Abstract
The utility of routine chimerism analysis as a prognostic indicator of subsequent outcomes after allogeneic hematopoietic cell transplantation (HCT) with myeloablative conditioning regimens remains controversial. To address this controversy, routine chimerism test results at 2 – 6 months after HCT with myeloablative conditioning regimens were evaluated for association with subsequent risks of chronic graft versus host disease (GVHD), non-relapse mortality (NRM), relapse and overall mortality. Only 70 (5%) of 1304 patients had <95% donor-derived cells in the marrow. Low donor chimerism in the marrow occurred predominantly among patients with low risk disease as compared to higher risk diseases and was significantly associated with a reduced risk of chronic GVHD. Among 673 patients tested, 164 (24%) had <85% donor-derived T cells in the blood. Low donor T cell chimerism occurred predominantly among patients with low risk disease as compared to higher risk diseases, among those who had conditioning with busulfan as compared to TBI, and among those with lower grades of acute GVHD. Low donor T cell chimerism in the blood was significantly associated with a reduced risk of chronic GVHD, but not with the risks of relapse, NRM or overall mortality. Routine testing of chimerism in the marrow and blood at 2 – 6 months after HCT with myeloablative conditioning regimens may be helpful in documenting engraftment in clinical trials but provides only limited prognostic information in clinical practice.
Keywords: Chimerism analysis, mixed chimerism, allogeneic hematopoietic cell transplantation
Introduction
Chimerism testing can be used to document engraftment after allogeneic hematopoietic cell transplantation (HCT) and can be highly useful in the diagnosis of rejection and recurrent malignancy. Lineage–specific analysis increases the sensitivity of the method and may provide more specific information [1,2]. Chimerism tests demonstrating the persistence of recipient cells after HCT with non-myeloablative regimens can also predict an increased risk of rejection or recurrent malignancy, but the role of chimerism analysis as a prognostic indicator of subsequent transplant outcomes after HCT with myeloablative conditioning regimens remains controversial. While Lamba et al. [3] reported higher relapse rates and lower overall survival (OS) in patients with mixed chimerism (MC) on day 90 after HCT, Doney et al. [4] found no correlation between persistence of recipient cells at 2 – 3 months after HCT and subsequent outcomes in patients who had myeloablative conditioning regimens. The results of Doney et al. are consistent with consensus recommendations that documentation of chimerism is not essential after HCT with myeloablative conditioning regimens and conventional GVHD prophylaxis [1].
In order to address this controversy, we reviewed results of routine chimerism tests at 2 – 6 months after HCT with myeloablative conditioning regimens in a large cohort of patients in order to evaluate whether test results were associated with subsequent risks of chronic graft versus host disease (GVHD), non relapse mortality (NRM), recurrent malignancy and survival.
Methods
The cohort included all patients who had a first allogeneic HCT with the use of a myeloablative conditioning regimen at the Fred Hutchinson Cancer Research Center, and had routine chimerism testing of the marrow (n = 1304) (Table 1) or both granulocytes and T cells in the blood (n = 673) (Table 2) with the use of sex markers or molecular markers as part of the departure evaluation before their care was transferred from the transplant center to the referring physician. Routine testing was done on a single occasion, and the choice of samples to be tested was dictated by institutional practices, which changed over time. Transplant for patients who had testing of the marrow were done between July 1988 and September 2006. Transplants for patients who had testing of blood cells were done between September 2000 and September 2006. Testing was done at a median of 79 (range, 53–188) days after HCT. Patients were excluded from further analysis if the departure testing showed evidence of recurrent malignancy. All patients included for consideration signed IRB-approved informed consent documents allowing medical information to be reviewed for research purposes, and the IRB approved the use of medical information for this retrospective study.
Table 1.
