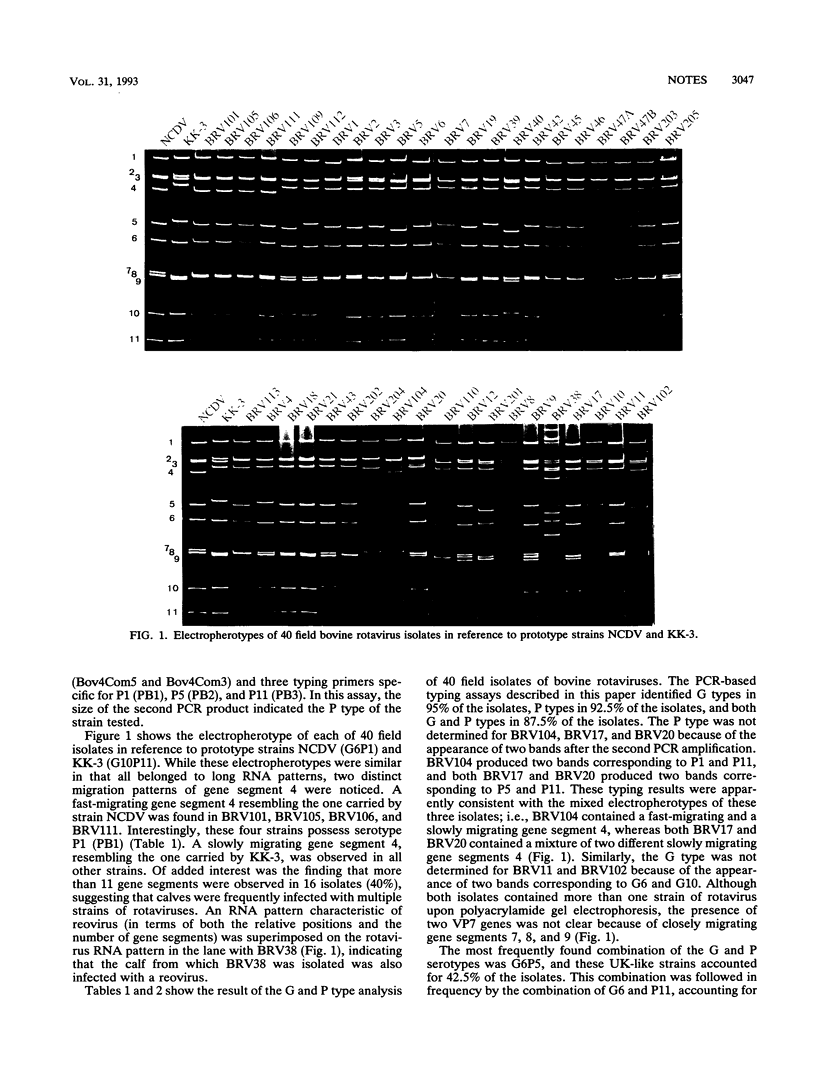
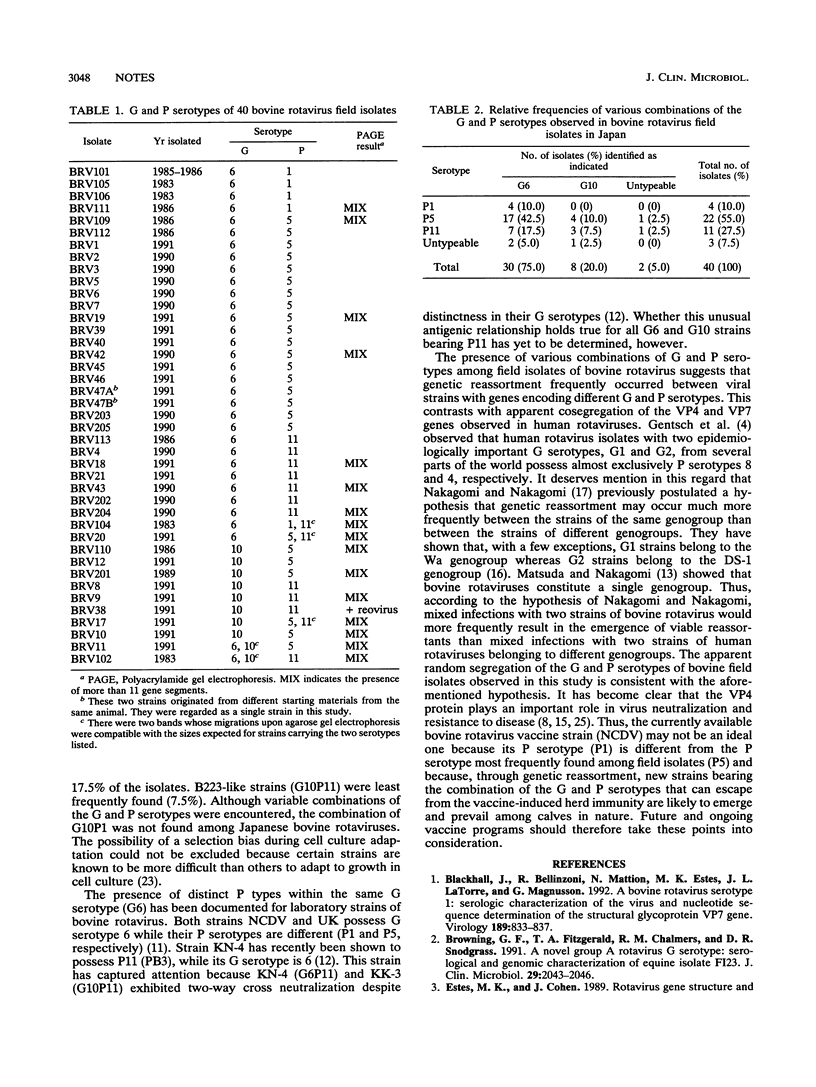
Abstract
The relative frequencies of both the G (VP7) and P (VP4) serotypes of 40 bovine rotaviruses isolated in cell culture from diarrheic calves in Japan between January 1983 and February 1991 were determined by recently developed polymerase chain reaction assays. Isolates with G serotype 6 and P serotype 5 (UK-like strains) were most frequently found (42.5%) followed by isolates with G6P11 (17.5%), G6P1 (10%), or G10P5 (10%). Isolates with G10P11 (B223-like strains) were least frequently found (7.5%). The presence of various combinations of G and P serotypes suggests frequent reassortment in nature among bovine rotaviruses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackhall J., Bellinzoni R., Mattion N., Estes M. K., La Torre J. L., Magnusson G. A bovine rotavirus serotype 1: serologic characterization of the virus and nucleotide sequence determination of the structural glycoprotein VP7 gene. Virology. 1992 Aug;189(2):833–837. doi: 10.1016/0042-6822(92)90617-x. [DOI] [PubMed] [Google Scholar]
- Browning G. F., Fitzgerald T. A., Chalmers R. M., Snodgrass D. R. A novel group A rotavirus G serotype: serological and genomic characterization of equine isolate FI23. J Clin Microbiol. 1991 Sep;29(9):2043–2046. doi: 10.1128/jcm.29.9.2043-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J. R., Glass R. I., Woods P., Gouvea V., Gorziglia M., Flores J., Das B. K., Bhan M. K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992 Jun;30(6):1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M. E., Gorziglia M., Woode G. N. Amino acid sequence analysis of bovine rotavirus B223 reveals a unique outer capsid protein VP4 and confirms a third bovine VP4 type. Virology. 1992 Nov;191(1):291–300. doi: 10.1016/0042-6822(92)90191-q. [DOI] [PubMed] [Google Scholar]
