Abstract
Acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1) is one of the four intestinal membrane bound acyltransferases implicated in dietary fat absorption. Recently, it was found that, in addition to acylating diacylglycerol (DAG), DGAT1 also possesses robust enzymatic activity for acylating monoacylglycerol (MAG) (Yen, C. L., Monetti, M., Burri, B. J., and Farese, R. V., Jr. (2005) J. Lipid Res. 46, 1502–1511). In the current paper, we have conducted a detailed characterization of this reaction in test tube, intact cell culture, and animal models. Enzymatically, we found that triacylglycerol (TAG) synthesis from MAG by DGAT1 does not behave according to classic Michaelis-Menten kinetics. At low concentrations of 2-MAG (<50 μm), the major acylation product by DGAT1 was TAG; however, increased concentrations of 2-MAG (50–200 μm) resulted in decreased TAG formation. This unique product/substrate relationship is similar to MGAT3 but distinct from DGAT2 and MGAT2. We have also found that XP620 is an inhibitor that selectively inhibits the acylation of MAG by DGAT1 (IC50 of human DGAT1: 16.6 ± 4.0 nm (MAG as substrate) and 1499 ± 318 nm (DAG as substrate); IC50 values of human DGAT2, MGAT2, and MGAT3 are >30,000 nm). Using this pharmacological tool, we have shown that ∼76 and ∼89% of the in vitro TAG synthesis initiated from MAG is mediated by DGAT1 in Caco-2 cell and rat intestinal mucosal membranes, respectively. When applied to intact cultured cells, XP620 substantially decreased but did not abolish apoB secretion in differentiated Caco-2 cells. It also decreased TAG and DAG syntheses in primary enterocytes. Last, when delivered orally to rats, XP620 decreased absorption of orally administered lipids by ∼50%. Based on these data, we conclude that the acylation of acylglycerols by DGAT1 is important for dietary fat absorption in the intestine.
Dietary fat absorption in mammals is critical for growth and development. In the small intestine, dietary triacylglycerol (TAG)2 is first hydrolyzed by pancreatic lipases into free fatty acid and 2-monoacylglycerol (MAG) that are readily taken up by the enterocytes. Upon appearance in the enterocytes, 2-MAG is first acylated by acyl coenzyme A:monoacylglycerol (MGAT) to form diacylglycerol (DAG); DAG is further acylated by acyl coenzyme A:diacylglycerol acyltransferase (DGAT) to re-synthesize TAG. TAG molecules are then packaged with other lipids, such as cholesteryl ester, retinyl ester, and phospholipids to form chylomicron lipoprotein particles, which are ultimately secreted into the lymph to serve as an energy source for the whole body (1–3).
Recently, multiple membrane-bound acyltransferases have been identified, which are implicated in the sequential MAG and DAG acylation reactions in the gut. Chronologically, DGAT1 was the first enzyme identified (4). It belongs to the acyl coenzyme A:cholesterol acyltransferase gene family (5), which possesses up to nine transmembrane domains (6). A detailed analysis of DGAT1 knock-out mice, which have been shown to be resistant to a high fat diet-induced obesity (7), revealed that DGAT1 deletion caused a substantial decrease, but not a total ablation, of chylomicron formation following an acute challenge of orally administered fat (8). Subsequently, DGAT2 and its homologues were identified as a seven-member gene family (9). Although DGAT1 and DGAT2 are capable of catalyzing the same DGAT enzyme reaction, they bear little sequence resemblance. In addition, a recent topology study indicates that DGAT2 has only two transmembrane domains, with the bulk of the protein facing the cytosol (10). In the DGAT2 gene family, three members were found to acylate MAG and, as a result, were called MGAT1, -2, and -3, respectively (11–14). MGAT1 is not expressed in the gut and therefore is unlikely to be involved in fat absorption (11). In contrast, MGAT2 and -3 are highly expressed in the small intestine and have been proposed to contribute to fat absorption (12, 14, 15). Interestingly, MGAT2 is expressed in both rodents and humans, whereas human MGAT3 does not have a rodent ortholog. Taken together, among the acyltransferases identified to date, DGAT1 (4), DGAT2 (9), MGAT2 (12, 13), and MGAT3 (14) are the four genes that are expressed in the gut and are therefore implicated in the monoacylglycerol pathway for the sequential acylation of MAG and DAG to form TAG in the small intestine.
Despite its original nomenclature, in addition to DAG, DGAT1 has recently been shown to also acylate MAG (16). In the current study, we found that XP620, a previously described DGAT1-selective inhibitor (17), can potently and selectively inhibit the acylation of MAG by DGAT1. This unique pharmacology tool allowed us to discern the importance of DGAT1-mediated acylation of acylglycerols in culture models and its contribution toward fat absorption in the whole animal.
EXPERIMENTAL PROCEDURES
Materials—Monoclonal anti-FLAG® M2 antibody, oleoyl coenzyme A, sn-2-monooleoylglycerol (2-MOG), 1,2-dioleoyl-sn-glycerol (1,2-DOG), and cholecystokinin-8 (CCK-8) were obtained from Sigma; the Bac-to-Bac® baculovirus expression system, Sf9, and High Five™ insect cells were obtained from Invitrogen; and a Caco-2 (human colon carcinoma) cell line was obtained from the American Type Culture Collection (ATCC). XP620 was synthesized by our colleagues in the Department of Medicinal Chemistry at Bristol-Myers Squibb Co.
DGAT and MGAT Assays—The expression of recombinant human DGAT1, DGAT2, MGAT2, and MGAT3 and the procedure for the membrane isolation were conducted according to methods described previously (14, 18). The DGAT/MGAT assays were based on a TLC method that was described previously (14), with slight modifications. For recombinant enzymes, each reaction contained 30 μg of membrane proteins in Assay Buffer 1 (100 mm potassium phosphate, pH 7.4), 50μm [14C]oleoyl coenzyme A, and 100 μm or other indicated concentrations of sn-2-monoacylglycerol or 1,2-dioleoyl-sn-glycerol (delivered in acetone, final acetone concentration <2%). For membranes of Caco-2 cells and rat intestinal mucosa, the reactions were carried out with the indicated amount of proteins in Assay Buffer 2 (150 mm potassium phosphate, pH 7.4, 5 mm dithiothreitol, 0.75 mg/ml bovine serum albumin), 50μm [14C]oleoyl coenzyme A, and 50μm or other indicated concentrations of sn-2-monoacylglycerol or 1,2-dioleoyl-sn-glycerol. The reactions were carried out at 37 °C for 10 min. After the reaction, the lipids were extracted by the termination solution of 4 ml of chloroform/methanol (2:1, v/v), dried, and separated by TLC with hexane/ethyl ether/acetic acid (85:15:0.5, v/v/v). Identities of TAG, DAG, MAG, free fatty acid, and other lipids were verified with lipid standards (Sigma) after staining with iodine vapor. The resultant chromatograms were analyzed using a STORM PhosphorImager. The quantification of final products in the TAG and DAG bands as the result of enzyme reactions was determined by the quantification of incorporation of [14C]oleoyl coenzyme A (20,000 dpm/nmol). For IC50 determinations, DGAT/MGAT assays were carried out using a SPA assay as described.3 Briefly, In each assay, 200 ng of recombinant DGAT or MGAT membrane preparation was incubated with 90 μm 1,2-dioleoyl-sn-glycerol or 15 μm sn-2-monoacylglycerol as acyl acceptor and 15 μm [3H]oleoyl-CoA as acyl donor in 100 mm potassium phosphate (pH 7.4) for 20 min with various concentrations of compound prepared in DMSO (final concentration at 1.6%) in a total volume of 30 μl. The assay was terminated with the addition of 20 μl of stopping solution (7.5 mg/ml yttrium oxide polylysine SPA beads, 3.3 mg/ml fraction V bovine serum albumin, and 200 μm mercuric chloride in 50 mm HEPES, pH 7.4). The signal was measured 1 h after quenching the reaction in a Gen IV LEADseeker™ imaging system for 5 min per 384-well microplate. The IC50 for a compound was calculated using a logistic 4 parameter fit equation, y = A + ((B – A)/(1 + ((C/x)D))) in XL-fit.
