Abstract
Activated protein C (APC) plays a critical anticoagulant role in vivo by inactivating procoagulant factor Va and factor VIIIa and thus down-regulating thrombin generation. In addition, APC bound to the endothelial cell protein C receptor can initiate protease-activated receptor-1 (PAR-1)-mediated cytoprotective signaling. Protein S constitutes a critical cofactor for the anticoagulant function of APC but is not known to be involved in regulating APC-mediated protective PAR-1 signaling. In this study we utilized a site-directed mutagenesis strategy to characterize a putative protein S binding region within the APC Gla domain. Three single amino acid substitutions within the APC Gla domain (D35T, D36A, and A39V) were found to mildly impair protein S-dependent anticoagulant activity (<2-fold) but retained entirely normal cytoprotective activity. However, a single amino acid substitution (L38D) ablated the ability of protein S to function as a cofactor for this APC variant. Consequently, in assays of protein S-dependent factor Va proteolysis using purified proteins or in the plasma milieu, APC-L38D variant exhibited minimal residual anticoagulant activity compared with wild type APC. Despite the location of Leu-38 in the Gla domain, APC-L38D interacted normally with endothelial cell protein C receptor and retained its ability to trigger PAR-1 mediated cytoprotective signaling in a manner indistinguishable from that of wild type APC. Consequently, elimination of protein S cofactor enhancement of APC anticoagulant function represents a novel and effective strategy by which to separate the anticoagulant and cytoprotective functions of APC for potential therapeutic gain.
Protein C is a vitamin K-dependent serine protease zymogen that circulates at a plasma concentration of ∼70 nm. It has a multidomain structure comprising an N-terminal γ-carboxyglutamic acid (Gla)3 domain (residues 1–45), two epidermal growth factor-like domains (EGF-1, residues 46–92, and EGF-2, residues 93–136) and a C-terminal serine protease domain (170–419). Protein C zymogen is activated by thrombin in complex with thrombomodulin on the surface of endothelial cells (1). Activated protein C (APC) generation is significantly enhanced by protein C binding to the endothelial cell protein C receptor (EPCR) (2). APC binds to anionic phospholipids on the endothelial cell surface and, in complex with its cofactor protein S inactivates procoagulant cofactors factor Va (FVa) (3) and factor VIIIa (4), thereby attenuating further thrombin generation and down-regulating coagulation. The physiological importance of the protein C anticoagulant pathway is well established. Individuals with homozygous protein C deficiency typically present shortly after birth with fulminant life-threatening thrombotic complications (5). Moreover, individuals heterozygous for protein C deficiency demonstrate significant lifelong increased risk of venous thromboembolism (6).
Recent studies have shown that APC bound to EPCR via its Gla domain can activate protease-activated receptor-1 (PAR-1) on endothelial cells, triggering complex intracellular signaling (7). The molecular mechanisms underlying the cytoprotective effects of APC have not been fully characterized but appear mediated independently of its anticoagulant function (8). Through mechanisms downstream of PAR-1 activation, APC has been shown to exhibit anti-inflammatory and anti-apoptotic properties via up-regulated gene expression of anti-apoptotic and anti-inflammatory mediators (9, 10), down-regulation of pro-inflammatory cytokines (10, 11), and stabilization of endothelial barrier function (12, 13).
In vivo beneficial effects of APC have been reported in a number of different animal injury models. For example, APC demonstrated significant neuroprotective effects in rat and murine stroke models (14–16) and was also recently shown to ameliorate experimental autoimmune encephalomyelitis in mice (17). Furthermore, APC was protective in a murine model of diabetic nephropathy by inhibition of endothelial and podocyte apoptosis, whereas anticoagulation with low molecular weight heparin had no such beneficial effect (18). Each of these animal studies demonstrated a consistent and critical role for the APC-EPCR-PAR-1 signaling axis.
The therapeutic potential of APC was highlighted in the activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study (19). Intravenous infusion of recombinant APC (24 μg/kg per h for 96 h) significantly reduced overall mortality (relative risk reduction, 19.4%) in adult patients with severe sepsis. In contrast, treatment with other anticoagulants (notably antithrombin (20) and tissue factor pathway inhibitor (21)) have no such effect on survival. The anticoagulant properties of APC are, however, associated with increased risk of bleeding complications and have thereby restricted its use in clinical practice. In the PROWESS study, serious bleeding complications were observed in 3.5% of the patients treated with APC compared with only 2% of the placebo group (19). Furthermore, recent post-marketing studies have reported even higher incidences of serious bleeding complications (6.5 and 6.7%, respectively) in patients treated with APC (22, 23). In this context, recent studies have sought to define the relative contributions of the cytoprotective and/or anticoagulant properties of APC toward mediating its therapeutic efficacy in different pathological settings. These studies have sought to design APC variants which retain beneficial cytoprotective actions but exhibit relative reduction in its anticoagulant properties and, thus, bleeding risk (24–26).
