Abstract
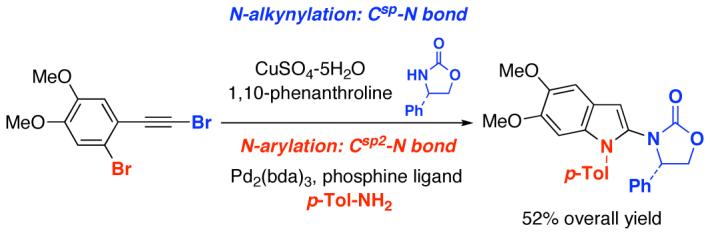
A sequential metal-catalyzed C-N bond formation employing ortho-haloaryl acetylenic bromides is described. The initial amidation is highly selective for Csp-N bond formation, leading to o-haloaryl-substituted ynamides that can be useful building blocks, while the overall sequence provides a facile construction of 2-amido-indoles.
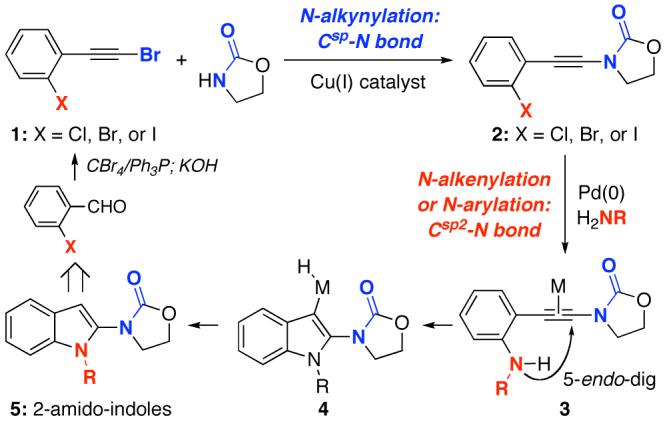
Given the importance of heterocyclic manifolds,1 we have been developing synthetic methods that feature ynamides2-3 en route to various heterocycles.4 These efforts led us to examine a possible entry for constructing amide substituted indoles.5-7 Specifically, as shown in Scheme 1, this pathway would commence with ortho-haloaryl acetylenic bromides 1 and adopt a consecutive metal-catalyzed C-N bond formation8 with the first involving the sp-hybridized carbon9-13 in an N-alkynylation manner, and the second one pertaining to an sp2-hybridized carbon in a N-arylation manner.8,14 The second C-N bond formation can also occur in a tandem manner with the ensuing indole formation promoted by the metal15,16 in a 5-endo-dig cyclization mode via 3. While copper can be employed to catalyze the Csp-N formation,9-13 we intend to utilize palladium for the Csp2-N formation.15 If this sequential C-N bond formation is selective, it would constitute a facile entry to de novo 2-amido-indoles17,18 5 from ortho-haloaryl acetylenes 1, which can be readily derived from aromatic aldehydes in two steps.19 We report here synthesis of 2-amido-indoles via a sequential metal-catalyzed C-N bond formation.
Scheme 1.
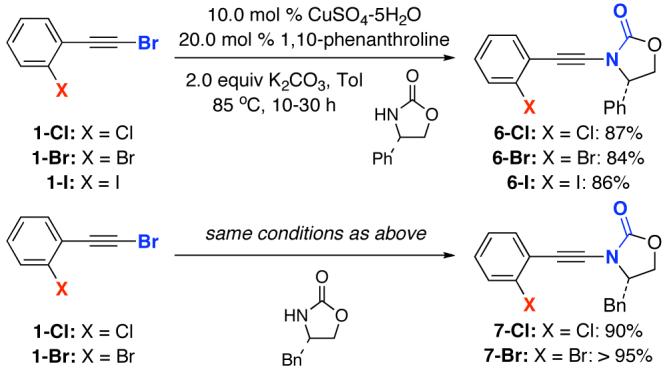
A selective amidative cross-coupling of ortho-haloaryl acetylenic bromides 120 could be readily established as shown in Scheme 2. By employing 10 mol% CuSO4-5H2O and 20 mol% of 1,10-phenanthroline,13 ynamide 6-Cl was attained in 87% yield from 1-Cl. The amidation remained selective when using 1-Br and even 1-I, leading to 6-Br and 6-I in 84% and 86% yield, respectively. Under the same conditions, ynamides 7-Cl and 7-Br were obtained also via a highly selective Csp-N formation.