Characteristics of patients who had testing of marrow cells
| Characteristic | Entire Cohort | <95% Donor Marrow Cells |
|---|---|---|
| Number of patients (%) | 1304 | 70 (5) |
| Patient age, median (range), y | 37 (0–66) | 41 (0–63) |
| Disease at transplantation, no. (%) | ||
| Myelodysplastic syndrome | 195 | 10 (5) |
| Acute myeloid leukemia | 300 | 3 (1) |
| Chronic myeloid leukemia | 443 | 40 (9) |
| Acute lymphoid leukemia | 182 | 4 (2) |
| Non-Hodgkin lymphoma or Hodgkin disease | 68 | 5 (7) |
| Other | 116 | 8 (7) |
| Pretransplant risk category, no. (%) | ||
| Low | 415 | 37 (9) |
| Intermediate | 628 | 25 (4) |
| High | 261 | 8 (3) |
| Donor age, median (range), y | 37 (0–81) | 40 (1–65) |
| Donor/recipient sex, no. (%)* | ||
| Male/male | 286 | 10 (4) |
| Male/female | 411 | 28 (7) |
| Female/male | 457 | 20 (4) |
| Female/female | 148 | 12 (8) |
| Donor type, no. (%) | ||
| HLA-identical related | 649 | 38 (6) |
| HLA-mismatched related | 108 | 8 (7) |
| HLA-matched unrelated | 293 | 13 (4) |
| HLA-mismatched unrelated | 254 | 11 (4) |
| Conditioning regimen, no. (%) | ||
| Cyclophosphamide and TBI | 676 | 26 (4) |
| Busulfan and cyclophosphamide | 375 | 27 (7) |
| Busulfan and TBI | 43 | 1 (2) |
| Busulfan, cyclophosphamide, and TBI | 59 | 1 (2) |
| Busulfan, cyclophosphamide, and ATG | 27 | 4 (15) |
| Cyclophosphamide, TBI, and ATG | 41 | 3 (7) |
| Other containing ATG | 55 | 4 (7) |
| Other | 28 | 4 (14) |
| Source of stem cells, no. (%) | ||
| Bone marrow | 1012 | 57 (6) |
| Mobilized blood | 279 | 10 (4) |
| Cord blood | 13 | 3 (23) |
| GVHD prophylaxis, no. (%) | ||
| Cyclosporine and methotrexate | 1061 | 63 (6) |
| Tacrolimus and methotrexate | 92 | 4 (4) |
| Methotrexate | 31 | 0 |
| Calcineurin inhibitor | 92 | 3 (3) |
| Calcineurin inhibitor and MMF | 21 | 0 |
| Other | 7 | 0 |
| Prior acute GVHD, no. (%)† | ||
| Grade 0 – I | 292 | 20 (7) |
| Grade II | 742 | 36 (5) |
| Grades III – IV | 269 | 14 (5) |
2 patients received cord blood transplants from 2 donors, one male and the other female.
GVHD could not be graded in one patient because of severe regimen-related toxicity.
Table 2.
Characteristics of patients who had testing of blood cells
| Characteristic | Entire Cohort | <95% Donor Granulocytes | <85% Donor T Cells |
|---|---|---|---|
| Number of patients (%) | 673 | 14 (2) | 164 (24) |
| Patient age, median (range), y | 39 (0–66) | 34 (1–57) | 42 (1–64) |
| Disease at transplantation, no. (%) | |||
| Myelodysplastic syndrome | 164 | 3 (2) | 57 (35) |
| Acute myeloid leukemia | 216 | 4 (2) | 36 (17) |
| Chronic myeloid leukemia | 118 | 3 (3) | 52 (44) |
| Acute lymphoid leukemia | 111 | 0 | 3 (3) |
| Other | 64 | 4 (6) | 16 (25) |
| Pretransplant risk category, no. (%) | |||
| Low | 144 | 4 (3) | 55 (38) |
| Intermediate | 434 | 8 (2) | 98 (23) |
| High | 95 | 2 (2) | 11 (12) |
| Donor age, median (range), y | 38 (1–76) | 30 (1–60) | 38 (5–65) |
| Donor/recipient sex, no. (%)* | |||
| Male/male | 204 | 5 (2) | 50 (25) |
| Male/female | 155 | 3 (2) | 42 (27) |
| Female/male | 160 | 2 (1) | 39 (24) |
| Female/female | 153 | 4 (3) | 33 (22) |
| Donor type, no. (%) | |||
| HLA-identical related | 288 | 10 (3) | 79 (27) |
| HLA-mismatched related | 15 | 0 | 2 (13) |
| HLA-matched unrelated | 225 | 3 (1) | 63 (28) |
| HLA-mismatched unrelated | 145 | 1 (1) | 20 (14) |
| Conditioning regimen, no. (%) | |||
| Cyclophosphamide and TBI | 250 | 2 (1) | 13 (5) |
| Busulfan and cyclophosphamide | 287 | 6 (2) | 106 (37) |
| Busulfan, cyclophosphamide, and ATG | 42 | 2 (5) | 6 (14) |
| Cyclophosphamide, TBI, and ATG | 25 | 0 | 3 (12) |
| Other containing ATG | 35 | 2 (6) | 20 (57) |
| Other | 34 | 1 (3) | 16 (47) |
| Source of stem cells, no. (%) | |||
| Bone marrow | 195 | 6 (3) | 62 (32) |
| Mobilized blood | 460 | 7 (2) | 101 (22) |
| Cord blood | 18 | 1 (6) | 1 (6) |
| GVHD prophylaxis, no. (%) | |||
| Cyclosporine plus methotrexate | 437 | 10 (2) | 120 (27) |
| Tacrolimus plus methotrexate | 157 | 3 (2) | 37 (24) |
| Methotrexate | 3 | 0 | 0 |
| Calcineurin inhibitor | 43 | 1 (2) | 6 (14) |
| Calcineurin inhibitor and MMF | 33 | 0 | 1 (3) |
| Prior acute GVHD, no. (%) | |||
| Grade 0 – I | 157 | 5 (3) | 56 (36) |
| Grade II | 426 | 8 (2) | 96 (23) |
| Grades III – IV | 90 | 1 (1) | 12 (13) |
1 patient received a cord blood transplant from 2 donors, one male and the other female.
