Abstract
Extracellular ATP, released at sites of inflammation or tissue damage, activates the P2X7 receptor, which in turn triggers a range of responses also including cell proliferation. In this study the ability of the human cathelicidin LL-37 to stimulate fibroblast growth was inhibited by commonly used P2X7 blockers. We investigated the structural requirements of the growth-promoting activity of LL-37 and found that it did not depend on helix sense (the all-d analog was active) but did require a strong helix-forming propensity in aqueous solution (a scrambled analog and primate LL-37 orthologs devoid of this property were inactive). The involvement of P2X7 was analyzed using P2X7-expressing HEK293 cells. LL-37 induced proliferation of these cells, triggered Ca2+ influx, promoted ethidium bromide uptake, and synergized with benzoyl ATP to enhance the pore and channel functions of P2X7. The activity of LL-37 had an absolute requirement for P2X7 expression as it was blocked by the P2X7 inhibitor KN-62, was absent in cells lacking P2X7, and was restored by P2X7 transfection. Of particular interest, LL-37 led to pore-forming activity in cells expressing a truncated P2X7 receptor unable to generate the non-selective pore typical of the full-length receptor. Our results indicate that P2X7 is involved in the proliferative cell response to LL-37 and that the structural/aggregational properties of LL-37 determine its capacity to modulate the activation state of P2X7.
P2X7 is an ATP-activated ion channel expressed by lymphocytes, dendritic cells, macrophages/microglia, and non-hematopoietic cells, including human primary fibroblasts (1). Activation of P2X7 causes massive calcium entry often followed by cell death (2). Cytotoxicity is likely due to the opening of a large, non-selective pore either intrinsic to or associated with P2X7, which permeabilizes the plasma membrane to molecules of mass up to 900 Da (3). Its triggering has been shown to enhance proliferation of lymphocytes (4), microglial cells (5), and adventitial fibroblasts (6). In the immune system, ATP released by infected or stressed cells at sites of inflammation acts as a danger or alert signal. Among the many cellular receptors activated by extracellular ATP, P2X7 has been implicated in inhibition of infection by intracellular pathogens (7), in the processing and release of cytokines such as IL-1β, and in the modulation of cell death via apoptotic and necrotic pathways (8). As such, it appears to be an important player in inflammation.
Antimicrobial host defense peptides have long been recognized as vital components of the innate immune system that provide an endogenous first line of defense against microbial infection by wielding a direct toxic activity against a wide range of microorganisms (9). This role has more recently broadened from that of endogenous antibiotics to multifunctional modulators of defense responses (10) and early warning signals, or “alarmins” (11). Two evolutionarily distinct families of such peptides in particular, the defensins and the cathelicidins, are found in mammals and are either constitutively expressed or readily inducible upon infection, inflammation, or injury (12, 13). They are diverse in length, sequence, and secondary structure, but all tend to adopt amphipathic conformations and are cationic at physiological pH (14). These features account for their ability to bind avidly to the negatively charged bacterial surface and to perturb membrane organization, thus leading to microbial killing.
hCAP-18/LL-37 is the only cathelicidin present in humans and is stored in the specific granules of neutrophils (15), in lymphocytes and macrophages (16), and in epithelial cells from skin (12) and of mucosal surfaces (17). It adopts a helical conformation in membrane-like environments (18), but more unusually, it also does so in solutions containing biologically relevant ions (19, 20). Although it exhibits a somewhat lower antibacterial potency than commonly used drugs in in vitro assays, it has been shown to effectively increase survival rates in animal models of infection and sepsis (21). This feature likely reflects the ability of LL-37 to boost mechanisms of innate immunity in addition to its direct killing of bacteria. Studies of the biological activities of LL-37 suggest that it is chemotactic for human peripheral blood monocytes, neutrophils, mast cells, and CD4 T lymphocytes (22, 23). It is involved in wound repair by stimulating angiogenesis (24) and re-epithelialization of human skin wounds (25). Chemotaxis and endothelial cell proliferation by LL-37 may be mediated via activation of formyl peptide receptor-like 1 (FPRL1)3 (23, 24), whereas the effect on keratinocytes requires the epidermal growth factor receptor and G-protein-coupled receptors (26–28). Recent reports suggest that LL-37 inhibits spontaneous apoptosis in neutrophils (29) and induces IL-1β release in lipopolysaccharide-primed monocytes (30) through activation of P2X7.
In this study we found that P2X7 was also involved in the growth-promoting activity of LL-37 as shown using murine fibroblasts and human embryonic kidney (HEK293) cells stably transfected with human P2X7 cDNA. By analyzing the functional interaction of LL-37 with P2X7, we found that LL-37 triggered Ca2+ influx and ethidium bromide uptake in HEK293 cells expressing P2X7 but not in mock-transfected cells. Furthermore it strongly potentiated the agonist-dependent responses in HEK293-hP2X7 cells. Activation of P2X7 by LL-37 was strictly dependent on the helix-forming propensity of LL-37 in aqueous solution and was independent of helix sense. Finally LL-37 restored pore-forming activity in cells expressing a truncated P2X7 receptor that is by itself unable to generate the non-selective pore typical of full-length P2X7. Our results indicate that expression of the P2X7 receptor is necessary for LL-37-dependent cell activation and suggest that several other reported activities of LL-37 are mediated by P2X7 activation.
EXPERIMENTAL PROCEDURES
Reagents—Derivatized (peptide amide linker-) polyethylene glycol-polystyrene resins, coupling reagents for peptide synthesis, and Fmoc-amino acids were purchased from Applied Biosystems, Novabiochem, and ChemImpex. Peptide synthesis grade N,N-dimethylformamide, dichloromethane, piperidine, and high pressure liquid chromatography grade acetonitrile were from Biosolve. Trifluoroacetic acid, N-methylmorpholine, and triethylamine were from Acros Chimica. ATP, BzATP, oxidized ATP, KN-62, BBG, PPADS, Reactive Blue, MTT, ethidium bromide (EB), and digitonin were purchased from Sigma-Aldrich. YO-PRO-1 and fura-2/AM were from Molecular Probes. Cell culture media and supplements were from Euroclone. All other reagents were of analytical grade. Buffers were prepared in double glass-distilled water.
Peptide Synthesis and Characterization—Peptides were chemically synthesized by Fmoc solid-phase peptide synthesis on a Milligen 9050 or Pioneer automated synthesizer (Applied Biosystems). Difficult coupling steps were carried out as described previously (20, 31). The correct identity of the peptides was confirmed by electrospray mass spectrometry using an API I instrument (PerkinElmer Sciex). Peptide concentrations were determined by measuring the absorbance at 257 nm of Phe residues (primate and scrambled LL-37 peptides) and the absorbance at 280 nm of Tyr and Trp residues for BMAP-28 and confirmed by the bicinchoninic acid protein assay (Pierce). The relative amphipathicity (μHrel) of peptides was calculated using the peptide sequence analysis tool HydroMCalc available on line.
Structuring was probed by circular dichroism spectroscopy at 20 μm peptide concentration using a Jasco J-600 spectropolarimeter and 2-mm-path length quartz cells. Peptides were dissolved in PIL buffer (113 mm NaCl, 24 mm NaHCO3, 0.6 mm MgCl2, 1.3 mm CaCl2, 3.9 mm KCl, pH 7.3), in DMEM, or in SDS (10 mm SDS in 10 mm sodium phosphate buffer, pH 7.4). All spectra are the mean of at least two trials, each with the accumulation of three scans. Helical content was estimated from the ellipticity at 222 nm according to Chen et al. (32).
Cell Cultures—NIH 3T3 murine fibroblastic cells were cultured under 5% CO2 at 37 °C as exponentially growing subconfluent monolayers in DMEM. HEK293 cells were purchased from the American Type Culture Collection and cultured in DMEM/Ham's F-12. All media were supplemented with 10% (v/v) FCS, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. HEK293 transfection with full-length or COOH-truncated P2X7 receptor lacking residues 415–595 was carried out with Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's recommendations. Selection was performed in the presence of G418.
Assessment of Cell Proliferation and Membrane Permeabilization—NIH 3T3 cells were seeded in 96-well plates at a density of 104 cells/cm2 and incubated for 24 h at 37 °C in complete DMEM. The cell culture medium was then replaced with DMEM containing 0.5% FCS, and the incubation was prolonged for 48 h. After this time, fewer than 5% of cells were found to actively replicate their DNA as assessed by bromodeoxyuridine incorporation (33). Growth-arrested cells were incubated in DMEM containing 2% FCS in the absence or presence of selected peptides. To determine cell numbers, 0.5 mg/ml MTT was added to the culture media after 24 h, and cells were incubated for an additional 3 h. Cells were then lysed with isopropanol, and the absorbance at 570 nm was recorded. Cell proliferation in the presence of each peptide was expressed as percentage of the control value, corresponding to untreated cells.