Scheme 2.
A diverse array of ortho-haloaryl acetylenic bromides could be subjected to this selective amidation to give ynamides 8-16 [Figure 1]. Moreover, a range of cyclic and acyclic amides including sulfonamides could be employed for the N-alkynylation to afford ynamides 17-23 in good yields.
Figure 1. N-Alkynylation Products.a.
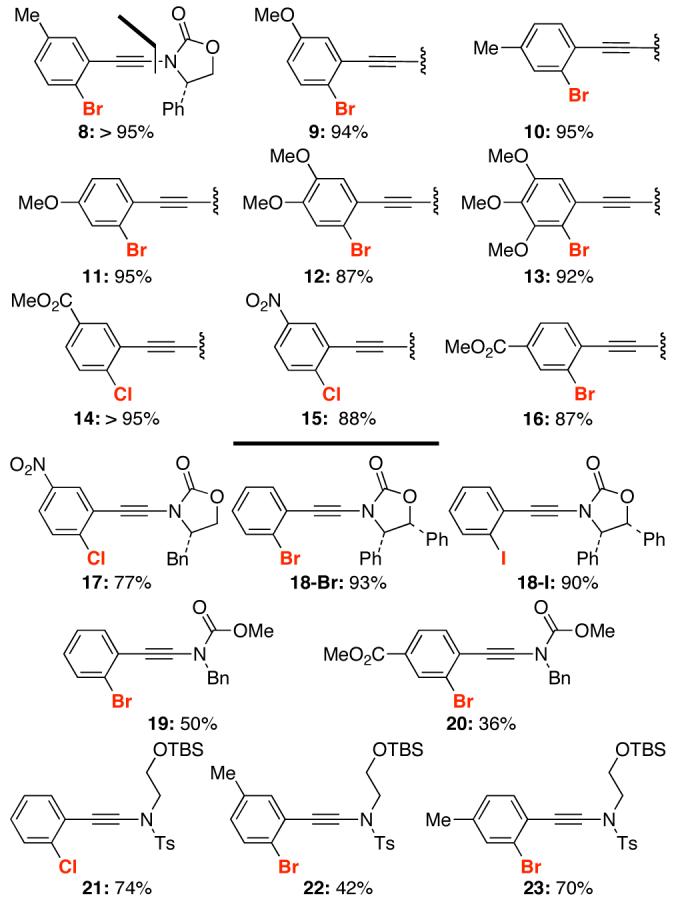
a. Reaction conditions are the same as those in Scheme 2. All are isolated yields.
Having established this selective amidation, we recognized that we have an excellent protocol to access o-haloaryl-substituted ynamides 6-23, which represent a new class of functionally rich building blocks that could be utilized in a number of transformations involving either the o-haloaryl or ynamido motif [Figure 2], leading to rapid assembly of structural complexity.
Figure 2. Synthetic Potential of o-Haloaryl Ynamides.

To illustrate such synthetic potential, we chose to pursue aminative cross-coupling of aryl halides8,14 to access 2-acetylenic anilines en route to 2-amido-indoles via metal-promoted 5-endo-dig cyclization.15-19,21 As shown in Scheme 3, when ynamide 7-Br was subjected to amination conditions employing 2.5 mol% Pd2(dba)3 and p-Tol-NH2, 2-amido-indole 24 was obtained in good yields when using either 5.0 mol% of van Leeuwen’s xantphos22 or Buchwald’s X-phos as ligands.23 Intriguingly, the use of X-phos appears to shorten the reaction time relative to xantphos while BINAP was not useful. On the other hand, amination of 7-Cl led to 24 in 78% yield only when using X-phos. It is noteworthy that amination of 6-I gave only 26% yield of corresponding indole [not shown], thereby suggesting that aryl chlorides and bromides are better suited in this operation than aryl iodides.
Scheme 3.