Microsomal Triglyceride Transfer Protein (MTP) Activity Assay—MTP activity was determined as described by Athar et al. (19). Briefly, different indicated concentrations of XP620 were incubated for 30 min with 1 μg of purified MTP using an MTP transfer activity assay kit (Chylo's Inc.). The known MTP inhibitor BMS-200150 was used as a positive control (20).
Studies with Cells—Caco-2 cells were cultured (75-cm2 flasks, Corning Glass) in Dulbecco's modified Eagle's medium containing high glucose supplemented with l-glutamine and antibiotic/antimycotic mixture (Dulbecco's modified Eagle's medium) and 10% fetal bovine serum as described before (21–24). For experiments, cells from 70–80% confluent flasks were plated on polycarbonate micropore membrane inserts (Transwells®, 6-well plate, 24-mm diameter, 3-μm pore size; Corning Costar Corp., Cambridge, MA) at a density of 1 × 105 cells/cm2. To induce differentiation, media were changed every other day for 14 days (21–24). Experiments were conducted by incubating cells in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and oleic acid/taurocholate (1.6:0.5 mm) in the absence or presence of XP620 (50 and 100 μm concentrations) on the apical side and 2 ml of serum-free medium on the basolateral side for 17 h. Basolateral conditioned media (1.6 ml) were subjected to sequential density gradient ultracentrifugation (see below), and the rest was used for the measurement of apolipoproteins.
Isolation of primary Enterocytes—Primary enterocytes were isolated from control mice as described previously (24–26). Briefly, overnight-fasted mice were anesthetized, and small intestines were removed; washed with 117 mm NaCl, 5.4 mm KCl, 0.96 mm NaH2PO4, 26.2 mm NaHCO3, and 5.5 mm glucose; filled with 67.5 mm NaCl, 1.5 mm KCl, 0.96 mm NaH2PO4, 26.2 mm NaHCO3, 27 mm sodium citrate, and 5.5 mm glucose; and bathed in oxygenated saline at 37 °C for 10 min. The buffer was discarded, and intestinal lumens were refilled with 67.5 mm NaCl, 1.5 mm KCl, 0.96 mm NaH2PO4, 26.2 mm NaHCO3, 27 mm sodium citrate, 5.5 mm glucose, 1.5 mm EDTA, and 0.5 mm dithiothreitol and incubated in 0.9% sodium chloride solution at 37 °C for 10 min. Lumenal contents were collected and centrifuged at 1,500 rpm for 5 min. All buffers were adjusted to pH 7.4, gassed with 95% O2, 5% CO2 for 20 min, and maintained at 37 °C prior to use. Enterocytes were resuspended in 4 ml of Dulbecco's modified Eagle's medium and incubated with micelles containing 1 μCi/ml of [3H]oleic acid, 0.14 mm sodium cholate, 0.15 mm sodium deoxycholate, 0.17 mm phosphatidylcholine, 1.2 mm oleic acid, and 0.19 mm monopalmitoylglycerol in the absence or presence of XP620 (100 and 200 μm concentrations) at 37 °C in a cell culture incubator with 5% CO2. Cells were gassed at 15-min intervals for 1 min with 95% O2, 5% CO2. After 3 h, enterocytes were centrifuged (3,000 rpm, 5 min), and conditioned media were collected. One ml of condition medium was used to extract total lipids, and the remaining 3 ml of media were subjected to density gradient ultracentrifugation as described below. The top three fractions containing apoB-containing lipoproteins and the bottom three fractions containing apoA1-containing lipoproteins were pooled together and used to extract lipids. Cell pellets were incubated overnight at 4 °C with 1 ml of isopropyl alcohol to isolate total lipids. After lipid extraction, 1 ml of 0.1 n NaOH was added to dissolve proteins. Proteins were measured by the Bradford method, using Coomassie reagent (Pierce).
Density Gradient Ultracentrifugation—Variable amounts of the conditioned media were brought to 4 ml with 1.006 g/ml density solution in SW41 ultracentrifuge tubes (21–24, 26, 27). To adjust the density to 1.12 g/ml, 0.565 g of KBr were added and dissolved by repeated pipetting. Media were sequentially overlaid with 3 ml each of 1.063 and 1.019 g/ml and 2 ml of 1.006 g/ml density solutions. The tubes were subjected to ultracentrifugation (SW41 rotor, 40,000 rpm, 33 min, 15 °C), and the top 1 ml represents large chylomicrons (Sf > 400). The gradients were then overlaid with 1 ml of 1.006 g/ml solution and centrifuged (40,000 rpm, 3 h and 30 min, 15 °C), and the top 1 ml was collected (fraction 2, small chylomicrons (CMs)). The tubes were replenished with 1.006 g/ml solutions and centrifuged (40,000 rpm, 15 °C, 17 h), and the top 1 ml was collected by aspiration. This fraction 3 represents the VLDL size particles (CMVLDL). The rest of the contents were fractionated into 1.5-ml fractions. ApoB and apoA1 were measured in each fraction in triplicate.
Extraction and Analysis of Lipids—Lipids were extracted from the media and fractions according to the method of Bligh and Dyer (28). To 1 ml of media or fractions was added 3.75 ml of chloroform/methanol (1:2, v/v), and these were mixed and incubated at room temperature with intermittent mixing. After 15 min, 1.25 ml of chloroform was added, mixed, and incubated for 1 min. Subsequently, 1.25 ml of water was added; the contents were thoroughly mixed and centrifuged (10 min, 5,000 rpm); and the lower phase was collected with a Pasteur pipette. The leftover upper phase was extracted again with 1.25 ml of chloroform and combined with the earlier extract. Organic extracts from cells, media, and fractions were dried under nitrogen, dissolved in 100 μl of chloroform, and separated by TLC on PE SIL G Silica gel plates (Whatman) using hexane/diethyl ether/glacial acetic acid (82:17:2, v/v/v). Bands corresponding to TAG, DAG, MAG, free fatty acid, and other lipids (phospholipids (PL) + MAG), verified with lipid standards (Sigma) after staining with iodine vapor, were scraped and counted by liquid scintillation counting.
