Abstract
Trypanosoma brucei, the causative agent of African sleeping sickness, encodes three cysteine homologues (Px I-III) of classical selenocysteine-containing glutathione peroxidases. The enzymes obtain their reducing equivalents from the unique trypanothione (bis(glutathionyl)spermidine)/tryparedoxin system. During catalysis, these tryparedoxin peroxidases cycle between an oxidized form with an intramolecular disulfide bond between Cys47 and Cys95 and the reduced peroxidase with both residues in the thiol state. Here we report on the three-dimensional structures of oxidized T. brucei Px III at 1.4Å resolution obtained by x-ray crystallography and of both the oxidized and the reduced protein determined by NMR spectroscopy. Px III is a monomeric protein unlike the homologous poplar thioredoxin peroxidase (TxP). The structures of oxidized and reduced Px III are essentially identical in contrast to what was recently found for TxP. In Px III, Cys47, Gln82, and Trp137 do not form the catalytic triad observed in the selenoenzymes, and related proteins and the latter two residues are unaffected by the redox state of the protein. The mutational analysis of three conserved lysine residues in the vicinity of the catalytic cysteines revealed that exchange of Lys107 against glutamate abrogates the reduction of hydrogen peroxide, whereas Lys97 and Lys99 play a crucial role in the interaction with tryparedoxin.
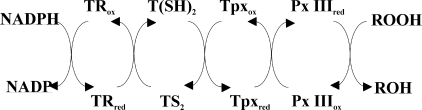
Trypanosomes and Leishmania, the causative agents of several tropical diseases, have a unique thiol redox metabolism that is based on trypanothione (N1,N8-bis(glutathionyl)spermidine) and the flavoenzyme trypanothione reductase (for reviews, see Refs. 1 and 2). The parasites lack catalases and selenocysteine-containing glutathione peroxidases. 2-Cys-peroxiredoxins and cysteine-containing glutathione peroxidase-type enzymes are responsible for hydroperoxide reduction acting both as tryparedoxin peroxidases (for a recent review, see Ref. 3). With NADPH as primary electron source, the reducing equivalents flow via trypanothione and tryparedoxin (Tpx),2 a distant relative of the thioredoxin protein family, onto the peroxidases which then reduce the hydroperoxide substrates (Scheme 1). In Trypanosoma brucei, the causative agent of African sleeping sickness, both the cytosolic 2-Cys peroxiredoxin and the glutathione peroxidase-type enzymes proved to be essential (4, 5).
SCHEME 1.
In African trypanosomes, hydroperoxides (ROOH) are detoxified by a cascade composed of trypanothione reductase (TR), trypanothione (T(SH)2), Tpx, and the Px III or a 2-Cys-peroxiredoxin.
Classical glutathione peroxidases (GPXs) are selenoproteins. Five distinct enzymes have been characterized in mammals, cytosolic GPX1, gastro-intestinal GPX2, plasma GPX3, phospholipid hydroperoxide peroxidase GPX4, and in humans, GPX6, which is restricted to the olfactory system (6). The high specificity of GPX1 for glutathione as reducing substrate gave the protein family its name (7). In the epididymis of rodents and monkeys, a GPX5 is expressed that contains an active site cysteine instead of the selenocysteine. Recently the structure of human GPX7, also a cysteine homologue, has been solved (PDB code 2P31). Expression of this gene is dramatically down-regulated in breast cancer cells (8). In general, cysteine-containing glutathione peroxidase-type enzymes are widespread in nature, and most of the enzymes, few of which are biochemically characterized, function as thioredoxin peroxidases (for reviews, see Refs. 9 and 10).
The selenoenzymes contain a catalytic triad. The selenoate of the peroxidatic selenocysteine is supposed to be stabilized by hydrogen bonds with a Gln and a Trp residue (11, 12). Site-directed mutagenesis (13) and computational studies (14, 15) supported the crucial role of the Gln and Trp residue for catalysis. Both residues are conserved over the entire family of glutathione peroxidase-type enzymes (Ref. 10; Fig. 1) with a few plant proteins having a Glu instead of Gln (16). This suggested that the cysteine-containing glutathione peroxidase-type enzymes are activated by a related mechanism (5, 16).
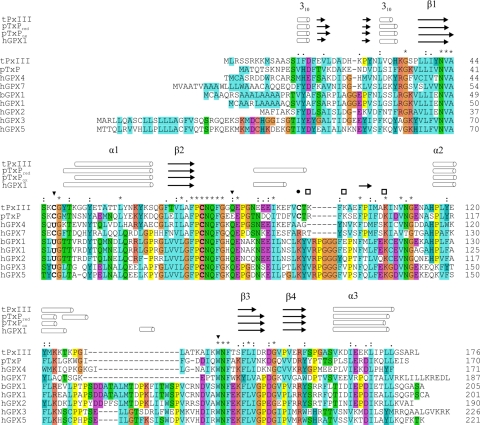
FIGURE 1.
Sequence alignment of T. brucei Px III with related peroxidases for which the three-dimensional structures are known. The residues SeCys (U) or Cys, Gln, and Trp that form a catalytic triad in glutathione peroxidases and several cysteine-containing relatives are marked by triangles. Cysteine 95, which is essential for catalytic activity of Px III, is marked by a filled circle. The three Lys residues, Lys97, Lys99, and Lys107, that were mutated in this work are marked by a square. α and 310 helices (rods) as well as β-sheet regions (arrows) above the alignment refer to the secondary structures of Px III in comparison to reduced and oxidized poplar TxP (pTxPred and pTxPox) as well as human GPX1 (hGPX1). Residues that are identical in all sequences are marked by an asterisk, and highly or partially conserved residues by are marked by colons and periods, respectively. Cysteine residues are given in bold letters. The alignment was generated with ClustalX (57). tPxIII, T. brucei Px III (17); pTxP, Populus trichocarpa glutathione peroxidase 5 (PDB codes 2P5R and 2P5Q (16)); hGPX4, human GPX4 (2GS3 and 2OBI (42)); hGPX7, human GPX7 (2P31); bGPX1, bovine GPX1 (1GP1 (12)); hGPX1, human GPX1 (2F8A); hGPX2, human GPX2 (2HE3); hGPX3, human GPX3 (11); hGPX5, human GPX-5 (2IY3).
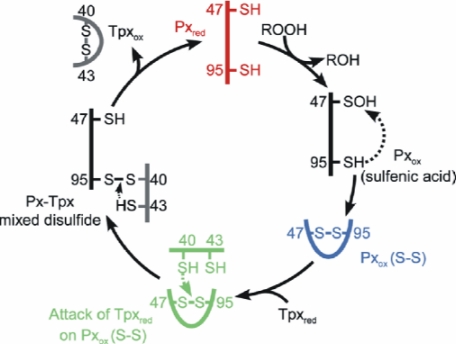
T. brucei encodes three nearly identical genes for cysteine-containing glutathione peroxidase-type tryparedoxin peroxidases (Px I-III) (17). The proteins occur in the cytosol and the mitochondrion of the parasites (5). In the mitochondrial Px III sequence, Cys47 replaces the selenocysteine residue. Substitution of Cys47 or Gln82 by a glycine residue resulted in inactive protein species, and the glycine mutant of Trp137 had only 12% wild type activity (18), which seemed to confirm the presence of the catalytic triad. Interestingly, in a related enzyme from Chinese cabbage, the respective glutamine to glycine mutation had no effect on peroxidase activity (19). Nearly all of the cysteine homologues, but not the selenoenzymes, possess a second cysteine (Cys95 in T. brucei Px III) that is essential for peroxidase activity (18, 19). In the first half reaction of catalysis, the thiolate form of Cys47 (reduced enzyme) reacts with the hydroperoxide substrate, resulting in the respective alcohol and probably a sulfenate at Cys47. Attack of Cys95 generates an intramolecular disulfide bond (oxidized enzyme) that is in the second half reaction reduced by interaction with Tpx (Scheme 2).
SCHEME 2.
Proposed reaction mechanism of Px III. In the first half reaction, reduced Px III (Pxred) reacts with the hydroperoxide substrate (ROOH), and Cys47 becomes oxidized to a sulfenic acid (Pxox (sulfenic acid)). Attack of Cys95 leads to formation of an intramolecular disulfide between Cys47 and Cys95 (Pxox (S-S)). In the second half-reaction, Cys40 of reduced Tpx (Tpxred) attacks Cys95 of the disulfide bond, resulting in an intermolecular disulfide between both proteins (Px-Tpx mixed disulfide). Cys43 of Tpx finally reacts with its vicinal Cys40 to release Pxred and oxidized Tpx (Tpxox). The three states of the catalytic cycle studied in this work, Pxred, Pxox (S-S), and the complex between Tpx and PxIII are depicted in red, blue, and green, respectively.