The anticoagulant function of APC requires the presence of its principal cofactor, protein S (27). Protein S may enhance APC anticoagulant activity by increasing its affinity for negatively charged phospholipid surfaces (27), re-positioning the APC active site for optimal substrate cleavage (28), or removing the protection of FVa in the prothrombinase complex conferred by FXa binding (29). Thus, protein S plays a critical role in mediating the anticoagulant properties of APC, but there is no evidence to date that it is important for the anti-inflammatory or anti-apoptotic effects of APC. The molecular basis through which protein S interacts with APC is not fully understood; however, a possible role for the APC Gla domain has recently been described (30). In this study, we have used a site-directed mutagenesis strategy to characterize the impact of individual amino acid residue substitutions in this putative protein S binding region upon APC anticoagulant function. We demonstrate that, surprisingly, a single amino acid substitution (L38D) is sufficient to entirely ablate the ability of protein S to function as a cofactor for this APC variant yet does not alter APC-mediated proteolysis of FVa in the absence of protein S. Consequently, in both protein S-dependent FVa proteolysis assays using purified coagulation proteins and in the plasma milieu, this APC Gla domain variant exhibited minimal residual anticoagulant activity compared with wild type APC. In addition, despite the fact that this key amino acid is located in the APC Gla domain, we also show that this APC variant interacts normally with EPCR and that it retains its ability to initiate PAR-1 protective signaling in an identical fashion to that of wild type APC.
EXPERIMENTAL PROCEDURES
Generation and Characterization of Recombinant Protein C Variants—Recombinant protein C Gla domain variants were generated by site-directed mutagenesis, expressed, and isolated from serum-free medium as previously described (30, 31). Recombinant protein C was characterized by Coomassie staining and immunodetection using a horseradish peroxidase-conjugated anti-protein C polyclonal antibody (Dako, Ely) and a mouse anti-Gla monoclonal antibody (American Diagnostica) according to standard procedures. To generate recombinant APC, wild type protein C and protein C variants were activated with Protac (Hyphen-BioMed). APC chromogenic substrate (BIOPHEN CS-21(66), Hyphen-Biomed) cleavage by each recombinant APC preparation was determined as previously described (30, 31).
Inhibition of APC in Plasma—Inhibition of APC variants in plasma was determined as previously described (32). Wild type and variant APC (20 nm) was incubated in pooled human citrated plasma at 37 °C. At designated time points, aliquots were removed, and the residual amidolytic activity was determined using a chromogenic substrate for APC (BIOPHEN CS2166). To examine the role of protein C inhibitor, the same experiments were performed in the presence of heparin (10 units/ml).
Determination of APC Anticoagulant Activity in Protein C-deficient Plasma—The anticoagulant function of wild type and variant APC upon protein C-deficient plasma was assessed using a Fluoroskan Ascent Plate Reader (Thermo Lab System, Helsinki, Finland) in combination with Thrombinoscope software (SYNAPSE BV) as previously described (30). 80 μl of protein C-deficient plasma (Hyphen-Biomed) was incubated with 20 μl of PPP reagent (Synapse) containing 5 pm tissue factor and 4 μm phospholipids (PC/PS/PE, 40%:20%:20%) in the presence or absence of wild type or variant APC (1.25–20 nm). Thrombin generation was initiated by automatic dispensation of fluorogenic thrombin substrate (Z-Gly-Gly-Arg-amidomethylcoumarin-HCl) and 100 mm CaCl2 into each well. Thrombin generation was determined using thrombin calibration standard (Synapse). Measurements were taken at 20-s intervals for 60 min or until thrombin generation was complete. The endogenous thrombin potential (ETP) of each reaction were then calculated. Experiments were performed in triplicate, and data are reported as the mean ETP ± S.E.
Protein S Enhancement of APC Anticoagulant Activity in Protein S-deficient Plasma—The enhancement of APC anticoagulant activity in protein S-deficient plasma was determined using a similar assay to that described above. Briefly, protein S-deficient plasma (Hyphen-Biomed) was incubated with wild type or variant recombinant APC (10 nm) in the presence or absence of plasma-purified protein S (0.125–1.5 μm, Hematologic Technologies Inc.). Thrombin generation was initiated and assessed using Thrombinoscope software as described previously. All experiments were performed in triplicate and data plotted as mean ETP ± S.E.
Determination of Protein S-independent APC-mediated Factor Va Proteolysis—FVa degradation by APC was assessed as previously described (30, 31). 0.32 nm APC was incubated at 37 °C with phospholipids vesicles (PC/PS/PE, 60%:20%:20%; Avanti Lipids) and 4 nm FVa (Hematologic Technologies, Inc.) in 40 mm Tris-HCl, 140 mm NaCl, 3 mm CaCl2, and 0.3% w/v bovine serum albumin (0.08 nm APC, 19 μm phospholipids, and 1 nm FVa, final concentration). Phospholipid vesicles were prepared as described previously (31). At the specified time points, 2-μl aliquots were removed and added to a prothrombinase mixture (25 μm phospholipids, 1 nm factor Xa, and 0.5 μm prothrombin (Hematologic Technologies Inc.) final concentrations) for 3 min. Each reaction was then stopped using 5 μl of ice-cold 0.5 m EDTA. 100 μl of the reaction mixture was removed and incubated with 100 μl of 2 mm thrombin chromogenic substrate Biophen CS-01(38) (Hyphen-Biomed) to assess thrombin generation. The rate of chromogenic substrate cleavage was measured at 405 nm using a plate reader. All experiments were performed in triplicate.
Determination of Protein S-enhanced Proteolysis of FVa by APC—Protein S-enhanced proteolysis of FVa by APC was determined as previously described (30). Human protein S (2.5–25 nm) was incubated with 0.8 nm wild type or variant APC, 8 nm FVa, and 75 μm phospholipids vesicles in 40 mm Tris HCl, pH7.4, 140 mm NaCl, 3 mm CaCl2, 0.3% (w/v) bovine serum albumin (0.2 nm APC, 2 nm FVa, and 19 μm phospholipids vesicles, final concentrations) for 2 min at 37 °C. After this incubation, a 2-μl aliquot was added to 0.3 nm FXa, 1.5 μm prothrombin, and 75 μm phospholipids vesicles (0.1 nm FXa, 0.5 μm prothrombin, and 25 μm phospholipids vesicles, final concentrations) at 37 °C for 3 min, then stopped with 5 μl of ice-cold 0.5 m EDTA. The rate of thrombin substrate cleavage by the consequent reaction mixture was then measured as before. Experiments were performed in triplicate, and data were plotted as the mean residual FVa cofactor activity ± S.E.