Blood cells were sorted according to expression of CD33 and CD3 by flow-cytometry before chimerism testing. Non-fractionated aspirated marrow and fractionated blood cells from patients with sex-mismatched donors were tested by fluorescent in situ hybridization with Y chromosome-specific probes or with both Y and X-chromosome-specific probes [5]. Beginning in 1998, DNA samples from patients with same-sex donors were tested by amplification and semi-quantitative analysis of variable number tandem repeat loci with informative polymorphisms. Amplified products were analyzed semi-quantitatively after electrophoresis and silver staining in polyacrylamide gels [6]. As assessed by mixing experiments, this method has between 0.1 – 5% sensitivity, depending on the relative size of the informative markers. Beginning in 2004, samples from patients with same-sex donors were tested by multiplex amplification of short tandem repeat loci with informative polymorphisms (PowerPlex 16; Promega, Madison, WI, USA). Amplified products were analyzed quantitatively by capillary electrophoresis (ABI 3130 × 1; Applied Biosystems, Foster City, CA, USA). This method has at least 0.5% sensitivity, as assessed by mixing experiments [7]. Pretransplant samples from the donor and recipient were routinely included as controls.
Survival probabilities were estimated according to the Kaplan-Meier method. Probabilities of recurrent malignancy, NRM and chronic GVHD were estimated by the cumulative incidence method. Follow-up for survival and recurrent malignancy was censored at the date of last contact or death. Follow-up for NRM and chronic GVHD was censored at the onset of recurrent malignancy or date of last contact, whichever occurred first. Chi-square test or Fisher’s exact test was used to estimate the statistical significance of categorical differences. Cox proportional hazards analysis was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for mortality, recurrent malignancy, NRM and chronic GVHD for patients with low donor chimerism compared to those without low donor chimerism. Adjustment factors considered were disease risk (low risk included chronic myeloid leukemia in chronic phase and refractory anemia; high risk included malignant disease in relapse, refractory anemia with excess blasts in transformation, and chronic myeloid leukemia in blast crisis; intermediate risk included all other diagnoses), conditioning (busulfan and cyclophosphamide, cyclophosphamide and total body irradiation, and all other regimens), grafting with growth factor-mobilized blood cells, and grades II – IV acute GVHD with onset before chimerism testing.
Results
Two factors were considered in defining a threshold for “low donor chimerism.” First, the threshold percentage of recipient cells had to be above the lower limit of sensitivity in reliably detecting these cells by the assays used for testing (5%) in order to avoid confounding caused by false-negative results. Second, the number of patients with low donor chimerism had to be high enough to allow reasonable statistical power for observing associations with clinical outcomes, if possible. Routine testing of aspirated marrow at 2 – 6 months after HCT showed >5% recipient cells (<95% donor cells) in only 70 (5%) of the 1304 patients tested (Table 1). Although this threshold does not provide optimal statistical power, the use of a less stringent definition of low donor chimerism was not feasible because of limits in the sensitivity of chimerism tests.
