Abstract
IFNγ, once called the macrophage-activating factor, stimulates many genes in macrophages, ultimately leading to the elicitation of innate immunity. IFNγ's functions depend on the activation of STAT1, which stimulates transcription of IFNγ-inducible genes through the GAS element. The IFN consensus sequence binding protein (icsbγ or IFN regulatory factor 8), encoding a transcription factor of the IFN regulatory factor family, is one of such IFNγ-inducible genes in macrophages. We found that macrophages from ICSBP−/− mice were defective in inducing some IFNγ-responsive genes, even though they were capable of activating STAT1 in response to IFNγ. Accordingly, IFNγ activation of luciferase reporters fused to the GAS element was severely impaired in ICSBP−/− macrophages, but transfection of ICSBP resulted in marked stimulation of these reporters. Consistent with its role in activating IFNγ-responsive promoters, ICSBP stimulated reporter activity in a GAS-specific manner, even in the absence of IFNγ treatment, and in STAT1 negative cells. Indicative of a mechanism for this stimulation, DNA affinity binding assays revealed that endogenous ICSBP was recruited to a multiprotein complex that bound to GAS. These results suggest that ICSBP, when induced by IFNγ through STAT1, in turn generates a second wave of transcription from GAS-containing promoters, thereby contributing to the elicitation of IFNγ's unique activities in immune cells.
IFNs are pleiotropic cytokines that play a major role in the host defense against microbial pathogens (1–3). Although IFNα and IFNβ are produced by many cell types and confer antiviral activities on them, IFNγ is produced by T lymphocytes and natural killer cells when stimulated by the macrophage-derived cytokine, IL-12. IFNγ elicits broad effects, particularly on cells of the immune system (1, 2). IFNγ was previously called the “macrophage activation factor,” and indeed, it plays particularly important roles in macrophages, which include elicitation of antipathogenic activity and antitumor activity, stimulation of chemokine/cytokine production, and enhanced antigen presentation. Activation of the JAK/STAT pathway is the first event in IFNγ signal transduction (4, 5). Binding of IFNγ to the receptor (IFNγR1 and IFNγR2; ref. 6) results in activation of JAK1 and JAK2 kinases, which phosphorylate the latent transcription factor STAT1 (7). STAT1 is then translocated into the nucleus to bind to the IFNγ activation site (GAS), after which transcriptional induction of IFNγ-responsive genes ensues (5). The GAS element is found in numerous IFNγ-inducible genes, many of which are expressed specifically in the immune system (8). Studies with stat1−/− mice as well as human cells lacking STAT1 expression have established that STAT1 is required for IFNγ-dependent transcription and for its biological activities (9–11). Transcription factors induced by STAT1, such as CIITA, in turn stimulate their own target genes, generating diversity in IFNγ-dependent gene expression patterns (12). Other factors including IFNγ receptors and additional proteins interacting with JAK signaling pathways also contribute to the variability in gene expression patterns in response to IFNγ (7, 13).
IFN consensus sequence binding protein (ICSBP), a member of the IFN regulatory factor (IRF) family (14), is an IFNγ-inducible, immune-system-specific transcription factor (15, 16). ICSBP is induced by IFNγ through the GAS sequence present in its promoter (17). Similar to several other members of the IRF family (18), ICSBP represses IFNα/β-inducible promoters through the IFN-stimulated responsive element (ISRE; refs. 19 and 20). However, studies of ICSBP knockout mice indicated that ICSBP is required for establishing IFNγ-mediated resistance to various pathogens (21–23). This unexpected deficiency in IFNγ-dependent host defenses prompted us to examine the role of ICSBP in IFNγ-dependent transcription. Herein, we report that ICSBP is capable of stimulating transcription from IFNγ-inducible promoters in a GAS-dependent manner. Our results raise the possibility that ICSBP is a late-acting activator of IFNγ-responsive genes involved in the elicitation of IFNγ's unique activities in the immune cells.
Materials and Methods
Transfection.