Assessment of APC Variant Binding to Soluble EPCR—APC binding to sEPCR was determined using a BIAcore X100 (GE Healthcare) as previously described (30, 31). Briefly, 10 μg/ml monoclonal anti-EPCR antibody, RCR-2 (kind gift of Dr. K. Fukudome, Saga Medical School), was immobilized on to both flow cells of a CM5 sensor chip. sEPCR (31) in HBS-P buffer (100 mm HEPES, pH7.4, 150 mm NaCl, 0.005% v/v surfactant P20) was bound to the surface of the test flow cell (400–800 response units). A reference flow cell with only RCR-2 bound was used to detect nonspecific binding. Wild type or variant APC (25–100 nm) was sequentially injected over both flow cells at a flow rate of 5 μl/min for 60 s. APC-EPCR binding was dissociated using HBS-EP buffer (HBS-P, but containing 3 mm EDTA; BIAcore) The RCR-2 surface was regenerated with 10 μl of 10 mm glycine-HCl (pH 2.5) after each experiment.
Measurement of Endothelial Cell Barrier Protection by APC—Endothelial cell barrier permeability was determined as described previously with minor modifications (13). Briefly, EAhy926 cells (kind gift of Dr. C. Edgell, University of North Carolina, Chapel Hill, NC) were grown to confluence on polycarbonate membrane transwells (Costar, 3 μm pore size, 12-mm diameter) and incubated with 20 nm wild type or variant APC. After 3 h the cells were treated with thrombin (Hematologic Technologies) in serum-free media for 10 min. The cells were washed and incubated with 0.67 mg/ml Evans Blue with 4% bovine serum albumin (Sigma). Changes in endothelial cell barrier permeability were determined by following the increase in absorbance at 650 nm in the outer chamber over time due to the transmigration of Evans Blue-bovine serum albumin. Experiments were performed in triplicate and plotted as the mean ± S.E.
Determination of APC-mediated Protection of Apoptotic Endothelial Cells—Confluent EAhy926 cells in 6-well plates were pretreated with either wild type or variant APC for 17 h. EAhy926 cell apoptosis was induced by staurosporine (20 μm, Sigma) treatment for 4 h. Cells were then trypsinized, and RNA was extracted using the RNeasy Mini kit (Qiagen). Reverse transcription was carried out (High Capacity cDNA reverse transcription kit, Applied Biosystems), then real time PCR was performed using bax (Hs00180269_m1), bcl-2 (Hs00153350_m1) and β-actin (Hs99999903_m1) Taqman® gene expression assays (Applied Biosystems) in a Applied Biosystems 7500 real time PCR system. Experiments were performed in triplicate and plotted as the mean bax/bcl-2 ratio ±S.E.
RESULTS
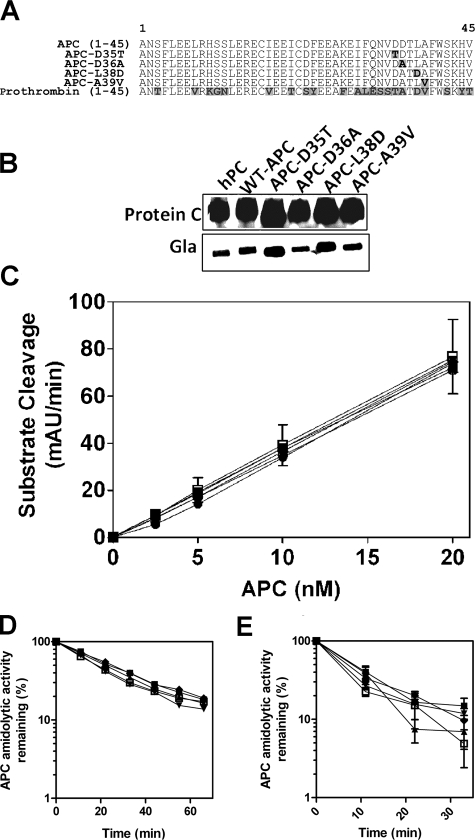
Characterization of Recombinant Protein C/APC Gla Domain Variants—To identify the critical amino acid residues in the APC Gla domain that mediate protein S cofactor enhancement of APC anticoagulant activity, recombinant protein C Gla domain variants were generated by site-directed mutagenesis spanning a region of the Gla domain proposed to mediate protein S cofactor function (Fig. 1A). Each of these variants (APC-D35T, APC-D36A, APC-L38D, and APC-A39V) contains single amino acid substitutions with the corresponding residue of the human prothrombin Gla domain. This approach enables analysis of APC-specific functions while maintaining Gla domain structural integrity (30). Each expressed recombinant protein C variant was characterized by Western blotting with an anti-protein C polyclonal antibody and an anti-Gla monoclonal antibody. Recombinant wild type and variant protein C migrated in a similar fashion to plasma-purified protein C under non-reducing conditions at the expected molecular mass of ∼65 kDa. Wild type and variant recombinant protein C exhibited similar band intensity when detected with both anti-protein C and anti-Gla antibodies to that observed with plasma-purified protein C, indicative of normal expression and post-translational modification of the recombinant proteins (Fig. 1B). For functional studies wild type and variant recombinant protein C were activated using Protac. The amidolytic activity of each recombinant APC variant was assessed and found to be identical to that of wild type APC (Fig. 1C).