HEK293 cells stably expressing human P2X7 (HEK293hP2X7) or mock-transfected cells (HEK293-mock) were plated in triplicate in a polylysine-pretreated 6-well plate at a density of 1 × 105 cells/well and incubated overnight at 37 °C in complete DMEM/Ham's F-12. HEK293-hP2X7 cells were transfected for the P2X7-silencing experiment 2 h after plating. The following day medium was changed with DMEM/Ham's F-12 in the absence of fetal calf serum, and cells were counted in phase-contrast light with a Nikon Eclipse TE 300 inverted microscope (Nikon) (considered as time 0) and stimulated with the indicated peptides. Cell proliferation was evaluated 24 h later as a percentage of the untreated cells taken as a control.
To monitor plasma membrane permeability changes, NIH 3T3 cells were incubated for 90 min at 37 °C in DMEM plus 2% FCS in the absence or presence of each peptide at 5 μm. The activity of lactate dehydrogenase, a cytosolic enzyme, in cell-free media and cell lysates, respectively, was measured using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega). Lactate dehydrogenase activity in the culture media was expressed as percentage of total cellular lactate dehydrogenase activity.
RNA Interference—Suppression of human pannexin-1 expression was obtained by transfecting HEK293 cells stably expressing truncated rat P2X7 (HEK293-rP2X7ΔC) with 150 pmol of a Silencer® Select Predesigned siRNA (identification number s24426, Ambion). The Silencer Select Negative Control siRNA (identification number 4390843, Ambion) was used as negative control.
RNA interference against human P2X7 was obtained by transfecting cells with 200 pmol of a custom siRNA purchased from Ambion (sense strand, 5′-CUACACCACGAGAAACAUCtt-3′; antisense strand, 5′-GAUGUUUCUCGUGGUGUAGtt-3′). A custom scrambled siRNA (scr hP2X7) (sense strand, 5′-CAACACCACUAGAAGCAUCtt-3′; antisense strand, 5′-GAUGCUUCUAGUGGUGUUGtt-3′) was used as negative control.
Transfections were performed using the Lipofectamine 2000 protocol. Briefly 5 × 106 cells were plated onto a 10-cm culture dish 2 h before siRNA transfections and incubated for 48 h at 37 °C before experiments. Protein expression levels of pannexin-1 and P2X7 were determined by Western blot.
Western Blot Analysis—Cells were resuspended in a lysis buffer containing 300 mm sucrose, 1 mm K2HPO4, 1 mm MgSO4, 5.5 mm glucose, 20 mm Hepes (pH 7.4), 1 mm benzamidine, 1 mm phenylmethylsulfonyl fluoride, 0.2 μg of deoxyribonuclease, 0.2 μg of ribonuclease and subjected to three freeze/thawing cycles. Proteins were separated on a 7.5% sodium dodecyl sulfate-polyacrylamide gel (pannexin-1 blots) or a 4–12% NuPAGE bis-Tris precast gel (Invitrogen) for P2X7 blots. The rabbit polyclonal anti-P2X7 receptor antibody (Sigma-Aldrich) was used at a dilution of 1:200, and the rabbit polyclonal antibody against human pannexin-1 (Abcam) was used at a dilution of 1:1000 in 10 mm Tris-HCl, 150 mm NaCl, pH 8.0. The primary antibody was revealed by protein A conjugated to horseradish peroxidase at a dilution of 1:3000. Densitometric analysis was done by Eastman Kodak Co. 1D Image Analysis Software. The density of bands was expressed as net intensity, which is the sum of the background-subtracted pixel value within the region of interest.
Ethidium Bromide and YO-PRO-1 Uptake—Ethidium bromide and YO-PRO-1 are commonly used markers of P2X7-mediated permeabilization of the plasma membrane to low molecular mass aqueous solutes. EB uptake was monitored by stimulating HEK293 cells (5 × 105/ml) in standard saline solution (125 mm NaCl, 5 mm KCl, 1 mm MgSO4, 1 mm NaH2PO4, 20 mm Hepes, 5.5 mm glucose, 5 mm NaHCO3, 1 mm CaCl2, pH 7.4), in the presence of 20 μm EB. At the end of the experiment 100 μm digitonin was added to obtain 100% membrane permeabilization. Changes in plasma membrane permeability were monitored at the excitation/emission wavelengths of 360/580 nm.
YO-PRO-1 uptake was measured by suspending NIH 3T3 cells in a low ionic strength saline solution (1 mm MgSO4, 1 mm K2HPO4, 5.5 mm glucose, 300 mm saccharose, 20 mm Hepes, pH 7.4) containing 250 μm sulfinpyrazone and 1 μm YO-PRO-1 and incubating cells at 37 °C in the absence or presence of P2X7 blockers. Cells were then challenged with BzATP for 30 min at 37 °C. After washing with saline containing 2% FCS, cells were suspended in FCS-free saline for analysis. Samples were analyzed with a flow cytometer equipped with the Cell Quest software using a 488 nm argon ion laser as the excitation source (FACScan, BD Biosciences). A minimum of 10,000 events per sample was analyzed.
Reverse Transcription-PCR Analysis—Total RNA was isolated from NIH 3T3 cells using the RNAqueous® kit (Ambion) according to the manufacturer's instructions. After treatment with DNA-free™ (Ambion) to remove contaminating DNA, 1 μg of total RNA was reverse transcribed for 2 h at 42 °C with 200 units of SuperScript II reverse transcriptase (Invitrogen) and oligo(dT) adaptor primers. PCR amplification was performed in a total volume of 25 μl including 0.5 units of EuroTaq DNA polymerase (Euroclone) and 5 μl of 1:10 diluted reverse transcription sample. After 10 min at 94 °C, 35 cycles were performed with the following program: 30 s at 94 °C, 40 s at 60 °C, and 1 min at 72 °C. Primer sequences, designed on the basis of the published sequences of the murine P2X7 and glyceraldehyde-3-phosphate dehydrogenase genes, were as follows: for P2X7, 5′-GGCAGTTCAGGGAGGAATCATGG-3′ (forward) and 5′-GAAGCGCCAGGTGGCGTAGCAC-3′ (reverse); and for glyceraldehyde-3-phosphate dehydrogenase, 5′-TGATGACATCAAGAAGGTGGTGGA-3′ (forward) and 5′-TCCTTGGAGGCCATGTAGGCCAT-3′ (reverse). PCR products were analyzed in a 1.5% agarose gel, and their sizes were compared with a 100-bp DNA ladder (New England Biolabs).
Intracellular Ca2+ Measurement—HEK293 cells (107/ml) were incubated at 37 °C for 20 min in standard saline solution containing 250 μm sulfinpyrazone in the presence of 4 μm fura-2/AM (34, 35). Cells were then washed in the same saline solution, and experiments were performed in a thermostatted, magnetically stirred cuvette by using a PerkinElmer Life Sciences LS50 fluorometer. Intracellular Ca2+ concentration ([Ca2+]i) transients were monitored using the 340/380 nm excitation ratio at an emission wavelength of 505 nm. [Ca2+]i changes are reported as [Ca2+]i increases over basal (Δ[Ca2+]i).
RESULTS
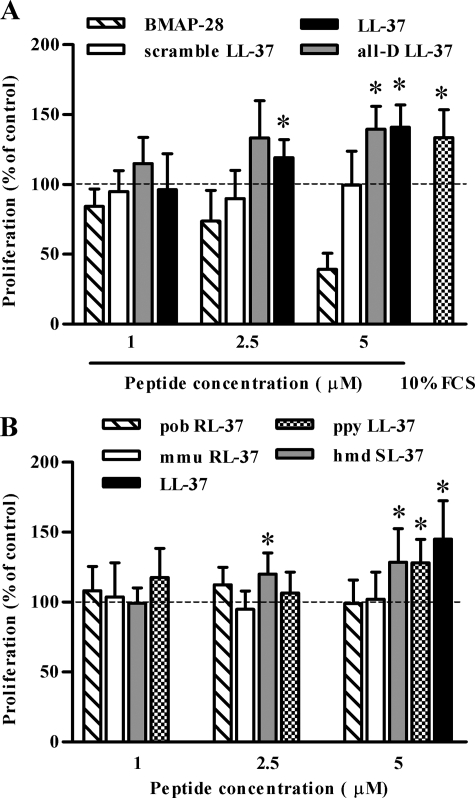
The Fibroblast Proliferation-inducing Activity of LL-37 Depends on the Propensity of LL-37 to Structure in Aqueous Solution—First we investigated the ability of LL-37 to induce fibroblast proliferation using murine NIH 3T3 fibroblasts. Growth-arrested NIH 3T3 cells were incubated for 24 h with LL-37 at peptide concentrations similar to those reported to promote keratinocyte proliferation (26, 36). Cell numbers increased in a peptide concentration-dependent manner; the highest activity was achieved at 5 μm LL-37. At this peptide concentration the cell number increment was comparable to that induced by 10% FCS (Fig. 1A). LL-37 was ineffective at 10 μm (not shown) possibly due to a significant cytotoxicity (over 20% release of the cytoplasmic enzyme lactate dehydrogenase) at this peptide concentration.