Murine macrophage-like RAW264.7 (RAW) cells and human 2fTGH, U3A, U4A, and γ1A cells (refs. 11 and 24; 105 cells) were transfected with 10–50 ng of luciferase reporter containing four copies of the wild-type (WT) GAS element from the ICSBP promoter or mutant (mt) GAS (see Fig. 1f) connected to the herpes virus thymidine kinase gene promoter (17) and 0.4–1.6 μg of ICSBP expression vector (19) or a control vector without insert (LK440) by using the SuperFect reagent (Qiagen, Chatsworth, CA). As controls, cells were transfected with a luciferase reporter containing the ISRE (25) and/or expression vectors for IRF-1 (pAct1) or IRF-2 (pAct2; ref. 18; both were gifts from T. Taniguchi, University of Tokyo, Tokyo) in the same manner, and luciferase activity was measured 16–24 h later. CL-2 cells (ICSBP−/−) were established from ICSBP−/− bone marrow, which expressed the Mac-1 (CD11b) but not GR-1 marker and which expressed IL-1α and IL-1β in response to IFNγ plus lipopolysaccharide as RAW cells (I.-M.W., C.C., A. Masumi, X. Ma., G. Trinchieri, and K.O., unpublished work). Reporter (10 μg) and expression vectors (30 μg) were transfected into 107 CL-2 cells by electroporation, and luciferase activity was measured 16 h later. When indicated, mouse or human recombinant (r)IFNγ (a gift from G. Adolf, Boehringer Ingelheim, Bender, Austria) was added at 200 units/ml 6–12 h before harvest. Reporter activity was normalized according to the activity of cotransfected β-galactosidase gene in the early stage and to protein concentrations in the later stage. ICSBP deletion constructs (120, 150, 250, and 350 in Fig. 2d) were constructed by cloning appropriate PCR fragments into the control vector LK440.
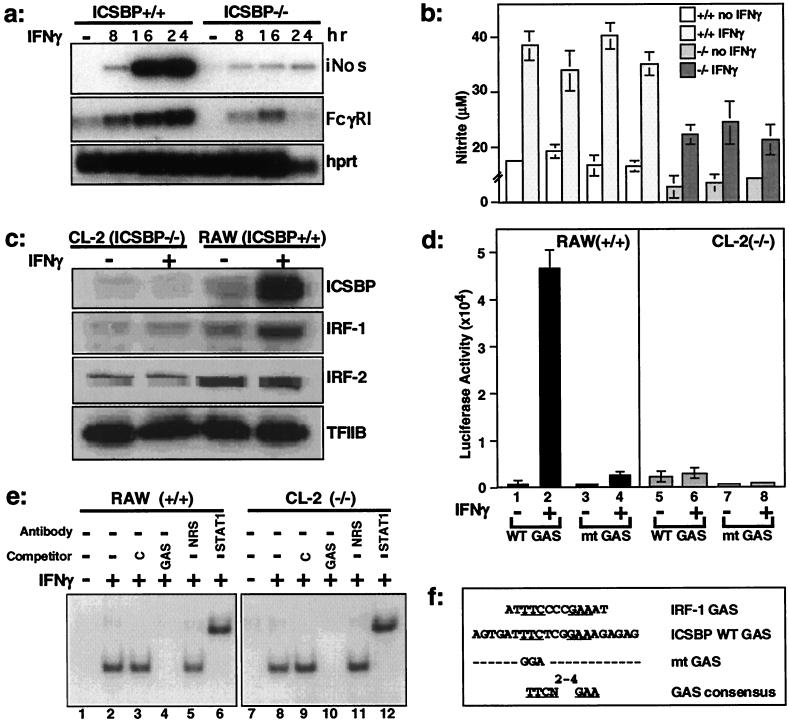
Figure 1.
Impaired IFNγ responsiveness in ICSBP−/− cells. (a) RNA expression of IFNγ-inducible genes was examined by quantitative reverse transcription–PCR in ICSBP+/+ or −/− peritoneal macrophages stimulated with IFNγ for indicated times. iNos, induction of nitric oxide synthase; FcγRI, Fcγ receptor I; hprt, hypoxanthine-guanine phosphoribosyl transferase. (b) NO production in ICSBP+/+ and −/− macrophages after stimulation with IFNγ for 72 h. The values represent the average of measurements from three independent pools of two to three animals ± SD. (c) IFNγ induction of IRF-1 and ICSBP in RAW and CL-2 cells. Immunoblotting was performed with cells treated with IFNγ for 12 h. (d) Impaired GAS reporter activity in ICSBP−/− cells. RAW and CL-2 cells were transiently transfected with luciferase reporters containing WT or mt GAS. Values represent five determinations ±SD. (e) Electrophoretic mobility-shift assay analysis was performed with 10 μg of nuclear extracts from RAW or CL-2 cells treated with IFNγ for 8 h by using 32P-labeled, single-copy WT-GAS oligonucleotide as a probe. (f) GAS sequences (8, 17); the ICSBP WT and mt sequences were used in this work.