- Hardy M. E., Woode G. N., Xu Z. C., Gorziglia M. Comparative amino acid sequence analysis of VP4 for VP7 serotype 6 bovine rotavirus strains NCDV, B641, and UK. J Virol. 1991 Oct;65(10):5535–5538. doi: 10.1128/jvi.65.10.5535-5538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Saif L. J., Sereno M. M., Chanock R. M., Kapikian A. Z. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J Virol. 1988 Mar;62(3):744–748. doi: 10.1128/jvi.62.3.744-748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isegawa Y., Nakagomi O., Nakagomi T., Ishida S., Uesugi S., Ueda S. Determination of bovine rotavirus G and P serotypes by polymerase chain reaction. Mol Cell Probes. 1993 Aug;7(4):277–284. doi: 10.1006/mcpr.1993.1041. [DOI] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Tregear G. W., Holmes I. H. Nucleotide sequence of UK bovine rotavirus segment 4: possible host restriction of VP3 genes. Virology. 1988 Oct;166(2):308–315. doi: 10.1016/0042-6822(88)90501-6. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Isegawa Y., Woode G. N., Zheng S., Kaga E., Nakagomi T., Ueda S., Nakagomi O. Two-way cross-neutralization mediated by a shared P (VP4) serotype between bovine rotavirus strains with distinct G (VP7) serotypes. J Clin Microbiol. 1993 Feb;31(2):354–358. doi: 10.1128/jcm.31.2.354-358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Nakagomi O. Antigenic and molecular characterization of bovine rotaviruses isolated in Japan. Res Virol. 1989 Jul-Aug;140(4):337–350. doi: 10.1016/s0923-2516(89)80114-1. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Nakagomi O., Offit P. A. Presence of three P types (VP4 serotypes) and two G types (VP7 serotypes) among bovine rotavirus strains. Arch Virol. 1990;115(3-4):199–207. doi: 10.1007/BF01310530. [DOI] [PubMed] [Google Scholar]
- Matsui S. M., Mackow E. R., Greenberg H. B. Molecular determinant of rotavirus neutralization and protection. Adv Virus Res. 1989;36:181–214. doi: 10.1016/s0065-3527(08)60585-0. [DOI] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T. Genetic diversity and similarity among mammalian rotaviruses in relation to interspecies transmission of rotavirus. Arch Virol. 1991;120(1-2):43–55. doi: 10.1007/BF01310948. [DOI] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T. Molecular evidence for naturally occurring single VP7 gene substitution reassortant between human rotaviruses belonging to two different genogroups. Arch Virol. 1991;119(1-2):67–81. doi: 10.1007/BF01314324. [DOI] [PubMed] [Google Scholar]