The generality of this tandem amination-5-endo-dig cyclization is shown in Table 1, featuring a range of different amines and o-chloroaryl- or o-bromoaryl-substituted ynamides in excellent yields for their respective reactions. X-ray crystallographic analysis of 2-amido-indole 28 reveals unique orthogonality of three planes: 2-Oxazolidone, the indole ring, and the para-tolyl ring [Figure 3]. Structures with related orthogonality have been shown24 to possess inhibitory activities against human peptidyl prolyl cis/trans isomerase [PPI] Pin-1,25 which catalyzes the isomerization of prolyl peptides from cis to trans26,27 and accommodates such orthogonality at its active site. We are currently investigating such potential biological activity.
Table 1. N-Alkenylation in the 2-Amido-Indole Synthesis.
| entry | ynamidesa,b | 2-amido-indoles | yield [%]c | ||
|---|---|---|---|---|---|
| 1 | 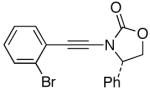 |
6-Br | 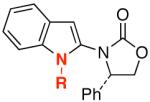 |
25: R = p-Tol | 91 |
| 2 | 6-Br | 26: R = o-Tol | 82 | ||
| 3 | 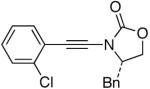 |
7-Cl | 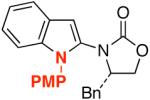 |
27d | 91 |
| 4 | 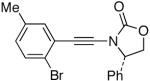 |
8 | 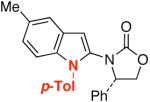 |
28 | 65 |
| 5 | 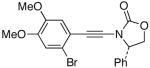 |
12 |  |
29 | 60 |
| 6 |  |
14 | 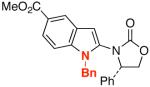 |
30 | 72 |
| 7 | 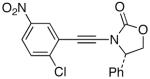 |
15 |  |
31 | 64 |
| 8 |  |
16 | 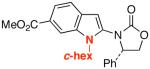 |
32 | 80 |
| 9 | 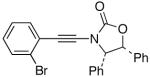 |
18-Br | 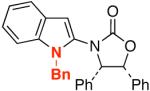 |
33 | 71 |
| 10 | 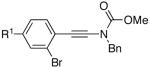 |
19 |  |
34: R1 = H | 82 |
| 11 | 20 | 35: R1 = CO2Me | 75 | ||
| 12 |  |
21 |  |
36 | 88 |
Reaction conditions: 2.5 mol % Pd2(bda)3, 5.0 mol % X-phos, 3.3 equiv Cs2CO3, 1.3 equiv R-NH2, 110 °C, 8-24 h.
Toluene was used as solvent in entries 4, 5, 7, and 9, and dioxane was used in entries 1-3, 6, 8, and 10-12.
Isolated yields.
PMP = para-methoxy-phenyl.
Figure 3. X-Ray Structure of 2-Amido-Indole 28.

We have described here a sequential metal-catalyzed C-N bond formation employing ortho-haloaryl acetylenic bromides. The initial amidation is highly selective for Csp-N bond, leading to o-haloaryl-substituted ynamides that can be useful building blocks. The overall sequence provides a facile construction of 2-amido-indoles possessing a unique structural manifold.
Supplementary Material
Acknowledgement
We thank UW-Madison and Cancer Center for funding. PYY and KZ thank the Cheung Kong Scholar Program for funding. We thank Dr. Haibing Song [Nankai University] for solving single-crystal X-ray structure. We also thank Dr. Yunfei Du [Tianjin University] for valuable suggestions.
References
- 1.For a review, see:Fan W-Q, Katritzky AR. In: Comprehensive Heterocyclic Chemistry. Katritzky AR, Rees CW, Scriven EFV, editors. Vol. 4. Pergamon Press; Oxford: 1996. pp. 101–126.
- 2.For reviews on ynamides, see:Zificsak CA, Mulder JA, Hsung RP, Rameshkumar C, Wei L-L. Tetrahedron. 2001;57:7575.Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379.Katritzky AR, Jiang R, Singh SK. Heterocycles. 2004;63:1455.