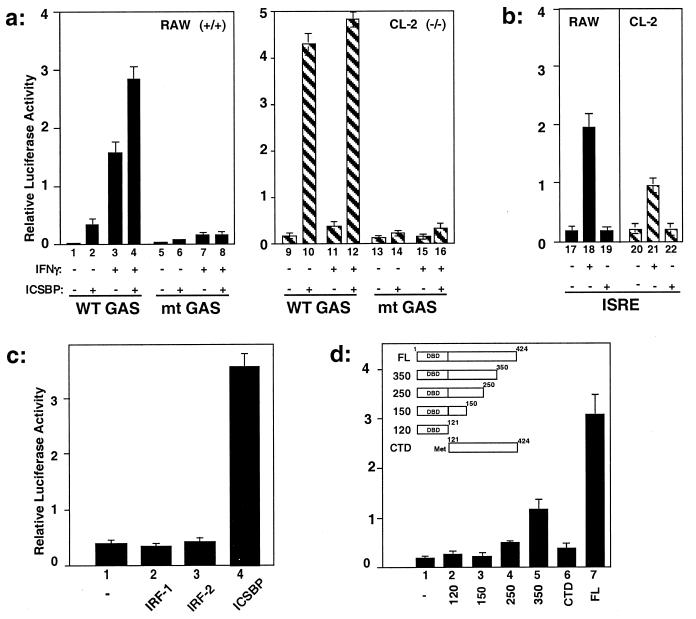
Figure 2.
Stimulation of GAS reporter activity by ICSBP. (a) RAW or CL-2 cells were cotransfected with the WT GAS or mt GAS reporter as described for Fig. 1d, along with the ICSBP vector or empty vector, and treated with or without IFNγ as described for Fig. 1. (b) Absence of ISRE-reporter stimulation by ICSBP. Cells were cotransfected and assayed as described for a, except that the ISRE luciferase reporter was used. (c) RAW cells were cotransfected with WT GAS reporter and empty vector, vector for IRF-1, IRF-2, or ICSBP, and then luciferase activity was measured as described for a. (d) ICSBP domain analysis. RAW cells were cotransfected with the WT GAS reporter and indicated deletion constructs and assayed as described for a. DBD, DNA-binding domain; CTD, C-terminal domain; FL, full length.
DNA Affinity Binding Assay (26).
A biotinylated DNA fragment containing four copies of the WT GAS (Fig. 1e) was synthesized from the luciferase reporter by PCR. Biotinylated DNA (2 μg; ≈20 pmol) was conjugated to 100 μg of Dynabeads (M-280 Streptavidin, Dynal, Great Neck, NY) in buffer containing 10 mM Tris⋅HCl (pH 8.0), 1 mM EDTA, and 0.1 M NaCl. GAS-conjugated beads (10 μl) were incubated with 500 μg of nuclear extracts from RAW or CL-2 cells treated with or without IFNγ (200 units/ml), prepared as described in ref. 25. When indicated, rICSBP or rIRF-1 produced in baculovirus vectors (100 ng; ref. 26) was added to the nuclear extracts. Beads, extracts, and recombinant proteins were incubated at 4°C for 2 h in the presence of 20 μg of herring sperm DNA (Sigma). Bound materials were eluted in 20 μl of buffer containing 0.5% SDS and 1 M NaCl and were separated by SDS/4–20% gradient PAGE. Bound proteins were detected by immunoblot assays with rabbit antibodies against ICSBP, IRF-1, TFIIB, or STAT (15, 25).
Quantitative PCR, Immunoblot Analysis, and NO Production.
Peritoneal macrophages from ICSBBP+/+ and −/− mice were allowed to adhere (>85% pure) and were treated with murine IFNγ (100 units/ml). cDNA was constructed from total RNA (23). Serially diluted cDNA was subjected to PCR by using appropriate primers: 5′-acaagctgcatgtgacatcg-3′ and 5′-ggcaaagatgagctcatcca-3′ for mouse iNos (27), 5′-gtcactttatggtggtggaggg-3′ and 5′-tccatccgtgacacctcaag-3′ for FcγRI (28), and 5′-gttggatacaggctttgttg-3′ and 5′-gattcaacttgcgctcatcttaggc-3′ for HPRT (23). PCRs (32 cycles) were performed for iNos and HPRT, and 35 cycles were performed for FcγRI. PCR products were fractionated on a 1.3% agarose gel and hybridized with appropriate 32P-labeled probes. Immunoblot analysis was performed as described (15). For NO production, adherent macrophages were treated with IFNγ (200 units/ml) for 70 h and then stimulated with phorbol 12-myristate 13-acetate for 2 h. The amount of NO was measured by a colorimetric reaction with the Total Nitric Oxide Assay kit (R & D Systems; ref. 29).
