Abstract
Objectives
To determine short-term outcome for children with acute liver failure (ALF) as it relates to etiology, clinical status, patient demographics and to determine prognostic factors.
Study design
A prospective, multi-center case study collecting demographic, clinical, laboratory and short-term outcome data on children from birth to 18 years with ALF. Patients without encephalopathy were included if the prothrombin time and INR remained ≥ 20 seconds and/or >2, respectively, despite vitamin K. Primary outcome measures three weeks after study entry were death, death after transplant, alive with native liver, alive with transplanted organ.
Results
The etiology of ALF in 348 children included acute acetaminophen toxicity (14%), metabolic disease (10%), autoimmune liver disease (6%), non-APAP drug-related hepatotoxicity (5%), infections (6%), other diagnosed conditions (10%); 49% were indeterminate. Outcome varied between patient sub-groups; 20% with non-acetaminophen ALF died or underwent liver transplantation and never developed clinical encephalopathy.
Conclusions
Etiologies of ALF in children differ from adults. Clinical encephalopathy may not be present in children. The high percentage of indeterminate cases provides an opportunity for investigation.
Acute liver failure (ALF) is a dramatic clinical syndrome in which previously healthy children rapidly lose hepatic function and become critically ill within days.(1, 2) Over the last 25 years, single center experiences (3–6) or general reviews(1, 2, 7)of pediatric ALF from Europe and North America identified a variety of infectious, metabolic, cardiovascular and drug-related causes, as well as indeterminate cases.(4–6) These pediatric studies utilized the adult definition of ALF, which requires the presence of hepatic encephalopathy (HE) within 8 weeks of the development of clinical jaundice. Unfortunately, HE is difficult to assess in many infants and children and may not be essential to the diagnosis of ALF in children.(3, 8)
The outcome for children with ALF remains poor for infants under one year of age(3), patients with accidental acetaminophen (APAP) overdose(9), and Wilson’s disease presenting with hepatic HE.(10) Spontaneous recovery (i.e., survival without transplantation) remains between 15–20% for those with severe HE.(5, 6) Given the ongoing shortage of donor livers(11), development of a reliable prognostic score will be useful in allocating organs to the most needy patients.
The Pediatric Acute Liver Failure (PALF) study group was formed in 1999 to develop a database that would facilitate an improved understanding of the etiopathogenesis, treatment and outcome of ALF in children. These data will also serve to identify factors that will help to predict the likelihood of death or need for liver transplant.
Methods
Organization
The PALF study group began as an adjunct to the National Institutes of Health (NIH) sponsored, adult-focused ALF Study Group (William Lee, M.D, Principal Investigator). The PALF study group now consists of 24 active pediatric sites, 21 within the United States, 1 in Canada and 2 in the United Kingdom. Working groups of pediatric hepatologists established definitions for ALF and various diagnostic categories. Representatives from all participating centers approved final recommendations from the working groups. Patient enrollment began in December, 1999.
Data Collection
Following informed consent from a parent or legal guardian, demographic, clinical and laboratory information were recorded daily for seven days. In most patients, an additional aliquot of serum or plasma was collected on each of the seven study days, frozen at −70° C and then shipped to the Data Coordinating Center (DCC) located at the University of Texas Southwestern Medical Center in Dallas, Texas. Diagnostic evaluation and medical management were consistent with the standard of care at each site. As in the adult study, our primary outcome measures determined at 3 weeks after entry into the study included death, death after transplant, alive with native organ, alive with transplanted organ. Completed data forms were forwarded under code to the DCC for review by the principal investigator for the pediatric study. If discrepancies were identified, the site was queried, and upon resolution, data were then entered into the PALF database. The NIH provided a Certificate of Confidentiality to the study and IRB approval was secured at each site prior to patient enrollment.
Subjects of the Study
Patients from birth through 18 years of age were eligible for enrollment if they met the following entry criteria for the PALF study: (1) children with no known evidence of chronic liver disease, (2) biochemical evidence of acute liver injury, and (3) hepatic-based coagulopathy defined as a prothrombin time (PT) ≥ 15 seconds or INR ≥ 1.5 not corrected by Vitamin K in the presence of clinical HE or a PT ≥20 seconds or INR ≥2.0 regardless of the presence or absence of clinical HE. A standard adult clinical coma grade scale was used for older children and a coma grade scale was adapted for infants and children < 4 years old.(12)(Table 1).
TABLE 1. Assessment of Encephalopathy for Young Children.