Oral Lipid Tolerance Test—Sprague-Dawley rats (300–350 g) were obtained from Charles River Laboratories with indwelling jugular vein catheters implanted by the supplier. The animals were habituated to handling for several days before use. Before the study, the rats were fasted for ∼16 h. On the day of the study, the distal ends of the catheters were exteriorized under isoflurane anesthesia. A base-line serum sample was obtained. The rats were allowed to recover from anesthesia before compound was given. Compounds were administered orally in 0.5% Methocel A4M Prem, 0.1% Tween 80 at 3 ml/kg. Doses of XP620 used were 10 and 30 mg/kg. Orlistat (8 mg/kg) was used as a positive control. Thirty min after compound administration, all animals received Intralipid® (30% fat; Abbott Laboratories) at 2 g/kg. Blood was collected into serum separators 1, 2, 4, and 6 h after Intralipid administration. TAG concentration (corrected for glycerol) of the serum was measured on a Roche Applied Science-Hitachi 917 Chemistry Auto-analyzer using Roche Applied Science TAG/GB reagent.
Gastric Emptying Assay—Male Sprague-Dawley rats (300–325 g) were obtained from Charles River Laboratories and were maintained on ad libitum laboratory chow. At the start of the experiment the rats were deprived of food for ∼16 h. One h before the test meal, the rats were deprived of water. Thirty min prior to the test meal, the rats were dosed by oral gavage either with vehicle (0.5% Methocel A4M Prem, 0.1% Tween 80 at 3 ml/kg) or with XP620 in the vehicle at 10 or 30 mg/kg or CCK-8 (5 μg/kg, in saline). CCK-8 was administered as an intraperitoneal injection. Thirty min. after dosing, 3 ml of a semisolid test meal (10% charcoal, 5% gum arabic, and 1% carboxymethylcellulose) was administered via oral gavage. Sixty min after the test meal, the rats were euthanized with CO2, and a rapid laparotomy was performed. The pylorus and the esophagus were clamped, and the stomach was removed and weighed. Next an incision was made along the greater curvature of the stomach, and the stomach itself was emptied, turned inside-out, rinsed, dried, and reweighed. The difference between the weight of the full stomach and the emptied stomach was recorded as the amount of the test meal remaining.
RESULTS
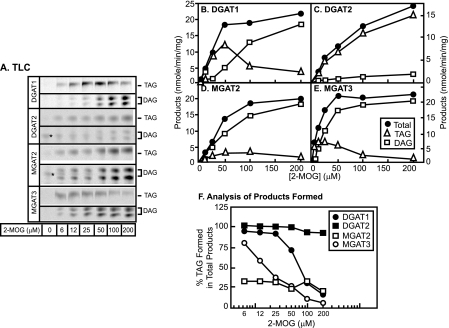
Fig. 1A illustrates the relative expression levels of the FLAG epitope tag fused to the NH2 termini of DGAT1, DGAT2, MGAT2, and MGAT3. When 1,2-DOG was used as the substrate, recombinant DGAT1, DGAT2, MGAT2, and MGAT3 exhibited specific activities of 4.88 ± 0.32, 2.35 ± 0.25, 1.01 ± 0.06, and 1.02 ± 0.03 nmol/min/mg protein, respectively, for the synthesis of TAG. These values are 11.0-, 5.2-, 2.2-, and 2.2-fold over the background levels as determined by the insect cell membrane that is mock-infected with the wild type baculovirus. When 2-MOG was used as the substrate, both DAG and TAG were generated as the enzymatic products. DGAT1, DGAT2, MGAT2, and MGAT3 exhibited specific activities of 11.2 ± 2.0, 17.4 ± 0.4, 7.4 ± 0.8, and 5.7 ± 1.8 nmol/min/mg protein for the synthesis of TAG and 4.5 ± 1.6, 0.8 ± 0.1, 11.7 ± 3.3, and 13.9 ± 0.8 nmol/min/mg protein for the synthesis of DAG, respectively. Because only 2-MOG was presented as the substrate, TAG product was the result of further acylation of DAG.
FIGURE 1.
A, immunoblot analysis of recombinant human DGAT1, DGAT2, MGAT2, and MGAT3. The full-length open reading frames of human DGAT1, DGAT2, MGAT2, and MGAT3 were fused in frame with the FLAG epitope tag at the NH2 terminus and expressed in the baculovirus expression system. Hi5 cells were infected with either wild type (WT) or higher titer recombinant virus for 48 h. Aliquots of 10 μg of membrane extracts were loaded for SDS-PAGE and immunoblot analysis with anti-FLAG IgG. B, TLC enzyme assays. Aliquots of 30-μg membrane extracts derived from Hi5 cells infected with wild type or recombinant baculovirus were subjected to the acyltransferase reactions for 10 min at 37 °C as previously described (14). One hundred μm 1,2-dioleoylglycerol or 2-monooleoylglycerol were used as the acyl acceptors, and 50 μm [14C]oleoyl coenzyme A was used as the acyl donor. FFA, free fatty acid band. C, quantitative analysis. Data shown are representative of four separate experiments. Points are means of duplicate determinations.
Of note, despite the fact that the total enzymatic products generated by these four enzymes using 2-MOG were similar (all ranging from 15 to 20 nmol/min/mg (Fig. 1C)), the relative proportions of TAG and DAG generated exhibited marked differences. Of the total products, DGAT1, DGAT2, MGAT2, and MGAT3 produced 71, 96, 39, and 29% TAG, respectively. It appears that DGAT2 has the biggest preference for generating TAG, whereas MGAT2 and MGAT3 preferentially generate DAG. DGAT1 appears to be between these two extremes. This result prompted us to investigate the detailed enzymatic behavior, by conducting a 2-MOG substrate titration experiment (Fig. 2). For all four enzymes, the total product (TAG + DAG) formation as a function of substrate 2-MOG concentration exhibited a classic Michaelis-Menten relationship (Fig. 2, B–E). However, the detailed analyses revealed different patterns of preference for TAG and DAG formation by DGAT1, DGAT2, MGAT2, and MGAT3 with different concentrations of 2-MOG (Fig. 2, A–E). For DGAT1, TAG formation exhibited a bellshaped curve with the maximum at 50 μm; in contrast, DAG formation exhibited a hyperbolic curve (Fig. 2B). For DGAT2 and MGAT2, both TAG and DAG formation exhibited hyperbolic saturation curves (Fig. 2, C and D). Notably, regardless of the concentration of 2-MOG, the majority of the product formed by DGAT2 was TAG, whereas the majority for MGAT2 was DAG. Last, for MGAT3 (Fig. 2E), TAG is the major product formed at low concentrations of 2-MOG, where high concentrations of 2-MOG DAG lead to DAG as the predominant product.
FIGURE 2.
2-Monooleoylglycerol concentration-dependent acylation by recombinant DGAT1, DGAT2, MGAT2, and MGAT3. Various concentrations of 2-MOG (from 0 to 200 μm) were provided for the enzyme assays that were conducted as described in the legend to Fig. 1. A, TAG and DAG bands captured from PhosphorImager plate. *, an unknown band that is not dependent on the exogenous 2-MOG substrate. B–E, the quantitative values (means of duplicate determinations) derived from experiments represented in A were plotted against the concentration of 2-MOG. F, analysis of percentage of TAG and DAG formed as a result of acylation of 2-MOG.
In order to illustrate the preference of product formation in a quantitative manner, in Fig. 2F, the percentage of TAG formed was plotted against 2-MOG substrate concentration. Regardless of the 2-MOG concentration, DGAT2 had a constitutively high preference (>95%) for the formation of TAG; MGAT2 had a constitutively low preference of TAG formation (<30%). DGAT1 and MGAT3 exhibited a different scenario. At low concentrations of 2-MOG, the majority of the product formed by both enzymes is TAG; however, when the concentration of 2-MOG increased, the proportion of TAG produced decreased. The switching point for the change of product preference appears distinct in MGAT3 and DGAT1. For MGAT3, the decline of percentage of TAG formation happens at a very low concentration of 2-MOG (at ∼10–50 μm), whereas for DGAT1, the decline of TAG formation occurred at higher concentrations of 2-MOG (at ∼50–200 μm).