Results
Impaired Induction of IFNγ-Responsive Genes in ICSBP−/− Macrophages.
IFNγ induction of iNos and FcγRI genes (27, 28) was tested in freshly isolated peritoneal macrophages from ICSBP−/− and ICSBP+/+ mice. These genes carry a functional GAS in their promoters (30, 31). As shown in Fig. 1a, induction of both genes was markedly reduced in ICSBP−/− cells relative to that in ICSBP+/+ cells. Accordingly, NO production was significantly reduced in IFNγ-treated ICSBP−/− macrophages compared with +/+ cells (Fig. 1b), confirming that the reduction in iNos mRNA is reflected in reduction of the gene products. We then tested IFNγ induction of IRF-1 and ICSBP proteins in two macrophage-like cell lines, CL-2 (ICSBP−/−) and RAW (ICSBP+/+) cells. CL-2 cells were established from ICSBP−/− bone marrow cells. In RAW cells, ICSBP and IRF-1 proteins were both induced by IFNγ. However, in CL-2 cells, IRF-1 was barely induced by IFNγ, whereas ICSBP was not expressed, as expected. Both IRF-1 and ICSBP carry a GAS element in their promoter (17, 32). IRF-2, tested as a control, was not induced by IFNγ, and its levels were comparable in the two types of cells. These results indicated that ICSBP−/− cells are deficient in inducing certain IFNγ-responsive genes.
To define the basis of this deficiency, we next tested, by transfection assays, whether IFNγ is capable of stimulating the activity of a luciferase reporter containing GAS elements in ICSBP−/− cells. As shown in Fig. 1d, IFNγ robustly stimulated reporter activity in RAW cells but only very weakly in CL-2 cells. Supporting the specificity of activation, IFNγ did not activate the mt GAS reporter (17) in either cell type (Fig. 1d). One explanation for these results was that IFNγ signaling was disrupted in ICSBP−/− cells. To assess the integrity of signal transduction in these cells, we tested STAT1 activation by IFNγ. As seen in Fig. 1e, an electrophoretic gel mobility-shift assay with a WT GAS probe showed comparable activation of STAT1 in both ICSBP+/+ and −/− cells. Phosphorylation analysis revealed that STAT1 was phosphorylated at the expected Tyr-701 and Ser-727 residues (33) in both cells (not shown). These results suggested that STAT1 activation is insufficient for IFNγ-stimulated promoter activity, and additional ICSBP-dependent steps are necessary for optimal IFNγ response.
Transfected ICSBP Stimulates Activity of GAS-Containing Reporters.
We tested whether reintroduction of ICSBP relieves the deficiency in ICSBP−/− macrophages. As seen in Fig. 2a, cotransfection with an ICSBP expression vector strongly stimulated the activity of the WT GAS reporter both in ICSBP+/+ and −/− cells, even in the absence of IFNγ, but did not affect the mt GAS reporter. In ICSBP+/+ cells, IFNγ treatment resulted in a further increase in promoter activity, whereas the treatment had little effect on ICSBP−/− cells. Several additional GAS-containing reporters, including one with the IRF-1 GAS (32), were also stimulated by cotransfected ICSBP (not shown). Activation was specific for GAS, because a luciferase reporter containing the ISRE element from the GBP gene, which could also be stimulated by IFNγ (25), was not stimulated by ICSBP (Fig. 2b). Experiments in Fig. 2c investigated whether other members of the IRF family are similarly capable of stimulating the GAS reporter. In contrast to ICSBP, IRF-1 and IRF-2 did not stimulate GAS reporter activity in the absence or presence of IFNγ. Although unable to stimulate GAS reporter activity, IRF-1 was able to stimulate ISRE-reporter activity, as expected (ref. 18; data not shown). To study whether GAS reporter stimulation depends on a specific domain of ICSBP, deletion constructs shown in Fig. 2d were tested in cotransfection assays. The C-terminal truncations 250, 150, and 120, containing the DNA-binding domain and increasing portions of the C-terminal region, failed to stimulate reporter activity, whereas the longer construct 350 weakly stimulated promoter activity. The C-terminal construct lacking the DNA-binding domain (CTD, C-terminal domain) also failed to stimulate reporter activity. These results suggest that both the DNA-binding and C-terminal domains of ICSBP are required for stimulation of GAS-containing promoter activity.