For children birth to age 3 years:
| Grade | Clinical | Asterixis/Reflexes | Neurological signs |
|---|---|---|---|
| Early (I and II) | Inconsolable crying, sleep reversal, inattention to task | Unreliable/ normal or hyperreflexic | Untestable |
| Mid (III) | Somnolence, stupor, combativeness | Unreliable/hyperreflexic | Most likely untestable |
| Late (IV) | Comatose, arouses with painful stimuli (IVa) or no response (IVb) | Absent | Decerebrate or decorticate |
Diagnostic Categories
Diagnostic criteria for acute APAP toxicity included: a toxic serum APAP level based on the Rumack nomogram(13) or a history of an acute ingestion of 100 mg/kg within a 24 hour period. The diagnosis of autoimmune hepatitis (AIH) was established if a patient had one or more positive autoantibody tests (anti-nuclear antibody ≥ 1:80, smooth muscle antibody ≥ 1:20, liver-kidney microsomal antibody ≥ 1:20) and no evidence of serologically defined viral hepatitis.(14) Non-APAP drug-induced hepatitis was diagnosed if a temporal relationship between exposure to a suspected drug and the onset of ALF was established and other common causes were excluded. Hepatitis A, B, or C infection was confirmed serologically or by polymerase chain reaction (PCR). Evidence of other viral infections required a positive IgM antibody, evidence of virus in liver tissue, or a positive PCR. Metabolic diseases were diagnosed by laboratory tests (e.g., alpha-1-antitrypsin phenotype of ZZ), analysis of liver tissue (e.g., mitochondrial enzyme defect), or analysis of cultured skin fibroblasts (e.g., fatty acid oxidation defect). If the site investigator suspected an infection or metabolic disease but lacked supporting evidence or if a specific diagnosis could not be established, the final diagnosis was registered as indeterminate.
Statistical Methods
Diagnostic categories were defined as APAP, indeterminate and all others in whom a specific diagnosis was determined. Age was dichotomized into patients less than 3 years of age and those 3 years of age and older. Race was dichotomized into Caucasians versus non-Caucasians.
All associations between pairs of dichotomous or dichotomized variables (2 way tables) were conducted using χ2 analyses. For those χ2 analyses found significant, post hoc Tukey-type multiple comparison tests for proportions (TTMC) were performed.(15) The two independent samples proportions test with correction was used to compare proportions for two groups. Two different logistic regression models were used to predict death or transplant in the two non-APAP groups using data available at admission (coma grade, PT, and total bilirubin) and peak measures within the first 7 days of hospitalization or before transplant (peak bilirubin, peak PT, maximum coma grade). These models also included gender (male versus female) and age (<3 versus ≥ 3 years).
SPSS V12.0 and SAS V9.1 were used in all analyses. The assumptions for all statistical tests were checked for violations. Statistical significance was set at p < 0.05.
Results
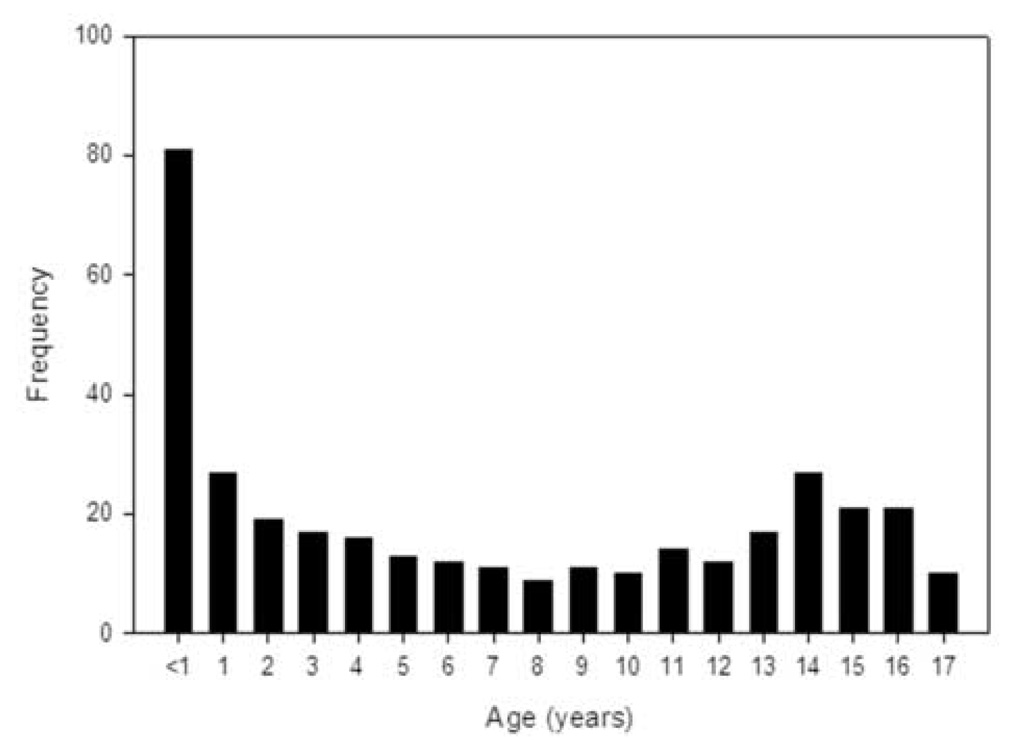
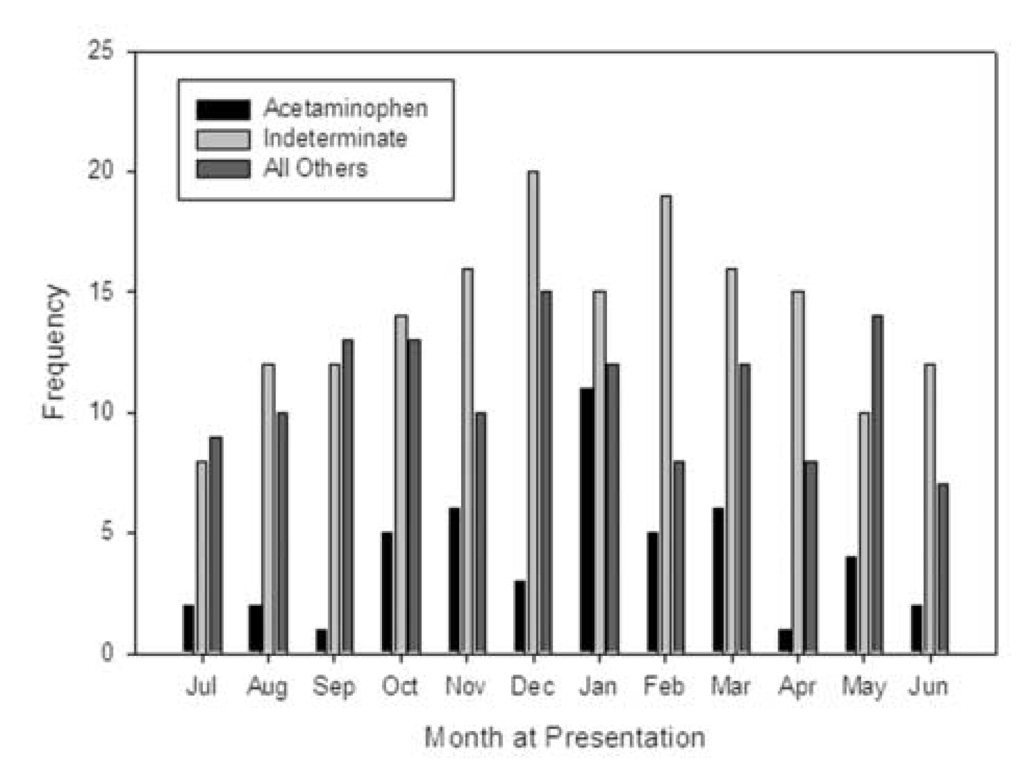