FIGURE 1.
Effect of LL-37 and related peptides on proliferation of NIH 3T3 fibroblasts. Growth-arrested NIH 3T3 cells were incubated in DMEM containing 2% FCS in the presence or absence of increasing concentrations of the indicated peptides. As a control of proliferative potential, cells were incubated with 10% FCS-containing medium. The number of cells after 24-h incubation was assessed by MTT assay and was expressed as percentage of cells incubated in the absence of LL-37. The mean of five experiments and the S.D. are reported. Asterisks denote a statistically significant difference to untreated cells (*, p < 0.05 as assessed by Student's t test).
The structural determinants of the cell growth-promoting activity of LL-37 were investigated by testing in parallel the all-d LL-37 enantiomer, a non-amphipathic scrambled version of LL-37 showing a residue content and net positive charge (+6) otherwise identical to LL-37, and the helical bovine cathelicidin BMAP-28 (37) (Table 1). Only all-d LL-37 exhibited proliferation-inducing activity comparable to that of the natural LL-37 peptide at concentrations up to 5 μm, whereas BMAP-28 was ineffective or toxic in the range of concentrations used (Fig. 1A).
TABLE 1.
Sequences and structuring properties of the peptides under study
Non-human primate peptides are from P. pygmaeus (ppy LL-37), H. moloch (hmd SL-37), M. mulatta (mmu RL-37), and P. obscura (pob RL-37). Relative amphipathicity (μHrel) was calculated using HydroMCalc. The percent helix was calculated based on the circular dichroism spectra of peptides at 20 μm in the indicated conditions. ND, not determined. Italic letters are d-amino acids.
|
Peptide
|
Sequence
|
Charge
|
μHrel
|
Percent helix
|
||||
|---|---|---|---|---|---|---|---|---|
| PIL | DMEM | SDS | ||||||
| % | ||||||||
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | +6 | 0.59 | 28.5 | 33.5 | 30.3 | ||
| ppy LL-37 | LLGDFFRKAREKIGEEFKRIVQRIKDFLRNLVPRTES | +4 | 0.59 | 41.4 | 51.7 | 31.5 | ||
| hmd SL-37 | SLGNFFRKARKKIGEEFKRIVQRIKDFLQHLIPRTEA | +6 | 0.60 | 24.9 | 26.3 | 36.8 | ||
| mmu RL-37 | RLGNFFRKVKEKIGGGLKKVGQKIKDFLGNLVPRTAS | +8 | 0.56 | <4 | <4 | ND | ||
| pob RL-37 | RLGNFFRKAKKKIGRGLKKIGQKIKDFLGNLVPRTES | +10 | 0.62 | <4 | <4 | 27.5 | ||
| All-d LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | +6 | 0.59 | 27.5 | 32.9 | 35.0 | ||
| Scrambled LL-37 | DKWVSSFIREILKRRGKKREKKPQNLGFFITDEFLRL | +6 | 0.20 | 5.4 | 8.5 | 16.0 | ||
| BMAP-28 | GGLRSLGRKILRAWKKYGPIIVPIIRI | +7 | 0.60 | 8.2 | 9.3 | 37.6 | ||
The results obtained with all-d LL-37 argued against a chiral specificity for binding to cellular target(s), whereas a degree of sequence specificity was indicated by the inability of scrambled LL-37 or BMAP-28 to induce cell proliferation. The latter two peptides showed a significantly lower helix-forming propensity in aqueous solutions compared with native and all-d LL-37. Based on circular dichroism measurements, LL-37 and all-d LL-37 adopted well defined α-helical structures in physiological salt medium (PIL) and in DMEM (Table 1) because of the stabilizing effects of intramolecular salt bridging (19). BMAP-28 instead assumed this secondary structure only in the presence of membrane-mimicking SDS micelles, and scrambled LL-37 showed scarce propensity to structure under any condition (Table 1).
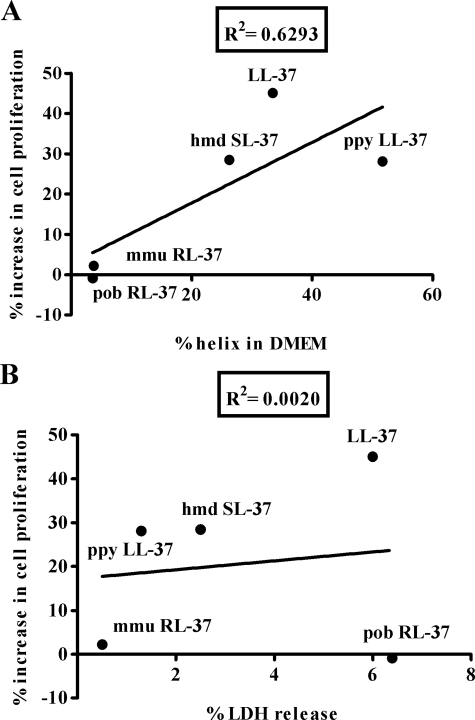
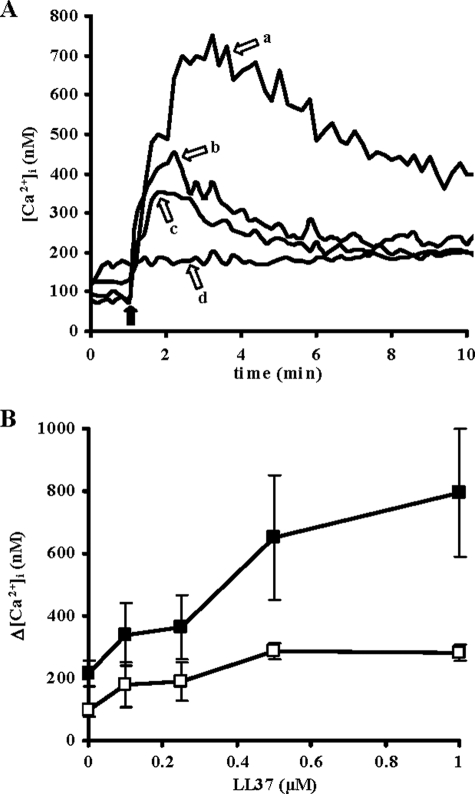
These observations suggested that the amino acid sequence and/or the stable conformation of LL-37 in solution are important determinants of its activity. We further investigated this issue by testing the proliferative potential of LL-37 orthologs from other primate species that showed subtle variations in sequence, charge, charged residue arrangement, and thus also helix-forming propensity (20). Among these, ppy LL-37 (orangutan, Pongo pygmaeus) and hmd LL-37 (gibbon, Hylobates moloch) resembled LL-37 in that they adopted amphipathic helical structures not only in the presence of membrane-mimicking environments but also in PIL and in DMEM. Conversely pob RL-37 (leaf monkey, Presbytis obscura) and mmu RL-37 (macaque, Macaca mulatta) only structured in the presence of membrane-mimicking solutions (Table 1). The results of proliferation assays indicated that only those peptides that showed structuring properties similar to LL-37, i.e. ppy LL-37 and hmd LL-37, enhanced proliferation of NIH 3T3 (Fig. 1B). Their structuring capacity was found to correlate with the proliferative activity (Fig. 2A) but not with direct membrane toxicity (Fig. 2B).
FIGURE 2.
Relationship between structure and activity of primate LL-37 orthologs. For each peptide, the percentage of helix in DMEM (A) and the percentage of lactate dehydrogenase (LDH) release (B) was plotted versus the corresponding increase in proliferation of NIH 3T3 cells. The ellipticity in DMEM was taken from Table 1. Lactate dehydrogenase release was calculated as the percentage of the lactate dehydrogenase activity in the cell supernatant over total cellular activity after 90-min incubation with each peptide (5 μm). The percent increase in cell proliferation after treatment with each peptide (5 μm) was calculated by the MTT assay as described above. Data are the mean of at least three separate experiments. Linear regression was applied to the data to allow calculation of correlation coefficients, R2.