Transfected ICSBP Can Stimulate GAS Reporter Activity in the Absence of JAK/STAT Pathway Activation.
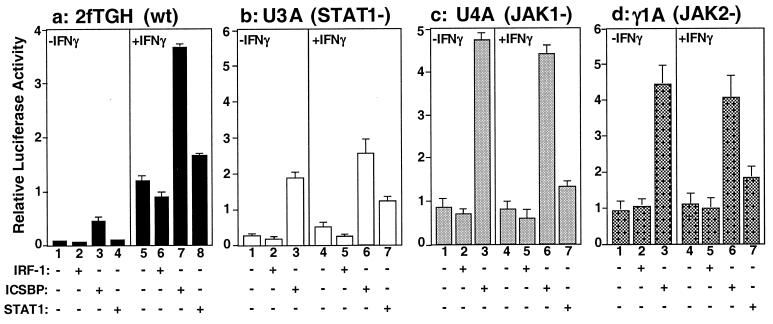
To determine whether ICSBP stimulation of GAS-containing reporter depends on the activation of the JAK/STAT1 pathway, mt cells lacking a discrete component of the pathway were tested in transfection assays. As shown in Fig. 3a, in the 2fTGH cells (parental line), which do not express ICSBP, GAS reporter activity was significantly enhanced by cotransfection of ICSBP (Fig. 3a, lanes 3 and 7 vs. lanes 2 and 6). As expected, transfection of STAT1 also led to an increase in reporter activity, albeit a modest one (Fig. 3a, lane 8). As shown in Fig. 3b, ICSBP also stimulated GAS reporter activity in U3A cells, which lack expression of STAT1 (34). IFNγ did not stimulate reporter activity in U3A cells, but transfection of STAT1 restored IFNγ-mediated reporter activation (Fig. 3b, lane 7). U4A and γ1A cells lack the JAK1 and JAK2 kinase, respectively; these kinases are activated by IFNγ and are required for activation of STAT1 (24, 30). Similar to the results obtained with U3A cells, the GAS reporter was not stimulated by IFNγ in these cells (Fig. 3 c and d, compare lane 1 vs. lanes 4 and 5). However, cotransfection of ICSBP, but not IRF-1, led to marked stimulation of reporter activity in either the presence or absence of IFNγ (Fig. 3 c and d, lanes 3 and 6). Little stimulation was observed when these cells were cotransfected with STAT1 and stimulated by IFNγ, as expected (Fig. 3 c and d, lane 7). Thus, ICSBP, when ectopically expressed, is capable of stimulating GAS reporter activity without requiring activation of the JAK/STAT signaling pathway.
Figure 3.
ICSBP stimulates GAS reporter activity in JAK/STAT-pathway-deficient cells. 2fTGH, U3A, U4A, and γ1A cells were cotransfected with the WT GAS reporter and expression vector for ICSBP, IRF-1, or STAT1 and then were treated with or without human IFNγ (200 units/ml) for 6 h before harvest. Values represent the average of five determinations ±SD.
Recruitment of ICSBP to the GAS Element.
