occurs in Syrian hamsters [14]. Whether this possible involvement of leptin is responsible for the SD-triggered changes in food hoarding remains to be tested explicitly.
Collectively, although other challenges resulting in decreases in body/lipid mass result in striking increases in food hoarding, and often foraging as well, that are above control or initial levels [16, 18, 21], here we found initial decreased food hoarding that increased back to initial levels when body mass (and likely lipid mass) were decreasing most rapidly and profoundly. Therefore, although somewhat consistent with the notion that decreases in body/lipid mass trigger increases in the appetitive ingestive behaviors of foraging/hoarding [16, 18, 21], these data are more consistent with the more general idea that energy flux (i.e., increases or decreases in energy balance), that may not be reflected in more molar measures of energy status such as body or WAT pad masses, can influence these behaviors. Finally, an alternative view of the essentially unchanged food hoarding accompanied by decreased food intake and decreased body/lipid mass in the SD-housed hamsters in the present study is that the relative hoard size actually increased (i.e., food hoard size per g hamster or per g body fat or per g lean body mass) promoting a food cache that could last longer because it is larger and because food intake ultimately is decreased (~>10–12 weeks; e.g., [11]) thereby potentially enhancing the chances of winter survival.
Acknowledgments
The authors thank Bruce Smith Jr., Dominiq Okoduwa, and Claudia Leitner for assistance with data collection. This research was supported by NIH R01 DK 78358 to TJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff DW, editor. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 93–156. [Google Scholar]
- 2.Turek FW, Campbell CS. Photoperiodic regulation of neuroendocrine-gonadal activity. Biol Reprod. 1979;20:32–50. doi: 10.1093/biolreprod/20.1.32. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Demas GE, Song CK. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones and the sympathetic nervous system. Exp Biol Med. 2002;227:363–376. doi: 10.1177/153537020222700601. [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Goldman BD. Mammalian pineal melatonin: A clock for all seasons. Experientia. 1989;45:939–945. doi: 10.1007/BF01953051. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- 7.Weiner J. Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp zool Soc Lond. 1987;57:167–187. [Google Scholar]
- 8.Figala J, Hoffmann K, Goldau G. Zur Jahresperiodik beim Dsungarischen Zwerghamster Phodopus sungorus Pallas. Oecologia. 1973;12:89–118. doi: 10.1007/BF00345511. [DOI] [PubMed] [Google Scholar]
- 9.Bartness TJ, Hamilton JM, Wade GN, Goldman BD. Regional differences in fat pad responses to short days in Siberian hamsters. Am J Physiol. 1989;257:R1533–R1540. doi: 10.1152/ajpregu.1989.257.6.R1533. [DOI] [PubMed] [Google Scholar]
- 10.Bartness TJ. Short day-induced depletion of lipid stores is fat pad- and gender-specific in Siberian hamsters. Physiol Behav. 1995;58:539–550. doi: 10.1016/0031-9384(95)00082-t. [DOI] [PubMed] [Google Scholar]
- 11.Wade GN, Bartness TJ. Effects of photoperiod and gonadectomy on food intake, body weight and body composition in Siberian hamsters. Am J Physiol. 1984;246:R26–R30. doi: 10.1152/ajpregu.1984.246.1.R26. [DOI] [PubMed] [Google Scholar]
- 12.Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol. 1994;266:R1111–R1117. doi: 10.1152/ajpregu.1994.266.4.R1111. [DOI] [PubMed] [Google Scholar]
- 13.Bartness TJ, Morley JE, Levine AS. Effects of food deprivation and metabolic fuel utilization on the photoperiodic control of food intake in Siberian hamsters. Physiol Behav. 1995;57:61–68. doi: 10.1016/0031-9384(94)00203-h. [DOI] [PubMed] [Google Scholar]
- 14.Schneider JE, Buckley CA. Food hoarding is increased by food deprivation and decreased by leptin treatment in Syrian hamsters. Am J Physiol. 2001;285:R1021–R1029. doi: 10.1152/ajpregu.00488.2002. [DOI] [PubMed] [Google Scholar]
- 15.Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992;16:235–272. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- 16.Bartness TJ. Food hoarding is increased by pregnancy, lactation and food deprivation in Siberian hamsters. Am J Physiol. 1997;272:R118–R125. doi: 10.1152/ajpregu.1997.272.1.R118. [DOI] [PubMed] [Google Scholar]
- 17.Wood AD, Bartness TJ. Partial lipectomy, but not PVN lesions, increases food hoarding by Siberian hamsters. Am J Physiol. 1997;272:R783–R792. doi: 10.1152/ajpregu.1997.272.3.R783. [DOI] [PubMed] [Google Scholar]
- 18.Dailey ME, Bartness TJ. Fat pad-specific effects of lipectomy on foraging, food hoarding and food intake. Am J Physiol. 2008;94:R321–R328. doi: 10.1152/ajpregu.00230.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood AD, Bartness TJ. Food deprivation-induced increases in hoarding by Siberian hamsters are not photoperiod-dependent. Physiol Behav. 1996;60:1137–1145. doi: 10.1016/0031-9384(96)00173-4. [DOI] [PubMed] [Google Scholar]