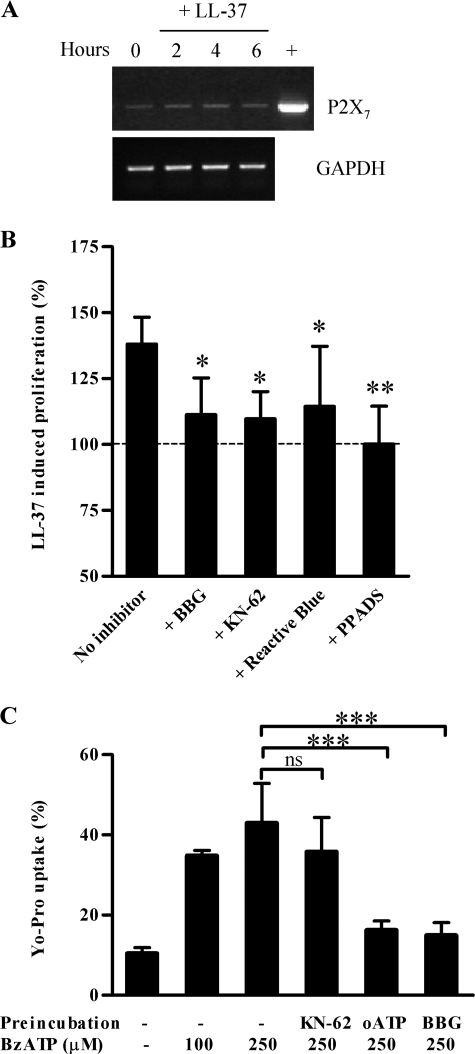
The Cell Growth-promoting Activity of LL-37 Is Inhibited by Antagonists of the Purinergic Receptor P2X7—The purinergic receptor P2X7 has been reported to mediate various cellular responses to LL-37 (29, 30) and has been implicated in the growth of various cell types also including adventitial fibroblasts (4–6). To investigate the involvement of P2X7 in the proliferation-inducing activity of LL-37 we first examined the expression levels of the P2X7 gene in NIH 3T3 cells by reverse transcription-PCR analysis of serum-starved cells. The gene was expressed in these cells and was not up-regulated after incubation for up to 6 h with 5 μm LL-37 (Fig. 3A). FPR2, the murine ortholog of FPRL1, another cellular receptor that is known to be activated by LL-37 (23), was not expressed in these cells (data not shown).
FIGURE 3.
Expression and functional analysis of P2X7 in NIH 3T3 fibroblasts. A, growth-arrested NIH 3T3 cells were incubated with 5 μm LL-37 in DMEM containing 2% FCS and collected at selected times for total RNA extraction. After reverse transcription of RNA, cDNAs were amplified by PCR using primers specific for P2X7 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). PCR included a positive cDNA sample (+) obtained from 264.7 RAW macrophages for P2X7 amplification. B, growth-arrested NIH 3T3 cells were preincubated with medium alone, BBG (500 nm), KN-62 (10 nm), Reactive Blue (50 μm), or PPADS (200 μm). After 30 min, 5 μm LL-37 was added to the culture medium, and cells were incubated at 37 °C for an additional 24 h. Cell numbers were assessed using the MTT assay. Cell proliferation under each condition was expressed as percent increase over cells incubated in the absence of LL-37. The mean of at least four experiments and the S.D. are reported. Asterisks denote a statistically significant difference to cells incubated with LL-37 in medium alone (*, p < 0.05; **, p < 0.01 as assessed by one-way analysis of variance and Bonferroni posthoc test). C, cells were incubated in saline solution in the absence or presence of KN-62 (100 nm, 30 min), oxidized ATP (oATP) (300 μm, 2 h), or BBG (500 nm, 30 min) before addition of 1 μm YO-PRO-1 and challenge with the indicated amount of BzATP. The percentage of YO-PRO-1-positive cells was then determined by flow cytometry. The mean of three experiments and the S.D. are reported. Asterisks denote a statistically significant difference to cells incubated in the absence of inhibitors (ns, not significant; ***, p < 0.001 as assessed by one-way analysis of variance and Bonferroni post hoc test).
Various antagonists of purinergic receptors that are commonly used to implicate P2X7, including BBG, KN-62, Reactive Blue, and PPADS (38, 39), were tested for the ability to inhibit LL-37-induced cell proliferation. All compounds markedly suppressed the growth of NIH 3T3 cells when added to the culture medium before cell incubation with LL-37 (Fig. 3B), suggesting that P2X7 could be involved. Yet when we tested the ability of the P2X7 agonist BzATP to induce cellular uptake of YO-PRO-1, a process that is considered to be a hallmark of P2X7 activation, the inhibition pattern of this response did not fully match the typical inhibition pattern reported for P2X7 antagonists. As shown in Fig. 3C, BzATP at 100 and 250 μm induced YO-PRO-1 uptake in 35.0 ± 1.4 and 42.9 ± 10.0% of total cells versus 10.4 ± 1.5% in the absence of agonist. When cells were treated with oxidized ATP (40), BBG, or KN-62 before incubation with BzATP, the stimulatory effect was significantly suppressed by oxidized ATP and BBG (Fig. 3C) but was hardly affected by KN-62. The latter finding is in line with published reports indicating that KN-62 is a weak antagonist for mouse P2X7 (41).
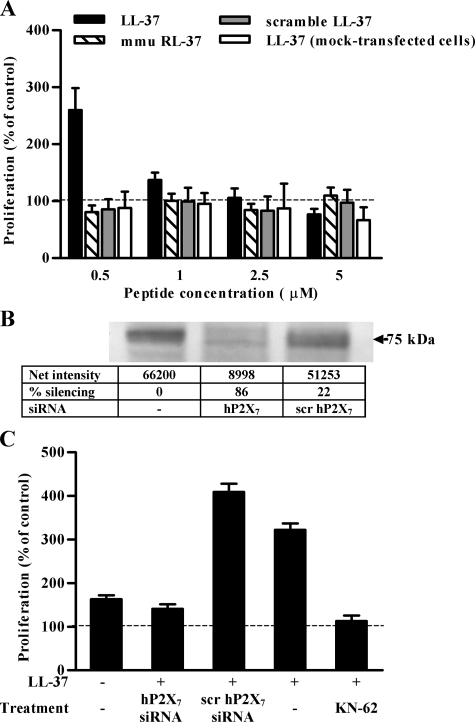
The results of this analysis prompted us to investigate the effects of LL-37 in cells ectopically expressing P2X7 gene to gather unambiguous information on the involvement of P2X7 in these activities. Specifically we utilized human HEK293, a cell line lacking endogenous P2X7, either stably expressing human P2X7 (HEK293-hP2X7) or mock-transfected (mock-HEK293). The LL-37 peptide had a marked growth-promoting effect at a concentration of 0.5 μm in HEK293-hP2X7 but not in mock-HEK293. This effect was not mimicked by either the scrambled LL-37 peptide or mmu RL-37 (Fig. 4A). Transfection with a P2X7 siRNA reduced P2X7 expression by 80% and obliterated the growth-promoting effect of LL-37 (Fig. 4, B and C). Scrambled siRNA had no effect. LL-37-stimulated growth was also inhibited by KN-62 (Fig. 4C).
FIGURE 4.
LL-37 induces P2X7-dependent proliferation of HEK293-hP2X7 cells. A, HEK293-hP2X7 or mock-HEK293 cells were incubated in serum-free DMEM/Ham's F-12 in the absence or presence of increasing concentrations of the indicated peptides. Cell proliferation was evaluated 24 h later by cell counting and was expressed as percentage of cells incubated in the absence of peptides. The mean of 10 experiments and the S.D. are reported. B, Western blot showing the efficiency of P2X7 silencing in HEK293-hP2X7 cells transiently transfected with siRNA targeting hP2X7 or with scrambled (scr) siRNA. C, proliferation of HEK293-hP2X7 cells in the presence of 0.5 μm LL-37 as determined in cells transfected with siRNA targeting hP2X7 or with scrambled siRNA or preincubated with 100 nm KN-62.