Here we present the crystal structure of oxidized T. brucei Px III and the NMR structures of the protein in oxidized and reduced form. Contrary to previous observations on poplar TxP (16), the closest homologue for which a three-dimensional structure has been published, T. brucei Px III does not undergo any significant structural rearrangement upon reduction of the intramolecular disulfide bridge. Furthermore, residues Gln82 and Trp137, the residues equivalent to the catalytic triad in classical GPXs, are located far away from the active site Cys47 in our structures, ruling out the presence of the catalytic triad. Thus, the catalytic mechanism of Px III appears to differ fundamentally from any of the currently characterized enzymes. Analysis of the active site identified three lysines in the vicinity of the redox active cysteines that could play a role during catalysis. Kinetic analysis of three lysine mutants revealed that two of them, Lys97 and Lys99, play a crucial role in the interaction with tryparedoxin, whereas Lys107 is involved in the first part of the reaction, the reduction of hydrogen peroxide.
EXPERIMENTAL PROCEDURES
Materials—Recombinant T. brucei Tpx (20) and Trypanosoma cruzi trypanothione reductase (21) were prepared as described. Trypanothione disulfide was purchased from Bachem, and H2O2 was from Merck. 15NH4Cl, [13C6]glucose and selectively labeled amino acids were obtained from Spectra Stable Isotopes.
Cloning, Expression, and Purification of Px III—For crystallization, the Ser76 mutant of Px III was expressed from the pQE30/c76s-px III plasmid which resulted in a protein species starting with Lys7. The protein was prepared as described (17). For NMR studies, the Px III gene was cloned into a modified pET 9d vector with an N-terminal thioredoxin-His6 tag cleavable by tobacco etch virus protease. The mitochondrial signal sequence of Px III was removed, and Cys76 was replaced by a serine residue which resulted in a fully active peroxidase (18) but excluded putative unspecific oxidation or dimerization. Details of the purification have been published elsewhere (22). The Ser47, Ser95, Glu97, Gln97, Glu99, Gln99, and Glu107 mutants were generated using the QuikChange® mutagenesis kit (Stratagene) and the pET9d/c76s-px III or pET9d/px III plasmid as template. Escherichia coli XL1-Blue cells were transformed and grown at 37 °C in Luria-Bertani (LB) medium with 50 μg/ml kanamycin. The plasmid DNA was purified using the Nucleobond® PC100 kit (Macherey-Nagel). The recombinant Px III mutants were purified as described (22).
Structure Determination of the Oxidized State by X-ray Crystallography—Purified Px III was dialyzed against 100 mm Tris, 1 mm EDTA, pH 7.6, and concentrated to 13 mg/ml. The protein was crystallized by the hanging drop vapor diffusion method at 4 °C using a droplet size of 4 μl of Px III solution and 4 μl of the reservoir solution of 2 m ammonium sulfate, 0.1 m sodium acetate, pH 4.6. A crystal with an approximate size of 50 × 50 × 400 μm3 was shock-frozen under liquid nitrogen using mother liquor containing 20% glycerol as cryo protectant. Data were collected on beamline ID14-EH4 at the European Synchrotron Radiation Facility, Grenoble, France, at a wavelength of λ = 0.9393 Å (Table 1). A total of 500 frames with 0.25° oscillation were collected (i.e. 125° total). Toward higher resolution, diffraction spots were split, pointing to a potential twinning problem not readily detectable from the optical appearance of the crystals. Autoindexing, as implemented in denzo (23), suggested a centered orthorhombic cell with one molecule per asymmetric unit. Possible lower symmetries were centered monoclinic and primitive monoclinic with two or primitive triclinic with four molecules per asymmetric unit. Merging R-factors from scalepack (23) were 7.7% (C222), 6.1% (C2), 5.8% (P2), and 3.4% (P1). Data manipulation was carried out with the CCP4 package (24).
TABLE 1.
Data collection and refinement statistics of the X-ray structure of oxidized Px III
HR, high resolution bin; Rsym = ∑h∑i|I(h) - I(h)i|/ ∑h∑iI(h)i, where I(h) is the mean intensity after rejections; Rwork = ∑h||Fobs(h)| - |Fcalc(h)|| /∑h|Fobs(h)|, where Fobs(h) and Fcalc(h) are the observed and calculated structure factors, respectively. 5% of the data were excluded to calculate Rfree.
| Data collection statistics | |
| Space group | P21 |
| Unit cell a, b, c (Å), b (°) | 42.8 × 105.9 × 42.8, 100.5 |
| Resolution (Å)/HR bin (Å) | 50-1.41/1.43-1.41 |
| Rsym (%)/HR (%) | 5.8/47.3 |
| I/σ(I)/HR | 14.9/2.1 |
| Completeness (%)/HR (%) | 99.2/96.8 |
| Redundancy/HR | 2.6/2.5 |
| Unique reflections | 71,308 |
| Average B (Å2) | 14.6 |
| Refinement statistics | |
| Rwork(%)/Rfree (%) | 12.6/19.4 |
| Residues in model | A and B, Met-9-Leu-171 |
| No of amino acids with double conformations | 32 |
| No of protein atoms/waters | 2,782/358 |
| r.m.s.d. bond length (Å)/angle (°) | 0.011/0.030 |
| Ramachandran statistics | |
| Most favored (residues/%) | 89.2 |
| Additional favored (residues/%) | 10.4 |
| Generously allowed (residues/%) | 0.4 |
| Disallowed (residues/%) | 0.0 |
We used the ARP/wARP procedure of free atom density modification with subsequent auto-building (25) in the P2 lattice. Due to twinning, the model had to be completed in three steps by iterated model building in Coot (26) and ARP/wARP autotracing and refmac5 refinement (27). Twin refinement using the twin operator (-l, -k, -h) in shelxl (28) immediately reduced the Rfree by more than 5% (Table 1); the twin fraction refined to 0.473. Model correction and introduction of anisotropic refinement reduced the Rfree by a further 3%. Model building and refinement were carried out similarly to the serine protease example given in the shelx manual. The initial 491 water molecules, assigned by the ARP/wARP procedure, were reduced in the course of refinement to 365 water molecules to avoid fitting of noise; in refinement cycles 3 and 5, the automated water-divining procedure of shelx was used with default/recommended settings.
NMR Experiments—The chemical shift assignment has been published elsewhere (22). For structure determination, two-dimensional NOESY in 100% D2O, 15N HSQC-NOESY, and 13C heteronuclear multiple quantum coherence-NOESY spectra were recorded at high field (800/900 MHz) with mixing times of 80 ms (oxidized form) and 100 ms (reduced form) at 22 °C and a protein concentration of 1 mm. Data were processed with NMRPIPE (29) and analyzed using NMRVIEW (30). Structures were calculated with ARIA1.2/CNS (31) on the basis of the experimentally derived NOE restraints (Table 2). Additional dihedral restraints derived from TALOS analysis (32) of the chemical shifts and hydrogen bonds identified on the basis of characteristic NOE patterns (Table 2) were added. The water-refined structures were examined with PROCHECK (33). Figures were prepared with MOLMOL (34) and PyMOL (35).
TABLE 2.
Structural statistics of the NMR structures of oxidized and reduced Px III
| <SAox>a | <SAoxwatref>b | <SAred>a | <SAredwatref>a | |
|---|---|---|---|---|
| Distance restraints | ||||
| All NOEs (unambiguous/ambiguous) | 4273/51 | 4627/46 | ||
| Intraresidual | 1328 | 1315 | ||
| Sequential (|i - j| = 1) | 791 | 868 | ||
| Medium range (1<|i - j| ≤ 4) | 619 | 792 | ||
| Long range (|i - j|>4) | 1596 | 1702 | ||
| Dihedral angles ϕψ | 89/89 | 89/89 | ||
| H bonds | 44 | 44 | ||
| r.m.s.d. (Å) from experimental restraintsc | ||||
| All distance restraints | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Dihedral anglesd | 2.63 ± 0.21 | 2.63 ± 0.28 | 2.78 ± 0.29 | 2.50 ± 0.26 |
| Coordinate precision (Å)e | ||||
| N, Cα, C′ | 1.02 ± 0.20 | 0.84 ± 0.20 | 1.36 ± 0.36 | 1.03 ± 0.12 |
| All heavy atoms | 1.59 ± 1.24 | 1.38 ± 0.22 | 2.02 ± 0.30 | 1.63 ± 0.23 |
| Structural qualityf, bad contacts | 14.75 ± 2.38 | 3.05 ± 0.76 | 12.00 ± 2.83 | 3.20 ± 0.70 |
| Ramachandran plot | ||||
| Residues in most favored region (%) | 69.3 ± 2.0 | 77.0 ± 2.0 | 68.1 ± 2.0 | 72.4 ± 2.3 |
| Residues in additionally | 24.1 ± 2.5 | 17.4 ± 2.2 | 24.0 ± 2.1 | 21.6 ± 2.5 |
| Allowed region (%) | ||||
| Residues in generously allowed region (%) | 4.3 ± 1.6 | 3.9 ± 1.2 | 5.0 ± 2.1 | 3.3 ± 1.5 |
| Residues in disallowed Region (%) | 2.3 ± 1.2 | 1.7 ± 1.0 | 2.9 ± 1.9 | 2.7 ± 1.3 |
<SAox> and <SAred> are ensembles of the 20 lowest energy solution structures (out of 20 calculated) of Px III in the oxidized and reduced state before water refinement, respectively.