We previously described that XP620, a dihydrothiopyrancarboxamide, possesses DGAT1-inhibitory activity (17). We examined if XP620 would inhibit the DGAT1 activity when 2-MOG is used as the substrate. Interestingly, XP620 had a higher inhibitory activity when DGAT1 was assayed with 2-MOG than with 1,2-DOG (Table 1). When 2-MOG was used as the substrate, the IC50 values were determined to be 16.6 ± 4.0, 2.9 ± 0.5, and 2.7 ± 0.9 nm for recombinant human, rat, and mouse DGAT1, respectively. However, when 1,2-DOG was used as the substrate, the IC50 values were determined to be 1499 ± 318, 176 ± 40, and 123 ± 4 nm for human, rat and mouse DGAT1, respectively. In all three species of DGAT1 recombinant enzymes, there is a ∼45–90-fold shift of IC50 values when 2-MOG and 1,2-DOG are used as the substrates. These data indicate that XP620 preferentially inhibits the acylation of MAG by DGAT1.
TABLE 1.
Potency of XP620 against various intestinal acyltransferases
|
Substrate
|
XP620 IC50
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
DGAT1
|
DGAT2
|
MGAT2
|
MGAT3
|
||||||||
| Human | Rat | Mouse | |||||||||
| nm | |||||||||||
| 1,2-DOG | 1499 ± 318 | 176 ± 40 | 123 ± 4 | >30,000 | NAa | NA | |||||
| 2-MOG | 16.6 ± 4.0 | 2.9 ± 0.5 | 2.7 ± 0.9 | >30,000 | >30,000 | >30,000 | |||||
| 1-Hexadecanol | 23.3 | 1.4 | |||||||||
NA, not available.
Yen et al. (16) described that DGAT1 can also catalyze the esterification of hexadecanol for the formation of wax esters. We hypothesize that XP620 may possess the ability to discriminate substrates with single or two long acyl chains. If this were the case, we would predict that the XP620 IC50 values against DGAT1 when using hexadecanol (which contains a single long chain as the substrate) would be closer to 2-MOG than 1,2-DOG. Indeed, the IC50 values against human and mouse DGAT1 were determined to be 23.3 and 1.4 nm when 1-hexadecanol was used as the substrate (Table 1). These values are much closer to those when 2-MOG was used as the substrate.
Last, we also examined the specificity of XP620 with respect to the other acyltransferases. Consistent with our earlier results (17), XP620 did not show inhibitory activities against DGAT2, MGAT2, and MGAT3.
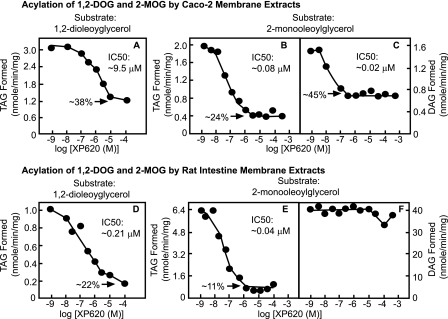
The unique characteristics of XP620 in differentially inhibiting the acylation of MAG and DAG by DGAT1 and its exquisite specificity versus DGAT2, MGAT2, and MGAT3 provide us with a unique opportunity to explore the importance of DGAT1 in the monoacylglycerol pathway. We first assessed the ability of XP620 to inhibit the in vitro 1,2-DAG acylation activity using membrane extracts derived from human Caco-2 cells (Fig. 3A) or rat intestinal mucosa (Fig. 3D). In Caco-2 membranes, XP620 only partially inhibited 1,2-DOG acylation, with an IC50 of ∼9.5 μm; at 100 μm, XP620 inhibited ∼62% of activity (Fig. 3A). In contrast, in rat intestinal membrane extracts, XP620 exhibited greater potency, with an IC50 of ∼0.21 μm; at ∼100 μm, it also achieved ∼78% inhibition (Fig. 3D). The potency increase from human Caco-2 to rat intestinal membrane extracts is consistent with the higher potency of XP620 found for recombinant rodent DGAT1 than the human counterpart (Table 1). Furthermore, the fact that the majority of the intestinal membrane 1,2-DAG acylation activity can be inhibited by XP620 is consistent with genetic data, in which DGAT1 gene deletion ablated the majority but not all of the intestinal in vitro 1,2-DAG acylation activity (7). These data, therefore, validate the current pharmacological approach of using XP620 to quantify the DGAT1 contribution in the overall membrane acyltransferase activity in vitro.
FIGURE 3.
Effects of XP620 on the in vitro acylation of 1,2-DOG and 2-MOG by Caco-2 and rat intestinal membrane extracts. Membrane fractions derived from Caco-2 cells differentiated for 2 weeks (A–C) or rat intestinal mucosal membranes (D–F) were used for enzymatic assays for acylation of 1,2-DOG (A and D) and 2-MOG (B, C, E, and F). Aliquots of 30 μg of Caco-2 cells or 5 μg of rat intestinal mucosal membrane proteins were incubated with 50 μm 1,2-DOG or 2-MOG and 50 μm [14C]oleoyl-CoA for 10 min in Assay Buffer 2 in the absence or presence of the indicated concentrations of XP620. The reactions were stopped by chloroform/methanol (2:1, v/v), and the organic extracts were subjected to TLC analysis as described under “Experimental Procedures.” The specific activities of 14C incorporated into the DAG and TAG bands were calculated as nmol/min/mg. Results shown are representative of three independent experiments with similar results. All points are means of duplicate determinations.
We next evaluated the effect of XP620 on the acylation of 2-MAG using membrane extracts from human Caco-2 (Fig. 3, B and C) and rat intestinal mucosa (Fig. 3, E and F). In Fig. 3, B and E, XP620 caused dose-dependent inhibition of TAG formation using both enzyme sources. For Caco-2 membrane, the maximal level of inhibition of TAG formed was ∼76%. The IC50 value for the inhibition of TAG was determined to be ∼0.08 μm. Consistent with this finding, for rat intestinal membrane, the maximal level inhibition was determined to be ∼89%, and the IC50 value for the inhibition of TAG was found to be ∼0.04 μm. Of note, for both cases when 2-MOG was used as substrate, the potencies of inhibiting the TAG formation were increased dramatically from those when 1,2-DOG was used as the substrate, consistent with the data obtained from recombinant DGAT1 (Table 1). These studies indicate that the majority of in vitro synthesis of TAG initiated with 2-MAG in Caco-2 cells and rat enterocytes may be attributed to DGAT1.
In contrast to its effect on TAG synthesis, XP620 was found to be less effective at inhibiting DAG synthesis (Fig. 3, C and F). In Caco-2 membrane, XP620 only inhibited ∼55% of DAG formation, whereas in rat intestinal membrane, at a concentration as high as 1 mm, XP620 did not have an inhibitory effect on the synthesis of DAG from 2-MOG. These results indicate that DGAT1 may contribute to approximately half of the in vitro 2-MAG acylation for the DAG synthesis in Caco-2 cell membrane, but it may contribute very little for the in vitro 2-MAG acylation for the synthesis of DAG in rat intestinal mucosa membrane.