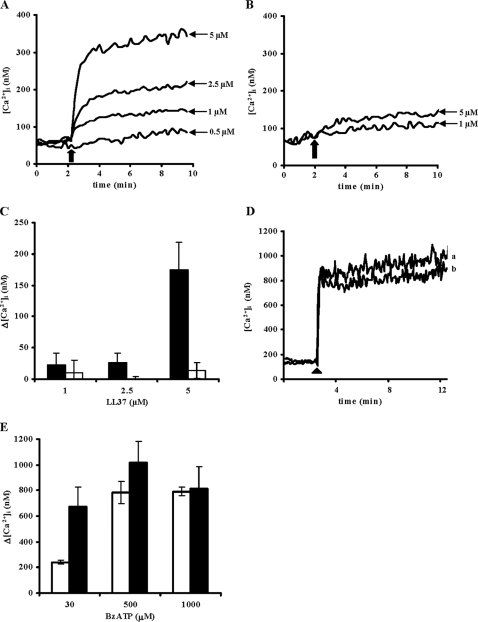
LL-37 Triggers [Ca2+]i Influx and Enhances BzATP-dependent P2X7 Activation in HEK293-hP2X7—The ability of LL-37 to modulate the channel activity of P2X7 was examined by loading HEK293-hP2X7 or mock-HEK293 with fura-2/AM and then stimulating them with increasing concentrations (0.5–5 μm) of LL-37. [Ca2+]i changes were measured 8 min after LL-37 addition. Cells incubated in the presence of up to 1 μm peptide showed minor to moderate [Ca2+]i changes, whereas higher doses (2.5 and 5 μm) triggered a fast and sustained increase (Fig. 5A). LL-37 had a much smaller effect on the mock-HEK293 (Fig. 5B). In the absence of extracellular Ca2+, the LL-37-dependent [Ca2+]i increase was fully abrogated, suggesting that the observed [Ca2+]i rise was because of extracellular ion influx and not to release from intracellular stores (not shown). Fig. 5C reports average [Ca2+]i increases triggered by increasing doses of LL-37 in HEK293-hP2X7 (closed bars) and mock-HEK293 (open bars). The peptide caused a slight [Ca2+]i rise in cells lacking P2X7, whereas in cells expressing this receptor it was over 10-fold higher. Furthermore addition of LL-37 to HEK293-hP2X7 cells maximally stimulated with BzATP caused no further increase in [Ca2+]i (Fig. 5, D and E).
FIGURE 5.
LL-37 induces Ca2+ influx in HEK293 stably transfected with human P2X7. A, HEK293-hP2X7 cells were loaded with 4 μm fura-2/AM as described under “Experimental Procedures.” Intracellular Ca2+ changes were monitored for 8 min after addition of increasing doses of LL-37. An arrow indicates the point of addition of the peptide. B, LL-37-stimulated [Ca2+]i changes in mock-HEK293 cells. C, [Ca2+]i transients induced by increasing doses of LL-37 in HEK293-hP2X7 (closed bars) and mock-HEK293 (open bars) cells. The averages ± S.D. of [Ca2+]i increases over basal (Δ[Ca2+]i) from three independent experiments are reported. D, HEK293-hP2X7 cells were loaded with fura-2/AM and stimulated with 1 mm BzATP (arrowhead) in the presence (trace a) or absence (trace b) of 1 μm LL-37. E, [Ca2+]i transients induced in HEK293-hP2X7 cells by increasing doses of BzATP in the presence (closed bars) or absence (open bars) of 1 μm LL-37.
There is some evidence that other natural antimicrobial compounds (e.g. polymyxin B) interact with P2X7 and modulate its responses to physiological or pharmacological ligands such as ATP or BzATP (42). We therefore checked whether LL-37 might also elicit this effect. To this end, fura-2/AM-loaded HEK293-hP2X7 cells were stimulated with BzATP in the absence or presence of 1 μm LL-37, a dose that induced a relatively small [Ca2+]i increase (see Fig. 5A). Stimulation with 30 μm BzATP alone induced a fast [Ca2+]i increase followed by a slowly declining plateau (Fig. 6A, trace c). Both the [Ca2+]i peak and the following plateau were strongly enhanced by a short (6-min) preincubation with 1 μm LL-37 (Fig. 6A, trace a). In three independent experiments, the peak Δ[Ca2+]i increase in the presence of BzATP plus 1 μm LL-37 was 577 ± 163 nm compared with BzATP alone (Fig. 6B, closed squares). The P2X7 blocker KN-62 fully obliterated this dramatic [Ca2+]i rise elicited by BzATP plus LL-37 (Fig. 6A, trace d). Conversely when the primate cathelicidin mmu RL-37 was examined in parallel as representative of a non-structuring peptide, it did not affect [Ca2+]i by itself nor did it affect the BzATP-mediated [Ca2+]i increase (Fig. 6A, trace b). This is entirely in agreement with the cell proliferation data shown in Fig. 1B and further supports the requirement for a preformed helical structure for activation of P2X7 by LL-37. Conversely LL-37 caused only a modest potentiation of ATP-stimulated Ca2+ release from intracellular stores, a response dependent on activation of P2Y G-protein-coupled receptors (not shown).
FIGURE 6.
LL-37 potentiates the [Ca2+]i rise mediated by BzATP in HEK293-hP2X7 cells. A, fura-2/AM-loaded HEK293-hP2X7 cells were incubated for 6 min with 1 μm LL-37 (trace a) or 1 μm mmu RL-37 (trace b) and then stimulated with 30 μm BzATP. As a control, BzATP was added in the absence of preincubation with either peptide (trace c). Cells pretreated with LL-37 were stimulated with BzATP also in the presence of 100 nm KN-62 (trace d). The black arrow indicates the point of BzATP addition. B, effect of increasing concentrations of LL-37 on 30 μm BzATP-mediated [Ca2+]i increase. The [Ca2+]i peak (closed squares) and the following plateau (open squares) were measured 2 and 8 min after BzATP addition, respectively. Data are averages ± S.D. of three independent experiments.
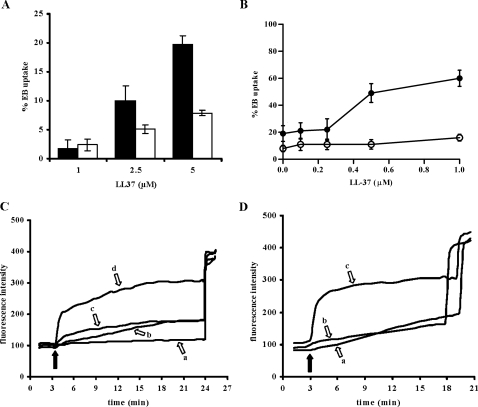
LL-37 Promotes Ethidium Bromide Uptake via P2X7—A hallmark of P2X7 activation is the permeabilization of the plasma membrane to low molecular mass (<900 Da) aqueous solutes due to opening of a non-selective pore (3). Thus we investigated whether LL-37 was able to cause uptake of the membrane-impermeant fluorescent dye ethidium bromide (314 Da) by HEK293-hP2X7 cells (Fig. 7). The fluorescent signal was measured 15 min after addition of LL-37, and dye uptake was calculated as percentage of total permeabilization induced by addition of 100 μm digitonin at the end of the experiment. Dye uptake triggered by LL-37 in mock-HEK293 accounted for nonspecific plasma membrane perturbation by this membrane-active peptide, but in the presence of P2X7 expression dye uptake was at least 2-fold higher (Fig. 7A). Furthermore at lower concentrations where this nonspecific effect is irrelevant, LL-37 still strongly potentiated pore opening triggered by BzATP with a threshold of dye uptake of 0.25 μm and an optimal concentration of 1 μm (Fig. 7B). KN-62 fully prevented EB uptake caused by BzATP and markedly reduced that induced by BzATP plus LL-37 (Fig. 7C). Similarly to Ca2+ influx, mmu RL-37 had very little effect on EB uptake triggered by BzATP (Fig. 7D). Altogether results on Ca2+ influx and EB uptake strongly suggest that LL-37 modulates agonist-dependent activation of both the channel and pore functions of P2X7. Antagonism by KN-62 was not reverted by increasing the LL-37 concentration up to 1 μm (Fig. 7B), suggesting that the two compounds do not share a common binding site.
FIGURE 7.
LL-37 induces ethidium bromide uptake and potentiates BzATP-stimulated plasma membrane permeabilization. A, HEK293-hP2X7 (closed bars) and mock-HEK293 (open bars) cells were stimulated with increasing doses of LL-37 in a standard saline solution containing 20 μm EB. EB uptake was monitored for 15 min, and at the end of the experiment 100 μm digitonin was added to achieve 100% plasma membrane permeabilization. B, HEK293-hP2X7 cells were pretreated with increasing doses of LL-37 and then stimulated with 300 μm BzATP in the presence (open circles) or absence (closed circles) of 100 nm KN-62. In A and B, data are means ± S.D. for three independent experiments. C, HEK293-hP2X7 cells were stimulated with 300 μm BzATP alone (trace b) or in the presence of 1 μm LL-37 (trace d), in the presence of 100 nm KN-62 (trace a), or in the presence of 1 μm LL-37 plus 100 nm KN-62 (trace c). D, HEK293-hP2X7 cells were stimulated with 300 μm BzATP alone (trace a) or together with 1 μm mmu RL-37 (trace b) or 1 μm LL-37 (trace c). In C and D, black arrows indicate the point of BzATP addition.