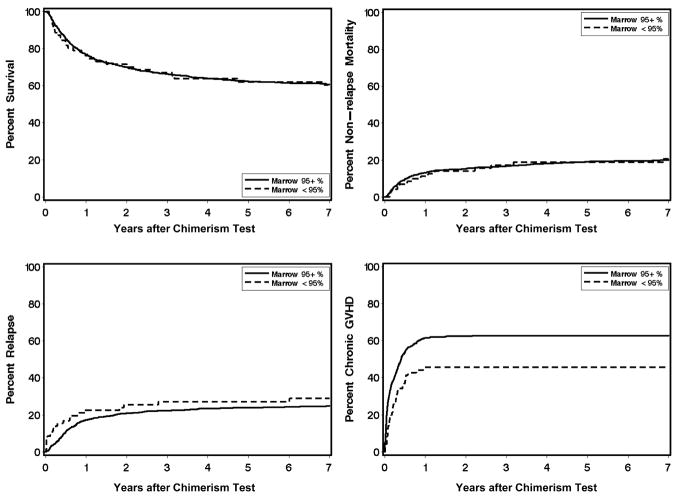
Low donor chimerism (<95% donor cells) in the marrow occurred more frequently among patients with low-risk diseases (predominantly chronic myeloid leukemia) as compared to higher–risk diseases (predominantly acute leukemia) (P < .001). Persistence of >5% recipient cells was not significantly associated with donor-recipient gender combination, relationship between the donor and recipient, the conditioning regimen, the use of growth factor mobilized blood versus marrow, the post-transplant immunosuppressive regimen or the severity of acute GVHD (Table 1). Correlation with clinical outcome showed a statistically significant association of low donor chimerism in the marrow with decreased subsequent risk of chronic GVHD (HR, 0.65; 95% C.I., 0.5 – 0.9; P = .02) but not with subsequent risks of recurrent malignancy, non-relapse mortality or overall mortality (Table 3, Figure 1).
Table 3.
Association of low donor chimerism with subsequent outcomes after HCT
| Overall Mortality | Relapse | Non-relapse Mortality | Chronic GVHD | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Donor chimerism in marrow | ||||||||
| ≥95% (n=1234) | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| <95% (n=70) | 0.97 (0.7–1.4) | .87 | 1.35 (0.9–2.1) | .17 | 0.97 (0.6–1.7) | .90 | 0.65 (0.5–0.9) | .02 |
| Donor chimerism in blood granulocytes | ||||||||
| ≥95% (n=659) | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| <95% (n=14) | 0.60 (0.2–1.9) | .38 | 0.59 (0.1–2.4) | .45 | 0.90 (0.2–3.6) | .88 | 0.67 (0.3–1.4) | .30 |
| Donor chimerism in blood T cells | ||||||||
| ≥85% (n=509) | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| <85% (n=164) | 0.66 (0.5–0.9) | .02 | 0.84 (0.6–1.2) | .38 | 0.62 (0.4–1.0) | .07 | 0.76 (0.6–1.0) | .02 |
Results do not reflect adjustment for other risk factors.
Figure 1.
Clinical outcomes according to level of donor chimerism in the marrow.
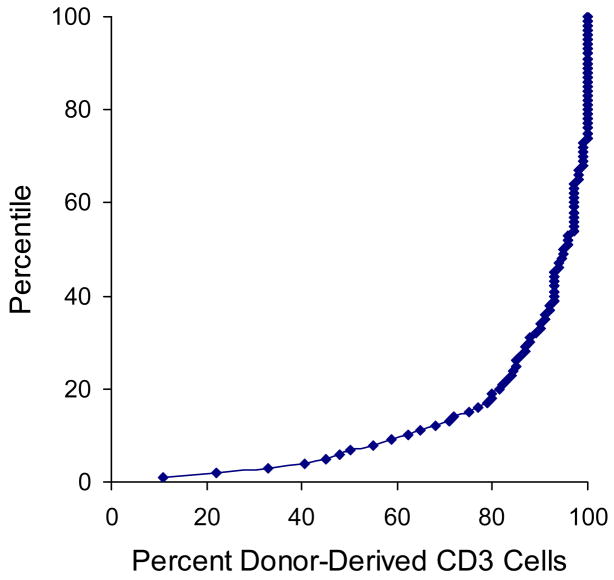
Based on the same considerations used to define low donor chimerism in the marrow, the presence of <95% donor-derived cells was used to define low donor chimerism in blood granulocytes. Only 14 (2%) of 673 patients had <95% donor-derived granulocytes in the blood. Low donor chimerism in blood granulocytes occurred too infrequently for meaningful analysis of correlation with transplant characteristics or clinical outcome (Tables 2 and 3). The percentile distribution of T cell chimerism suggested that the presence of <85% donor-derived cells could be used to define low donor chimerism (Figure 2). By this definition, low donor chimerism (<85%) was observed in 164 (24%) of the 673 patients (Table 2). Low T cell chimerism occurred more frequently among patients with chronic myeloid leukemia or myelodysplastic syndrome as compared to acute lymphoid leukemia (P < .001), among patients with low-risk diseases as compared to high-risk diseases (P < .001), among patients who had conditioning with busulfan as compared to TBI (P < .001) and among patients with grades 0 – 1 acute GVHD as compared to grades III – IV GVHD (P < .001). Low T cell chimerism occurred less frequently when the donor and recipient were HLA-mismatched (P < .001) and also among patients who received a combination of calcineurin inhibitor with mycophenolate mofetil as compared to methotrexate after the transplant (P = .003). Low T cell chimerism was not significantly associated with donor recipient gender combination, the relationship between the donor and recipient, or the source of stem cells (Table 2).