In order to further investigate if species difference would cause variations in the acylglycerol acylations in vitro, we isolated mouse intestinal membrane and tested its acyltransferase sensitivities toward XP620. The results are consistent with the rat intestinal data as shown in Fig. 3, D–F. When 2-MOG was used as the substrate, the XP620 IC50 value in inhibiting the TAG formation was ∼0.03 μm, whereas there was no measurable inhibition toward the DAG formation; when 1,2-DOG was used as the substrate, the IC50 value for XP620 to inhibit TAG formation was determined to be ∼0.34 μm.
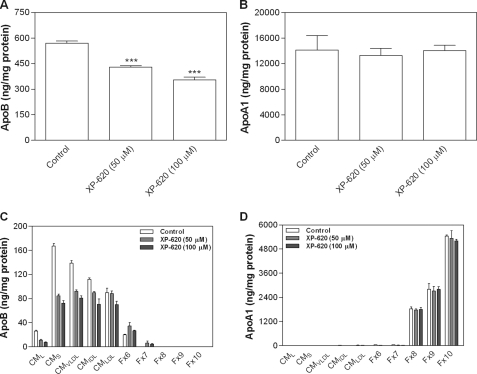
We then investigated the effect of XP620 on lipoprotein biosynthesis in intestinal cells as a way to determine the importance of TAG synthesis by DGAT1 in TAG-rich lipoprotein assembly. Treatment of differentiated Caco-2 cells with XP620 resulted in a significant decrease in the secretion of apoB-containing lipoproteins (Fig. 4A) without having any effect on apoAI secretion (Fig. 4B). Next, we separated the lipoproteins by density gradient ultracentrifugation and found that XP620 decreased the secretion of larger apoB particles (Fig. 4C). Again, there was no change in the secretion of apoAI particles (Fig. 4D).
FIGURE 4.
Inhibition of large apoB-containing lipoprotein secretion. Caco-2 cells were differentiated as described under “Experimental Procedures” and treated with either 50 or 100 μm XP620 for 17 h. The basolateral conditioned media were used to measure total apoB (A) and apoA1 (B) lipoproteins by enzyme-linked immunosorbent assay (35, 36). Conditioned media were also subjected to density gradient ultracentrifugation, and fractions were used to determine apoB (C) and apoA1 (D) lipoprotein levels. Data shown are the means ± S.D. of triplicate determinations. ***, p < 0.001 as compared with control.
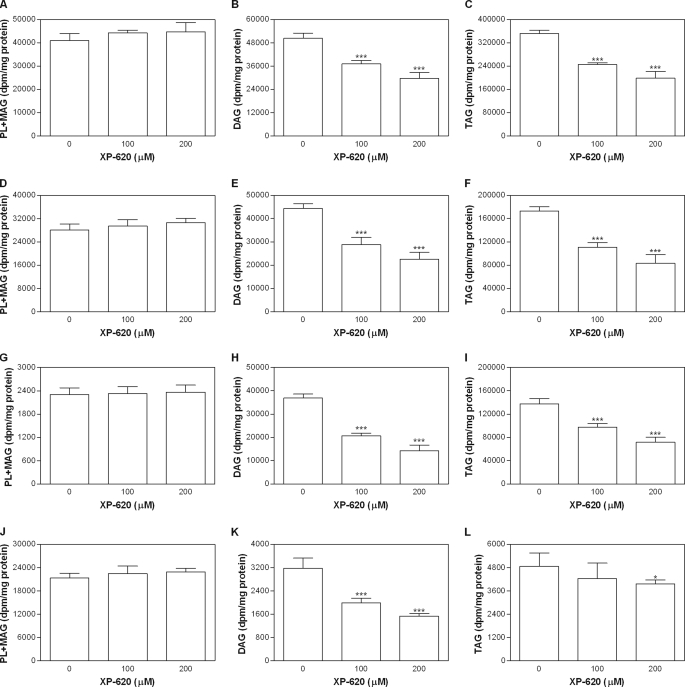
To determine whether XP620 affects the synthesis and secretion of lipids in the mice primary enterocytes, we labeled the enterocytes with [3H]oleic acid in the presence of XP620 and then determined the levels of lipids in the cells and conditioned media. Incubation with XP620 had no effect on the synthesis of PL in the mice primary enterocytes (Fig. 5A). However, there was a significant decrease in the cellular levels of DAG (Fig. 5B) and TAG (Fig. 5C) in the presence of XP620. Next, we examined secreted lipids. Secretion of phospholipids was not inhibited by XP620 treatment (Fig. 5D), but XP620 significantly reduced the secretion of DAG (Fig. 5E) and TAG (Fig. 5F). The lipoproteins were then separated by density gradient ultracentrifugation by pooling the first three fractions (apoB lipoproteins) and last three fractions (non-apoB lipoproteins). Lipids were isolated from the pooled fractions and separated on a TLC plate. There was no change in secretion of PLs with apoB-containing lipoproteins (Fig. 5G). However, XP620 significantly decreased the secretion of DAG (Fig. 5H) and TAG (Fig. 5I) with apoB-containing lipoproteins. As expected, most of the secreted PLs were associated with apoAI-containing lipoproteins, and treatment with XP620 had no effect on their secretion (Fig. 5J). ApoAI lipoproteins contain very small amounts of DAG and TAG compared with apoB lipoproteins, but still there was a decrease in the secretion of DAG (Fig. 5K). TAG in the apoAI lipoproteins was decreased only at the higher concentration of XP620 used (Fig. 5L). These data indicate that XP620 specifically inhibits the secretion of DAG and TAG as part of chylomicrons in cultured enterocytes. We interpret these data to suggest that DGAT1 is a major contributor of TAG during the assembly and secretion of larger chylomicron particles.
FIGURE 5.
Inhibition of DAG and TAG synthesis by XP620 in mouse primary enterocytes. Mouse primary enterocytes were isolated as described under “Experimental Procedures” and incubated with micelles containing 1 μCi/ml [3H]oleic acid in the absence or presence of either 100 or 200 μm XP620 for 3 h. Cellular lipids were extracted by adding isopropyl alcohol and separated by TLC (A, PL + MAG; B, DAG; C, TAG). The conditioned media were used either to extract lipids and separated on TLC (D, PL + MAG; E, DAG; F, TAG) or subjected to density gradient ultracentrifugation to separate lipoproteins. The top three fractions (apoB-containing lipoproteins PL + MAG (G), DAG (H), and TAG (I)) and bottom three fractions (non-apoB-containing lipoproteins PL + MAG (J), DAG (K), and TAG (L)) were pooled. Lipids were extracted from pooled fractions and subjected to TLC analysis. Data shown are the means ± S.D. of triplicate determinations. *, p < 0.05; ***, p < 0.001 as compared with control.