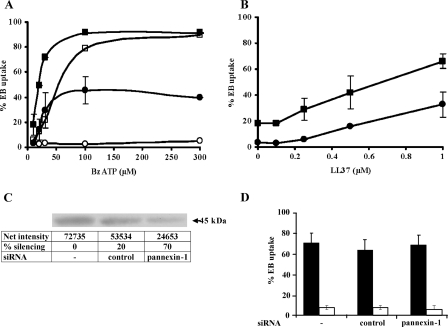
LL-37 Triggers Ethidium Bromide Uptake in HEK293 Stably Transfected with Truncated P2X7 (HEK293-rP2X7ΔC)—To gain further insight into the molecular basis of the LL-37-P2X7 receptor interaction, we took advantage of a truncated form of the rat P2X7 receptor deleted from residue position 415. This incomplete receptor, which lacks the cytosolic C-terminal tail, is reported to maintain an intact channel function but to lack the non-selective pore function (43). BzATP in fact caused no EB uptake by HEK293-rP2X7ΔC (Fig. 8A, open circles), whereas it was fully active on HEK293 expressing full-length rat P2X7 (HEK293-rP2X7)(open squares). Preincubation in the presence of LL-37 strongly potentiated EB uptake not only in cells expressing the full-length (closed squares) but also in those expressing the truncated receptor (closed circles). Moreover preincubation of cells with increasing concentrations (0.1–1 μm) of LL-37 before BzATP addition showed a similar dose-dependent potentiation of EB uptake in HEK293-rP2X7 and HEK293-rP2X7ΔC (Fig. 8B). These results strongly suggest that the cytosolic C-terminal tail of P2X7 is not necessary for LL-37 activity. To further explore the mechanism by which LL-37 restored pore function of truncated P2X7 receptor, we inhibited pannexin-1 function by RNA interference (Fig. 8, C and D). By using a specific siRNA we were able to reduce pannexin-1 expression by 70%, yet EB uptake was minimally affected. Transfection with an unrelated siRNA is shown as control.
FIGURE 8.
LL-37 triggers pore formation in HEK293-rP2X7ΔC. A, HEK293-rP2X7 (squares) or HEK293-rP2X7ΔC (circles) were incubated for 6 min in the absence (open symbols) or presence (closed symbols) of 1 μm LL-37 and then stimulated with increasing concentrations of BzATP. B, HEK293-rP2X7 (closed squares) and HEK293-rP2X7ΔC (closed circles) were preincubated for 6 min with increasing doses of LL-37, and plasma membrane permeabilization was monitored for 15 min after addition of 30 μm BzATP. Data are expressed as averages ± S.D. of determinations performed in triplicate. Where not shown, S.D. bars are smaller than the symbol size. C, Western blot and quantification of human pannexin-1 protein levels in HEK293-rP2X7ΔC cells transiently transfected with siRNA targeting pannexin-1 or with a control siRNA. D, percentage of EB uptake in HEK293-rP2X7ΔC transiently transfected with siRNA targeting pannexin-1 or with a control siRNA and incubated with 100 μm BzATP in the presence (closed bars) or absence (open bars) of 1 μm LL-37. Data are means ± S.D. for three independent experiments.
DISCUSSION
Host defense peptides are multifunctional effector molecules that protect the host from infections by simultaneously killing pathogens and boosting endogenous defense responses (10). However, what the precise roles are in host defense and the relative significance of each in diverse physiological or pathological contexts still remain poorly understood. These are key considerations in their study both as endogenous defense agents and as lead compounds for the development of novel antimicrobial drugs. Anti-infective therapies based on multifunctional host defense peptides would have many potential benefits, including a reduced risk of selecting antibiotic-resistant strains (44), but their effectiveness depends on a thorough understanding of the complexity of their interactions with host cells as host defense peptides exert various effects in distinct phases of the immune response and on different target cells.
The immunomodulatory effects of the human cathelicidin LL-37 have been ascribed to activation of multiple surface receptors. LL-37 is chemotactic for human leukocytes through FPRL1 or other (as yet undefined) G-protein-coupled receptors (22, 23), and it stimulates proliferation of endothelial cells via activation of FPRL1 (24) and of keratinocytes via epidermal growth factor receptor and G-protein-coupled receptors (26–28). Other proinflammatory responses to LL-37, such as induction of IL-1β release from monocytes (30) and inhibition of spontaneous apoptosis in neutrophils (29), appear to be mediated by the purinergic receptor P2X7. Although all these findings convincingly demonstrate that LL-37 acts in a receptor-dependent manner, it is currently still unclear whether it does so by interacting with various specific cellular receptors in a canonical manner or by indirectly modulating receptor activity (45).
In this study we have shown that the ability of LL-37 to promote cell proliferation was inhibited by P2X7 antagonists. In line with these results, LL-37 potentiated the cellular influx of calcium and uptake of EB induced by P2X7 agonists in P2X7-transfected HEK293 cells, whereas it only marginally affected the intracellular calcium mobilization induced in the presence of ATP by members of the P2Y family of G-protein-coupled receptors. The idea that P2X7 might have a strong survival/growth-promoting effect is increasingly acknowledged. In previous studies we investigated this issue in detail, dissecting the intracellular biochemical pathways involved (5, 46). The controlled increase in intramitochondrial Ca2+ and the consequent augmented efficiency of oxidative phosphorylation leading to an overall increase in cellular ATP are clearly crucial steps in this P2X7-activated chain of events.
LL-37 exhibits a strong helix-forming propensity that is derived from a specific distribution of positively and negatively charged residues allowing for formation of intramolecular salt bridges (19). This appears to be a positively selected trait in the evolution of primate cathelicidin (20). Our current results indicate that the cell growth-promoting activity of LL-37 was strictly dependent on its structuring features because only those primate LL-37 analogs that displayed helix-forming propensity similar to that of LL-37 had a comparable proliferation-inducing activity. On the other hand, the non-structured macaque ortholog mmu RL-37 failed to induce cell proliferation, to boost calcium influx through P2X7, and to induce pore formation in HEK293-hP2X7 cells.
It was reported previously that, unlike mmu RL-37, LL-37 can insert deeply within the hydrophobic/hydrophilic interface of eukaryotic membranes (47) and affect membrane integrity and morphology of human lymphocytes (20). Similarly a model helical peptide (P3) showing an intrinsic propensity to assume a helical conformation in solution binds to phosphatidylcholine/cholesterol bilayers with higher affinity compared with unstructured variants because of its ability to insert deeply into the membrane (48). Overall these data suggest that the structural/aggregational properties of LL-37 may affect its capacity to modulate the activation state of P2X7 by first affecting its mode of interaction with the cell membrane.
Importantly no correlation was found between proliferation-inducing potential and membrane toxicity (Fig. 2B), suggesting that cell proliferation was not associated with gross membrane perturbation. The involvement of non-lytic alterations of the membrane properties appears plausible. In fact P2X7 activity is sensitive to modifications in the membrane environment as indicated by the finding that the agonist potency toward P2X7 is modulated by a variety of lipids possibly through changes in membrane elasticity (49). In line with this hypothesis, LL-37 has recently been proposed to alter cell functions through selective interaction with the cell membrane (45). Our current observation that, unlike P2X7, P2Y receptors were barely affected by LL-37 supports the view that the alterations induced by the peptide at the membrane level are quite specific, allowing for selective activation of certain receptors.
No structural information is currently available to substantiate a direct interaction of LL-37 with P2X7 or with other known LL-37 receptors. We reasoned on potential LL-37 binding domain(s) and focused on hydrophobic domains in view of the amphipathic nature of LL-37. Each receptor subunit has two putative membrane-spanning segments separated by an ectodomain and an additional hydrophobic region (residues 512–536) that is part of the intracellular C-terminal domain. Importantly the C-terminal region of P2X7, spanning residues 353–595, may directly associate with proteins and/or lipids (50) and is involved in several functions of the receptor also including pore formation. In fact truncation at residue 415 abolishes the pore-forming activity while preserving the channel activity (43). Our results indicate that LL-37 leads to pore-forming activity in cells expressing a C-terminally truncated P2X7, thus showing that the C-terminal domain of the receptor is not necessary for LL-37 activity and is not involved in LL-37 binding. Most importantly, the fact that LL-37 restores a pore-forming function to the truncated P2X7 implies that under the proper conditions of activation the C-terminal tail may not be required for pore formation.
Although the opening of a large conductance pore is generally taken as the hallmark of P2X7 activation, the molecular identity of its constituents and the mechanism of activation of such a non-selective plasma membrane pathway are largely unknown. Two opposite hypotheses have been put forward: (a) the large conductance pore is intrinsic to the receptor, which under sustained stimulation undergoes a channel-to-pore transition (51), or (b) the pore is a separate membrane structure activated by the receptor possibly via the C-terminal tail (52). Recently Pelegrin and Surprenant (53) have shown that the ubiquitous plasma membrane channel pannexin-1 mediates dye uptake triggered by P2X7 activation, thus providing some evidence supporting the hypothesis that the large pore is separate from the receptor. The ability of LL-37 to induce EB uptake via the truncated P2X7 demonstrates that the C-terminal tail is not an absolute requirement for pore formation. In the case of a separate pore, our data suggest that LL-37 is able to replace the tail as a bridge to the pore. If alternatively the pore is intrinsic to the receptor, LL-37 might simply favor the channel-to-pore transition.