Figure 2.
Percentile distribution of donor T cell chimerism in the blood.
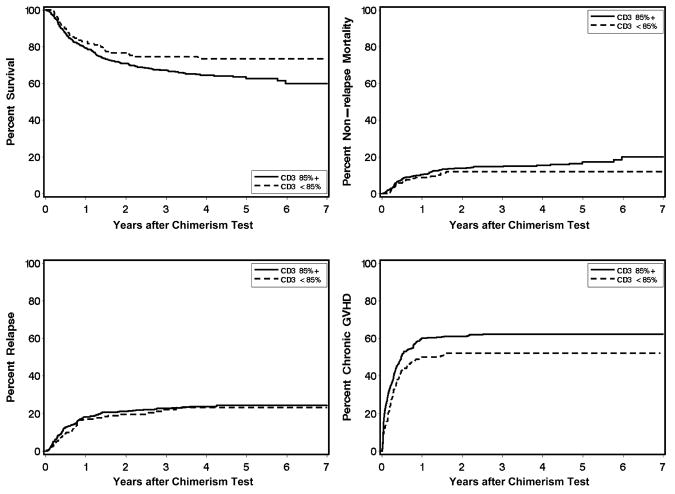
Correlation with clinical outcome showed a statistically significant association between low T cell chimerism and a reduced subsequent risk of chronic GVHD (HR, 0.76; 95% C.I., 0.6 – 1.0; P = .02) (Table 3 and Figure 3). This association was slightly attenuated after adjustment for differences in pretransplant risk category, conditioning regimen, use of mobilized blood cells, and prior grades II – IV GVHD (HR, 0.80; 95% C.I., 0.6 – 1.0, P = .09). In univariate analysis, low T cell chimerism was significantly associated with lower overall mortality (HR, 0.66; 95% C.I. 0.5 – 0.9; P = .02) (Table 3, P Figure 3), but after adjustment for differences in disease risk group and conditioning regimen, this association was no longer statistically significant (HR, 0.77; 95% C.I. 0.5 – 1.1; = .16). Low T cell chimerism showed a trend for association with a lower risk of NRM (HR, 0.62; 95% C.I. 0.4 – 1.0; P = .07) but not with an increased risk of recurrent malignancy (Table 3).
Figure 3.
Clinical outcomes according to level of donor T cell (CD3) chimerism in the blood.
To some extent, the association of low donor chimerism in the marrow with a reduced risk of chronic GVHD could reflect low donor T cell chimerism in the blood, since aspirated marrow contains an appreciable number of T cells from the blood. To test this hypothesis, we evaluated the correlation between donor chimerism levels in the marrow and the blood in 173 patients who had both tests. Low donor marrow chimerism was observed in 6 of 47 (12.8%) patients with low T cell chimerism, compared to 3 of 126 (2.4%) patients with high donor T cell chimerism (P = .01, Fisher’s exact test). Among these patients, low donor T cell chimerism was significantly associated with a reduced risk of chronic GVHD (HR, 0.51; 95% C.I., 0.3 – 0.9; P = .02), but low donor marrow chimerism was not (HR, 0.97; 95% C.I. 0.4 – 2.7; P = .96).
None of the 509 patients with ≥ 85% donor T cell chimerism had a second transplant for treatment of graft failure, and only 2 of the 164 (1.2%) patients with low donor T cell chimerism had a second transplant because of poor graft function. In one case, the results on day 81 after the first transplant showed 5 – 15% donor-derived T cells in the blood with 95 – 99% donor-derived granulocytes, and results on day 160 showed 1 – 5% donor-derived T cells in the blood again with 95 – 99% donor-derived granulocytes. In the other case, the results on day 81 after the first transplant showed 60% donor-derived T cells in the blood with 77% donor-derived granulocytes, and results on day 111 were similar.