<SAoxwatref> and <SAredwatref> are the <SA> ensembles after refinement in a shell of water (31). The CNS Erepel function was used to simulate van der Waals interactions with an energy constant of 25 kcal mol−1 Å−4 using PROLSQ van der Waals radii; r.m.s.d. for bond length, bond angles, and improper dihedral angels are 0.0034 (±0.0001) Å, 0.581 (±0.015)°, and 0.578 (±0.021)° before and 0.0052 (±0.0002) Å, 0.697 (±0.020)°, and 2.24 (±0.11)° after water refinement (oxidized form) and 0.0047 (±0.0001) Å, 0.657 (±0.011)°, and 0.634 (±0.017)° before and 0.0063 (±0.0002) Å, 0.761 (±0.016)°, and 2.27 (±0.10)° after water refinement (reduced form).
Distance restraints were employed with a soft square-well potential using an energy constant of 50 kcal mol−1 Å−2. No distance restraint in the <SAox> and <SAred> was violated by more than 0.2 Å.
Dihedral angle restraints derived from TALOS (32) were applied to ϕ,ψ backbone angles using energy constants of 200 kcal mol−1 rad−2.
Coordinate precision is given as the Cartesian coordinate r.m.s.d. of the 20 lowest energy structures in the NMR ensemble with respect to their mean structure for residues 15-179 of Px III.
Structural quality was analyzed using PROCHEK.
To analyze the two conformations of the reduced enzyme, 300 μm Px III in 50 mm sodium phosphate, 100 mm KCl, pH 6.8, was treated with 10 mm dithiothreitol, and the NMR-tube was purged with argon to prevent reoxidation. Constant reducing conditions were verified by subjecting an aliquot of the solution to thiol determination with 5,5-dithiobis(2-nitrobenzoate). The difference in chemical shift Δδ was calculated with the formula Δδ =√(δH2 + (δN/10)2). One-dimensional 1H NMR spectra were recorded at 600 MHZ between 29 and 4 °C in D2O and H2O. To evaluate if the appearance of two conformations was pH-dependent, the Px III solution was titrated to pH 5.0, 6.8, and 9.5 with 1 m HCl or NaOH. The role of Cys47 and Cys95 was studied by alkylating the protein at pH 8.0 with 15 mm iodoacetamide in the presence of 2 mm dithiothreitol.
Kinetic Analysis of the Px III Lysine Mutants—Peroxidase activity was measured at 25 °C in a total volume of 150 μl of 0.1 m Tris, 5 mm EDTA, pH 7.6, in the presence of 240 μm NADPH, 100 μm trypanothione disulfide, 150 milliunits of trypanothione reductase, 10 μm Tpx, and 0.1-0.9 μm concentrations of the different Px III species. The reaction was started by adding 100 μm H2O2, and NADPH consumption was followed at 340 nm (5, 17). The kinetic parameters were determined by Dalziel kinetics as described for the wild type enzyme (17). The Tpx concentration was varied between 2 and 10 μm in the presence of 0.2 and 0.6 μm Px III. The reactions were started by the addition of 60 μm H2O2.
Modeling of the Complex between Px III and Tpx—The 10 lowest energy NMR structures of oxidized Px III and the x-ray structure of oxidized Trypanosoma brucei Tpx (PDB code 1O73 (36)) were used to model the complex structure. The docking was performed using HADDOCK 2.0 (37, 38). The solvated docking was carried out with the recommended parameters of HADDOCK. Cys40 of Tpx and Cys95 of Px III were defined as active residues. During the rotational and translational rigid body minimization docking, 1000 structures were calculated with solvation. The best 200 solutions according to intermolecular energy were used for the semi-flexible annealing in torsion angle space. This was followed by a final refinement with explicit modeling of hydrating water molecules. The resulting 200 structures were clustered on the basis of the intermolecular van der Waals, electrostatic, and restraint energy terms, buried surface area, desolvation energy, and binding energy as combined in the HADDOCK score versus the backbone r.m.s.d. from the lowest HADDOCK score structure. The HADDOCK score is a weighted sum of various energy terms obtained in different phases of the docking. The energy terms include van der Waals energy, electrostatic energy, ambiguous distance restraints energy, buried surface area, binding energy and desolvation energy. The structure with the smallest weighted sum, that is, with the smallest HADDOCK score, is ranked first. Solvent surface accessibility was determined by NACCES 2.1.1 (39). Intermolecular interactions were analyzed with LIGPLOT (40).
RESULTS
Overall Structure of T. brucei Px III—We determined the structure of oxidized T. brucei Px III both by x-ray crystallography at a resolution of 1.4 Å and by NMR spectroscopy (Fig. 2). For the structural analyses, Cys76 of Px III was replaced by a serine residue which resulted in a fully active enzyme species (18). The structure of the reduced form was obtained only by NMR spectroscopy. The statistics of the structure determinations by x-ray and NMR are presented in Tables 1 and 2, respectively.
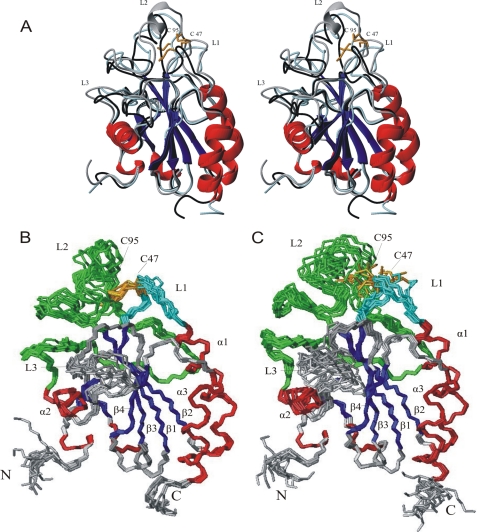
FIGURE 2.
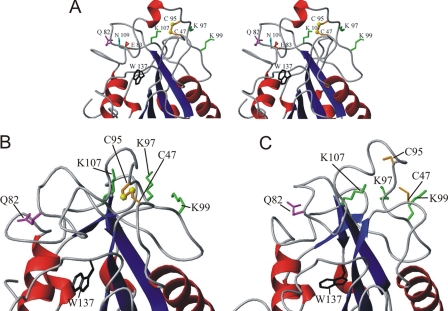
Structures of oxidized and reduced Px III. A, stereo image of the superposition of the structures of oxidized Px III obtained by x-ray and NMR analysis (gray and black, respectively) and the NMR-structure of reduced Px III (light blue). α-Helices and 310-helices are colored in red, and β-sheets are in blue. The two catalytically active cysteine residues Cys47 and Cys95 are depicted in orange. The α-helix in loop 2 only present in the crystal structure is shown in gray. B and C, ensemble of the 10 lowest energy NMR structures of 200 calculated structures after water refinement for the oxidized and reduced protein, respectively. The three most extended loops are given in cyan (L1, loop 1; Ala44-Lys51), green (L2, loop 2; Ser76-His116) and gray (L3, loop 3; Lys126-Thr140). N and C mark the N and the C terminus of the protein.
The x-ray and NMR structures of oxidized Px III (Fig. 2A) are highly similar, with a r.m.s.d. over the backbone atoms of 0.63 Å. The crystal structure revealed a dimer in the asymmetric unit. The buried surface has been determined with CNS (41) to be 1160 Å2. The interface comprises the loops from Gly81 to Glu88 and from Gly128 to Trp137 as well as Asn111. Trp137 is involved in hydrophobic interactions in the dimer; in classical GPXs, this residue is in hydrogen-bonding distance to the catalytic selenocysteine instead. The localization of Trp137 at the dimer interface leads to two different conformations for the indole ring, neither of which corresponds to the conformation observed by NMR. To assay whether the dimer occurs at higher concentrations or is driven by crystal packing, 1H T2 spin-echo relaxation measurements and pulsed-field-gradient NMR diffusion measurements were performed. They yielded a relaxation time and diffusion coefficient typical for a monomeric protein at 1 mm concentration (14.1 ± 1.5 ms at 21 °C, Ds = 0.953 ± 0.008 × 10-6 cm2s-1, supplemental Figs. 1 and 2, respectively) corroborating previous gel chromatography results in which Px III eluted as a monomer (17).