Between December 1999 and December 2004, 348 children were enrolled; patient demographics are outlined in Table 2. The median dose of APAP ingested was 183 mg/kg (range 19.2 to 734.1). There was an association between etiology and gender (p=0.0002) with the percentage of females significantly higher in the APAP group compared to both non-APAP groups (TTMC p<0.05, 79% versus 46% or versus 60%, respectively). The association between the three etiologic categories and race (Caucasians versus non-Caucasians) was found to be significant (p=0.0275) with more Caucasians in the APAP vs. the indeterminate group (TTMC p < 0.05, 67% vs. 47%; respectively). Children under three years of age accounted for 36.5% (127/348) of patients.(Figure 1) ALF due to APAP and those of indeterminate cause appear to occur more commonly during the cooler months, but this did not reach statistical significance (p=0.2240).(Figure 2)
Table 2.
Patient Demographics
| Acetaminophen (%) | Indeterminate (%) | All Others (%) | χ2 p-value |
||
|---|---|---|---|---|---|
| Total | (N=348) | 48 (14) | 169 (49) | 131 (38) | |
| Gender | Female (N=181) | 38 (79) | 78 (46) | 65 (50) | 0.0002 |
| Male (N=167) | 10 (21) | 91 (54) | 66 (50) | ||
| Age | < 3.0 (N=127) | 2 (4) | 68 (40) | 57 (44) | <0.0001 |
| > 3.0 (N=221) | 46 (96) | 101 (60) | 74 (56) | ||
| Race | African American (N=54) | 5 (10) | 32 (19) | 17 (13) | 0.02751 |
| Asian (N=30) | 5 (10) | 14 (8) | 11 (8) | ||
| Hispanic (N=53) | 3 (6) | 32 (19) | 18 (14) | ||
| Native American (N=4) | 0 (0) | 2 (1) | 2 (2) | ||
| Other (N=21) | 3 (6) | 10 (6) | 8 (6) | ||
| White (N=186) | 32 (67) | 79 (47) | 75 (57) |
Test of Caucasian versus Non-Caucasian
Figure 1.
Age of the patient at entry into the PALF study.
Figure 2.
Calendar month when patients were entered into the PALF study. Patients were divided into three diagnostic cagtegories: acetaminophen toxicity, indeterminate, and all others with an established diagnosis.
HE at admission to the study and peak HE during the subsequent 7 days stratified by age and diagnosis is captured in Table 3. HE was present more frequently in the combined non-APAP groups than the APAP patients (56.6% [164/290] vs. 39.6% [19/48]; p=0.0425). In addition, the number of patients who developed HE during the seven day study period was greater in those with non-APAP ALF compared to the APAP group (68.4% [203/297] vs. 39.6% [19/48]; p=0.0002). Patients in all diagnostic and age categories experienced worsening HE, although this occurred more commonly in older patients (24.0% [29/121] vs. 34.6% [75/217]). Other clinical and management features stratified by diagnosis and age are listed in Table 4. Development of ascites, need for ventilator and blood pressure support, and requirement for red blood cell and plasma infusions were more likely to develop in patients within the two non-APAP groups than in APAP patients. In comparisons by age category, those in the younger age group were more likely to develop ascites, require ventilator support, and infusions of red blood cells and fresh frozen plasma.
Table 3.
