Abstract
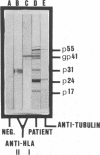
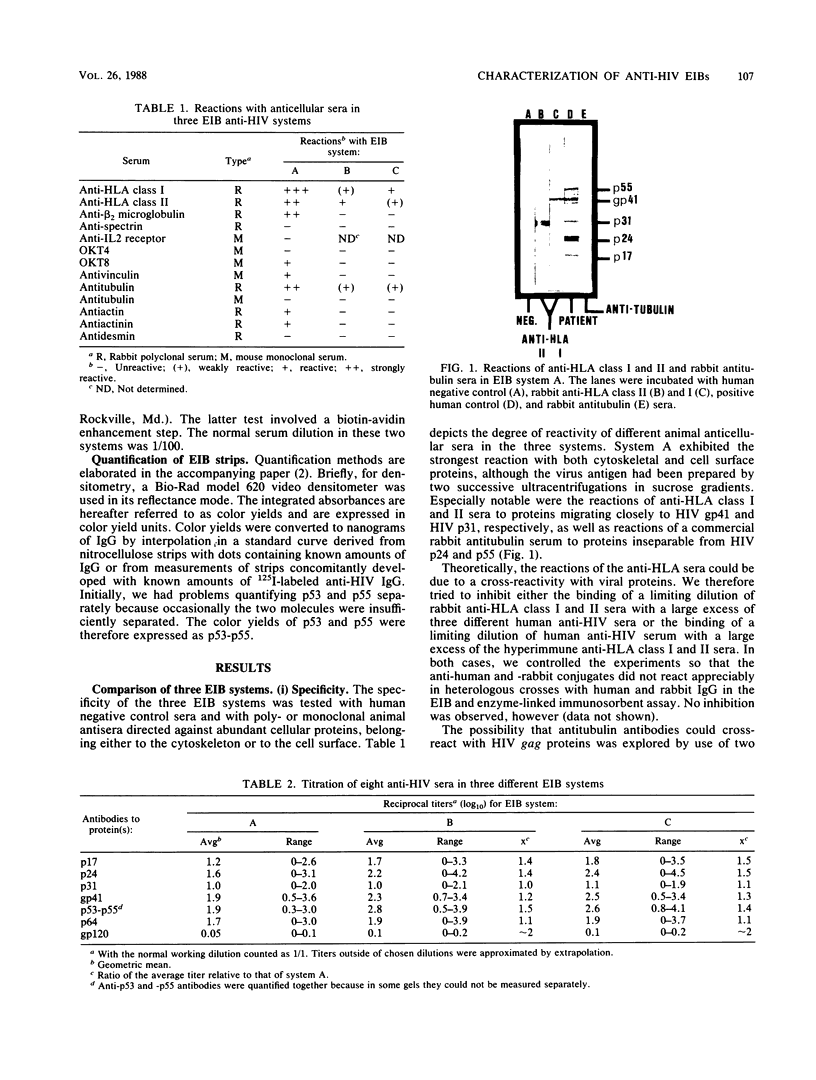
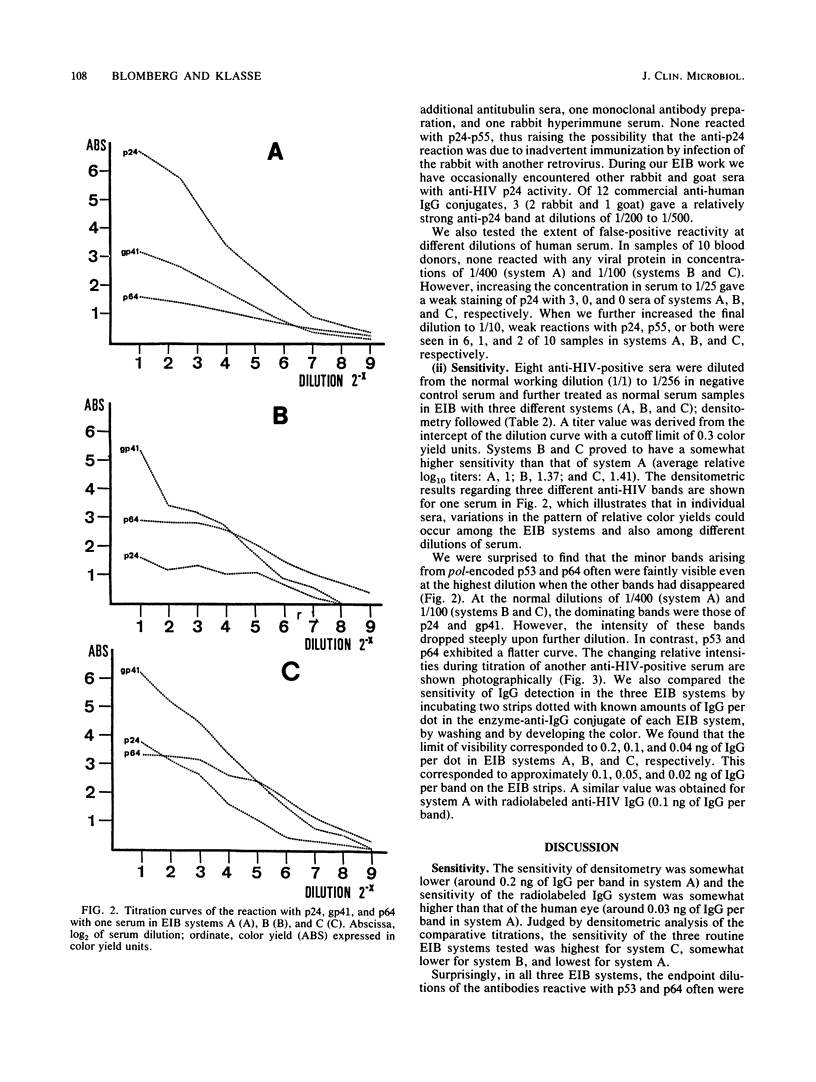
Electrophoretic immunoblotting (EIB [Western blotting]) has emerged as the major method for verification of seropositivity for human immunodeficiency virus (HIV) and therefore needs to be thoroughly characterized. The specificities of three EIB systems, our own and two commercial systems, were studied with anticellular sera and serial dilutions of human sera. We demonstrated that in one system, anti-HLA classes I and II gave bands comigrating with viral proteins, which can be controlled by EIB with uninfected H9 cells. In addition, animal antisera, including anti-immunoglobulin enzyme conjugates, occasionally reacted with HIV gag proteins, necessitating appropriate controls. Whereas none of 10 blood donors reacted at the standard dilution in serum (1/100 or 1/400) in any of the three systems, 6, 1, and 2 of 10 donors reacted with p24, p55, or both at a dilution of 1/10 for the three systems tested. Thus, nonspecific reactions can arise in several ways and justify critical EIB interpretation. The sensitivity of the three systems was studied by comparative titrations and direct quantification of bound immunoglobulin G (IgG). In the titrations with all three, the minor anti-HIV bands p53 and p64, coded from pol, were often detectable in higher dilutions than were antibodies to any other HIV protein. The minimum visible amounts of IgG bound per HIV protein band estimated by extra- and interpolation in densitometric curves and liquid scintillation counting of radiolabeled patient IgG were approximately 0.1, 0.05, and 0.02 ng per band in the three systems. One of the commercial systems had both the highest sensitivity and highest specificity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld G., Bredberg-Rådén U., Böttiger B., Putkonen P. O., Blomberg J., Juto P., Wadell G. Blood donor sera with false-positive Western blot reactions to human immunodeficiency virus. Lancet. 1986 Aug 2;2(8501):289–290. doi: 10.1016/s0140-6736(86)92111-2. [DOI] [PubMed] [Google Scholar]