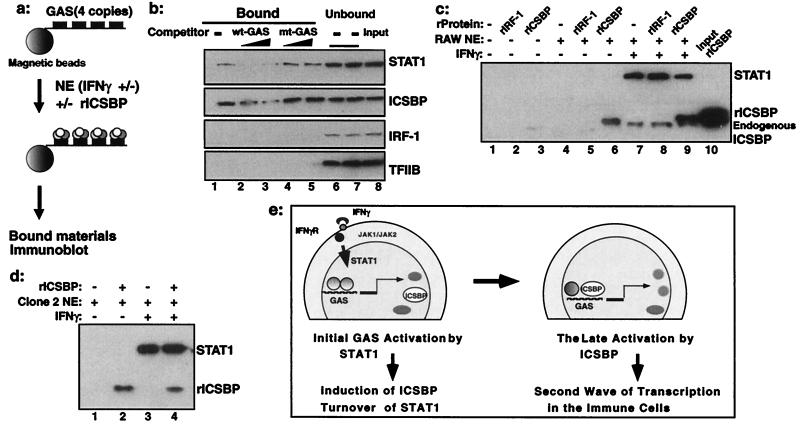
Given the strong stimulation of GAS reporter activity by ICSBP, it was of importance to test whether ICSBP binds to this element. Initial electrophoretic mobility-shift assays failed to reveal ICSBP binding to GAS probes (not shown); therefore, we employed the DNA affinity binding assay depicted in Fig. 4a (26). A biotinylated DNA fragment containing the WT GAS element (Fig. 1e) was conjugated to magnetic beads and incubated with nuclear extracts from IFNγ-treated RAW cells; bound proteins were detected by immunoblotting. Both STAT1 and ICSBP, present in RAW cell extracts, were found on the GAS-conjugated beads indicating their recruitment to GAS, whereas IRF-1 and TFIIB were not. Recruitment of ICSBP and STAT1 was abolished by excess competitor oligomer for the WT but not mt GAS (Fig. 4b). To study whether ICSBP is recruited to GAS by direct binding or through protein–protein interaction, baculovirus-derived rICSBP was tested alone or mixed with extracts from RAW cells. As a control, rIRF-1 was tested in the same manner. As seen in Fig. 4c (lanes 2 and 3), neither rICSBP nor rIRF-1 alone bound to the GAS-conjugated beads. However, rICSBP, but not rIRF-1, was found on the GAS-conjugated beads when mixed with extracts from RAW cells (Fig. 4c, lane 6). When extracts from IFNγ-treated RAW cells were tested, both rICSBP and endogenous ICSBP were recruited to GAS (Fig. 4c, lanes 8 and 9); endogenous ICSBP was distinguished from rICSBP by the slightly smaller size. In addition, STAT1 was recruited to GAS when extracts from IFNγ-treated RAW cells were tested, as expected (Fig. 4c, lanes 7–9). Recruitment of rICSBP to GAS was also observed with extracts from ICSBP−/− CL-2 cells (Fig. 4d). These results indicate that ICSBP is recruited to the GAS element in cooperation with another factor (or other factors) present in the cells.
Figure 4.
Recruitment of endogenous and rICSBP to the GAS. (a) Diagram of DNA affinity binding assay. GAS-conjugated beads were incubated with nuclear extracts (NE) with or without rICSBP, and bound materials were detected in immunoblot assays. (b) Binding of endogenous ICSBP to the GAS element: competition analysis. GAS-conjugated beads were incubated with nuclear extracts from RAW cells treated with IFNγ in the absence (lane 1) or presence (lanes 2–5) of a 100- or 25-fold molar excess of WT-GAS or mt-GAS oligomers. Bound (lanes 1–5) or unbound (lane 6, WT GAS competitor; lane 7, mt GAS competitor) fractions were analyzed for ICSBP and STAT1 proteins. (c) Binding assays were performed by using rICSBP or rIRF-1 in the absence (lanes 1–3) or presence of extracts from RAW cells treated without (lanes 5 and 6) or with (lanes 8 and 9) rIFNγ. (d) rICSBP binding in the presence of extracts from CL-2 cells treated without (lanes 1 and 2) or with (lanes 3 and 4) IFNγ. (e) A model for IFNγ action. IFNγ-responsive genes including ICSBP are first activated by the classic JAK/STAT pathway through GAS. ICSBP is recruited to GAS through protein–protein interaction, providing a second wave of transcription from certain IFNγ-inducible genes in an immune-cell-specific manner.
Discussion
Consistent with the initial observations that ICSBP−/− macrophages were defective in inducing certain IFNγ-responsive genes, transfection of ICSBP led to a marked stimulation of transcription from GAS-containing promoters. In light of the established role for STAT1 in activating GAS-dependent transcription (3–5, 7), stimulation of GAS reporter activity by ICSBP may seem somewhat unexpected. However, the fact that ICSBP itself is an IFNγ-inducible gene (17) provides a ready meaning to our observations. ICSBP is specifically expressed in the immune system, primarily in macrophages, but also in T cells (15, 16); its induction by IFNγ is downstream of STAT1 activation and depends on the GAS element in the promoter (17). Therefore, the stimulation of GAS reporter activity observed by ICSBP is a post-STAT1 event in vivo and depends on activation of the JAK/STAT1 pathway.