Minor differences between the x-ray and the NMR structure were observed for loop 1 (Ala44-Lys51) and loop 3 (Lys126-Thr140, Fig. 2A). Well defined NOE contacts from Ser45 to Pro75 and Ser76 lead to an altered orientation of loop 1 in the NMR structure. Because loop 1 and loop 2 (Ser76-His116) are connected via the intramolecular disulfide between Cys47 and Cys95, loop 2 and the entire catalytic region are shifted as a consequence (Fig. 2A). Loop 3 is involved in the hydrogen bonding contacts between monomers in the crystal, which leads to a different conformation.
In the x-ray structure, Glu88-Glu92 form an α-helical turn (Fig. 2A), which coincides with α-helix 2 in GPXs (Fig. 1) and reduced poplar TxP (16). Because NMR data reflect the average of all possible conformations, it is conceivable that this part of loop 2 has some potential of α-helix formation.
The monomeric state of Px III is in agreement with the fact that it lacks a long stretch (inserted between Ile129 and Leu130) that forms the subunit interface in the tetrameric GPXs (Refs. 11 and 12; Fig. 1). This loop is not present in the monomeric human GPX4 (PDB code 2GS3 and 2OBI (42)) as well as in most of the cysteine-containing glutathione peroxidase-type proteins including poplar TxP (16). Nevertheless, the latter forms a homodimer (42, 43). This protein dimerizes through the C-terminal region (16). Most of the residues localized in the interface are conserved in related plant proteins but not in Px III.
Px III displays a high loop content of about 60% (Figs. 1 and 2). The overall fold of Px III is 310-β-β-310-β1-α-β2-α-β3-β4-α instead of the common 310-β-β-310 -β1-α-β2-α-β-α-β3-β4-α-fold of other GPX-type proteins, because it lacks an α-helix and a short β-sheet found in distinct GPX structures between β-sheet 2 and α-helix 2. This region with the redox active Cys95 forms a loop of more than 30 residues (loop 2; Cys76-Leu118).
Active Site Structure of Px III—Cys47 and Cys95 form the redox active dithiol/disulfide couple, which in the oxidized enzyme forms an intramolecular disulfide (Ref. 18; Figs. 2A and 3A). In both oxidized and reduced Px III, the peroxidatic Cys47 is located on the first loop (L1) between β-strand 1 and α-helix 1, whereas the resolving Cys95 is part of the long loop 2 (L2) between β-sheet 2 and α-helix 2 that is relatively disordered in the bundle of NMR structures (Figs. 2, B and C). Both residues are surface-exposed and are, thus, freely accessible to large hydroperoxide substrates as well as to tryparedoxin. Px III contains three solvent-exposed aromatic residues (Tyr49, Phe79, and Phe93). Two of them (Tyr49 and Phe93) are located close to the redox active cysteines. Because aromatic residues usually pack into the core of a protein, this may indicate that these residues are involved in the binding of protein partners. An aromatic residue at position 93 is found in most of the monomeric members of the protein family (Ref. 10, Fig. 1), whereas Tyr49 is not conserved. Interestingly, Cys47 and Cys95 of Px III are located in the center of a negatively and positively charged surface patch formed by Glu87, Glu88, Glu89, Glu92 and Lys46, Lys97, Lys99, Lys107, respectively. The residues of these charged patches are partially conserved (Fig. 1).
FIGURE 3.
Details of the catalytic site. A, stereo image of the x-ray structure of oxidized Px III showing the details of the active site. Trp137 (black) does not interact with Cys47. Lys97, Lys99, and Lys107 that were mutated in this work are depicted in green. Gln82 (magenta) on loop 2 is 16.7 Å apart from the disulfide bridge and is, thus, unable to interact with the cysteines. Instead, its side chain amide is in hydrogen bond distance to the carboxylate group of Glu83 (red), which in turn is 2.9 Å distance from the side chain amide of Asn109 (cyan). Thus, Gln82 is part of a network of hydrogen bonds and thereby may stabilize the orientation of Cys95. B and C, details of the redox active part in a representative NMR structure of the oxidized (B) and reduced (C) Px III, respectively.
In the selenocysteine-containing peroxidases, the active site selenoate is in hydrogen bonding distance to the indole NεH of the tryptophan and the NεH amide group of the glutamine of the catalytic triad. The corresponding residues are conserved throughout the entire protein family (Fig. 1) (11-13, 42). However, in all of our three structures of Px III, Trp137 and Gln82 are not found in the immediate vicinity of the cysteines (the distance between the Cα atom of Cys47 and the side chain nitrogen atoms of Trp137 or Gln82 are 16 and 16.7 Å; Fig. 3, A-C). According to the NMR data, Trp137 is surface-exposed, with only a few NOEs to Leu130, Lys136, and Ser155. In the x-ray structure, the side chain amide of Gln82 is in hydrogen-bonding distance to the carboxylate group of Glu83, which forms a hydrogen bond with the side chain amide of Asn109 (Fig. 3A), concomitantly stabilizing loop 2 that harbors Cys95. In the NMR structures of both the oxidized and reduced protein, the side chain orientation of Gln82 could not be determined to high precision due to spectral overlap of its resonances, but the rough orientation of Gln82 defined by a few isolated NOEs in both structures superimposed well with the crystallographic data (Fig. 3, A-C). Thus, despite the conservation of Gln82 and Trp137 and the fact that the Gly82 and Gly137 mutants of T. brucei Px III had 2 and 12% wild type activity, respectively (18), in Px III the two residues are too far away from the redox active cysteine residues to contribute to a stabilization of the thiolate anion of Cys47.
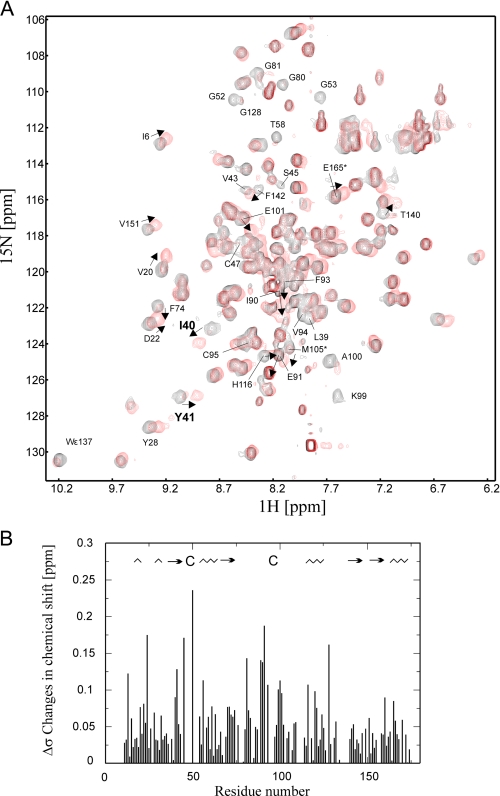
Structure of Reduced Px III—Superposition of the 1H,15N HSQC fingerprint spectra of the reduced and oxidized protein revealed that numerous residues underwent significant chemical shift changes, and several resonances disappeared due to exchange broadening (Fig. 4A). Most of these are located close to the redox active cysteines (Fig. 4B). To identify which peaks disappeared due to exchange broadening and which ones shifted into highly overlapping regions, selectively labeled samples of [15N]Ala/[15N]Lys, [15N]Thr, [15N]Phe, [15N]Tyr, and [13C,15N]Cys were prepared and analyzed. For the selectively labeled [13C,15N]Cys protein, two resonances were detected as expected in the oxidized protein. Upon reduction, both signals disappeared completely. Removal of dithiothreitol restored the cysteine signals (supplemental Fig. S3).
FIGURE 4.
Comparison of the 1H15N HSQC spectra of oxidized
and reduced Px III. A, superposition of the
1H15N HSQC spectra of oxidized (black) and
reduced (red) Px III. Several residues (marked by arrows)
are shifted upon reduction or experience line-broadening. B, chemical
shift difference between oxidized and reduced Px III plotted onto the
sequence. The secondary structure of Px III is shown at the top of
the figure ( , α- and
310-helices; →, β-sheets).
, α- and
310-helices; →, β-sheets).