FIGURE 1.
Characterization of recombinant APC Gla domain variants. A, recombinant protein C Gla domain variants were generated in which protein C residues at positions 35, 36, 38, and 39 were replaced with the corresponding residues in human prothrombin. Protein C variants were expressed in HEK 293 cells and then isolated from conditioned serum-free medium. B, plasma-purified, recombinant wild type (WT) and variant protein C preparations were assessed by Western blotting with sheep anti-protein C polyclonal and mouse anti-Gla monoclonal antibodies. hPC, human PC. C, each protein C preparation was activated using Protac, and amidolytic activity of each recombinant APC was assessed using a short APC chromogenic substrate. mAU, milliabsorbance units. Inhibition of wild type and variant APC in normal pooled plasma was assessed in the absence (D) and presence (E) of 10 units/ml heparin (wild type, □; APC-D35T, ▪; APC-D36A, ▴; APC-L38D, ▾; APC-A39V, ♦; all 20 nm). Samples were removed at specified time points, and APC amidolytic activity was tested using a chromogenic substrate. The data shown represents the mean of three independent determinations ± S.D.
The rate of inhibition of each APC variant by serpins was determined in normal plasma. Inhibition of both wild type and variant APC was closely comparable (Fig. 1D). To examine the role of protein C inhibitor (PCI), the same experiment was performed in the presence of heparin, which accelerates PCI inhibition of APC. Accordingly, inhibition of wild type APC was enhanced 3-fold, as previously described (32). Each APC variant was inhibited in a similar manner to that of wild type APC in this system (Fig. 1E). Furthermore, subsequent assays indicated that APC inhibition kinetics in the presence of purified α1-antitrypsin and protein C inhibitor were not affected by the presence of any of the APC variants tested (data not shown). Therefore, the residue substitutions present in each APC variant do not alter APC inhibition by its known plasma inhibitors.
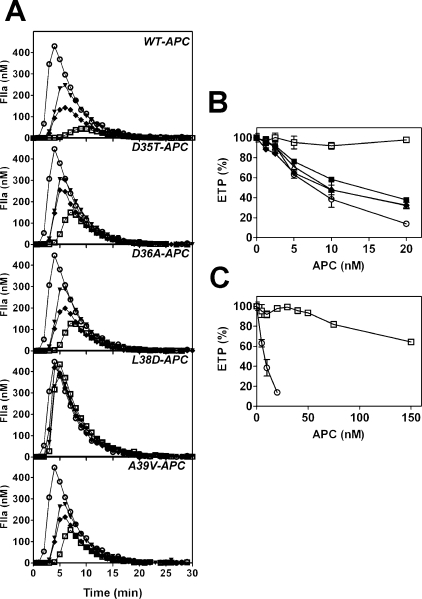
Determination of Recombinant APC Gla Domain Variant Anticoagulant Activity—To identify the specific APC Gla domain residue(s) responsible for mediating APC anticoagulant activity in plasma, the ability of wild type and variant APC to inhibit tissue factor-induced thrombin generation was assessed. Wild type APC diminished thrombin generation in a concentration-dependent manner, as previously described (30) (Fig. 2A). Variants APC-D35T, APC-D36A, and APC-A39V exhibited mildly impaired anticoagulant activity, with <2-fold reduced ETP compared with wild type APC (Fig. 2, A and B). Variant APC-L38D, however, exhibited severely impaired anticoagulant activity (Fig. 2, A and B). APC-L38D was unable to achieve half-maximal inhibition of thrombin generation (ETP) at concentrations as high as 150 nm APC (Fig. 2C), compared with half-maximal inhibition observed with wild type APC (7.2 nm; Fig. 2B). These data indicate that APC-L38D is at least 20-fold less active than wild type APC in plasma.
FIGURE 2.
Variant APC-L38D has negligible anticoagulant activity in protein C-deficient plasma. The ability of recombinant APC variants to inhibit tissue factor-induced thrombin generation was assessed. A, APC (1.25–20 nm) was incubated with protein C-deficient plasma, and thrombin generation initiated with tissue factor, phospholipid vesicles, and CaCl2 (see “Experimental Procedures”). Thrombin generation in protein C-deficient plasma in the absence of APC (○) was compared with 5 nm (▾), 10 nm (♦), and 20 nm (□) of each recombinant APC. B, the ETP for each experiment was determined and expressed as % of thrombin generated in the absence of APC (wild type APC, (○); APC-D35T, (▪); APC-D36A, ▵; APC-L38D, □; APC-A39V, ♦. C, high concentrations (up to 150 nm) of APC-L38D (□) was compared with wild type APC (○) in the same assay.