Encephalopathy on Admission and at Peak during the Seven Day Study Period Stratified by Age and Diagnosis Category
| Admission | Acetaminophen (%) | Indeterminate (%) | All Others (%) | |||
|---|---|---|---|---|---|---|
| Coma Grade | Age < 3.0 | ≥3.0 yr | Age < 3.0 | ≥ 3.0 yr | Age < 3.0 | ≥ 3.0 yr |
| 0 (N=155) | 0 (0) | 29 (63) | 27 (42) | 38 (38) | 30 (55) | 31 (44) |
| 1–2 (N=138) | 0 (0) | 12 (26) | 34 (53) | 45 (45) | 20 (36) | 27 (38) |
| 3 (N=31) | 1 (50) | 4 (9) | 2 (3) | 12 (12) | 5 (9) | 7 (10) |
| 4 (N=14) | 1 (50) | 1 (2) | 1 (2) | 5 (5) | 0 (0) | 6 (8) |
| Peak | Acetaminophen (%) | Indeterminate (%) | All Others (%) | |||
| Coma Grade | < 3.0 | ≥3.0 | < 3.0 | ≥ 3.0 | < 3.0 | ≥ 3.0 |
| 0 (N=123) | 0 (0) | 29 (63) | 21 (31) | 25 (25) | 26 (46) | 22 (30) |
| 1–2 (N=134) | 0 (0) | 12 (26) | 36 (54) | 40 (40) | 21 (38) | 25 (34) |
| 3 (N=39) | 1 (50) | 1 (2) | 4 (6) | 15 (15) | 8 (14) | 10 (14) |
| 4 (N=49) | 1 (50) | 4 (9) | 6 (9) | 21 (21) | 1 (2) | 16 (22) |
Table 4.
Clinical Characteristics by diagnostic category and age category
| Acetaminophen | Diagnosis Indeterminate |
All Others | χ2 | Age Group | χ2 | ||
|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | <3 yr (%) | ≥3 yr (%) | |||
| n=48 | n=169 | n=131 | p-value | n=127 | n=221 | p-value | |
| Ascites (N=78) | 2 (4) | 35 (21) | 41 (31) | 0.0004 | 42 (33) | 36 (16) | 0.0003 |
| Seizure (N=23) | 0 (0) | 13 (8) | 10 (8) | 0.1394 | 6 (5) | 17 (8) | 0.2833 |
| Ventilation Support (N=145) | 8 (17) | 75 (44) | 62 (47) | 0.0007 | 62 (49) | 83 (38) | 0.0402 |
| Pressor Support (N=82) | 5 (10) | 33 (20) | 44 (34) | 0.0012 | 33 (26) | 49 (22) | 0.4198 |
| Hemofiltration (N=33) | 3 (6) | 11 (7) | 19 (15) | 0.0457 | 8 (6) | 25 (11) | 0.1244 |
| Plasmapheresis (N=35) | 3 (6) | 21 (12) | 11 (8) | 0.3301 | 8 (6) | 27 (12) | 0.0772 |
| Red cell transfusion (N=146) | 7 (15) | 81 (48) | 58 (44) | 0.0002 | 79 (62) | 67 (30) | <0.0001 |
| Fresh frozen plasma (N=221) | 20 (42) | 122 (72) | 79 (60) | 0.0003 | 90 (71) | 131 (59) | 0.0306 |
Overall, a specific etiology of ALF was not identified in 49% of patients and 54% of children less than 3 years of age. (Table 5; available at www.jpeds.com) APAP toxicity accounted for only 14% of all patients, with 96% of these cases occurring in older patients. Specific viruses, drugs, toxins and metabolic disorders are also listed in Table 5. Interestingly, only three patients with acute hepatitis A infection, one patient with hepatitis C, and no patients with hepatitis B were identified in this cohort.
Table 5.
Final Diagnosis in Children with ALF
| Age Category | |||
|---|---|---|---|
| Diagnosis | < 3 (%) | ≥3 (%) | Total (%) |
| Acetaminophen (N=48) | 2 (2) | 46 (21) | 48 (14) |
| Indeterminate (N=169) | 68 (54) | 101 (46) | 169 (49) |
| Autoimmune (N=22) | 6 (5) | 16 (7) | 22 (6) |
| Infectious (N=20) | 9 (7) | 11 (5) | 20 (6) |
| Adenovirus (N=2) | 1 (1) | 1 (0) | 2 (1) |
| Cytomegalovirus (N=1) | 1 (1) | 0 (0) | 1 (0) |
| Epstein-Barr virus (N=6) | 1 (1) | 5 (2) | 6 (2) |
| Enterovirus (N=1) | 1 (1) | 0 (0) | 1 (0) |
| Hepatitis A (N=3) | 0 (0) | 3 (1) | 3 (1) |
| Hepatitis C (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Herpes simples virus (N=6) | 5 (4) | 1 (0) | 6 (2) |
| Non-APAP drug induced liver disease (N=17) | 1 (1) | 16 (7) | 17 (5) |
| Mushroom (N=2) | 0 (0) | 2 (1) | 2 (1) |
| Anesthetic (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Bactrim (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Cylert (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Cytoxan/Dilantin (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Dilantin (N=1) | 0 (0) | 1 (0) | 1 (0) |
| INH (N=2) | 0 (0) | 2 (1) | 2 (1) |
| Iron (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Methotrexate (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Minocycline (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Pravastatin (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Valproate (N=3) | 1 (1) | 2 (1) | 3 (1) |