The results shown in Fig. 8, C and D, suggest that pore formation by the truncated P2X7 receptor in the presence of LL-37 occurs via a mechanism different from that of the full-length P2X7 receptor. As illustrated in Fig. 8D, a reduction of pannexin-1 expression of at least 70% caused no decrease in EB uptake, indicating that pannexin-1 was likely not implicated in this process. In comparison, in macrophages this same siRNA reduced pannexin-1 expression to approximately the same level, and in parallel dye uptake was decreased by 70–80% (51).
Given the capacity of LL-37 to insert deeply into host cell membranes, it may reasonably be assumed that the functional interaction between LL-37 and P2X7 involves transmembrane segment-mediated binding. Such binding would explain the activity of all-d LL-37 considering that the hydrophobic environment of the membrane allows for specific interactions to be formed between polypeptides irrespective of their chirality or helix sense. It has been reported that all-d peptides corresponding to transmembrane domains of the Escherichia coli aspartate receptor Tar or of the T cell receptor inhibit the activity of the corresponding full-length proteins in a similar manner to that in their natural counterparts (54, 55). This type of interaction could be energetically favored within the membrane and is compatible with the proposed role of the membrane in mediating interaction with the receptor.
P2X7 displays a relatively low affinity for ATP compared with other purinergic receptors, and the high concentrations of ATP that are required for in vitro activation are not normally found in the extracellular milieu. On this basis it has been speculated that endogenous molecules are needed to sensitize the receptor to respond to physiological levels of ATP, thus acting as a supporting danger signal for cells. For example, lysophosphatidylcholine has been shown to potentiate calcium flux, pore formation, and p44/42 mitogen-activated protein kinase phosphorylation mediated by P2X7 in mouse microglial cells (56), and arachidonic acid has a similar activity in astrocytes (57). It is feasible that LL-37 plays such a role by cooperating with ATP for full activation of the receptor at sites of inflammation or tissue damage. This is further supported by the finding that LL-37 is produced by human skin upon wounding (25), and the ATP concentration is also likely to be increased in this setting as a result of cellular injury.
LL-37 has been involved in various biological activities that are important for tissue repair, such as leukocyte chemotaxis, angiogenesis, and wound closure by re-epithelialization. Direct evidence of a role of cathelicidins in repair of injured tissue comes from the observation that neovascularization at the wound edge is reduced in cathelicidin-deficient mice as compared with wild type animals (24). In the present study we show that LL-37 can induce fibroblast proliferation. Fibroblasts have a key role in tissue repair because they change their phenotype during the late phase of repair and begin to proliferate and synthesize huge amounts of extracellular matrix, which is crucial for wound resolution. Our results suggest that the fibroblast growth-promoting activity is a possible mechanism by which LL-37 contributes to the healing process.
This work was supported by the Italian Ministry of Education, University and Research (Progetti di Ricerca di Interesse Nazionale 2005 Grants 2005068150_001 and 2005051341_004), Regione Friuli-Venezia Giulia (Grant art.23 L.R. 26/2005), the Italian Association for Cancer Research, Telethon of Italy (Grant GGP06070), the Italian Space Agency (Agenzia Spaziale Italiana-Osteoporosi e Atrofia Muscolare), the Commission of European Communities (7th Framework Program HEALTH-F2-2007-202231), and institutional funds from the University of Ferrara. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: FPRL1, formyl peptide receptor-like 1; BzATP, 2′,3′-(4-benzoyl)-benzoyl-ATP; KN-62, [4-[2-(isoquinolin-5-ylsulfonylmethylamino)-3-oxo-3-(4-phenylpiperazin-1-yl)propyl]phenyl] isoquinoline-5-sulfonate; BBG, Coomassie Brilliant Blue G; PPADS, pyridoxal phosphate-6-azophenyl-2′,4′-disulfonate; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; EB, ethidium bromide; fura-2/AM, fura 2-acetoxymethyl ester; HEK293 cells, human embryonic kidney cells; Fmoc, 9-fluorenylmethyloxycarbonyl; DMEM, Dulbecco's modified Eagle's medium; FCS, fetal calf serum; siRNA, small interfering RNA; bis-Tris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; [Ca2+]i, intracellular Ca2+ concentration.
References
- 1.Solini, A., Chiozzi, P., Morelli, A., Fellin, R., and Di Virgilio, F. (1999) J. Cell Sci. 112 297–305 [DOI] [PubMed] [Google Scholar]
- 2.Ferrari, D., Chiozzi, P., Falzoni, S., Dal Susino, M., Collo, G., Buell, G., and Di Virgilio, F. (1997) Neuropharmacology 36 1295–1301 [DOI] [PubMed] [Google Scholar]
- 3.Steinberg, T. H., Newman, A. S., Swanson, J. A., and Silverstein, S. C. (1987) J. Biol. Chem. 262 8884–8888 [PubMed] [Google Scholar]
- 4.Baricordi, O. R., Melchiorri, L., Adinolfi, E., Falzoni, S., Chiozzi, P., Buell, G., and Di Virgilio, F. (1999) J. Biol. Chem. 274 33206–33208 [DOI] [PubMed] [Google Scholar]
- 5.Bianco, F., Ceruti, S., Colombo, A., Fumagalli, M., Ferrari, D., Pizzirani, C., Matteoli, M., Di Virgilio, F., Abbracchio, M. P., and Verderio, C. (2006) J. Neurochem. 99 745–758 [DOI] [PubMed] [Google Scholar]
- 6.Gerasimovskaya, E. V., Ahmad, S., White, C. W., Jones, P. L., Carpenter, T. C., and Stenmark, K. R. (2002) J. Biol. Chem. 277 44638–44650 [DOI] [PubMed] [Google Scholar]
- 7.Darville, T., Welter-Stahl, L., Cruz, C., Sater, A. A., Andrews, C. W., Jr., and Ojcius, D. M. (2007) J. Immunol. 179 3707–3714 [DOI] [PubMed] [Google Scholar]
- 8.Lister, M. F., Sharkey, J., Sawatzky, D. A., Hodgkiss, J. P., Davidson, D. J., Rossi, A. G., and Finlayson, K. (2007) J. Inflamm. (Lond.) 4 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zasloff, M. (2002) Nature 415 389–395 [DOI] [PubMed] [Google Scholar]
- 10.Zanetti, M. (2004) J. Leukoc. Biol. 75 39–48 [DOI] [PubMed] [Google Scholar]
- 11.Oppenheim, J. J., and Yang, D. (2005) Curr. Opin. Immunol. 17 359–365 [DOI] [PubMed] [Google Scholar]
- 12.Frohm, M., Agerberth, B., Ahangari, G., Stahle-Backdahl, M., Liden, S., Wigzell, H., and Gudmundsson, G. H. (1997) J. Biol. Chem. 272 15258–15263 [DOI] [PubMed] [Google Scholar]
- 13.Dorschner, R. A., Pestonjamasp, V. K., Tamakuwala, S., Ohtake, T., Rudisill, J., Nizet, V., Agerberth, B., Gudmundsson, G. H., and Gallo, R. L. (2001) J. Investig. Dermatol. 117 91–97 [DOI] [PubMed] [Google Scholar]
- 14.Tomasinsig, L., and Zanetti, M. (2005) Curr. Protein Pept. Sci. 6 23–34 [DOI] [PubMed] [Google Scholar]
- 15.Sorensen, O., Arnljots, K., Cowland, J. B., Bainton, D. F., and Borregaard, N. (1997) Blood 90 2796–2803 [PubMed] [Google Scholar]