- Nakagomi T., Akatani K., Ikegami N., Katsushima N., Nakagomi O. Occurrence of changes in human rotavirus serotypes with concurrent changes in genomic RNA electropherotypes. J Clin Microbiol. 1988 Dec;26(12):2586–2592. doi: 10.1128/jcm.26.12.2586-2592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwani A. V., Rosen B. I., Flores J., McCrae M. A., Gorziglia M., Saif L. J. Detection and differentiation of bovine group A rotavirus serotypes using polymerase chain reaction-generated probes to the VP7 gene. J Vet Diagn Invest. 1992 Apr;4(2):148–158. doi: 10.1177/104063879200400206. [DOI] [PubMed] [Google Scholar]
- Parwani A. V., Rosen B. I., McCrae M. A., Saif L. J. Development of cDNA probes for typing group A bovine rotaviruses on the basis of VP4 specificity. J Clin Microbiol. 1992 Oct;30(10):2717–2721. doi: 10.1128/jcm.30.10.2717-2721.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Green K. Y. Human rotavirus strain 69M has a unique VP4 as determined by amino acid sequence analysis. Virology. 1991 May;182(1):407–412. doi: 10.1016/0042-6822(91)90691-4. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Fitzgerald T., Campbell I., Scott F. M., Browning G. F., Miller D. L., Herring A. J., Greenberg H. B. Rotavirus serotypes 6 and 10 predominate in cattle. J Clin Microbiol. 1990 Mar;28(3):504–507. doi: 10.1128/jcm.28.3.504-507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Hoshino Y., Fitzgerald T. A., Smith M., Browning G. F., Gorziglia M. Identification of four VP4 serological types (P serotypes) of bovine rotavirus using viral reassortants. J Gen Virol. 1992 Sep;73(Pt 9):2319–2325. doi: 10.1099/0022-1317-73-9-2319. [DOI] [PubMed] [Google Scholar]
- Xu Z., Hardy M. E., Williams J. D., Woode G. N., Ramig R. F. Immunodominant neutralizing antigens depend on the virus strain during a primary immune response in calves to bovine rotaviruses. Vet Microbiol. 1993 May;35(1-2):33–43. doi: 10.1016/0378-1135(93)90114-m. [DOI] [PubMed] [Google Scholar]