| Metabolic (N=36) | 23 (18) | 13 (6) | 36 (10) |
| Alpha-1 antitrypsin (N=1) | 1 (1) | 0 (0) | 1 (0) |
| Fatty acid oxidation defect (N=4) | 4 (3) | 0 (0) | 4 (1) |
| Galactosemia (N=2) | 2 (2) | 0 (0) | 2 (1) |
| Fructose intolerance (N=1) | 1 (1) | 0 (0) | 1 (0) |
| Mitochondrial disorder (N=4) | 2 (2) | 2 (1) | 4 (1) |
| Niemann-Pick type C (N=1) | 1 (1) | 0 (0) | 1 (0) |
| Respiratory chain defect (N=7) | 7 (6) | 0 (0) | 7 (2) |
| Reyes syndrome (N=1) | 0 (0) | 1 (0) | 1 (0) |
| Tyrosinemia (N=4) | 4 (3) | 0 (0) | 4 (1) |
| Urea cycle defect (N=2) | 1 (1) | 1 (0) | 2 (1) |
| Wilson disease(N=9) | 0 (0) | 9 (4) | 9 (3) |
| Other (N=20) | 11 (9) | 9 (4) | 20 (6) |
| Budd-Chiari (N=2) | 0 (0) | 2 (1) | 2 (1) |
| Hemophagocytic syndrome (N=4) | 2 (2) | 2 (1) | 4 (1) |
| Leukemia (N=2) | 1 (1) | 1 (0) | 2 (1) |
| Neonatal iron storage disease (N=6) | 6 (5) | 0 (0) | 6 (2) |
| Veno-occlusive disease (N=6) | 2 (2) | 4 (2) | 6 (2) |
| Shock (N=16) | 7 (6) | 9 (4) | 16 (5) |
| Total | 127 (36) | 221 (64) | 348 (100) |
The short-term outcome for each diagnostic category is described in Table 6. When all cases are considered, the association between outcome and gender was significant (p=0.0428) with spontaneous recovery for females higher than for males (60% vs. 46%, respectfully), but this association disappears when APAP patients are removed from the analysis. Survival and need for liver transplant varied depending upon the diagnosis. Spontaneous recovery was greatest in children with APAP toxicity (45/48; 94%), worst for those with non-APAP drug-induced liver injury (7/17; 41%) and with indeterminate etiology (73/169; 43%). Patient outcomes based upon admission and peak HE are outlined in Table 7. Patients who never developed HE were more likely to experience spontaneous recovery than those who did develop HE (78.9% versus 40.1%; p<0.0001). In contrast, patients who developed Stage III or IV HE had a spontaneous recovery rate of only 33% and 22% respectively. Logistic regression analysis (Table 8) to predict death or liver transplant identified total bilirubin ≥ 5 mg/dl, INR ≥ 2.55, and HE to be risk factors if present on admission. The logistic regression model using peak values was similar to that for admission with increasing predictive values (odds ratios) for all variables.
Table 6.
Short-term (21 days) Outcome of Children with ALF
| Not Transplanted | Transplanted | χ2 | ||||
|---|---|---|---|---|---|---|
| Alive | Dead | Alive | Dead | p-value | ||
| Age | < 3.0 (N=127) | 67 (53) | 24 (19) | 33 (26) | 3 (2) | 0.2141 |
| ≥3.0 (N=221) | 119 (54) | 25 (11) | 72 (33) | 5 (2) | ||
| Gender | Female (N=181) | 109 (60) | 19 (10) | 50 (28) | 3 (2) | 0.0428 |
| Male (N=167) | 77 (46) | 30 (18) | 55 (33) | 5 (3) | ||
| Diagnosis | Acetaminophen (N=48) | 45 (94) | 1 (2) | 1 (2) | 1 (2) | <0.0001 |
| Other Dx Categories (N=300) | 141 (47) | 48 (16) | 104 (35) | 7 (2) | ||
| Dx details | Acetaminophen (N=48) | 45 (94) | 1 (2) | 1 (2) | 1 (2) | |
| Indeterminate (N=169) | 73 (43) | 18 (11) | 71 (42) | 7 (4) | ||
| Autoimmune (N=22) | 12 (55) | 3 (14) | 7 (32) | 0 | ||
| Infectious (N=20) | 10 (50) | 5 (25) | 5 (25) | 0 | ||
| Non-APAP drug induced liver disease | ||||||
| (N=17) | 7 (41) | 5 (29) | 5 (29) | 0 | ||
| Metabolic (N=36) | 16 (44) | 8 (22) | 12 (33) | 0 | ||
| Other (N=20) | 10 (50) | 6 (30) | 4 (20) | 0 | ||
| Shock (N=16) | 13 (81) | 3 (19) | 0 | 0 | ||
| For the Non-APAP patients | ||||||
| Not Transplanted | Transplanted | χ2 | ||||
| Alive | Dead | Alive | Dead | p-value | ||
| Age | < 3.0 (N=125) | 66 (53) | 23 (18) | 33 (26) | 3 (2) | 0.0884 |
| ≥3.0 (N=175) | 75 (43) | 25 (14) | 71 (41) | 4 (2) | ||
| Gender | Female (N=143) | 74 (52) | 18 (13) | 49 (34) | 2 (1) | 0.2274 |
| Male (N=157) | 67 (43) | 30 (19) | 55 (35) | 5 (3) | ||
Table 7.