- 3.For recent references on chemistry of ynamides, see:Zhang X, Hsung RP, Li H, Zhang Y, Johnson WL, Figueroa R. Org. Lett. 2008;10:3477. doi: 10.1021/ol801257j.Al-Rashid ZF, Hsung RP. Org. Lett. 2008;10:661. doi: 10.1021/ol703083k.Istrate FM, Buzas AK, Jurberg ID, Odabachian Y, Gagosz F. Org. Lett. 2008;10:925. doi: 10.1021/ol703077g.Kim JY, Kim SH, Chang S. Tetrahedron Lett. 2008;49:1745.Martínez-Esperón MF, Rodríguez D, Castedo L, Saá C. Tetrahedron. 2008;64:3674.Yavari I, Sabbaghan M, Hosseini N, Hossaini Z. Synlett. 2007;20:3172.Oppenheimer J, Hsung RP, Figueroa R, Johnson WL. Org. Lett. 2007;9:3969. doi: 10.1021/ol701692m.You L, Al-Rashid ZF, Figueroa R, Ghosh SK, Li G, Lu T, Hsung RP. Synlett. 2007:1656.Hashimi ASK, Salathe R, Frey W. Synlett. 2007:1763.Rodríguez D, Martínez-Esperón MF, Castedo L, Saá C. Synlett. 2007:1963.Couty S, Meyer C, Cossy J. Synlett. 2007:2819.Tanaka K, Takeishi K. Synthesis. 2007:2920.Tanaka K, Takeishi K, Noguchi K. J. Am. Chem. Soc. 2006;128:4586. doi: 10.1021/ja060348f.Couty S, Meyer C, Cossy J. Angew. Chem. Int. Ed. 2006;45:6726. doi: 10.1002/anie.200602270.
- 4.(a) Li H, You L, Zhang X, Johnson WL, Figueroa R, Hsung RP. Heterocycles. 2007;74:553. [Google Scholar]; (b) Zhang X, Hsung RP, Li H. Chem. Commun. 2007:2420. doi: 10.1039/b701040k. [DOI] [PubMed] [Google Scholar]; (c) Oppenheimer J, Johnson WL, Tracey MR, Hsung RP, Yao P-Y, Liu R, Zhao K. Org. Lett. 2007;9:2361. doi: 10.1021/ol0707362. [DOI] [PubMed] [Google Scholar]; (d) Zhang X, Li H, You L, Tang Y, Hsung RP. Adv. Syn. Cat. 2006;348:2437. [Google Scholar]; (e) Zhang X, Hsung RP, You L. Org. Biomol. Chem. 2006;6:2679. doi: 10.1039/b606680a. [DOI] [PubMed] [Google Scholar]
- 5.For reviews on indole chemistry, see:Sundberg RL. Indoles. Academic; London: 1996. Katritzky AR, Pozharskii AF. Handbook of Heterocyclic Chemistry. Pergamon; Oxford: 2000. Chapter 4.Li JJ, Gribble GW. Palladium in Heterocyclic Chemistry. Pergamon; Oxford: 2000. Chapter 3.Gribble GW. J. Chem. Soc., Perkin Trans. 1. 2000:1045.
- 6.For recent reviews on indole-containing natural products, see:Crich D, Banerjee A. Acc. Chem. Res. 2007;40:151. doi: 10.1021/ar050175j.Somei M, Yamada F. Nat. Prod. Rep. 2005;22:73. doi: 10.1039/b316241a.
- 7.For a leading reference on biological activities of indoles, see:Van Zandt MC, Jones ML, Gunn DE, Geraci LS, Jones JH, Sawicki DR, Sredy J, Jacot JL, DiCioccio AT, Petrova T, Mitschler A, Podjarny AD. J. Med. Chem. 2005;48:3141. doi: 10.1021/jm0492094.
- 8.For reviews, see:Dehli JR, Legros J, Bolm C. Chem. Commun. 2005:973. doi: 10.1039/b415954c.Ley SV, Thomas AW. Angew. Chem. Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594.Hartwig JF. Angew. Chem. Int. Ed. 1998;37:2046. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L.Wolfe JP, Wagaw S, Marcoux J-F, Buchwald SL. Acc. Chem. Res. 1998;31:805.
- 9.For a recent elegant account on copper-catalyzed Csp-N bond formation under oxidative conditions, see:Hamada T, Ye X, Stahl SS. J. Am. Chem. Soc. 2008;130:833. doi: 10.1021/ja077406x.