Discussion
In this study, we found that at 2 – 6 months after HCT with myeloablative conditioning regimens, only a small minority of patients had less than 95% donor-derived cells in the marrow and in blood granulocytes. Low donor chimerism in the marrow occurred predominantly among patients with low-risk diseases such as chronic phase CML and was associated with a reduced risk of chronic GVHD. Approximately 25% of the patients in this study had < 85% donor-derived T cells in the blood at 2 – 6 months after HCT with myeloablative conditioning regimens. Low donor chimerism in blood T cells occurred predominantly among patients with low-risk diseases and was associated with the absence of TBI in the conditioning regimen and the absence of acute GVHD. Low donor chimerism in the marrow and in blood T cells at 2 – 6 months after HCT with myeloablative conditioning regimens was significantly associated with a reduced risk of chronic GVHD but not with the risks of recurrent malignancy, non-relapse mortality, overall mortality, or graft failure leading to a second transplant. Routine testing of chimerism in the marrow and blood at 2 – 6 months after HCT with myeloablative conditioning regimens may be helpful in documenting engraftment in clinical trials but provides only limited prognostic information in clinical practice.
The threshold of < 85% donor-derived T cells used to define low donor chimerism in our study was selected empirically and may differ from the values used in other studies. The percentile distribution of T cell chimerism in our study showed a smooth progression from low values to high values, with no obvious break point. The 85% threshold approximates the inflection of the curve and had no a priori biological significance.
The absence of prior myelosuppressive treatment before referral for HCT most likely accounts for the association of low-risk diseases with low donor marrow chimerism and low donor T cell chimerism at 2 – 6 months after HCT with myeloablative conditioning regimens. Low risk diseases included chronic myeloid leukemia in chronic phase and refractory anemia, which are almost never treated with high-dose myelosuppressive treatment. High risk diseases included malignant disease in relapse, refractory anemia with excess blasts in transformation, and chronic myeloid leukemia in blast crisis, and intermediate risk diseases included all other diagnoses. We acknowledge that some patients in the intermediate risk and high risk categories did not receive prior myelosuppressive treatment. We used the low and higher-risk categories as a surrogate for the respective absence or presence of prior myelosuppressive treatment, although we do not have direct data demonstrating this correlation. A similar explanation has been invoked for the observation that the incidence of graft rejection after HCT with nonmyeloablative conditioning regimens is higher among patients with low-risk diseases than among those with higher-risk diseases [8]. These results support the hypothesis that previous exposure to multiple cycles of myelosuppressive chemotherapy increases the susceptibility of recipient hematopoietic cells and T cells to the effects of both myeloablative and nonmyeloablative pretransplant conditioning regimens.
The observation that low donor T cell chimerism occurred more frequently than low donor myeloid chimerism suggests that prior myelosuppressive treatment, the pretransplant conditioning regimen and the effects of acute GVHD have a greater cumulative effect on myeloid cells than on T cells in the recipient. Mattsson et al. [9] reported similar lower levels of T cell chimerism compared to myeloid chimerism. The results of chimerism testing at 2 – 6 months after HCT suggest that the fractionated TBI exposures and doses of busulfan used in this study had equivalent cumulative effects on recipient myeloid cell but different effects on recipient T cells. These results indicate that fractionated TBI has a more potent immunosuppressive effect than busulfan, as demonstrated by other studies [9]. The association of HLA-mismatching and acute GVHD with decreased proportions of persisting recipient T cells and higher levels of donor T cell chimerism is consistent with the hypothesis that the targets of acute GVHD include recipient T cells, as well as basal epithelial cells in the skin, bile duct epithelial cells in the liver, and crypt epithelial cells in the gastrointestinal tract.
Our results demonstrating that persistence of recipient cells in the marrow or blood T cells beyond 2 months after HCT is associated with a reduced risk of chronic GVHD after allogeneic HCT with myeloablative conditioning regimens are consistent with results of other studies. McCann et al. [10] showed that patients with mixed chimerism had a decreased risk of chronic GVHD after HCT for treatment of aplastic anemia, while Balon et al. [11] showed that development of complete chimerism within the first 3 months after HCT was associated with an increased risk of chronic GVHD. To some extent, low donor chimerism in aspirated marrow specimens could be explained by low T cell chimerism in the blood. The reduced risk of chronic GVHD associated with low donor chimerism in the blood and not with low donor chimerism in the marrow among patients who had both tests suggests that low donor T cell chimerism (or persistence of recipient T cells) represents the dominant association. Recipient T cells could have suppressive or veto effects on donor cells that cause chronic GVHD, thereby inducing a state of tolerance. Alternatively, the association of high donor T cell chimerism with an increased risk of chronic GVHD could reflect the activity of some other factor that simultaneously eliminates recipient T cells and also induces chronic GVHD.