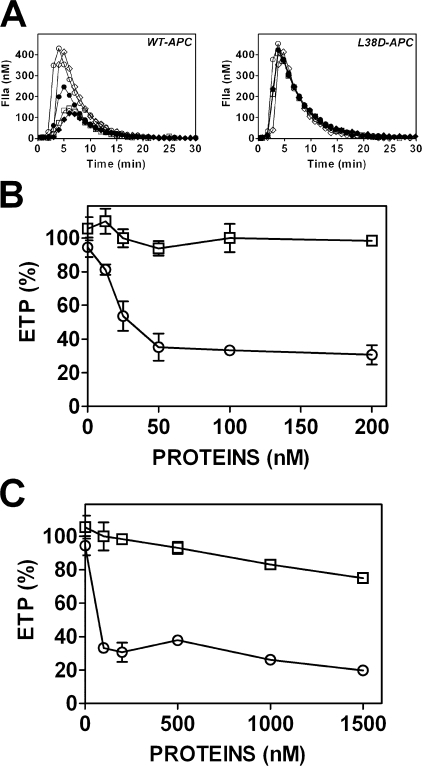
Leu-38 Is Required for Protein S-dependent APC Anticoagulant Function in Protein S-deficient Plasma—To clarify the role of Leu-38 in facilitating APC anticoagulant function, the ability of APC-L38D to be stimulated in protein S-deficient plasma reconstituted with plasma purified protein S was determined. Wild type APC had no anticoagulant function in protein S-deficient plasma, as previously observed (33). However, co-incubation of wild type APC with increasing protein S concentrations (12.5–200 nm) caused a corresponding reduction in thrombin generation (ETP) (IC50 = 24 nm protein S) (Fig. 3, A and B). Variant APC-L38D, however, was almost entirely unresponsive to protein S (Fig. 3, A and B). At the highest protein S concentration tested (1.5 μm), corresponding to 10-fold plasma free protein S concentration, thrombin generation was reduced only 22 ± 2% compared with thrombin generated in the absence of protein S. Therefore, residue Leu-38 mediates APC anticoagulant function in plasma by facilitating critical protein S cofactor function.
FIGURE 3.
Variant APC-L38D is not stimulated by protein S in protein S-deficient plasma. A, the ability of recombinant wild type APC (○) and variant APC-L38D (□) (both 10 nm) to inhibit tissue-factor induced thrombin generation in protein S deficient plasma was assessed in the presence of increasing plasma purified protein S concentrations; no APC/protein S (⋄), 10 nm APC only (○), APC + 25 nm protein S (•), APC + 50 nm protein S (□), and APC + 100 nm protein S (♦). B, the thrombin generation (ETP, %) in the protein S enhancement of variant APC-L38D (□) was compared with wild type APC (○) in the presence of protein S concentrations (B) (6–200 nm) and (C) (0.125–1.5 μm) in the same assay.
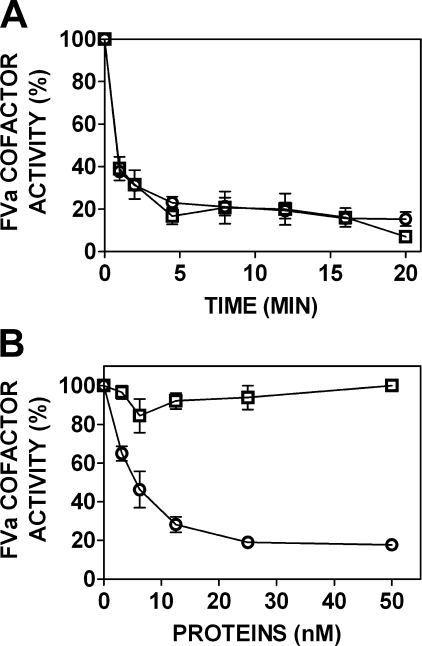
APC-L38D Exhibits Normal Protein S-Independent but Severely Impaired Protein S-dependent FVa Proteolysis—To assess the mechanism by which the impaired response to protein S by APC-L38D in plasma occurs, the rate of FVa proteolysis by APC in the presence and absence of protein S was determined. Using a phospholipid-dependent FVa proteolysis time course assay, the ability of APC-L38D to inactivate FVa in the absence of protein S was determined. Both wild type APC and APC-L38D rapidly reduced FVa cofactor activity (Fig. 4, upper panel), indicating that the observed impaired anticoagulant activity in plasma observed for APC-L38D is not mediated by impaired interaction with anionic phospholipids or FVa. A modified version of this assay was used to evaluate FVa proteolysis of wild type and variant APC in response to protein S. Wild type APC-mediated FVa proteolysis was rapidly enhanced by protein S (Fig. 4, lower panel). Half-maximal inhibition of FVa cofactor activity was observed at 5 nm protein S (Fig. 4, lower panel). In contrast, APC-L38D exhibited no protein S-enhanced FVa proteolysis at each protein S concentration tested. Therefore, APC-L38D loss of protein S-dependent APC anticoagulant activity observed in plasma occurs via impaired protein S-mediated FVa proteolysis.
FIGURE 4.
Protein S-dependent FVa proteolysis by APC requires residue Leu-38 in the APC Gla domain. Upper panel, FVa (1 nm) was incubated with wild type APC (○) or variant APC-L38D (□) (0.2 nm) in the presence of 25 μm phospholipid vesicles (40% PC, 20% PS, 20% PE). The FVa cofactor activity at specified time points was determined by prothrombinase assay (see “Experimental Procedures”). B, human plasma purified protein S (3–50 nm) was incubated with wild type APC (○) or variant APC-L38D (□) (0.4 nm), FVa (8 nm), and 25 μm phospholipid vesicles (40% PC, 20% PS, 20% PE). After 2 min of incubation, an aliquot was removed and added to a prothrombinase assay to assess FVa cofactor activity.