- 16.Agerberth, B., Charo, J., Werr, J., Olsson, B., Idali, F., Lindbom, L., Kiessling, R., Jornvall, H., Wigzell, H., and Gudmundsson, G. H. (2000) Blood 96 3086–3093 [PubMed] [Google Scholar]
- 17.Frohm Nilsson, M., Sandstedt, B., Sorensen, O., Weber, G., Borregaard, N., and Stahle-Backdahl, M. (1999) Infect. Immun. 67 2561–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tossi, A., Sandri, L., and Giangaspero, A. (2000) Biopolymers 55 4–30 [DOI] [PubMed] [Google Scholar]
- 19.Johansson, J., Gudmundsson, G. H., Rottenberg, M. E., Berndt, K. D., and Agerberth, B. (1998) J. Biol. Chem. 273 3718–3724 [DOI] [PubMed] [Google Scholar]
- 20.Zelezetsky, I., Pontillo, A., Puzzi, L., Antcheva, N., Segat, L., Pacor, S., Crovella, S., and Tossi, A. (2006) J. Biol. Chem. 281 19861–19871 [DOI] [PubMed] [Google Scholar]
- 21.Cirioni, O., Giacometti, A., Ghiselli, R., Bergnach, C., Orlando, F., Silvestri, C., Mocchegiani, F., Licci, A., Skerlavaj, B., Rocchi, M., Saba, V., Zanetti, M., and Scalise, G. (2006) Antimicrob. Agents Chemother. 50 1672–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niyonsaba, F., Iwabuchi, K., Someya, A., Hirata, M., Matsuda, H., Ogawa, H., and Nagaoka, I. (2002) Immunology 106 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De, Y., Chen, Q., Schmidt, A. P., Anderson, G. M., Wang, J. M., Wooters, J., Oppenheim, J. J., and Chertov, O. (2000) J. Exp. Med. 192 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koczulla, R., von Degenfeld, G., Kupatt, C., Krotz, F., Zahler, S., Gloe, T., Issbrucker, K., Unterberger, P., Zaiou, M., Lebherz, C., Karl, A., Raake, P., Pfosser, A., Boekstegers, P., Welsch, U., Hiemstra, P. S., Vogelmeier, C., Gallo, R. L., Clauss, M., and Bals, R. (2003) J. Clin. Investig. 111 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heilborn, J. D., Nilsson, M. F., Kratz, G., Weber, G., Sorensen, O., Borregaard, N., and Stahle-Backdahl, M. (2003) J. Investig. Dermatol. 120 379–389 [DOI] [PubMed] [Google Scholar]
- 26.Heilborn, J. D., Nilsson, M. F., Jimenez, C. I., Sandstedt, B., Borregaard, N., Tham, E., Sorensen, O. E., Weber, G., and Stahle, M. (2005) Int. J. Cancer 114 713–719 [DOI] [PubMed] [Google Scholar]
- 27.Tokumaru, S., Sayama, K., Shirakata, Y., Komatsuzawa, H., Ouhara, K., Hanakawa, Y., Yahata, Y., Dai, X., Tohyama, M., Nagai, H., Yang, L., Higashiyama, S., Yoshimura, A., Sugai, M., and Hashimoto, K. (2005) J. Immunol. 175 4662–4668 [DOI] [PubMed] [Google Scholar]
- 28.Braff, M. H., Hawkins, M. A., Di Nardo, A., Lopez-Garcia, B., Howell, M. D., Wong, C., Lin, K., Streib, J. E., Dorschner, R., Leung, D. Y., and Gallo, R. L. (2005) J. Immunol. 174 4271–4278 [DOI] [PubMed] [Google Scholar]
- 29.Nagaoka, I., Tamura, H., and Hirata, M. (2006) J. Immunol. 176 3044–3052 [DOI] [PubMed] [Google Scholar]
- 30.Elssner, A., Duncan, M., Gavrilin, M., and Wewers, M. D. (2004) J. Immunol. 172 4987–4994 [DOI] [PubMed] [Google Scholar]
- 31.Giacometti, A., Cirioni, O., Del Prete, M. S., Skerlavaj, B., Circo, R., Zanetti, M., and Scalise, G. (2003) J. Antimicrob. Chemother. 51 843–847 [DOI] [PubMed] [Google Scholar]
- 32.Chen, Y. H., Yang, J. T., and Chau, K. H. (1974) Biochemistry 13 3350–3359 [DOI] [PubMed] [Google Scholar]
- 33.Tomasinsig, L., Skerlavaj, B., Papo, N., Giabbai, B., Shai, Y., and Zanetti, M. (2006) J. Biol. Chem. 281 383–391 [DOI] [PubMed] [Google Scholar]
- 34.Di Virgilio, F., Milani, D., Leon, A., Meldolesi, J., and Pozzan, T. (1987) J. Biol. Chem. 262 9189–9195 [PubMed] [Google Scholar]
- 35.Di Virgilio, F., Steinberg, T. H., and Silverstein, S. C. (1990) Cell Calcium 11 57–62 [DOI] [PubMed] [Google Scholar]
- 36.Niyonsaba, F., Ushio, H., Nakano, N., Ng, W., Sayama, K., Hashimoto, K., Nagaoka, I., Okumura, K., and Ogawa, H. (2007) J. Investig. Dermatol. 127 594–604 [DOI] [PubMed] [Google Scholar]
- 37.Skerlavaj, B., Gennaro, R., Bagella, L., Merluzzi, L., Risso, A., and Zanetti, M. (1996) J. Biol. Chem. 271 28375–28381 [DOI] [PubMed] [Google Scholar]
- 38.Humphreys, B. D., Virginio, C., Surprenant, A., Rice, J., and Dubyak, G. R. (1998) Mol. Pharmacol. 54 22–32 [DOI] [PubMed] [Google Scholar]
- 39.Jiang, L. H., Mackenzie, A. B., North, R. A., and Surprenant, A. (2000) Mol. Pharmacol. 58 82–88 [PubMed] [Google Scholar]
- 40.Murgia, M., Hanau, S., Pizzo, P., Rippa, M., and Di Virgilio, F. (1993) J. Biol. Chem. 268 8199–8203 [PubMed] [Google Scholar]
- 41.Hibell, A. D., Thompson, K. M., Xing, M., Humphrey, P. P., and Michel, A. D. (2001) J. Pharmacol. Exp. Ther. 296 947–957 [PubMed] [Google Scholar]
- 42.Ferrari, D., Pizzirani, C., Adinolfi, E., Forchap, S., Sitta, B., Turchet, L., Falzoni, S., Minelli, M., Baricordi, R., and Di Virgilio, F. (2004) J. Immunol. 173 4652–4660 [DOI] [PubMed] [Google Scholar]
- 43.Surprenant, A., Rassendren, F., Kawashima, E., North, R. A., and Buell, G. (1996) Science 272 735–738 [DOI] [PubMed] [Google Scholar]
- 44.Hancock, R. E., and Sahl, H. G. (2006) Nat. Biotechnol. 24 1551–1557 [DOI] [PubMed] [Google Scholar]
- 45.Di Nardo, A., Braff, M. H., Taylor, K. R., Na, C., Granstein, R. D., McInturff, J. E., Krutzik, S., Modlin, R. L., and Gallo, R. L. (2007) J. Immunol. 178 1829–1834 [DOI] [PubMed] [Google Scholar]
- 46.Adinolfi, E., Callegari, M. G., Ferrari, D., Bolognesi, C., Minelli, M., Wieckowski, M. R., Pinton, P., Rizzuto, R., and Di Virgilio, F. (2005) Mol. Biol. Cell 16 3260–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henzler-Wildman, K. A., Martinez, G. V., Brown, M. F., and Ramamoorthy, A. (2004) Biochemistry 43 8459–8469 [DOI] [PubMed] [Google Scholar]
- 48.Zelezetsky, I., Pacor, S., Pag, U., Papo, N., Shai, Y., Sahl, H. G., and Tossi, A. (2005) Biochem. J. 390 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michel, A. D., and Fonfria, E. (2007) Br. J. Pharmacol. 152 523–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denlinger, L. C., Fisette, P. L., Sommer, J. A., Watters, J. J., Prabhu, U., Dubyak, G. R., Proctor, R. A., and Bertics, P. J. (2001) J. Immunol. 167 1871–1876 [DOI] [PubMed] [Google Scholar]
- 51.Di Virgilio, F., Ferrari, D., Falzoni, S., Chiozzi, P., Munerati, M., Steinberg, T. H., and Baricordi, O. R. (1996) Ciba Found. Symp. 198 290–305 [DOI] [PubMed] [Google Scholar]
- 52.North, R. A. (2002) Physiol. Rev. 82 1013–1067 [DOI] [PubMed] [Google Scholar]
- 53.Pelegrin, P., and Surprenant, A. (2006) EMBO J. 25 5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sal-Man, N., Gerber, D., and Shai, Y. (2004) J. Mol. Biol. 344 855–864 [DOI] [PubMed] [Google Scholar]
- 55.Gerber, D., Quintana, F. J., Bloch, I., Cohen, I. R., and Shai, Y. (2005) FASEB J. 19 1190–1192 [DOI] [PubMed] [Google Scholar]
- 56.Takenouchi, T., Sato, M., and Kitani, H. (2007) J. Neurochem. 102 1518–1532 [DOI] [PubMed] [Google Scholar]
- 57.Alloisio, S., Aiello, R., Ferroni, S., and Nobile, M. (2006) Mol. Pharmacol. 69 1975–1983 [DOI] [PubMed] [Google Scholar]