The absence of a correlation between low donor T cell chimerism and the subsequent risk of recurrent malignancy is somewhat unexpected, since persistence of recipient T cells was associated with a decreased risk of chronic GVHD and since development of chronic GVHD has been associated with a decreased risk of recurrent malignancy. Any increment in risk of recurrent malignancy associated with the observed decrease in risk of chronic GVHD among patients with persisting recipient T cells could have been too small to measure in a cohort of patients with a variety of malignant diseases. The absence of a correlation between low donor marrow chimerism or low donor T cell chimerism and the subsequent risk of recurrent malignancy in our study is consistent with results of several previous studies [4,12,13,14]. Other studies, however, have shown that persistence of recipient myeloid cells [15,16] or T cells [17] after unmanipulated [15,16] or T cell-depleted [16,17] HCT was associated with an increased risk of recurrent malignancy among patients with CML. Associations between persistence of recipient cells and an increased risk of recurrent malignancy have also been reported among patients with acute leukemia [3,18]. Chimerism assays can certainly be used as a diagnostic indicator of recurrent malignancy, but their utility as a prognostic indicator of recurrent malignancy depends on the nature of the malignancy, the intensity of the conditioning regimen [8] and the methods used to prevent GVHD.
Contrary to results of Lamba et al. [3], our data do not support an association between survival and low donor T cell chimerism at 2 – 6 months after HCT with myeloablative conditioning. On the other hand, we observed a trend for an association of low donor T cell chimerism with a reduced subsequent risk of non-relapse mortality. This association is at least partly explained by the reduced risk of chronic GVHD associated with persistence of recipient T cells, since chronic GVHD is the primary cause of late non-relapse mortality after allogeneic HCT.
Our current results do not support an association between low donor T cell chimerism and a subsequent risk of graft failure after T cell-replete HCT with myeloablative conditioning regimens, although other studies have shown such associations [2]. Although graft rejection can be caused by recipient T cells surviving the conditioning regimen, the presence of a particular cell population does not allow direct inferences concerning its function capabilities [19]. Hence, our results emphasize that the persistence of recipient T cells after HCT does not necessarily indicate that rejection is likely to occur. Chimerism assays can certainly be used as a diagnostic indicator of rejection, but their utility as a prognostic indicator of rejection depends on other factors that affect the risk of rejection, such as the intensity of the conditioning regimen and the use of T cell depletion to prevent GVHD.
To some extent, the association between donor T cell chimerism and the subsequent risk of chronic GVHD could be used in making decisions about the withdrawal of immunosuppressive treatment or enrolment of patients in clinical trials testing new approaches for prevention of chronic GVHD. Routine chimerism testing at 2 – 6 months after HCT with myeloablative conditioning regimens can also be used to document engraftment in clinical trials. Our results showing the absence of strong correlations with risks of recurrent malignancy, non-relapse mortality and overall survival apply only for chimerism testing in patients who have had HCT with myeloablative conditioning regimens and should not be extrapolated to patients who have had non-myeloablative conditioning regimens [8]. Although our results apply to the use of routine chimerism testing for prognostic purposes, they have no relevance regarding the use of chimerism tests for diagnostic purposes.
Acknowledgments
We thank Gary Schoch for assistance with data management. We thank Mari Malkki and Dr. Effie Petersdorf for information related to HLA-matching of donors and recipients.