Last, we administered XP620 to rats orally and examined its potential to inhibit dietary fat absorption in the oral lipid tolerance test. In this experiment, rats were fasted overnight. Thirty min after administration of XP620, a bolus of lipid (Intralipid) was gavaged, and the appearance of TAG in the serum was used as an indication of intestinal absorption. In the same experiment, orlistat, a pancreatic lipase inhibitor, which is known to inhibit dietary fat absorption, was used as a control. As shown in Fig. 6, upon the bolus challenge of oral lipid, there was a robust rise of TAG in the serum at the 1 h time point. This level of TAG was maintained after an additional 3 h. At 6 h, however, there was a significant drop in plasma TAG. XP620, at both 10 and 30 mg/kg, significantly blocked the appearance of the TAG at both the 1 and 2 h time points (p < 0.05 versus vehicle). However, TAG levels started to rise at 4 h and remained high at 6 h. We interpret these data to suggest that XP620 reduced the initial rapid phase of lipid absorption but allowed a low absorption over a longer period. Orlistat caused a significant (p < 0.05 versus vehicle) decrease in TAG concentration at 2, 4, and 6 h. The area under the curve for serum TAG was significantly decreased versus vehicle for XP620 at 30 mg/kg (p = 0.0209) and for orlistat (p = 0.0018). Taken together, these data indicate that DGAT1 inhibition delays and decreases but does not abolish dietary fat absorption.
FIGURE 6.
A and B, oral lipid tolerance test in rats. The study was conducted as described under “Experimental Procedures.” The time course (A) and area under the curve (AUC)(B) of the initial 6 h of TAG detected in the serum was plotted. For each group, n = 6. Data shown are means ± S.E. *, p < 0.05 as compared with vehicle control. C, gastric emptying assay in rats. The study was conducted as detailed under “Experimental Procedures.” XP620 at 10 and 30 mg/kg did not affect gastric emptying, whereas 5 μg of CCK-8 significantly reduced emptying of the test meal. For each group, n = 10. Data shown are means ± S.E. **, p < 0.01 as compared with vehicle control. D, MTP in vitro assay. Data shown are means ± S.D. of triplicate determinations.
Given the delayed and decreased fat absorption we observed in the oral lipid tolerance test, we sought to determine if XP620 delayed gastric emptying, which would delay the appearance of TAG in the intestine, the primary site of absorption. In this experiment, rats were fasted overnight. Thirty min after administration of vehicle or XP620, 3 ml of a nonnutritive test meal was administered. Sixty min later, stomachs were removed, and their contents were weighed to determine the amount of the test meal that had been emptied. In the same experiment, CCK-8, a well documented inhibitor of gastric emptying (29), was used as a positive control. It is important to note that the 60 min time point matches a time point in the oral lipid tolerance test at which XP620 significantly reduced the appearance of TAG in the blood.
As shown in Fig. 6C, vehicle-treated rats had about 0.45 g of the test meal remaining in the stomach at the time of sacrifice. XP620 produced results similar to vehicle; the 10 mg/kg group had about 0.54 g remaining, whereas the 30 mg/kg group had about 0.43 g remaining. In contrast, the CCK-8-treated group had an average of 0.87 g remaining in the stomach. Only the CCK-8-treated group significantly differed from the vehicletreated group (p < 0.01). These data suggest that the reduced/delayed absorption of TAG observed in the oral lipid tolerance test was not due to reduced gastric emptying.
The decreased TAG serum appearance in the oral lipid tolerance test could be due to the effect of XP620 on other enzymes involved in the fat absorption pathway, such as MTP. To test this possibility directly, we assessed the effect of XP620 in an in vitro MTP activity assay. As shown in Fig. 6D, in contrast to BMS-200150, a known MTP inhibitor (20), XP620 did not have an effect on MTP at concentrations up to 100 μm.
DISCUSSION
In the current study, we characterized the enzymatic acylation of MAG by DGAT1 and compared its characteristics with the other three intestinal acyltransferases DGAT2, MGAT2, and MGAT3. All four of these enzymes are capable of catalyzing both acylation steps that lead to the synthesis of TAG from MAG (Figs. 1 and 2). Therefore, the substrate recognition sites of these four enzymes all possess the flexibility to accommodate both MAG and DAG. Unexpectedly, we found that TAG formation by DGAT1 and MGAT3 does not follow a Michaelis-Menten relationship when 2-MAG is used as a substrate. These two enzymes mainly synthesize TAG at low 2-MAG concentrations. AT high 2-MAG concentrations, TAG synthesis was reduced (Fig. 2). In contrast, the respective proportions of TAG formed by DGAT2 and MGAT2 were maintained at a similar level regardless of the 2-MAG concentration (Fig. 2). The difference among the latter two appears to be that DGAT2 prefers to synthesize TAG and MGAT2 prefers to synthesize DAG.
The simplest explanation of this observation might be that the rank order of the relative affinity toward DAG and MAG (which could be best described by the ratio of the affinityDAG/affinityMAG) is DGAT2 > DGAT1 > MGAT3 > MGAT2. According to this model, MAG and DAG may compete for the same active site. For DGAT2, when affinityDAG/affinityMAG is extremely high, DAG, once formed, is further acylated regardless of the 2-MAG concentration (Fig. 2, C and F). This is because, according to the model, 2-MAG does not possess enough affinity to compete with DAG. For DGAT1 and MGAT3, we propose that affinityDAG/affinityMAG is at an intermediate level, so that when 2-MAG concentration increases, it can compete against the DAG that is formed by the single acylation. As a result, despite the fact that more DAG was formed at high concentration of 2-MAG, the TAG synthesis was decreased (Fig. 2, B, E, and F). In addition, DGAT1 may possess higher affinityDAG/affinityMAG than MGAT3. Therefore, the decline of TAG formation happens at a higher 2-MAG concentration in DGAT1 than MGAT3. Last, at another extreme, affinityDAG/affinityMAG is extremely low for MGAT2, and once DAG is formed by MGAT2 as the result of a single acylation of MAG, the majority dissociates without being further acylated (Fig. 2, D and F). It should be emphasized that the low level of DAG formation in DGAT2, as shown in Fig. 2C, does not imply that it has low acylation activity for 2-MAG. To the contrary, the result of high levels of TAG formation indicates that DGAT2 strongly acylates 2-MAG, but unique to DGAT2, it also has a strong ability to conduct the second acylation step regardless of 2-MAG concentration.
The 2-MOG substrate used in this manuscript was obtained from Sigma (catalog number M2787). According to the manufacturer's information, it was >95% pure. However, in Figs. 1 and 2, the DAG products all contained similar levels of 1,3-DAG and 1,2-DAG. Since 1,3-DAG could only be derived from the acylation of 1-MOG, 2-MOG may have gone through a spontaneous acyl migration to 1-MOG. Because of the similar levels of 1,2-DAG and 1,3-DAG that were formed, it appears that 1-MAG can serve as a substrate similar to 2-MAG for all of these acyltransferases.
Inasmuch as DGAT1 (4) and DGAT2 (9) are highly expressed in liver and adipose tissues, our findings that both enzymes can acylate 2-MAG also helps explain the earlier observations where MAG acyltransferase activity was detected in vitro in liver (30) and adipose tissue (31). Recently, Cao et al. (32) asserted that MGAT3 might be a putative TAG synthase based on their observation that ample TAG was synthesized when MAG was provided as the substrate. The data presented in Figs. 1 and 2 in the current study are consistent and expand their findings.