The fact that ICSBP was able to stimulate promoter activity in a GAS-specific manner, even in the absence of IFNγ treatment and in STAT1/JAK-negative cells, indicates that, once ICSBP is induced, it can stimulate transcription without requiring STAT1. The ability to stimulate GAS reporter activity was characteristic of ICSBP but was not observed with other IRF members such as IRF-1 and IRF-2. One can envisage that ICSBP might generate a second wave of transcription from IFNγ-responsive promoters, which would lead to amplification of IFNγ's effects in an immune-cell-specific manner (model in Fig. 4e). Because STAT1 is labile and rapidly degraded on activation by phosphatases and proteasomes (35, 36), ICSBP may help prolong transcription from respective promoters after STAT1 activity declines, thereby sustaining the effect of IFNγ. These findings are of interest, because ICSBP has been thought to act as a repressor of IFNα/β inducible genes (19), providing an example in which a transcription factor can act either as an activator or repressor, depending on promoter elements.
ICSBP's remarkable ability to stimulate GAS reporter activity is compatible with the fact that ICSBP−/− macrophages are profoundly defective in inducing certain IFNγ-responsive genes, such as iNos and FCγRI, even though IFNγ activation of STAT1 is normal in these cells (Fig. 1). In this light, it may not be surprising that the most striking defects found in ICSBP−/− mice are those involving macrophage functions (22, 23, 37). Because IFNγ is shown to play a role in tumor surveillance (38), the mechanism described here may partly account for the increased tendency of ICSBP−/− mice to develop leukemia (39).
In the course of this study, we noted that not all IFNγ-inducible genes are defective in ICSBP−/− cells, e.g., the mig gene and MHC class II are normally induced in these cells (C.C., unpublished work; ref. 23). It is of note that GAS sequences vary considerably among different IFNγ-inducible genes (8). ICSBP seems capable of stimulating only a certain set of GAS elements but not others. This variability may result in a pattern of IFNγ responses different from that initiated by STAT1. The way in which ICSBP regulates expression of IFNγ-inducible genes may be affected by additional components, such as chromatin structure and recruitment of other cofactors. These additional components would in turn provide further complexity to the activities of ICSBP. In a DNA affinity binding assay (Fig. 4 a–d), endogenous ICSBP was found to be recruited to GAS. Because rICSBP did not bind to GAS by itself but was found on the GAS only after incubation with nuclear extracts, its recruitment is likely to depend on protein–protein interaction. At present, we do not know the nature of the protein complex nor the mechanism by which ICSBP is recruited. Because all ICSBP deletion constructs failed to stimulate GAS reporter activity (Fig. 2d), ICSBP may interact with multiple factors through multiple surfaces. In summary, our results suggest that IFNγ sequentially activates STAT1 and ICSBP in macrophages, thereby augmenting transcription of certain IFNγ-responsive genes whose functions are relevant to IFNγ's unique role in these cells.
Acknowledgments
We are grateful to Drs. G. Adolf and T. Taniguchi for reagents; Drs. J. Blanco, A. Masumi, and J. Lu for discussions; and Dr. I. Dawid for reading the manuscript. This work was partly supported by a International Union Against Cancer (UICC) Translational Cancer Research Fellowship funded by Novartis (Bern, Switzerland) to L.G.
Abbreviations
- ICSBP
IFN consensus sequence binding protein
- IRF
IFN regulatory factor
- RAW
RAW264.7
- WT
wild type
- mt
mutant
- iNos
inducible nitric oxide synthase
- FcγRI
Fcγ receptor I
- hprt
hypoxanthine-guanine phosphoribosyl transferase
- r
recombinant
- GAS
IFNγ activation site
- ISRE
IFN-stimulated responsive element
References
- 1.Billiau A. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 2.Boehm U, Klamp T, Groot M, Howard J C. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 4.Darnell J E, Jr, Kerr I M, Stark G R. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.Darnell J E., Jr Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 6.Pestka S, Kotenko S V, Muthukumaran G, Izotova L S, Cook J R, Garotta G. Cytokine Growth Factor Rev. 1997;8:189–206. doi: 10.1016/s1359-6101(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 7.Leonard W J, O'Shea J J. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 8.Decker T, Kovarik P, Meinke A. J Interferon Cytokine Res. 1997;17:121–134. doi: 10.1089/jir.1997.17.121. [DOI] [PubMed] [Google Scholar]