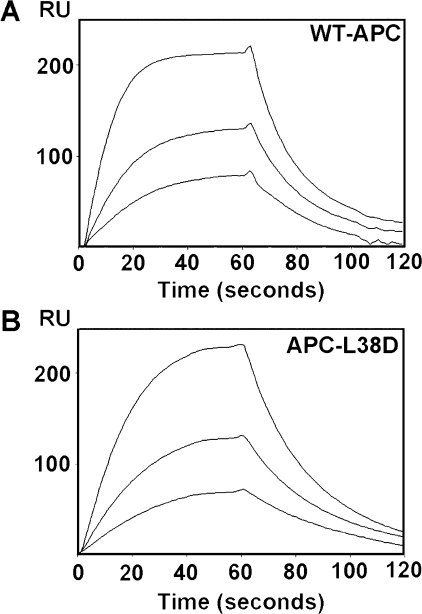
APC-L38D Binds sEPCR with Equal Affinity to Wild Type APC—APC-mediated PAR-1 activation is dependent upon APC binding to EPCR. To ascertain whether APC-L38D interacts normally with sEPCR, the binding affinity of this variant compared with wild type APC was tested by surface plasmon resonance. After sEPCR immobilization, wild type APC and APC-L38D (12.5–100 nm) were exposed to the sEPCR surface. APC-L38D bound sEPCR normally (KD = 112 ± 25 nm), similar to that previously described for wild type PC/APC (30, 31) (Fig. 5).
FIGURE 5.
APC-L38D has normal affinity for sEPCR. Recombinant APC variant binding to sEPCR was measured by surface plasmon resonance technology. sEPCR (500–600 response units (RU)) was immobilized on to the surface of a CM5 sensor chip via a monoclonal anti-EPCR antibody (RCR-2) (see “Experimental Procedures”). An RCR-2 only flow cell was used to detect nonspecific binding. 25–100 nm wild type APC (A) and APC-L38D (B) was passed over the sEPCR surface at a flow rate of 5 μl/min. Minor differences in APC levels are due to small differences in sEPCR bound to the sensor chip surface. Binding was assessed using BIAevaluation software package.
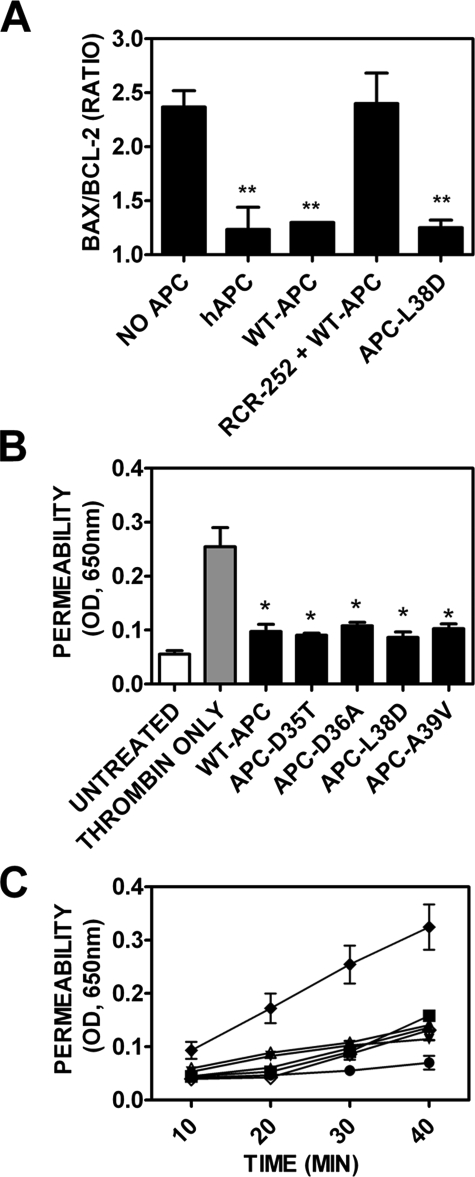
APC-L38D Exhibits Identical Anti-inflammatory and Antiapoptotic Activity to Wild Type APC—As APC-L38D bound sEPCR normally, the anti-inflammatory and anti-apoptotic properties of APC-L38D were evaluated. APC can regulate expression of both pro- and anti-apoptotic genes via EPCR-mediated PAR-1 signaling (10) to confer a net anti-apoptotic phenotype on endothelial cells. The ability of APC-L38D to protect EAhy926 cells from apoptosis was assessed by quantification of pro- (bax) and anti- (bcl-2) apoptotic gene expression after incubation with staurosporine, a well characterized inducer of apoptosis in endothelial cells. Staurosporine, as expected, significantly increased (2.3-fold) the bax/bcl-2 ratio compared with untreated EAhy926 cells (p < 0.005) (Fig. 6A). Preincubation with either plasma-purified or wild type APC, however, significantly ameliorated the effect of the staurosporine, as indicated by the large reduction in bax/bcl-2 ratio in APC-treated cells (p < 0.005) compared with those treated with staurosporine alone. The EPCR dependence of this anti-apoptotic activity was confirmed by incubation of an inhibitory anti-EPCR antibody in conjunction with wild type APC, which led to ablation of the APC-associated protective effect. Of note, APC-L38D significantly reduced the bax/bcl-2 ratio compared with staurosporine only treated cells (p < 0.005) to the same extent as wild type APC (Fig. 6). APC activation of PAR-1 signaling has also previously been described to protect against thrombin-induced endothelial cell barrier hyperpermeability. Using an in vitro assay to assess endothelial cell barrier function, thrombin was found to significantly increase endothelial cell barrier permeability (Fig. 6, B and C) as previously reported (13). Prior incubation with wild type APC, however, significantly attenuated thrombin-induced endothelial barrier permeability (Fig. 6, B and C). Each of the APC variants conferred an identical and significant level of endothelial barrier protection to that of wild type APC compared with thrombin-only treated EAhy926 cells (Fig. 6, B and C). Therefore, APC variants APC-D35T, APC-D36A, APC-L38D, and APC-A39V protect endothelial barrier function to the same extent as wild type APC. These results show that the anti-inflammatory and antiapoptotic functions of APC mediated by EPCR-PAR-1 signaling are entirely conserved in the APC-L38D variant despite significant loss of anticoagulant function caused by the severely impaired response to protein S.