The switch from TAG to DAG produced by DGAT1 and MGAT3 at higher concentrations of MAG may have physiological implications. Since MAG is the major end product of pancreatic lipase and the starting point of the monoacylglycerol pathway, its level may represent the nutrient state. It is likely that after a high fat meal, MAG concentration reaches a high level within enterocytes. This creates a condition necessary for efficient chylomicron synthesis. Since phospholipid is also an important component of chylomicrons, not only is efficient TAG synthesis important, but robust phospholipid synthesis is also necessary. According to our result, under such circumstances, DAG can be synthesized by DGAT1 and MGAT3, which in turn can serve as a starting point for not only synthesizing TAG by other enzymes, such as DGAT2 and diacylglycerol transacylase, but for phospholipids as well. Therefore, the enzymatic feature of DGAT1 and MGAT3 described here may help explain the balance of TAG and phospholipid in the chylomicron.
In the experiments assessing the XP620 inhibition of in vitro acyltransferase activities derived from differentiated Caco-2 cells or rat intestinal mucosa membranes (Fig. 3), we found that the majority of TAG synthesis either as a result of monoacylation of 1,2-DOG or diacylation of 2-MOG can be inhibited by XP620. Because of the exquisite specificity of XP620 (Table 1), we attribute the in vitro acyltransferase activities in differentiated Caco-2 cells or rat intestinal mucosa membranes to DGAT1. Interestingly, the DAG synthesis as a result of 2-MOG acylation was only partially inhibited in Caco-2 membrane and not at all in rat intestinal membranes. We hypothesize that the majority of these XP620-insensitive activities might be MGAT2 in rat intestine and a mixture of MGAT2/MGAT3 in Caco-2. This is based on the following reasons. 1) MGAT2 is highly expressed in rat intestine; there is no MGAT3 counterpart in rats. In contrast, the differentiation of Caco-2 cells for 2 weeks caused dramatic up-regulation of MGAT2 and MGAT3 expression by 18- and 9-fold, respectively, without a change in DGAT2.4 2) When 2-MOG was used as the substrate, the specific activity of DAG formation in rat intestinal mucosa membrane was determined to be ∼42.5 nmol/min/mg (Fig. 3F), whereas the specific activity for TAG formation was determined to be ∼6.4 nmol/min/mg (Fig. 3E). The preference of DAG formation is consistent with the enzyme characteristics of recombinant MGAT2 (Fig. 2). Our data for the ratio of DAG/TAG formation as the result of acylation of MAG either by recombinant MGAT2 or by the intestinal membrane extract are consistent with earlier findings by Lockwood et al. (33). Our data in Fig. 3, C and F, also are consistent with results by Trotter and Storch (34), supporting the notion that Caco-2 cells contain little MGAT activity and may not be the best model system for the MGAT pathway.
In cultured mouse enterocytes, treatment of XP620 decreased DAG synthesis by ∼30% (Fig. 5B). In contrast, XP620 did not cause significant inhibition of DAG formation by rat and mouse intestinal membrane extracts (Fig. 3, F and G). This might represent differences in the kinetics of synthesis of DAG and TAG in cells and isolated membrane systems. It is possible that in the enterocyte, DGAT1 prefers to synthesize more DAG than TAG due to differences in intracellular DAG concentrations. Our finding that the synthesis of DAG and TAG by DGAT1 can be modulated by different concentrations of 2-MOG (Fig. 2B) supports this possibility. We have not attempted to create precise cellular DAG levels in isolated membranes, because it is difficult to measure active DAG concentrations that are seen by membrane bound DGAT1. Alternatively, the in vitro enzyme assay condition may favor MGAT2 so that its contribution toward the synthesis of DAG is overrepresented in vitro. Both of these two scenarios may potentially contribute to the underestimation of DAG formation by DGAT1 and its resilience toward XP620 inhibition in the membrane extracts and explain the discrepancies with the data obtained from cultured enterocytes and isolated membranes.
In the cell culture model, we also showed that XP620 significantly inhibited the synthesis and secretion of TAG in primary enterocytes. Thus, DGAT1 might be the major enzyme contributing to the transport of dietary fat. It has been shown that newly synthesized TAG is preferentially utilized for the assembly of chylomicrons (21). We reasoned that if DGAT1 is the major enzyme involved in TAG synthesis, then its inhibition might affect the assembly and secretion of chylomicrons. Indeed, we found that XP620 specifically reduced the assembly and secretion of TAG-rich lipoproteins (CML, CMS, and CMVLDL) by Caco-2 cells. Therefore, we conclude that TAG synthesis by DGAT1 is an important determinant in the assembly and secretion of chylomicrons.
Last, when delivered orally, XP620 was able to significantly decrease the appearance of TAG in the serum derived from orally administered lipid in normal rats for several h. The area under the curve of the TAG curve was ∼50% lower compared with vehicle control for 6 h. XP620 has a poor oral bioavailability. When administered at 30 and 100 mg/kg, per os in rats, the plasma concentration after 3 h (∼Cmax) was only 0.19 and 0.74 μm, respectively. Based on the data from intact cell assays, XP620 only exerts significant function in inhibiting TAG synthesis when median concentration exceeds 5 μm (Figs. 4 and 5). The systemic exposure levels of orally administered XP620 at both 30 and 100 mpk, therefore, are unlikely to impact the TAG synthesis in liver and adipose tissue.
Therefore, our hypothesis is that XP620 inhibits the formation of TAG from fatty acids and MAG in the enterocytes. Alternatively, XP620 might block or slow the emptying of the lipid bolus from the stomach, thereby reducing and slowing the appearance of TAG in the serum. To test this hypothesis, we evaluated the effects of XP620 on gastric emptying after 1 h of feeding. XP620 did not reduce the emptying of the test meal. However, CCK-8 significantly reduced emptying. These results support our hypothesis that XP620 does not reduce gastric motility.
In order to evaluate further the specificity of XP620, we also assessed the effect of XP620 on MTP, a critical protein downstream of MAG acylation, in fat absorption. XP620 had no effect on MTP activity (Fig. 6D), indicating that XP620 decreases the TAG absorption by inhibiting TAG synthesis in the gut.
Of note, our pharmacological data are consistent with results from DGAT1 knock-out mice (8). In both cases, TAG absorption of orally administered lipids was attenuated but not totally abolished. Since our pharmacological experiment in Fig. 6 relies on a single dose and only lasted for 6 h, it rules out the possibility that the residual lipid absorption is a result of compensation of DGAT1 inhibition. Rather, alternative mechanisms, such as DGAT2, MGAT2, and diacylglycerol transacylase may provide means to support the remaining lipid absorption.
Acknowledgments
We thank Drs. Simeon Taylor and Robert Zahler for encouragement and stimulating discussions and Dr. Rajajit Srivastava for helping in the oral lipid tolerance test. We also thank Dr. Ruth Wexler for the supply of XP620 and Dr. Leonard Adam for critically reading the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TAG, triacylglycerol; DAG, diacylglycerol; MAG, monoacylglycerol; 1,2-DOG, 1,2-dioleoyl-sn-glycerol; 2-MOG, sn-2-monooleoylglycerol; DGAT, acyl coenzyme A:diacylglycerol acyltransferase; MGAT, acyl coenzyme A:monoacylglycerol acyltransferase; CCK-8, cholecystokinin-8; MTP, microsomal triglyceride transfer protein; CM, chylomicron; VLDL, very low density lipoprotein; PL, phospholipids.
Seethala, R., Peterson, T., Deng, J., Chu, C.-H., Chen, L., Golla, R., Ma, Z., Panemangalore, R., Lawrence, R. M., and Cheng, D. (2008) Anal. Biochem., in press.