- 9.Durbin J E, Hackenmiller R, Simon M C, Levy D E. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 10.Meraz M A, White J M, Sheehan K C, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, et al. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 11.Muller M, Laxton C, Briscoe J, Schindler C, Improta T, Darnell J E, Jr, Stark G R, Kerr I M. EMBO J. 1993;12:4221–4228. doi: 10.1002/j.1460-2075.1993.tb06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mach B, Steimle V, Martinez-Soria E, Reith W. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 13.Soh J, Donnelly R J, Kotenko S, Mariano T M, Cook J R, Wang N, Emanuel S, Schwartz B, Miki T, Pestka S. Cell. 1994;76:793–802. doi: 10.1016/0092-8674(94)90354-9. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen H, Hiscott J, Pitha P M. Cytokine Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 15.Nelson N, Kanno Y, Hong C, Contursi C, Fujita T, Fowlkes B J, O'Connell E, Hu-Li J, Paul W E, Jankovic D, et al. J Immunol. 1996;156:3711–3720. [PubMed] [Google Scholar]
- 16.Politis A D, Ozato K, Coligan J E, Vogel S N. J Immunol. 1994;152:2270–2278. [PubMed] [Google Scholar]
- 17.Kanno Y, Kozak C A, Schindler C, Driggers P H, Ennist D L, Gleason S L, Darnell J E, Jr, Ozato K. Mol Cell Biol. 1993;13:3951–3963. doi: 10.1128/mcb.13.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada H, Willison K, Sakakibara J, Miyamoto M, Fujita T, Taniguchi T. Cell. 1990;63:303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- 19.Nelson N, Marks M S, Driggers P H, Ozato K. Mol Cell Biol. 1993;13:588–599. doi: 10.1128/mcb.13.1.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisz A, Kirchhoff S, Levi B Z. Int Immunol. 1994;6:1125–1131. doi: 10.1093/intimm/6.8.1125. [DOI] [PubMed] [Google Scholar]
- 21.Fehr T, Schoedon G, Odermatt B, Holtschke T, Schneemann M, Bachmann M F, Mak T W, Horak I, Zinkernagel R M. J Exp Med. 1997;185:921–931. doi: 10.1084/jem.185.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giese N A, Gabriele L, Doherty T M, Klinman D M, Tadesse-Heath L, Contursi C, Epstein S L, Morse H C., III J Exp Med. 1997;186:1535–1546. doi: 10.1084/jem.186.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. J Exp Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watling D, Guschin D, Muller M, Silvennoinen O, Witthuhn B A, Quelle F W, Rogers N C, Schindler C, Stark G R, Ihle J N, et al. Nature (London) 1993;366:166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- 25.Wang I M, Blanco J C, Tsai S Y, Tsai M J, Ozato K. Mol Cell Biol. 1996;16:6313–6324. doi: 10.1128/mcb.16.11.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masumi A, Wang I-M, Lefebvre B, Yang X-J, Nakatani Y, Ozato K. Mol Cell Biol. 1999;19:1810–1820. doi: 10.1128/mcb.19.3.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowenstein C J, Glatt C S, Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1992;89:6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sears D W, Osman N, Tate B, McKenzie I F, Hogarth P M. J Immunol. 1990;144:371–378. [PubMed] [Google Scholar]
- 29.Ding A H, Nathan C F, Stuehr D J. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 30.Gao J, Morrison D C, Parmely T J, Russell S W, Murphy W J. J Biol Chem. 1997;272:1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 31.Pearse R N, Feinman R, Shuai K, Darnell J E, Jr, Ravetch J V. Proc Natl Acad Sci USA. 1993;90:4314–4318. doi: 10.1073/pnas.90.9.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pine R, Canova A, Schindler C. EMBO J. 1994;13:158–167. doi: 10.1002/j.1460-2075.1994.tb06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen Z, Zhong Z, Darnell J E., Jr Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 34.Muller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur A G, Barbieri G, Witthuhn B A, Schindler C, et al. Nature (London) 1993;366:129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 35.Kim T K, Maniatis T. Science. 1996;273:1717–1719. doi: 10.1126/science.273.5282.1717. [DOI] [PubMed] [Google Scholar]
- 36.Haspel R L, Salditt-Georgieff M, Darnell J E., Jr EMBO J. 1996;15:6262–6268. [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C-Y, Maeda H, Contursi C, Ozato K, Seder R A. J Immunol. 1999;162:807–812. [PubMed] [Google Scholar]
- 38.Kaplan D H, Shankaran V, Dighe A S, Stockert E, Aguet M, Old L J, Schreiber R D. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch K P, Gabriele L, Waring J F, et al. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]