- 20.Day DE, Mintz EM, Bartness TJ. Diet self-selection and food hoarding after food deprivation by Siberian hamsters. Physiol Behav. 1999;68:187–194. doi: 10.1016/s0031-9384(99)00167-5. [DOI] [PubMed] [Google Scholar]
- 21.Day DE, Bartness TJ. Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol Behav. 2003;78:655–668. doi: 10.1016/s0031-9384(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 22.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool. 2001;289:162–171. [PubMed] [Google Scholar]
- 23.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- 24.Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. I. Role of the gonads and pituitary. J Exp Zool. 1984;230:89–95. doi: 10.1002/jez.1402300112. [DOI] [PubMed] [Google Scholar]
- 25.Puchalski W, Lynch GR. Evidence for differences in the circadian organization of hamsters exposed to short day photoperiod. J Comp Physiol. 1986;159:7–11. doi: 10.1007/BF00612490. [DOI] [PubMed] [Google Scholar]
- 26.Horton TH, Buxton OM, Losee-Olson S, Turek FW. Twenty-four-hour profiles of serum leptin in Siberian and golden hamsters: photoperiodic and diurnal variations. Horm Behav. 2000;37:388–398. doi: 10.1006/hbeh.2000.1592. [DOI] [PubMed] [Google Scholar]
- 27.Bartness TJ, Wade GN. Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci Biobehav Rev. 1985;9:599–612. doi: 10.1016/0149-7634(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 28.Scherbarth F, Petri I, Steinlechner S. Effects of wheel running on photoperiodic responses of Djungarian hamsters (Phodopus sungorus) J Comp Physiol [B] 2008;178:607–615. doi: 10.1007/s00360-007-0251-7. [DOI] [PubMed] [Google Scholar]
- 29.Thomas EM, Jewett ME, Zucker I. Torpor shortens the period of Siberian hamster circadian rhythms. Am J Physiol. 1993;265:R951–R956. doi: 10.1152/ajpregu.1993.265.4.R951. [DOI] [PubMed] [Google Scholar]
- 30.Bartness TJ, Wade GN. Body weight, food intake and energy regulation in exercising and melatonin-treated Siberian hamsters (Phodopus sungorus sungorus) Physiol Behav. 1985;35:805–808. doi: 10.1016/0031-9384(85)90415-9. [DOI] [PubMed] [Google Scholar]
- 31.Freeman DA, Goldman BD. Evidence that the circadian system mediates photoperiodic nonresposiveness in Siberian hamsters: The effect of running wheel access on photoperiodic responsiveness. J Biol Rhythms. 1997;12:100–109. doi: 10.1177/074873049701200202. [DOI] [PubMed] [Google Scholar]
- 32.Freeman DA, Teubner BJ, Goldman BD. The thalamic intergeniculate leaflet mediates locomotor activity-induced reversal of phenotype in photoperiod nonresponsive Siberian hamsters. J Biol Rhythms. 2006;21:206–213. doi: 10.1177/0748730406287996. [DOI] [PubMed] [Google Scholar]
- 33.Keen-Rhinehart E, Bartness TJ. MTII attenuates ghrelin- and food deprivation-induced increases in food hoarding and food intake. Horm Behav. 2007;52:612–620. doi: 10.1016/j.yhbeh.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1728–R1737. doi: 10.1152/ajpregu.00597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;289:R29–R36. doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- 36.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. Am J Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- 37.Day DE, Bartness TJ. Agouti-related protein increases food hoarding, but not food intake by Siberian hamsters. Am J Physiol. 2004;286:R38–R45. doi: 10.1152/ajpregu.00284.2003. [DOI] [PubMed] [Google Scholar]
- 38.Mercer JG, Tups A. Neuropeptides and anticipatory changes in behaviour and physiology: seasonal body weight regulation in the Siberian hamster. Eur J Pharmacol. 2003;480:43–50. doi: 10.1016/j.ejphar.2003.08.091. [DOI] [PubMed] [Google Scholar]
- 39.Tups A, Ellis C, Moar KM, Logie TJ, Adam CL, Mercer JG, Klingenspor M. Photoperiodic regulation of leptin sensitivity in the Siberian hamster, Phodopus sungorus, is reflected in arcuate nucleus SOCS-3 (suppressor of cytokine signaling) gene expression. Endocrinology. 2004;145:1185–1193. doi: 10.1210/en.2003-1382. [DOI] [PubMed] [Google Scholar]
- 40.Klingenspor M, Niggemann H, Heldmaier G. Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus. J Comp Physiol [B] 2000;170:37–43. doi: 10.1007/s003600050005. [DOI] [PubMed] [Google Scholar]
- 41.Tups A, Barrett P, Ross AW, Morgan PJ, Klingenspor M, Mercer JG. The suppressor of cytokine signalling 3, SOCS3, may be one critical modulator of seasonal body weight changes in the Siberian hamster, Phodopus sungorus. J Neuroendocrinol. 2006;18:139–145. doi: 10.1111/j.1365-2826.2005.01394.x. [DOI] [PubMed] [Google Scholar]
- 42.Munzberg H, Bjornholm M, Bates SH, Myers MG., Jr Leptin receptor action mechanisms of leptin resistance. Cell Mol Life Sci. 2005;62:642–652. doi: 10.1007/s00018-004-4432-1. [DOI] [PubMed] [Google Scholar]
- 43.Ahima RS, Qi Y, Singhal NS. Adipokines that link obesity and diabetes to the hypothalamus. Prog Brain Res. 2006;153:155–174. doi: 10.1016/S0079-6123(06)53009-2. [DOI] [PubMed] [Google Scholar]
- 44.Keen-Rhinehart E. Leptin inhibits food-deprivation-induced increases in food intake and food hoarding. 2008 doi: 10.1152/ajpregu.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]