L. Chen and D. Cheng, unpublished results.
References
- 1.Hussain, M. M. (2000) Atherosclerosis 148 1–15 [DOI] [PubMed] [Google Scholar]
- 2.Hussain, M. M., Kancha, R. K., Zhou, Z., Luchoomun, J., Zu, H., and Bakillah, A. (1996) Biochim. Biophys. Acta 1300 151–170 [DOI] [PubMed] [Google Scholar]
- 3.Hussain, M. M., Kedees, M. H., Singh, K., Athar, H., and Jamali, N. Z. (2001) Front. Biosci. 6 D320–D331 [DOI] [PubMed] [Google Scholar]
- 4.Cases, S., Smith, S. J., Zheng, Y. W., Myers, H. M., Lear, S. R., Sande, E., Novak, S., Collins, C., Welch, C. B., Lusis, A. J., Erickson, S. K., and Farese, R. V., Jr. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 13018–13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, T. Y., Chang, C. C., and Cheng, D. (1997) Annu. Rev. Biochem. 66 613–638 [DOI] [PubMed] [Google Scholar]
- 6.Guo, Z. Y., Lin, S., Heinen, J. A., Chang, C. C., and Chang, T. Y. (2005) J. Biol. Chem. 280 37814–37826 [DOI] [PubMed] [Google Scholar]
- 7.Smith, S. J., Cases, S., Jensen, D. R., Chen, H. C., Sande, E., Tow, B., Sanan, D. A., Raber, J., Eckel, R. H., and Farese, R. V., Jr. (2000) Nat. Genet. 25 87–90 [DOI] [PubMed] [Google Scholar]
- 8.Buhman, K. K., Smith, S. J., Stone, S. J., Repa, J. J., Wong, J. S., Knapp, F. F., Jr., Burri, B. J., Hamilton, R. L., Abumrad, N. A., and Farese, R. V., Jr. (2002) J. Biol. Chem. 277 25474–25479 [DOI] [PubMed] [Google Scholar]
- 9.Cases, S., Stone, S. J., Zhou, P., Yen, E., Tow, B., Lardizabal, K. D., Voelker, T., and Farese, R. V., Jr. (2001) J. Biol. Chem. 276 38870–38876 [DOI] [PubMed] [Google Scholar]
- 10.Stone, S. J., Levin, M. C., and Farese, R. V., Jr. (2006) J. Biol. Chem. 281 40273–40282 [DOI] [PubMed] [Google Scholar]
- 11.Yen, C. L., Stone, S. J., Cases, S., Zhou, P., and Farese, R. V., Jr. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 8512–8517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao, J., Burn, P., and Shi, Y. (2003) J. Biol. Chem. 278 25657–25663 [DOI] [PubMed] [Google Scholar]
- 13.Yen, C. L., and Farese, R. V., Jr. (2003) J. Biol. Chem. 278 18532–18537 [DOI] [PubMed] [Google Scholar]
- 14.Cheng, D., Nelson, T. C., Chen, J., Walker, S. G., Wardwell-Swanson, J., Meegalla, R., Taub, R., Billheimer, J. T., Ramaker, M., and Feder, J. N. (2003) J. Biol. Chem. 278 13611–13614 [DOI] [PubMed] [Google Scholar]
- 15.Cao, J., Hawkins, E., Brozinick, J., Liu, X., Zhang, H., Burn, P., and Shi, Y. (2004) J. Biol. Chem. 279 18878–18886 [DOI] [PubMed] [Google Scholar]
- 16.Yen, C. L., Monetti, M., Burri, B. J., and Farese, R. V., Jr. (2005) J. Lipid Res. 46 1502–1511 [DOI] [PubMed] [Google Scholar]
- 17.Orland, M. D., Anwar, K., Cromley, D., Chu, C. H., Chen, L., Billheimer, J. T., Hussain, M. M., and Cheng, D. (2005) Biochim. Biophys. Acta 1737 76–82 [DOI] [PubMed] [Google Scholar]
- 18.Cheng, D., Meegalla, R. L., He, B., Cromley, D. A., Billheimer, J. T., and Young, P. R. (2001) Biochem. J. 359 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Athar, H., Iqbal, J., Jiang, X. C., and Hussain, M. M. (2004) J. Lipid Res. 45 764–772 [DOI] [PubMed] [Google Scholar]
- 20.Jamil, H., Gordon, D. A., Eustice, D. C., Brooks, C. M., Dickson, J. K., Jr., Chen, Y., Ricci, B., Chu, C. H., Harrity, T. W., Ciosek, C. P., Jr., Biller, S. A., Gregg, R. E., and Wetterau, J. R. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 11991–11995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anwar, K., Kayden, H. J., and Hussain, M. M. (2006) J. Lipid Res. 47 1261–1273 [DOI] [PubMed] [Google Scholar]
- 22.Luchoomun, J., and Hussain, M. M. (1999) J. Biol. Chem. 274 19565–19572 [DOI] [PubMed] [Google Scholar]
- 23.Nayak, N., Harrison, E. H., and Hussain, M. M. (2001) J. Lipid Res. 42 272–280 [PubMed] [Google Scholar]
- 24.Iqbal, J., Anwar, K., and Hussain, M. M. (2003) J. Biol. Chem. 278 31610–31620 [DOI] [PubMed] [Google Scholar]
- 25.Anwar, K., Iqbal, J., and Hussain, M. M. (2007) J. Lipid Res. 48 2028–2038 [DOI] [PubMed] [Google Scholar]
- 26.Iqbal, J., and Hussain, M. M. (2005) J. Lipid Res. 46 1491–1501 [DOI] [PubMed] [Google Scholar]
- 27.Fatma, S., Yakubov, R., Anwar, K., and Hussain, M. M. (2006) J. Lipid Res. 47 2422–2432 [DOI] [PubMed] [Google Scholar]
- 28.Bligh, E. G., and Dyer, W. J. (1959) Can. J. Biochem. Physiol. 37 911–917 [DOI] [PubMed] [Google Scholar]
- 29.Moran, T. H., and McHugh, P. R. (1988) Am. J. Physiol. 254 R628–R632 [DOI] [PubMed] [Google Scholar]
- 30.Coleman, R. A., and Haynes, E. B. (1986) J. Biol. Chem. 261 224–228 [PubMed] [Google Scholar]
- 31.Jamdar, S. C., and Cao, W. F. (1992) Arch. Biochem. Biophys. 296 419–425 [DOI] [PubMed] [Google Scholar]
- 32.Cao, J., Cheng, L., and Shi, Y. (2007) J. Lipid Res. 48 583–591 [DOI] [PubMed] [Google Scholar]
- 33.Lockwood, J. F., Cao, J., Burn, P., and Shi, Y. (2003) Am. J. Physiol. 285 E927–E937 [DOI] [PubMed] [Google Scholar]
- 34.Trotter, P. J., and Storch, J. (1993) J. Biol. Chem. 268 10017–10023 [PubMed] [Google Scholar]
- 35.Hussain, M. M., Zhao, Y., Kancha, R. K., Blackhart, B. D., and Yao, Z. (1995) Arterioscler. Thromb. Vasc. Biol. 15 485–494 [DOI] [PubMed] [Google Scholar]
- 36.Bakillah, A., Zhou, Z., Luchoomun, J., and Hussain, M. M. (1997) Lipids 32 1113–1118 [DOI] [PubMed] [Google Scholar]