Overall, the structures of the oxidized and reduced protein superimposed well (Fig. 2A). The r.m.s.d. over the backbone atoms between reduced and oxidized structures was 0.59 Å. Thus, most of the secondary chemical shifts do not originate from structural changes, e.g. the signals of Ile40 and Tyr41 (Fig. 4A) shift although they are located in the core of the protein. Large secondary chemical shifts can be attributed to the flanking regions of the two cysteines (Fig. 4B). Inspection of the structure revealed that they are essentially caused by the formation of the disulfide bridge and probably a slight rotation of the neighboring aromatic side chains of Phe93 and Phe98. Substantial structural rearrangements were not observed for the catalytic region in accordance with comparable NOE patterns in the oxidized and reduced protein for residues of loop 1 and 2. The residues between Gln82 and Lys97 do not have long range NOEs either in oxidized or in reduced Px III, and their 13C Cα- and Cβ chemical shifts were comparable in both redox states. This rules out that loop 2, rather separated from the core of the protein in our structures, rearranges into an α-helix in reduced Px III, as found for poplar TxP (16).
Trp137 and Gln82 are likewise unaffected by the redox state of the protein as both the NOE pattern and especially the more sensitive chemical shifts did not differ between oxidized and reduced protein. In the reduced protein signals obtained for Trp137 were exchange broadened (but still detectable), indicating higher flexibility. The tryptophan fluorescence emission spectra of reduced and oxidized Px III were identical (supplemental Fig. S4), corroborating that the protein environment around the single tryptophan residue does not change significantly. The spectra of the Ser47 and Ser95 mutants showed the same emission maximum, further highlighting that the environment of Trp137 is unaffected by formation of the intramolecular disulfide bridge. The fluorescence emission maximum at 344 nm is in accordance with a rather solvent-exposed position of Trp137. In the presence of 6 m guanidinium hydrochloride, the emission maximum of the reduced protein was 360 nm (not shown). Thus, denaturing only slightly further increases the hydrophilicity around Trp137.
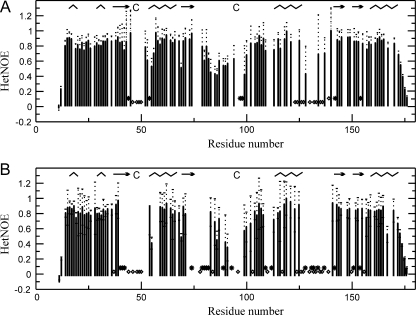
The Three Loops and the Adjacent Regions Are Flexible—In the oxidized Px III, the HN/N resonances of several residues in the vicinity of the catalytic cysteines or Trp137 were line-broadened, some of them (Lys46, Gly48 to Lys51, Asn77, Gln78, Thr96, Ile110, Gly112, Leu130, Lys133, Lys136 to Phe139) beyond detection. In the reduced state, these regions extend to further residues covering the entire loop 1 and parts of loop 2 and loop 3 (Ala44-Cys47, Gly52, Gly53, Ala57, Gly81, Val94, Cys95, Phe98, and Gly128 are additionally broadened) (Fig. 4A). To rule out that an overlap of the signals in crowded regions of the spectrum prevented their detection, Px III was labeled with either [15N]Ala/[15N]Lys, [15N]Thr, [15N]Phe, [15N]Tyr, or [13C,15N]Cys. In the spectra of the selectively labeled samples simplified that way (1H,15N HSQC, 15N-edited NOESY), the HN/N resonances were indeed missing, proving that they are exchange-broadened. Such a phenomenon is often caused by motion in the micro- or millisecond range. To compare the motion in both oxidized and reduced protein, we studied the backbone dynamics. The 1H,15N HetNOE values (Fig. 5) of the residues at the edges of the exchange-broadened loops 2 and 3, which contain Cys95 and Trp137, respectively, displayed lower values (down to 0.4) compared with the overall HetNOE of 0.8-0.9 in both oxidized and reduced protein. Unfortunately, line-broadening effects and also spectral overlap prevented analysis of the motion in more detail.
FIGURE 5.
The three loops are mobile in both oxidized and reduced Px III.
HetNOE analysis of oxidized (A) and reduced (B) Px III are
shown. Residues where line-broadening affected accurate determination of the
HetNOE value are marked by stars. Residues that were
exchange-broadened beyond detection are depicted as diamonds. Empty
fields denote that the residues cannot be analyzed due to spectral overlap.
The secondary structure of Px III is shown at the top of the figure
( , α- and
310-helices; →, β-sheets).
, α- and
310-helices; →, β-sheets).
Thus, the reason that the loops 2 and 3 are not well defined in both NMR structures is not only due to the fact that the respective residues have a low number of long range NOEs but reflects true mobility. It is not surprising that opening of the disulfide bridge leads to an increase in the mobility of the loops. This could also account for the overall low stability of the reduced protein. However, a significant motion is already present in the oxidized form and appears to be an intrinsic feature of the enzyme.
Evidence for Two Conformations in the Reduced Protein—When analyzing the two-dimensional NOESY spectra of reduced Px III acquired at high field (900 MHz, 22 °C), we observed a duplication of at least 20 resonances that showed an exactly identical twin NOE pattern originating from two conformations. The intensities of the two conformations were comparable. Residues affected by the duplication extended well into the core of the protein. One-dimensional spectra acquired at different pH values or after carboxyamidomethylation of the cysteines revealed that the duplication arose from the redox active cysteine (supplemental Fig. S5). The analysis of the Ser47 and Ser95 mutants confirmed this. The ratio of the two conformations varied with temperature (studied range 4-29 °C), pH, and H2O versus D2O, e.g. at 4 °C, one conformation was dominant, whereas at 29 °C both populations were of similar intensity. Thus, instead of two irreversibly modified distinct species, we observed two interchanging conformations. Although the phenomenon awaits further accurate exploration, our data suggest that the two conformations observed in the reduced protein are caused by protonation/deprotonation of the cysteines, which might be relevant for the catalytic mechanism of the enzyme.
Conserved Lysine Residues Specifically Affect Catalysis—In the three structures of Px III, Lys97, Lys99 (both conserved in trypanosomatid and related plant proteins), and Lys107 (conserved throughout the entire protein family) are located in the vicinity of the redox active dithiol/disulfide on the surface of the protein pointing outward with their NzH3 groups (Figs. 3, A-C). To elucidate if these residues play a role in catalysis, Lys97 and Lys99 were replaced by glutamine and all three lysine residues by glutamate. One-dimensional spectra confirmed that the mutations did not affect the overall protein structure.
Gln99, Glu99, and Gln97 Px III showed 40-60% and Glu97 Px III 12% remaining activity, whereas Glu107 Px III had only 2% wild type activity left (Table 3). The expression system introduced in this work yielded Px III species that were significantly more stable than the previously prepared recombinant protein (5, 17, 18). In addition, the wild type enzyme obtained here exerted a three times higher specific activity and displayed saturation kinetics. To investigate which of the two half reactions, namely the reduction of H2O2 by reduced Px III or the regeneration of reduced Px III by Tpx (Scheme 2), is affected by the replacement of the lysines, wild type, Glu97, Glu99, and Glu107 Px III were subjected to a detailed kinetic analysis (as shown for Glu97 and wild type Px III in supplemental Fig. S6). The Km values and the apparent second order rate constant k1′ of wild type, Glu97, and Glu99 Px III were in the same order of magnitude, whereas the k2′ value of Glu97 and Glu99 Px III was 10 and 5 times lower than that of the wild type enzyme, respectively (Table 3). This suggests that Lys97 and Lys99 play a role in the interaction between oxidized Px III and reduced Tpx.
TABLE 3.