Patient Outcome based upon Admission and Peak Encephalopathy
| Not Transplanted | Transplanted | χ2 | |||
|---|---|---|---|---|---|
| Alive (%) | Dead (%) | Alive (%) | Dead (%) | p-value1 | |
| Admission Coma Grade | |||||
| 0 (N=155) | 102 (66) | 14 (9) | 35 (23) | 4 (3) | 0.0002 |
| 1–2 (N=138) | 59 (43) | 20 (14) | 57 (41) | 2 (1) | |
| 3 (N=31) | 11 (35) | 9 (29) | 10 (32) | 1 (3) | |
| 4 (N=14) | 8 (57) | 3 (21) | 2 (14) | 1 (7) | |
| Peak Coma Grade | |||||
| 0 (N=123) | 97 (79) | 9 (7) | 16 (13) | 1 (1) | <0.0001 |
| 1–2 (N=134) | 65 (49) | 12 (9) | 55 (41) | 2 (1) | |
| 3 (N=39) | 13 (33) | 8 (21) | 18 (46) | 0 (0) | |
| 4 (N=49) | 11 (22) | 18 (37) | 15 (31) | 5 (10) | |
Test compares Spontaneous Recovery (Alive-Not Transplanted) to the other 3 groups combined
Table 8.
Logistic Regression Results predicting Death or Liver Transplant at 3 weeks Based upon Admission and Peak Measures
| Admission Values | ||||
|---|---|---|---|---|
| 95.0% C.I. for OR | Wald | |||
| Odds Ratio | Lower | Upper | p-value | |
| Overall Test - Coma | 0.0012 | |||
| Coma Grade 1–2 versus rest | 2.83 | 1.55 | 5.16 | 0.0007 |
| Coma Grade 3–4 versus rest | 2.96 | 1.22 | 7.16 | 0.0160 |
| INR ≥2.55 | 2.04 | 1.15 | 3.62 | 0.0150 |
| Total Bilirubin ≥5.0 mg/dL | 10.69 | 5.49 | 20.85 | <0.0001 |
| Model fit statistic: Hosmer-Lemeshow p = 0.30 | ||||
| Peak Values | 95.0% C.I.for OR | Wald | ||
| Odds Ratio | Lower | Upper | p-value | |
| Overall Test for Max Coma | <0.0001 | |||
| Max Coma 1–2 versus rest | 3.60 | 1.95 | 6.66 | <0.0001 |
| Max Coma 3–4 versus rest | 6.92 | 3.40 | 14.07 | <0.0001 |
| Max INR ≥2.55 | 3.36 | 1.91 | 5.91 | <0.0001 |
| Max Total Bilirubin ≥5.0 mg/dL | 8.62 | 4.28 | 17.34 | <0.0001 |
| Model fit statistic: Hosmer-Lemeshow p = 0.91 | ||||
Discussion
This report of the first 348 children in the PALF data set highlights a number of important observations: 1) HE is not an absolute requirement to establish the diagnosis of ALF in children; 2) a specific diagnosis was not made in almost half of all infants and children; 3) the etiologies of ALF in children differ from those seen in adults,(16) with children having more indeterminate cases and fewer APAP and viral-induced cases; and 4) short-term outcome varied among diagnostic groups.
HE is difficult to assess in children and, in fact, may never become clinically apparent in the setting of ALF.(3) However, coagulopathy is an independent risk factor for death or need for liver transplantation in ALF.(17) Therefore, we chose to include children without HE in our study, but only when a significant uncorrectable coagulopathy was present. HE remains an important predictor of outcome(18) and, similar to other studies in the post-liver transplant era, only 25% of our children with a peak HE of grade 3–4 had a spontaneous recovery. However, it is equally important to note that of 79 children with non-APAP ALF who never developed clinically detectable HE, death (8/79) or liver transplant (8/79) occurred in 20%. Our data support a definition of pediatric ALF that does not require HE.
An indeterminate cause of ALF was assigned to 54% of children < 3 years of age and 49% overall. Factors that may influence the intensity of the diagnostic evaluation in children with ALF include prioritization of the etiologic possibilities, blood volumes required for diagnostic studies, and the rapid evolution of disease to transplant or death. Thus, all potential diagnostic studies were not performed on each patient. The indeterminate group may include patients who were “under-evaluated” for known causes of ALF as well as those with novel infectious, immune, autoimmune, metabolic or genetic disorders.
An infectious agent was identified in only 6% of patients in this series. Herpes simplex virus and Epstein Barr virus were the most common identifiable infections in children <3 years and ≥3 years, respectively. Hepatitis A and B are commonly associated with ALF in adults(19), however we identified only three cases of Hepatitis A, one case of Hepatitis C and no cases of Hepatitis B. Nevertheless, these infections are common causes of ALF in children living in endemic areas where hepatitis A can represent up to 40% of ALF cases.(20) Respiratory viruses, enterovirus, or perhaps medications used for symptomatic treatment of these conditions might be implicated given the surge of cases in the winter months however, these viruses were rarely identified.