- Blomberg J., Klasse P. J., Kjelle'n L., Daniel M. D. Anti-STLV-IIImac reactivity in HIV seropositive individuals in Sweden. Lancet. 1986 Aug 9;2(8502):336–337. doi: 10.1016/s0140-6736(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Blomberg J., Klasse P. J. Quantification of immunoglobulin on electrophoretic immunoblot strips as a tool for human immunodeficiency virus serodiagnosis. J Clin Microbiol. 1988 Jan;26(1):111–115. doi: 10.1128/jcm.26.1.111-115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. S., Redfield R. R. False-positive Western blot tests for antibodies to HTLV-III. JAMA. 1986 Jul 18;256(3):347–347. [PubMed] [Google Scholar]
- Esteban J. I., Shih J. W., Tai C. C., Bodner A. J., Kay J. W., Alter H. J. Importance of western blot analysis in predicting infectivity of anti-HTLV-III/LAV positive blood. Lancet. 1985 Nov 16;2(8464):1083–1086. doi: 10.1016/s0140-6736(85)90683-x. [DOI] [PubMed] [Google Scholar]
- Fang C. T., Darr F., Kleinman S., Wehling R. H., Dodd R. Y. Relative specificity of enzyme-linked immunosorbent assays for antibodies to human T-cell lymphotrophic virus, type III, and their relationship to Western blotting. Transfusion. 1986 Mar-Apr;26(2):208–209. doi: 10.1046/j.1537-2995.1986.26286152918.x. [DOI] [PubMed] [Google Scholar]
- Hunter J. B., Menitove J. E. HLA antibodies detected by ELISA HTLV-III antibody kits. Lancet. 1985 Aug 17;2(8451):397–397. doi: 10.1016/s0140-6736(85)92544-9. [DOI] [PubMed] [Google Scholar]
- Michail-Merianou V., Tzivaras A., Piperi-Lowes L., Kattamis C., Ladis V., Papadakou-Lagogianni R. False-positive HTLV-III antibody tests in multitransfused patients with thalassaemia. Lancet. 1986 Mar 22;1(8482):678–678. doi: 10.1016/s0140-6736(86)91748-4. [DOI] [PubMed] [Google Scholar]
- Mortimer P. P., Parry J. V., Mortimer J. Y. Which anti-HTLV III/LAV assays for screening and confirmatory testing? Lancet. 1985 Oct 19;2(8460):873–877. doi: 10.1016/s0140-6736(85)90136-9. [DOI] [PubMed] [Google Scholar]
- Näher H., Tilgen W., Tilz G., Petzoldt D. Falsch-positive Ergebnisse beim Nachweis von Anti-LAV/HTLV-III-Antikörpern im Enzymimmunoassay. Hautarzt. 1986 Jun;37(6):338–340. [PubMed] [Google Scholar]