Kinetic constants for the reduction of hydrogen peroxide by wild type and mutant Px III species
The Dalziel coefficient Φ0 (the ordinate intercept in the secondary plot, see supplemental Fig. S6C) equals E0/Vmax that is 1/kcat, Φ1 (the slope of the primary plot, supplemental Fig. S6, A and B) corresponds to 1/k1′, and Φ2 (the slope of the secondary plot, supplemental Fig. S6C) corresponds to 1/k2′, The k1′ and k2′ values refer to the overall rate constants for the oxidation and reduction of the peroxidase, respectively. Km1 (Φ1/Φ0) and Km2 (Φ2/Φ0) represent the Km values for hydrogen peroxide and tryparedoxin, respectively. The values are the mean of three independent series of experiments. ND, not determined.
| Px III | Activity | Φ0 | Φ1 | Φ2 | kcat | k1′ | k2′ | Km1 | Km2 |
|---|---|---|---|---|---|---|---|---|---|
| % | s | μm s | μm s | s−1 | μm−1 s−1 × 104 | μm−1 s−1 × 105 | μm | μm | |
| Wild type | 100 | 0.63 | 10.3 ± 2.4 | 2.0 | 1.7 | 9.7 ± 3.0 | 5.1 | 18 ± 4 | 3.4 |
| Glu97 | 12 | 1.93 | 15.9 ± 7.0 | 20.5 | 0.5 | 6.3 ± 5.0 | 0.5 | 8 ± 4 | 10.7 |
| Glu99 | 36 | 1.41 | 17.4 ± 7.0 | 10.6 | 0.7 | 6.3 ± 6.0 | 0.9 | 12 ± 7 | 7.5 |
| Glu107 | 2 | 15.5 | 3000 ± 320 | 2.3 | 0.1 | 0.0003 | 4.4 | 1930 ± 20 | 0.1 |
| Gln97 | 58 | ND | ND | ND | ND | ND | ND | ND | ND |
| Gln99 | 59 | ND | ND | ND | ND | ND | ND | ND | ND |
Strikingly, replacement of Lys107 did not influence the interaction with Tpx (k2′) but caused a drop of the k1′-value by more than 3 orders of magnitude and, thus, severely affected the reduction of H2O2. Because Glu107 Px III slowly reduced H2O2, the disulfide bond between Cys47 and Cys95 can still be formed. Otherwise Cys47 should have been irreversibly over-oxidized. As shown previously, reaction of Ser95 Px III with a stoichiometric concentration of H2O2 generates a sulfinic acid at Cys47 (18). Probably the nucleophilic attack of Cys47 on the peroxide substrate is impaired.
Because our structures do not reveal direct contact between Cys47 and Lys107, structural changes in loop 1 or loop 2 may account for the observed changes. In 14 of the 20 lowest energy NMR structures of reduced Px III, Lys107 interacts with the backbone or side chain hydroxyl group of Ser45 and/or Thr96 and, thus, stabilizes the conformation of these loops. Ser45 in its turn could activate Cys47 by another hydrogen bond. However, since the side chain resonances of Lys107 are found in highly overlapping areas of the NOESY spectra, this hypothesis needs a final confirmation by mutational analysis.
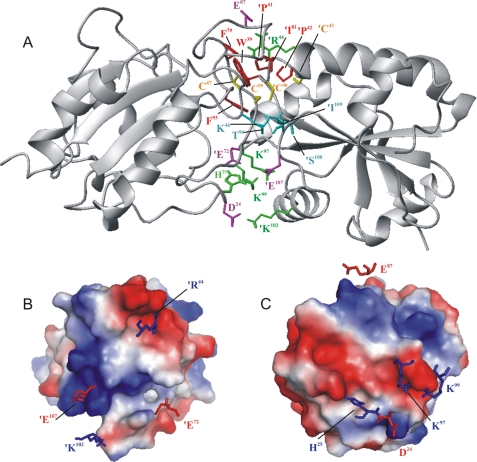
Modeling of the Interactions between Px III and Tpx—The structure of the complex between Px III and Tpx was modeled using HADDOCK (37, 38). The starting structures were the lowest energy NMR structure of the oxidized Px III and the x-ray structure of oxidized T. brucei Tpx (PDB code 1O73 (36)). In the absence of a structure of reduced Tpx, this approach seems justified because reduction of Tpx from C. fasciculata has only minor effects (36, 44). The expected structural change between oxidized and reduced Tpx was taken into account by defining the active site WCPPC motif fully flexible during the second stage of the docking.
Mass spectrometry data revealed that the intermolecular disulfide bridge is formed between Cys95 of Px III and Cys40 of Tpx (18). The two cysteines were, hence, treated as the center of the interaction. To create a tightly connected complex structure, the thiol hydrogen atoms of the reactive cysteines were removed, leaving more space for the sulfur atoms to approach each other. Lys97 and Lys99 of Px III were also incorporated in the interaction surface because of the mutagenesis results (Table 3).
The complex structure (Fig. 6A) contains 4 salt bridges, 1 hydrogen bond, and 21 hydrophobic contacts as identified by LIGPLOT (40). The buried surface area at the contact between the two protein molecules is 1350.46 Å2. The HADDOCK score achieved was -140 units as calculated from the weighted average of different parameters as described under “Experimental Procedures.” Vacuum electrostatic charges on the contacting surface are shown in Figs. 6, B and C. The cross-sections of the molecules revealed complementary electrostatic charges at the binding interface. Lys97 and Lys99 of Px III, identified by our mutational screen to interact with Tpx, are involved in a salt bridge with Glu107 of Tpx. This could explain why the Gln mutants retained a higher activity than the respective Glu mutants. Glutamine residues could still interact with the glutamate of Tpx through formation of hydrogen bonds, whereas the proximity of glutamate residues should lead to electrostatic repulsion.
FIGURE 6.
Model of the complex between Px III and Tpx. The complex between Px III (left) and Tpx (right, primed residues) as derived from the docking calculation (A) is shown. Potential intermolecular contacts are shown. Yellow, cysteines; green, positively (green); magenta, negatively charged residues involved in salt bridges (Lys97 and Lys99 of Px III with Glu107 of Tpx, Asp24 of Px III with Lys102 of Tpx, Glu83, Glu87, and Glu88 of Px III with Arg44 of Tpx); cyan, residues involved in hydrogen bonds (Thr96 of Px III with the backbone of Ile109 of Tpx); red, residues undergoing hydrophobic contacts (Phe79, Val94, and Phe93 of Px III with Trp39, Pro41, and Pro42 of Tpx). B and C, electrostatic surface of the proteins calculated with PyMOL (35) showing the electrostatic interactions between charged residues at the interface. B, the side chains of Tpx that interact with Px III are displayed together with a surface representation of Px III. C, same for side chains of Px III that interact with Tpx.
DISCUSSION
Eight structures of glutathione peroxidase-type enzymes are currently available. The majority of them (bovine GPX1 and human GPX1-4) represent the classical selenoenzymes, either the authentic proteins or mutants where the selenocysteine is replaced by different residues. The other two structures show cysteine-containing homologues (human GPX5 and GPX7), but these proteins lack a second redox active cysteine and, thus, are unable to form an intramolecular disulfide bridge. Thioredoxin-dependent poplar TxP is the only peroxidase that, as does Px III, cycles between a reduced dithiol form and an oxidized form containing an intramolecular disulfide bridge (16).
Poplar TxP undergoes a remarkable structural rearrangement upon oxidation previously observed for both typical and atypical 2-Cys peroxiredoxins (Refs. 45 and 46, and for a comprehensive review, see Ref. 47). The reduced form shows a catalytic triad with Glu82 and Trp133 stabilizing the peroxidatic cysteine analogous to GPXs (16). Upon oxidation, α-helix 3 unwinds, which leads to a long loop that extends from the core of the protein and allows the peroxidatic cysteine to approach the resolving one to form a disulfide bridge, disrupting the arrangement of the catalytic triad. Although the monomeric T. brucei Px III and the dimeric poplar TxP show 48% overall sequence identity, our structural data revealed that Px III does not undergo such a dramatic conformational change. Reduced and oxidized Px III have essentially the same structure, and the conserved Gln82 and Trp133 do not form a catalytic triad with Cys47. This was demonstrated by the unchanged chemical shifts for both residues and identical NOE patterns, especially for Trp137, loop 1, and loop 2.
Treatment of reduced Px III with 0.5 mm glutathione disulfide leads to glutathionylation of either Cys47 or Cys95 but not of both residues in the same protein molecule (48). Modification of one cysteine probably interferes sterically with the modification of the second one in accordance with the structural data that also in the reduced form of Px III both cysteines lie in close proximity. This would allow the rapid condensation between the sulfenic acid formed at Cys47 upon hydroperoxide reduction and the thiol of Cys95 generating the intramolecular disulfide bond of the oxidized enzyme.
The different catalytic mechanism of T. brucei Px III and the poplar TxP is further corroborated by the tryptophan fluorescence of the proteins. Whereas the spectra of poplar TxP reflect a switch from a hydrophobic environment in the reduced state to an exposed conformation in the oxidized state (43), the tryptophan fluorescence emission spectra of oxidized and reduced Px III are identical and in accordance with an exposed position of Trp137 in both forms (supplemental Fig. S4).