FIGURE 6.
APC variants 35–39 exhibit normal ability to inhibit apoptosis and endothelial barrier protection. A, confluent EAhy926 cells were incubated with Protac-generated plasma purified APC, recombinant wild type APC, and APC Gla domain variants for 17 h. A rat monoclonal anti-EPCR antibody (RCR-252) was added (400 μm) alongside wild type APC to determine EPCR dependence. Apoptosis was induced in EAhy926 cells by incubation with 20 μm staurosporine for 4 h. RNA was extracted and reverse-transcribed as described under “Experimental Procedures.” Reverse transcription-PCR was performed using specific Bax, Bcl-2, and β-actin primers. Experiments were performed in triplicate, and data are presented as the mean ± S.E. Unpaired two-tailed t tests were used to determine significance (**, p < 0.005 compared with staurosporine-only treated EAhy926 cells). hAPC, human APC. B, the protective effect of APC on the endothelial cell barrier was determined for wild type APC and each Gla domain variant. EAhy926 cells were preincubated with 20 nm wild type or variant APC (black bars) for 3 h. Untreated cells (white bar) were used as a negative control. EAhy926 cells were then treated with 5 nm thrombin in serum-free media for 10 min (cells treated with thrombin only, gray bar), and endothelial barrier permeability was assessed after 30 min using Evans Blue-bovine serum albumin (see “Experimental Procedures”). Unpaired two-tailed t tests were used to determine significance (*, p < 0.05 compared with thrombin-only treated EAhy926 cells). C, endothelial barrier protection was assessed over time as described above: untreated EAhy926 cells, •; 5 nm thrombin, ♦; WT-APC, ▴; APC-D35T, ▪; APC-D36A, ▵; APC-L38D, ⋄; APC-A39V, ▾.
DISCUSSION
Protein S is essential for normal anticoagulant function of APC in plasma, acting as a cofactor to enhance proteolysis of APC substrates factor Va and factor VIIIa. Although several putative APC binding sites have been described on protein S (34–40), the corresponding binding sites on APC are poorly defined. However, a previous study has demonstrated that substitution of the APC Gla domain amino acid residues 25–45 with the corresponding amino acid residues in human prothrombin prevented protein S enhancement of APC anticoagulant activity without adversely affecting anionic phospholipid or FVa interactions (41). Furthermore, a subsequent report highlighted the importance of APC Gla domain residues between 33–39, with particular significance assigned to residues Asp-35, Asp-36, Leu-38, and Ala-39 (30).
In this study we have generated and tested individual APC variants in which each of these Gla domain residues was individually substituted with the corresponding prothrombin amino acid residues. Interestingly, only the anticoagulant activity of APC-L38D was severely impaired, exhibiting minimal anticoagulant activity in protein C-deficient plasma compared with wild type APC, even at extremely high concentrations (only 35 ± 1% reduction in ETP observed at 150 nm APC-L38D; Fig. 2). Furthermore, when the protein S-dependent anticoagulant activity of this variant was assessed in protein S-deficient plasma (Fig. 3) and in FVa proteolysis assays (Fig. 4), APC-L38D exhibited effectively no response to protein S, even at free protein S concentrations 10-fold higher than that found in normal plasma (Fig. 3B). In contrast, the anticoagulant effects of APC-D35T, APC-D36A, and APC-A39V were only moderately impaired, exhibiting <2-fold impaired anticoagulant activity in plasma compared with wild type APC (Fig. 2). These findings suggest that APC Gla domain residues 35, 36, and 39 make a relatively minor contribution to the protein S interaction with the APC Gla domain in this region.
Protein S binding to APC has historically proved refractory to meaningful assessment in the absence of phospholipid vesicles, making it difficult at this stage to ascertain whether Leu-38 constitutes a key residue as part of a protein S binding site on APC or is critical for a protein S-mediated conformational change that facilitates enhanced APC substrate proteolysis. Further studies will be required to unravel the precise role of Leu-38 in regulating protein S cofactor enhancement.
Accumulating evidence from different animal disease models suggests that the cytoprotective signaling activity of APC may be of greater significance than its anticoagulant function in protection against various disease states, including severe sepsis, inflammatory bowel disease, and ischemic stroke (16, 19, 42). Nevertheless, the anticoagulant properties of APC are important in that APC administration is associated with a significant increased bleeding risk (19). Although protein S constitutes a critical cofactor for the anticoagulant function of APC, there is no suggestion that it plays any role in regulating PAR-1-mediated anti-inflammatory or anti-apoptotic effects. Thus, disrupting the interplay between APC and protein S constitutes an attractive and novel strategy that may be exploited to generate APC variants with discrepant anticoagulant to cytoprotective properties. However, previous studies have demonstrated that mutations in other regions of APC may influence the ability of the APC Gla domain to effectively bind EPCR, which is a prerequisite for PAR-1 signaling (24). As we have demonstrated, despite having almost entirely lost the ability to interact with protein S, the APC-L38D variant bound sEPCR with the same affinity as wild type APC (Fig. 5). Furthermore, APC-L38D exhibited PAR-1-mediated cytoprotective properties that were indistinguishable from those of wild type APC (Fig. 6).