AIH presenting as ALF accounted for 6% of patients, occurred in all age groups and should therefore be considered early in the diagnostic evaluation to enable timely initiation of corticosteroid treatment.(21) A metabolic cause for ALF was established in 18% of children <3 years of age. Unfortunately, diagnostic criteria for several conditions are not well-established and special attention to proper collection and transport of biological specimens to specialized research laboratories is needed. Wilson disease and defects or deficiencies in mitochondrial function and metabolism (i.e., mitochondrial hepatopathies) were the most common metabolic conditions identified in our study.
Acute APAP toxicity is the most common identifiable cause of ALF in children ≥ 3 y/o (21%), but the frequency is even higher in adults (40%).(22) Instances involving prolonged or inappropriate dosing, so-called therapeutic misadventures,(23) are not easily captured by this study. APAP-protein adducts are formed when the usual mechanisms of APAP metabolism and excretion are exhausted and the reactive APAP metabolite binds to important intracellular proteins resulting in cell death. Detection of these adducts in serum may serve as a biomarker of APAP toxicity.(24)
Non-APAP drug-related ALF was recognized only in the older age group in our series. Drug-related hepatotoxicity is relatively common in children, particularly those taking neuroleptic medications, yet ALF is rare.(25, 26) The mechanism of injury leading to ALF is thought to be an idiosyncratic reaction in most cases; however, children with ALF related to valproic acid should be evaluated for an underlying mitochondrial disorder.(27)In addition, polymorphisms of genes associated with drug detoxification or cytokine expressions may enhance a patient’s susceptibility to liver injury. (28, 29)
Patient outcome was influenced by a number of factors including age, diagnosis, the degree of HE, and severity of the coagulopathy. The risk of death or liver transplant was highest among children <3 years of age. While the numbers are relatively small, patients with Grade IV HE at enrollment experienced a higher rate of spontaneous recovery than those who progressed to Grade IV during the course of the study (50% vs. 20%). At the same time, 20% of children who never experienced clinical HE either died or received a liver transplant. Logistic regression analysis identified total bilirubin ≥ 5 mg/dl, INR ≥ 2.55 and HE to be risk factors to predict death or liver transplant.
In summary, this multicenter, multinational database has confirmed that APAP induced ALF has an excellent outcome when HE is absent(30), demonstrated the etiologies of ALF in children are age-dependent and differ from those in adults, and identified AIH as an important cause of ALF in all ages of children. Unfortunately, the majority of cases of ALF in children are indeterminate. Therefore, improvement in diagnosis will require a focused search for treatable causes that prioritizes diagnostic conditions known to cause ALF. Newer techniques to identify children with an underlying metabolic disease, APAP toxicity, and immune dysregulation will likely improve our ability to establish a diagnosis in these seriously ill children.
Acknowledgments
Grant Support: This study was supported by NIH grant RO1-DK58369-01; and by M01-RR00069, M01-RR00037, and M01 RR08084 from the General Clinical Research Center Program of the National Center for Research Resources of the NIH.
Site co-investigators: Dominic Dell Olio (Birmingham, UK), Emre Sukru (Mt. Sinai)
Study Coordinators: Beverly Bernard (Pittsburgh), Jeanna Zalsos (Mt. Sinai), Susan Krug (Cincinnati), Sue Kelly (Northwestern), Terri Fisher (Denver), Hazel Senz (Denver), Rachel Taylor (Kings College), Lily Luu (San Francisco), Melissa Young (Seattle), Sharon Kochanowicz (Omaha), Nadia Tayeh (Ann Arbor), Kurt Freer (Drexel), Pam Davis (Birminham, AL), Laura Krawczuk (Boston), Rosemary Nagy (St. Louis), Mary Kay Alford (Baltimore), Stephanie Johnson (Los Angeles), Zana Parman (San Diego),Jaymee Scott (Houston)
DSMB Members: Michael W. Fried (Chair), John Barnard, Katryn Furuya, Edmund A. Gehan, Caroline A. Riley, Lewis W. Teperman
National Institutes of Health-NIDDK: Patricia Robuck, Jay H. Hoofnagle, Eduwad Doo, Rebecca Torrance.