Is it possible that despite a sequence identity of nearly 50%, the structures differ so considerably? The paradigm of homology modeling efforts and structural genomic initiatives is that related sequences lead to homologous structures (49) hoping that prediction can mostly replace tedious structure determination. In general, a sequence identity of 50% is regarded as a good basis for a relatively safe prediction. However, a recent publication indicates that this goal is not always achievable. Bryan and co-workers inter-converted all residues of a GA and a GB domain of protein G, a cell wall protein from Streptococcus. 5 of 45 residues were shown to be sufficient to change the structure of an 3-α-helix-fold into an α/β-fold (50). Likewise, the insertion of three further residues into the sequence converts Gal3p, a protein of the GAL pathway in Saccharomyces cerevisiae, from a transcriptional inhibitor to an activator of the pathway, changing it into a galactokinase (51). Thus, small changes in the protein sequence can lead to huge and crucial differences in the three-dimensional structure or function that render predictions not entirely reliable.
The NMR structure of oxidized and reduced Px III revealed that the three loops containing Cys47, Cys95, and Trp137 are highly flexible, especially in the reduced protein. In particular, the residues between Gln82 and Phe98 do not show long range NOEs, in accordance with loop 2 not being intimately connected to the core of the protein. Previous studies of Akke and Kern and co-workers (52) have revealed that enzymes may not have to rearrange their enzymatic pockets in an induced fit but that the substrate appears to select the correct one among many allowed conformations. A similar mechanism could be the case for Px III. Binding of a substrate could trap one conformation leading to a stabilization of the loops. Unfortunately, hydroperoxides are rather unstable, so that this could not be investigated by NMR or x-ray analyses. The fact that several residues show line-broadening in the 1H15N HSQC spectra corroborates the idea that these loops and adjacent regions undergo micro- or millisecond motions. Upon formation of the disulfide bridge, the entire structure is stabilized, which is reflected in the higher thermal stability and the overall higher quality of the NMR spectra. When the disulfide bridge opens, the loops may undergo a swaying motion.
In the structures of Px III, Cys47, Gln82, and Trp137 are located far apart from each other and not in direct contact, excluding the formation of the canonical catalytic triad. The specific role of these residues is also not easy to analyze in other members of the family because they are not necessarily conserved, at least in the cysteine-containing glutathione peroxidase-type enzymes. In TxP, the glutamine residue of the catalytic triad is replaced by a glutamate (Glu79), but the structural arrangement is comparable with that in the selenocysteine enzymes. A respective mutation in GPX4 severely affected the catalytic efficiency of the protein (13, 42), but in the TxP of Chinese cabbage (19), a glutamine to glycine mutation resulted in a fully active enzyme species.
One interesting aspect is how the peroxidatic Cys47 is activated. In many cases the low pK value of a specific cysteine residue is difficult to explain. Despite a number of known structures and detailed biochemical studies, it is still not clear why the N-terminal Cys of the CXXC motif in glutaredoxins has a pK value significantly lower than that of a free cysteine, whereas the pK value of the C-terminal active site Cys has a pK value higher than that of free cysteine (53). A common hypothesis is that conserved basic residues (Arg26 and Lys19 in pig glutaredoxin) (54) or an arginine in peroxiredoxins (55) stabilize the thiolate of the more N-terminal cysteine (54). However, a recent molecular dynamics simulation suggests that cationic side chains contribute little to the activation. Instead, the thiolate appears to be stabilized by the backbone NH groups of the two following residues (53). The structures of Px III did not reveal any positively charged residue in the vicinity of the active site thiolate, and one may speculate that the thiolate of Cys47 in reduced Px III is also stabilized by interaction with the backbone. However, one should bear in mind that due to a lack of unambiguous NOEs of the residues close to Cys47, the exact orientation of several residues in the active site could not be determined by NMR.
The active site of Px III shows a very open conformation. The immediate environment of the redox active dithiol/disulfide is formed exclusively by residues of the loops 1 and 2 that contain Cys47 and Cys95, respectively. In comparison, the active sites of poplar TxP as well as of GPX1, GPX3, and GPX4 also comprise residues of the loops between α-helix 2 and β-strand 3 as well as between β-strand 4 and α-helix 3 in Px III (11, 16). Furthermore, the active site of these enzymes is shielded by an adjacent subunit. The exposed redox active cysteines of Px III would render the protein perfectly suited for interactions with bulky substrates and may also be a prerequisite for an efficient reduction by Tpx.
Glutathione peroxidase-type peroxidase-3 in yeast does not act only as a hydroperoxide scavenger but also as a hydroperoxide sensor (56). The sulfenic acid residue generated in the peroxidase reacts with a cysteine of the transcription factor Yap1. This intermolecular disulfide is then transposed to an intramolecular disulfide in Yap1, causing its activation. Future work will reveal if Px III is part of a redox signaling cascade in trypanosomes.
The PDB coordinates have been deposited under accession numbers 3dwv (oxidized protein, x-ray structure), 2rm5 (oxidized protein, NMR structure), 2rm6 (reduced protein, NMR structure).
Note Added in Proof—While this manuscript was under revision, Fairlamb and colleagues published the crystal structure of reduced T. brucei Px II (2VUP; Alphey, M. S., König, J., and Fairlamb, A. H. (2008) Biochem. J. 414, 375-381). This structure is similar to the crystal structure of the poplar TxP but differs from our solution structure in the details of the catalytic site. Future work is needed to resolve the discrepancies between the structures.
Supplementary Material
Acknowledgments
The modified pET-vector was kindly provided by Gunter Stier, EMBL. We thank Dr. Tanja Schlecker for helping with the crystallization of the peroxidase. We further thank staff at the European Synchrotron Radiation Facility, Grenoble, France, Dr. Klemens Wild, BZH Heidelberg for help with X-ray data collection, and Dr. Thomas Barends, MPI for Medical Research, Heidelberg, for helpful discussions on crystal twinning. High field NOESY spectra were recorded at the Frankfurt Facility for Biomolecular NMR, which is funded by the European Union project “EU-NMR-European Network of Research Infrastructures for Providing Access and Technological Advancement in Bio-NMR” (FP-2005-RII3 Contract 026145).
The atomic coordinates and structure factors (codes 3dwv, 2rm5, and 2rm6) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported by Deutsche Forschungsgemeinschaft SFB 544, project B3 (to L. K.-S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S6.
Footnotes
The abbreviations used are: Tpx, tryparedoxin; HSQC, heteronuclear single quantum correlation spectroscopy; GPX, classical selenocysteine-containing glutathione peroxidase; NOESY, nuclear Overhauser effect (NOE) spectroscopy; HetNOE, heteronuclear NOE; Px, glutathione peroxidase-type tryparedoxin peroxidase; r.m.s.d., root mean square deviation; TxP, glutathione peroxidase-type thioredoxin peroxidase.