Supported in part by CA18029 and HL36444 from the NIH, DHHS, Bethesda, MD and by the US-Egypt Joint Fund ID-Code BIO7-002-002, Contract No. 188, USDA, Washington, DC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antin JH, Childs R, Filipovich AH, Giralt S, Mackinnon S, Spitzer T, Weisdorf D. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2001;7:473–485. doi: 10.1053/bbmt.2001.v7.pm11669214. [DOI] [PubMed] [Google Scholar]
- 2.Lion T. Detection of impending graft rejection and relapse by lineage-specific chimerism analysis. Methods Mol Med. 2007;134:197–216. doi: 10.1007/978-1-59745-223-6_14. [DOI] [PubMed] [Google Scholar]
- 3.Lamba R, Abella E, Kukuruga D, et al. Mixed hematopoietic chimerism at day 90 following allogeneic myeloablative stem cell transplantation is a predictor of relapse and survival. Leukemia. 2004;18:1681–1686. doi: 10.1038/sj.leu.2403468. [DOI] [PubMed] [Google Scholar]
- 4.Doney KC, Loken MR, Bryant EM, Smith AG, Appelbaum FR. Lack of utility of chimerism studies obtained 2–3 months after myeloablative hematopoietic cell transplantation for ALL. Bone Marrow Transplant. 2008 doi: 10.1038/bmt.2008.155. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewald GW, Schad CR, Christensen ER, et al. Fluorescence in situ hybridization with X and Y chromosome probes for cytogenetic studies on bone marrow cells after opposite sex transplantation. Bone Marrow Transplant. 1993;12:149–154. [PubMed] [Google Scholar]
- 6.Smith AG, Martin PJ. Analysis of amplified variable number tandem repeat loci for evaluation of engraftment after hematopoietic stem cell transplantation. Rev Immunogenetics. 1999;1:255–264. [PubMed] [Google Scholar]
- 7.Thiede C, Bornhauser M, Oelschlagel U, et al. Sequential monitoring of chimerism and detection of minimal residual disease after allogeneic blood stem transplantation (BSCT) using multiplex PCR amplification of short tandem repeat-markers. Leukemia. 2001;15:293–302. doi: 10.1038/sj.leu.2401953. [DOI] [PubMed] [Google Scholar]
- 8.Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006;20:1690–1700. doi: 10.1038/sj.leu.2404335. [DOI] [PubMed] [Google Scholar]
- 9.Mattsson J, Uzunel M, Remberger M, Hassan M. Fractionated TBI correlates with less T cell mixed chimerism but increased risk of relapse compared to busulphan in patients with haematological malignancies after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003;32:477–483. doi: 10.1038/sj.bmt.1704154. [DOI] [PubMed] [Google Scholar]
- 10.McCann S, Passweg J, Bacigalupo A, et al. The influence of cyclosporin alone, or cyclosporin and methotrexate, on the incidence of mixed haematopoietic chimaerism following allogeneic sibling bone marrow transplantation for severe aplastic anaemia. Bone Marrow Transplant. 2007;39:109–114. doi: 10.1038/sj.bmt.1705552. [DOI] [PubMed] [Google Scholar]
- 11.Balon J, Ha aburda K, Bieniaszewska M, et al. Early complete donor hematopoietic chimerism in peripheral blood indicates the risk of extensive graft-versus-host disease. Bone Marrow Transplant. 2005;35:1083–1088. doi: 10.1038/sj.bmt.1704962. [DOI] [PubMed] [Google Scholar]
- 12.van Leeuwen JE, van Tol MJ, Joosten AM, et al. Persistence of host-type hematopoiesis after allogeneic bone marrow transplantation for leukemia is significantly related to the recipient’s age and/or the conditioning regimen, but it is not associated with an increased risk of relapse. Blood. 1994;83:3059–3067. [PubMed] [Google Scholar]
- 13.Mattsson J, Uzunel M, Tammik L, Aschan J, Ringdén O. Leukemia lineage-specific chimerism analysis is a sensitive predictor of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplantation. Leukemia. 2001;15:1976–1985. doi: 10.1038/sj.leu.2402311. [DOI] [PubMed] [Google Scholar]
- 14.Boeck S, Hamann M, Pihusch V, et al. Kinetics of dendritic cell chimerism and T cell chimerism in allogeneic hematopoietic stem cell recipients. Bone Marrow Transplant. 2006;37:57–64. doi: 10.1038/sj.bmt.1705217. [DOI] [PubMed] [Google Scholar]
- 15.Román J, Serrano J, Jiménez A, et al. Myeloid mixed chimerism is associated with relapse in bcr-abl positive patients after unmanipulated allogeneic bone marrow transplantation for chronic myelogenous leukemia. Haematologica. 2000;85:173–180. [PubMed] [Google Scholar]
- 16.Serrano J, Roman J, Sanchez J, et al. Molecular analysis of lineage-specific chimerism and minimal residual disease by RT-PCR of p210(BCR-ABL) and p190(BCR-ABL) after allogeneic bone marrow transplantation for chronic myeloid leukemia: increasing mixed myeloid chimerism and p190(BCR-ABL) detection precede cytogenetic relapse. Blood. 2000;95:2659–2665. [PubMed] [Google Scholar]
- 17.Mackinnon S, Barnett L, Heller G, O’Reilly RJ. Minimal residual disease is more common in patients who have mixed T-cell chimerism after bone marrow transplantation for chronic myelogenous leukemia. Blood. 1994;83:3409–3416. [PubMed] [Google Scholar]
- 18.Bader P, Beck J, Frey A, et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant. 1998;21:487–495. doi: 10.1038/sj.bmt.1701119. [DOI] [PubMed] [Google Scholar]
- 19.Martin PJ. Documentation of engraftment and characterization of chimerism following hematopoietic cell transplantation. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. Blackwell; Oxford, UK: 2008. in press. [Google Scholar]