Acknowledgments available at www.jpeds.com
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riely CA. Acute hepatic failure in children. Yale J Biol Med. 1984;57(2):161–184. [PMC free article] [PubMed] [Google Scholar]
- 2.Russell GJ, Fitzgerald JG, Clark JH. Fulminant Hepatic Failure. J Pediatr. 1987;111:313–319. doi: 10.1016/s0022-3476(87)80446-8. [DOI] [PubMed] [Google Scholar]
- 3.Durand P, Debray D, Mandel R, Baujard C, Branchereau S, Gauthier F, et al. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139(6):871–876. doi: 10.1067/mpd.2001.119989. [DOI] [PubMed] [Google Scholar]
- 4.Psacharopoulos HT, Mowat AP, Davies M, Portmann B, Silk DB, Williams R. Fulminant hepatic failure in childhood: an analysis of 31 cases. Arch Dis Child. 1980;55(4):252–258. doi: 10.1136/adc.55.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devictor D, Desplanques L, Debray D, Ozier Y, Dubousset AM, Valayer J, Houssin D, Bernard O. Huault. Emergency liver transplantation for fulminant liver failure in infants and children. Hepatology. 1992;16:1156–1162. [PubMed] [Google Scholar]
- 6.Rivera-Penera T, Moreno J, Skaff C, McDiarmid S, Vargas J, Ament ME. Delayed encephalopathy in fulminant hepatic failure in the pediatric population and the role of liver transplantation. J Pediatr Gastroenterol Nutr. 1997;24(2):128–134. doi: 10.1097/00005176-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Whitington PF, Alonso EM. Fulminant hepatitis in children: evidence for an unidentified hepatitis virus. J Pediatr Gastroenterol Nutr. 2001;33(5):529–536. doi: 10.1097/00005176-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Baker A, Alonso ME, Aw MM, Ciocca M, Porta G, Rosenthal P. Hepatic failure and liver transplant: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39 Suppl 2:S632–S639. doi: 10.1097/00005176-200406002-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gyamlani GG, Parikh CR. Acetaminophen toxicity: suicidal vs. accidental. Crit Care. 2002;6(2):155–159. doi: 10.1186/cc1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loudianos G, Gitlin JD. Wilson's disease. Semin Liver Dis. 2000;20(3):353–364. doi: 10.1055/s-2000-9389. [DOI] [PubMed] [Google Scholar]
- 11.Ojo AO, Heinrichs D, Emond JC, McGowan JJ, Guidinger MK, Delmonico FL, et al. Organ donation and utilization in the USA. Am J Transplant. 2004;4 Suppl 9:27–37. doi: 10.1111/j.1600-6135.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 12.Whitington PF, Alonso AE. Fulminant hepatitis and acute liver failure. In: DA D, editor. Paediatric Liver Disease. Blackwell: Oxford; 2003. pp. 107–126. [Google Scholar]
- 13.Rumack BH. Acetaminophen overdose in children and adolescents. Pediatr Clin North Am. 1986;33(3):691–701. doi: 10.1016/s0031-3955(16)36050-3. [DOI] [PubMed] [Google Scholar]
- 14.Gregorio GV, Portmann B, Reid F, Donaldson PT, Doherty DG, McCartney M, et al. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997;25(3):541–547. doi: 10.1002/hep.510250308. [DOI] [PubMed] [Google Scholar]
- 15.Zar JH. Biostatistical Analysis. Uper Saddle River: Prentice Hall; 1999. [Google Scholar]
- 16.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 17.Huo TI, Wu JC, Sheng WY, Chan CY, Hwang SJ, Chen TZ, et al. Prognostic factor analysis of fulminant and subfulminant hepatic failure in an area endemic for hepatitis B. J Gastroenterol Hepatol. 1996;11(6):560–565. doi: 10.1111/j.1440-1746.1996.tb01703.x. [DOI] [PubMed] [Google Scholar]
- 18.Dhiman RK, Seth AK, Jain S, Chawla YK, Dilawari JB. Prognostic evaluation of early indicators in fulminant hepatic failure by multivariate analysis. Dig Dis Sci. 1998;43(6):1311–1316. doi: 10.1023/a:1018876328561. [DOI] [PubMed] [Google Scholar]
- 19.Schiodt FV, Davern TJ, Shakil AO, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol. 2003;98(2):448–453. doi: 10.1111/j.1572-0241.2003.t01-1-07223.x. [DOI] [PubMed] [Google Scholar]
- 20.Shah U, Habib Z, Kleinman RE. Liver failure attributable to hepatitis A virus infection in a developing country. Pediatrics. 2000;105(2):436–438. doi: 10.1542/peds.105.2.436. [DOI] [PubMed] [Google Scholar]
- 21.Mieli-Vergani G, Vergani D. Autoimmune hepatitis in children. Clin Liver Dis. 2002;6(3):335–346. doi: 10.1016/s1089-3261(02)00020-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23(3):217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 23.Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: hepatoxicity after multiple doses in children. J Pediatr. 1998;132(1):22–27. doi: 10.1016/s0022-3476(98)70479-2. [DOI] [PubMed] [Google Scholar]
- 24.Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, et al. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30(4):446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 25.Arnon R, DeVivo D, Defelice AR, Kazlow PG. Acute hepatic failure in a child treated with lamotrigine. Pediatr Neurol. 1998;18(3):251–252. doi: 10.1016/s0887-8994(97)00196-3. [DOI] [PubMed] [Google Scholar]
- 26.Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169–183. doi: 10.1055/s-2002-30102. [DOI] [PubMed] [Google Scholar]
- 27.Schwabe MJ, Dobyns WB, Burke B, Armstrong DL. Valproate-induced liver failure in one of two siblings with Alpers disease. Pediatr Neurol. 1997;16(4):337–343. doi: 10.1016/s0887-8994(97)00030-1. [DOI] [PubMed] [Google Scholar]
- 28.Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JB, Alexander G, et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology. 2004;39(5):1430–1440. doi: 10.1002/hep.20205. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe I, Tomita A, Shimizu M, Sugawara M, Yasumo H, Koishi R, et al. A study to survey susceptible genetic factors responsible for troglitazone-associated hepatotoxicity in Japanese patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2003;73(5):435–455. doi: 10.1016/s0009-9236(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 30.Bernal W, Wendon J, Rela M, Heaton N, Williams R. Use and outcome of liver transplantation in acetaminophen-induced acute liver failure. Hepatology. 1998;27:1050–1055. doi: 10.1002/hep.510270421. [DOI] [PubMed] [Google Scholar]