- 10.Tracey MR, Hsung RP, Antoline JE, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb Steve M., editor. Georg Thieme Verlag KG; 2005. Chapter 21.4. [Google Scholar]
- 11.(a) Dunetz JR, Danheiser RL. Org. Lett. 2003;5:4011. doi: 10.1021/ol035647d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kohnen AL, Dunetz JR, Danheiser RL. Organic Syn. 2007;84:88. [PMC free article] [PubMed] [Google Scholar]
- 12.Riddell N, Villeneuve K, Tam W. Org. Lett. 2005;7:3681. doi: 10.1021/ol0512841. [DOI] [PubMed] [Google Scholar]
- 13.(a) Frederick MO, Mulder JA, Tracey MR, Hsung RP, Huang J, Kurtz KCM, Shen L, Douglas CJ. J. Am. Chem. Soc. 2003;125:2368. doi: 10.1021/ja021304j. [DOI] [PubMed] [Google Scholar]; (b) Zhang Y, Hsung RP, Tracey MR, Kurtz KCM, Vera EL. Org. Lett. 2004;6:1151. doi: 10.1021/ol049827e. [DOI] [PubMed] [Google Scholar]; (c) Zhang X, Zhang Y, Huang J, Hsung RP, Kurtz KCM, Oppenheimer J, Petersen ME, Sagamanova IK, Tracey MR. J. Org. Chem. 2006;71:4170. doi: 10.1021/jo060230h. [DOI] [PubMed] [Google Scholar]; (d) Sagamanova IK, Kurtz KCM, Hsung RP. Organic Syn. 2007;84:359. [Google Scholar]
- 14.For some examples, see:Chechik-Lankin H, Livshin S, Marek I. Synlett. 2005:2098.Han C, Shen R, Su S, Porco JA., Jr. Org. Lett. 2004;6:27. doi: 10.1021/ol0360041.Pan X, Cai Q, Ma D. Org. Lett. 2004;6:1809. doi: 10.1021/ol049464i.Brice JL, Meerdink JE, Stahl SS. Org. Lett. 2004;6:1845. doi: 10.1021/ol0494360.Langner M, Bolm C. Angew. Chem. Int. Ed. 2004;43:5984. doi: 10.1002/anie.200460953.Dehli JR, Bolm C. J. Org. Chem. 2004;69:8518. doi: 10.1021/jo0485583.Jiang L, Job GE, Klapars A, Buchwald SL. Org. Lett. 2003;5:3667. doi: 10.1021/ol035355c.Shen R, Lin CT, Porco JA., Jr. J. Am. Chem. Soc. 2002;124:5650. doi: 10.1021/ja026025a.
- 15.For leading references on Pd- or Cu-catalyzed cyclizations of 2-alkynylanilines, see:Majumdar KC, Mondal S. Tetrahedron Lett. 2008;49:2418.Ohno H, Ohta Y, Oishi S, Fujii N. Angew. Chem. Int. Ed. 2007;46:2295. doi: 10.1002/anie.200604342.Tang S, Xie Y-X, Li J-H, Wang N-X. Synthesis. 2007;12:1841.Cacchi S, Fabrizi G, Goggiamani A. Adv. Synth. Catal. 2006;348:1301.Lu BZ, Zhao W, Wei H-X, Dufour M, Farina V, Senanayake CH. Org. Lett. 2006;8:3271. doi: 10.1021/ol061136q.Abbiati G, Arcadi A, Beccalli E, Bianchi G, Marinelli F, Rossi E. Tetrahedron. 2006;62:3033.Nishikawa T, Koide Y, Kanakubo A, Yoshimura H, Isobe M. Org. Biomol. Chem. 2006;4:1268. doi: 10.1039/b516282c.
- 16.For references using other metals, see:Zhang Y, Donahue JP, Li C-J. Org. Lett. 2007;9:627. doi: 10.1021/ol062918m.Ambrogio I, Arcadi A, Cacchi S, Fabrizi G, Marinelli F. Synlett. 2007;11:1775.Terrasson V, Michaux J, Gaucher A, Wehbe J, Marque S, Prim D, Campagne J-M. Eur. J. Chem. 2007:5332.Yin Y, Ma W, Chai Z, Zhao G. J. Org. Chem. 2007;72:5731. doi: 10.1021/jo070681h.Kurisaki T, Naniwa T, Yamamoto H, Imagawa H, Nishizawa M. Tetrahedron Lett. 2007;48:1871.Trost BM, McClory A. Angew. Chem. Int. Ed. 2007;46:2074. doi: 10.1002/anie.200604183.