References
- 1.Fairlamb, A. H., and Cerami, A. (1992) Annu. Rev. Microbiol. 46 695-729 [DOI] [PubMed] [Google Scholar]
- 2.Krauth-Siegel, R. L., Bauer, H., and Schirmer, R. H. (2005) Angew. Chem. Int. Ed. Engl. 44 690-715 [DOI] [PubMed] [Google Scholar]
- 3.Krauth-Siegel, R. L., Comini, M. A., and Schlecker, T. (2007) Subcell. Biochem. 44 231-251 [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson, S. R., Horn, D., Prathalingam, S. R., and Kelly, J. M. (2003) J. Biol. Chem. 278 31640-31646 [DOI] [PubMed] [Google Scholar]
- 5.Schlecker, T., Schmidt, A., Dirdjaja, N., Voncken, F., Clayton, C., and Krauth-Siegel, R. L. (2005) J. Biol. Chem. 280 14385-14394 [DOI] [PubMed] [Google Scholar]
- 6.Brigelius-Flohé, R. (2006) Biol. Chem. 387 1329-1335 [DOI] [PubMed] [Google Scholar]
- 7.Brigelius-Flohé, R., and Flohé, L. (2003) Biofactors 17 93-102 [DOI] [PubMed] [Google Scholar]
- 8.Utomo, A., Jiang, X., Furuta, S., Yun, J., Levin, D. S., Wang, Y. C., Desai, K. V., Green, J. E., Chen, P. L., and Lee, W. H. (2004) J. Biol. Chem. 15 43522-43529 [DOI] [PubMed] [Google Scholar]
- 9.Herbette, S., Roeckel-Drevet, P., and Drevet, J. R. (2007) FEBS J. 274 2163-2180 [DOI] [PubMed] [Google Scholar]
- 10.Maiorino, M., Ursini, F., Bosello, V., Toppo, S., Tosatto, S. C., Mauri, P., Becker, K., Roveri, A., Bulato, C., Benazzi, L., De Palma, A., and Flohé, L. (2007) J. Mol. Biol. 365 1033-1046 [DOI] [PubMed] [Google Scholar]
- 11.Ren, B., Huang, W., Akesson, B., and Ladenstein, R. (1997) J. Mol. Biol. 268 869-885 [DOI] [PubMed] [Google Scholar]
- 12.Epp, O., Ladenstein, R., and Wendel, A. (1983) Eur. J. Biochem. 133 51-69 [DOI] [PubMed] [Google Scholar]
- 13.Maiorino, M., Aumann, K. D., Brigelius-Flohé, R., Doria, D., van den Heuvel, J., McCarthy, J., Roveri, A., Ursini, F., and Flohé, L. (1995) Biol. Chem. 376 651-660 [DOI] [PubMed] [Google Scholar]
- 14.Aumann, K. D., Bedorf, N., Brigelius-Flohé, R., Schomburg, D., and Flohé, L. (1997) Biomed. Environ. Mass Spectrom. 10 136-155 [PubMed] [Google Scholar]
- 15.Prabhakar, R., Vreven, T., Morokuma, K., and Musaev, D. G. (2005) Biochemistry 44 11864-11871 [DOI] [PubMed] [Google Scholar]
- 16.Koh, C. S., Didierjean, C., Navrot, N., Panjikar, S., Mulliert, G., Rouhier, N., Jacquot, J. P., Aubry, A., Shawkataly, O., and Corbier, C. (2007) J. Mol. Biol. 370 512-529 [DOI] [PubMed] [Google Scholar]
- 17.Hillebrand, H., Schmidt, A., and Krauth-Siegel, R. L. (2003) J. Biol. Chem. 278 6809-6815 [DOI] [PubMed] [Google Scholar]
- 18.Schlecker, T., Comini, M. A., Melchers, J., Ruppert, T., and Krauth-Siegel, R. L. (2007) Biochem. J. 405 445-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung, B. G., Lee, K. O., Lee, S. S., Chi, Y. H., Jang, H. H., Kang, S. S., Lee, K., Lim, D., Yoon, S. C., Yun, D. J., Inoue, Y., Cho, M. J., and Lee, S. Y. (2002) J. Biol. Chem. 277 12572-12578 [DOI] [PubMed] [Google Scholar]
- 20.Lüdemann, H., Dormeyer, M., Sticherling, C., Stallmann, D., Follmann, H., and Krauth-Siegel, R. L. (1998) FEBS Lett. 431 381-385 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan, F. X., and Walsh, C. T. (1991) Mol. Biochem. Parasitol. 44 145-147 [DOI] [PubMed] [Google Scholar]
- 22.Melchers, J., Krauth-Siegel, R. L., and Muhle-Goll, C. (2008) Biomol. NMR Assignments 2 65-68 [DOI] [PubMed] [Google Scholar]
- 23.Otwinowski, Z., and Minor, W. (1997) Methods Enzymol. 276 307-326 [DOI] [PubMed] [Google Scholar]
- 24.Collaborative Computational Project (1994) Acta Crystallogr. 50 760-763 [Google Scholar]
- 25.Perrakis, A., Morris, R., and Lamzin, V. S. (1999) Nat. Struct. Biol. 6 458-463 [DOI] [PubMed] [Google Scholar]
- 26.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 27.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 28.Sheldrick, G. M. (2008) Acta Crystallogr. 64 112-122 [DOI] [PubMed] [Google Scholar]
- 29.Delaglio, F., Grzesiek, S., Vuister, G. W., Zhu, G., Pfeifer, J., and Bax, A. (1995) J. Biomol. NMR 6 277-293 [DOI] [PubMed] [Google Scholar]
- 30.Johnson, B. A., and Blevins, R. A. (1994) J. Biomol. NMR 4 603-614 [DOI] [PubMed] [Google Scholar]
- 31.Linge, J. P., O'Donoghue, S. I., and Nilges, M. (2001) Methods Enzymol. 339 71-90 [DOI] [PubMed] [Google Scholar]
- 32.Cornilescu, G., Delaglio, F., and Bax, A. (1999) J. Biomol. NMR 13 289-302 [DOI] [PubMed] [Google Scholar]
- 33.Laskowski, R. A., Rullmannn, J. A., MacArthur, M. W., Kaptein, R., and Thornton, J. M. (1996) J. Biomol. NMR 8 477-486 [DOI] [PubMed] [Google Scholar]
- 34.Koradi, R., Billeter, M., and Wüthrich, K. (1996) J. Mol. Graph. 14 29-32 [DOI] [PubMed] [Google Scholar]
- 35.DeLano, W. L. (2002) DeLano Scientific, Palo Alto, CA
- 36.Alphey, M. S., Gabrielsen, M., Micossi, E., Leonard, G. A., McSweeney, S. M., Ravelli, R. B., Tetaud, E., Fairlamb, A. H., Bond, C. S., and Hunter, W. N. (2003) J. Biol. Chem. 278 25919-25925 [DOI] [PubMed] [Google Scholar]
- 37.Dominguez, C., Boelens, R., and Bonvin, A. M. (2003) J. Am. Chem. Soc. 125 1731-1737 [DOI] [PubMed] [Google Scholar]
- 38.de Vries, S. J., van Dijk, A. D., Krzeminski, M., van Dijk, M., Thureau, A., Hsu, V., Wassenaar, T., and Bonvin, A. M. (2007) Proteins 69 726-733 [DOI] [PubMed] [Google Scholar]
- 39.Hubbard, S. J., Campbell, S. F., and Thornton, J. M. (1991) J. Mol. Biol. 220 507-530 [DOI] [PubMed] [Google Scholar]
- 40.Wallace, A. C., Laskowski, R. A., and Thornton, J. M. (1995) Prot. Eng. 8 127-134 [DOI] [PubMed] [Google Scholar]
- 41.Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T., and Warren, G. L. (1998) Acta Crystallogr. D. Biol. Crystallogr. 54 905-921 [DOI] [PubMed] [Google Scholar]
- 42.Scheerer, P., Borchert, A., Krauss, N., Wessner, H., Gerth, C., Höhne, W., and Kuhn, H. (2007) Biochemistry 46 9041-9049 [DOI] [PubMed] [Google Scholar]
- 43.Navrot, N., Collin, V., Gualberto, J., Gelhaye, E., Hirasawa, M., Rey, P., Knaff, D. B., Issakidis, E., Jacquot, J. P., and Rouhier, N. (2006) Plant Physiol. 142 1364-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann, B., Budde, H., Bruns, K., Guerrero, S. A., Kalisz, H. M., Menge, U., Montemartini, M., Nogoceke, E., Steinert, P., Wissing, J. B., Flohé, L., and Hecht, H. J. (2001) Biol. Chem. 382 459-471 [DOI] [PubMed] [Google Scholar]
- 45.Hirotsu, S., Abe, Y., Okada, K., Nagahara, N., Hori, H., Nishino, T., and Hakoshima, T. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 12333-12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evrard, C., Capron, A., Marchand, C., Clippe, A., Wattiez, R., Soumillion, P., Knoops, B., and Declercq, J. P. (2004) J. Mol. Biol. 337 1079-1090 [DOI] [PubMed] [Google Scholar]
- 47.Karplus, P. A., and Hall, A. (2007) Subcell. Biochem. 44 41-60 [DOI] [PubMed] [Google Scholar]
- 48.Melchers, J., Dirdjaja, N., Ruppert, T., and Krauth-Siegel, R. L. (2007) J. Biol. Chem. 282 8678-8694 [DOI] [PubMed] [Google Scholar]
- 49.Burley, S. K., and Bonanno, J. B. (2002) Curr. Opin. Struct. Biol. 12 383-391 [DOI] [PubMed] [Google Scholar]
- 50.Alexander, P. A., He, Y., Chen, Y., Orban, J., and Bryan, P. N. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 11963-11968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platt, A., Ross, H. C., Hankin, S., and Reece, R. J. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 3154-3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisenmesser, E. Z., Bosco, D. A., Akke, M., and Kern, D. (2002) Science 295 1520-1523 [DOI] [PubMed] [Google Scholar]
- 53.Foloppe, N., and Nilsson, L. (2007) J. Mol. Biol. 372 798-816 [DOI] [PubMed] [Google Scholar]
- 54.Yang, Y. F., and Wells, W. W. (1991) J. Biol. Chem. 266 12759-12765 [PubMed] [Google Scholar]
- 55.Wood, Z. A., Schröder, E., Robin Harris, J., and Poole, L. B. (2003) Trends Biochem. Sci. 28 32-40 [DOI] [PubMed] [Google Scholar]
- 56.Delaunay, A., Pflieger, D., Barrault, M. B., Vinh, J., and Toledano, M. B. (2002) Cell 111 471-481 [DOI] [PubMed] [Google Scholar]
- 57.Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997) Nucleic Acids Res. 24 4876-4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.