- Quinn T. C., Mann J. M., Curran J. W., Piot P. AIDS in Africa: an epidemiologic paradigm. Science. 1986 Nov 21;234(4779):955–963. doi: 10.1126/science.3022379. [DOI] [PubMed] [Google Scholar]
- Saxinger W. C., Levine P. H., Dean A. G., de Thé G., Lange-Wantzin G., Moghissi J., Laurent F., Hoh M., Sarngadharan M. G., Gallo R. C. Evidence for exposure to HTLV-III in Uganda before 1973. Science. 1985 Mar 1;227(4690):1036–1038. doi: 10.1126/science.2983417. [DOI] [PubMed] [Google Scholar]
- Sayers M. H., Beatty P. G., Hansen J. A. HLA antibodies as a cause of false-positive reactions in screening enzyme immunoassays for antibodies to human T-lymphotropic virus type III. Transfusion. 1986 Jan-Feb;26(1):113–115. doi: 10.1046/j.1537-2995.1986.26186124012.x. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Popovic M., Gilden R. V., Gonda M. A., Sarngadharan M. G., Gallo R. C. Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science. 1984 May 4;224(4648):503–505. doi: 10.1126/science.6200937. [DOI] [PubMed] [Google Scholar]
- Weiss S. H., Mann D. L., Murray C., Popovic M. HLA-DR antibodies and HTLV-III antibody ELISA testing. Lancet. 1985 Jul 20;2(8447):157–157. doi: 10.1016/s0140-6736(85)90261-2. [DOI] [PubMed] [Google Scholar]
- Wendler I., Schneider J., Gras B., Fleming A. F., Hunsmann G., Schmitz H. Seroepidemiology of human immunodeficiency virus in Africa. Br Med J (Clin Res Ed) 1986 Sep 27;293(6550):782–785. doi: 10.1136/bmj.293.6550.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Marzo Veronese F., Copeland T. D., DeVico A. L., Rahman R., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of highly immunogenic p66/p51 as the reverse transcriptase of HTLV-III/LAV. Science. 1986 Mar 14;231(4743):1289–1291. doi: 10.1126/science.2418504. [DOI] [PubMed] [Google Scholar]
- van der Poel C. L., Reesink H. W., Tersmette T., Lelie P. N., Huisman H., Miedema F. Blood donations reactive for HIV in Western blot, but non-infective in culture and recipients of blood. Lancet. 1986 Sep 27;2(8509):752–753. doi: 10.1016/s0140-6736(86)90273-4. [DOI] [PubMed] [Google Scholar]