- 17.For recent reviews on the synthesis of indoles, see:Zeni G, Larock RC. Chem. Rev. 2004;104:2285. doi: 10.1021/cr020085h.Alonso F, Beletskaya IP, Yus M. Chem. Rev. 2004;104:3079. doi: 10.1021/cr0201068.Cacchi S, Fabrizi G. Chem. Rev. 2005;105:2873. doi: 10.1021/cr040639b.Humphrey GR, Kuethe JT. Chem. Rev. 2006;106:2875. doi: 10.1021/cr0505270.Battistuzzi G, Cacchi S, Fabrizi G. Eur. J. Org. Chem. 2002:2671.Gilchrist TL. J. Chem. Soc., Perkin Trans. 1. 2001:2491.Ackermann L. Synlett. 2007:507.
- 18.For leading references on the synthesis of 2-aminoindoles, see:Roy S, Gribble GW. Tetrahedron Lett. 2007;48:1003.Witulski B, Alayrac C, Tevzadze-Saeftel L. Angew. Chem. Int. Ed. 2003;42:4257. doi: 10.1002/anie.200351977.Fayol A, Fang Y-Q, Lautens M. Org. Lett. 2006;8:653. doi: 10.1021/ol061374l.
- 19.For leading references, see:Ackermann L. Org. Lett. 2005;7:439. doi: 10.1021/ol047649j.Kaspar LT, Ackermann L. Tetrahedron. 2005;61:11311.Tang Z-Y, Hu Q-S. Adv. Synth. Catal. 2006;348:846.Sanz R, Castroviejo MP, Guilarte V, Perez A, Fananas FJ. J. Org. Chem. 2007;72:5113. doi: 10.1021/jo070643y.For references on related indole synthesis from ortho-gem-dihalovinyl anilines, see:Fang Y-Q, Lautens M. J. Org. Chem. 2008;73:538. doi: 10.1021/jo701987r.Nagamochi M, Fang Y-Q, Lautens M. Org. Lett. 2007;9:2955. doi: 10.1021/ol071370w.
- 20.See Supporting Information.
- 21.For earlier examples of indole synthesis via 2-alkynylanilines, see:Stevens RD, Castro CE. J. Org. Chem. 1963;28:3313.Castro CE, Gaughan EJ, Owsley DC. J. Org. Chem. 1966;31:4071.Castro CE, Havlin R, Honward VK, malte A, Moje S. J. Am. Chem. Soc. 1969;91:6464. doi: 10.1021/ja01054a063.Fujiwara J, Fukutani Y, Sano H, Maruoka K, Yamamoto H. J. Am. Chem. Soc. 1983;105:7177.Rudisill DE, Stille JK. J. Org. Chem. 1989;54:5856.
- 22.For a leading reference on xantphos, see:Kranenburg M, van der Burgt YEM, Kamer PCJ, van Leeuwen PWNM. Organometallics. 1995;14:3081.
- 23.For a leading reference on X-phos, see:Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2003;125:6653. doi: 10.1021/ja035483w.
- 24.Daum S, Erdmann F, Fischer G, F aux de Lacroix B, Hessamian-Alinejad A, Houben S, Frank W, Braun M. Angew. Chem. Int. Ed. 2006;45:7454. doi: 10.1002/anie.200601569. [DOI] [PubMed] [Google Scholar]
- 25.For reviews, see:Bao L, Kimzey A, Sauter G, Sowadski JM, Lu K, Wang D. American Journal of Pathology. 2004;164:1727. doi: 10.1016/S0002-9440(10)63731-5.Lu KP. Cancer Cell. 2003;4:175. doi: 10.1016/s1535-6108(03)00218-6.Hamdane M, Smet C, Sambo A-V, Leroy A, Wieruszeski JM, Delobel P, Maurage C-A, Ghestem A, Wintjens R, Begard S, Sergeant N, Delacourte A, Horvath D, Landrieu I, Lippens G, Buee L. J. Mol. Neurosci. 2002;19:275. doi: 10.1385/JMN:19:3:275.
- 26.For reviews, see:Fischer G. Angew. Chem. Int. Ed. Engl. 1994;33:1415.Fischer G. Chem. Soc. Rev. 2000;29:119.
- 27.Fischer G, Bang H, Mech C. Biomed. Biochim. Acta. 1984;43:1101. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