Recombinant APC variants possessing APC modifications that have significantly reduced anticoagulant activity but retained significant PAR-1 signaling function have previously been generated using alternative strategies. Mosnier et al. (25) demonstrated that clustered alanine mutations (R229A/R230A) and (K191A/K192A/K193A) in two surface loops of the APC serine protease domain disrupted the anion binding site on APC for FVa exosite binding. Consequently, when these five alanine substitutions were combined (5A-APC), APC anticoagulant activity was markedly reduced (<3% residual APTT clotting assay) (26). Because the APC serine protease exosite for PAR-1 is distinct from that of FVa, this combination of mutations did not influence the cytoprotective properties of 5A-APC, which were identical to those of wild type APC. Thus the anticoagulant and anti-inflammatory profiles of this 5A-APC variant are comparable with that exhibited by the APC-L38D variant described herein despite the fact that the respective mutations occur at opposite ends of the APC molecule, are located in distinct structural domains, and target two different specific APC functional interactions.
Using an alternative approach, Bae et al. (24) engineered a disulfide bond (Cys67–Cys82; chymotrypsin numbering) within the APC serine protease domain, which prevented Ca2+ binding to the functionally critical Ca2+ binding 70–80 loop. As a result, the anticoagulant activity of the variant Cys67–Cys82APC was again dramatically impaired, but it was shown to retain PAR-1 activation mediated anti-apoptotic, anti-inflammatory, and endothelial barrier protective functions. However, in contrast to 5A-APC and the APC-L38D variant, the cytoprotective effects of Cys67–Cys82-APC required a 2-fold higher concentration of mutant APC to produce equivalent effects to wild type APC. Interestingly this reduction in the cytoprotective properties of Cys67–Cys82APC was subsequently shown to result from a reduced affinity of the variant APC for EPCR.
On the basis of their specifically reduced anticoagulant activity, one would anticipate that these two previously described recombinant APC variants together with the novel APC-L38D variant should all be associated with significantly reduced bleeding risk in vivo. Because of their reduced bleeding risk, these variants may permit the use of significantly higher APC doses and also longer duration APC administration. Such altered APC therapeutic regimens may be particularly relevant in severe sepsis where overall mortality in the group of patients treated with wild type APC in the PROWESS trial still exceeded 24% (19). A recent in vivo study has confirmed for the first time that APC variants with reduced or minimal anticoagulant activity may confer important therapeutic benefit. In a murine model, Kerschen et al. (8) demonstrate that 5A-APC was as effective as wild type APC in reducing overall mortality after LPS challenge.
In addition to the complete ablation of anticoagulant activity for APC-L38D, we observed a 2-fold reduced anticoagulant activity for each of the variants APC-D35T, APC-D36A, and APC-A39V respectively. Each of these variants also retained entirely normal PAR-1-mediated cytoprotective properties. Thus, these three variants demonstrate anticoagulant/cytoprotective phenotypes that are intermediate between wild type APC and the APC-L38D variant. Consequently, these variants may prove useful adjuncts for defining the relative importance of the anticoagulant and cytoprotective effects of APC in different disease states. Moreover, such APC variants with reduced but residual anticoagulant activity may also offer novel therapeutic opportunities. For example, a beneficial effect of APC has recently been described in a murine model of experimental autoimmune encephalomyelitis (17). In this animal model of multiple sclerosis, it was clearly demonstrated that both the cytoprotective and the anticoagulant properties of APC were required for maximal therapeutic efficacy. These findings suggest that second generation therapeutic recombinant APC variants that retain some residual anticoagulant activity may constitute the treatment of choice in specific disease settings. Further in vivo studies will be necessary to fully dissect the relative contributions of APC anticoagulant versus cytoprotective properties across such a wide variety of pathophysiological processes.
In conclusion, we have demonstrated that a single amino acid substitution (L38D) in the Gla domain of APC is sufficient to almost entirely ablate APC anticoagulant activity due to severely impaired protein S cofactor response. This variant, however, retains normal EPCR binding and PAR-1 signaling properties, suggesting a novel mechanism by which the anticoagulant and cytoprotective properties of APC can be separated for potential therapeutic gain.
This work was supported by a Health Research Board Fellowship PD/2006/24 (to R. J. S. P.), an Irish Heart Foundation grant (to J. S. O. D.), a Children's Medical Research Foundation Award (to F. N. A. and O. P. S.), and Science Foundation Ireland President of Ireland Young Researcher Award 06/Y12/0925 (to J. S. O. D.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Gla,γ-carboxyglutamic acid; APC, activated protein C; EPCR, endothelial cell protein C receptor; sEPCR, soluble EPCR; Fva, factor Va; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; ETP, endogenous thrombin potential; PAR-1, protease-activated receptor-1; PROWESS, activated Protein C Worldwide Evaluation in Severe Sepsis.
References
- 1.Esmon, C. T. (1989) J. Biol. Chem. 264 4743–4746 [PubMed] [Google Scholar]
- 2.Stearns-Kurosawa, D. J., Kurosawa, S., Mollica, J. S., Ferrell, G. L., and Esmon, C. T. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 10212–10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker, F. J., Sexton, P. W., and Esmon, C. T. (1979) Biochim. Biophys. Acta 571 333–342 [DOI] [PubMed] [Google Scholar]
- 4.Fay, P. J., Smudzin, T. M., and Walker, F. J. (1991) J. Biol. Chem. 266 20139–20145 [PubMed] [Google Scholar]
- 5.Seligsohn, U., Berger, A., Abend, M., Rubin, L., Attias, D., Zivelin, A., and Rapaport, S. I. (1984) N. Engl. J. Med. 310 559–562 [DOI] [PubMed] [Google Scholar]
- 6.Reitsma, P. H., Poort, S. R., Bernardi, F., Gandrille, S., Long, G. L., Sala, N., and Cooper, D. N. (1993) Thromb. Haemostasis 69 77–84 [PubMed] [Google Scholar]
- 7.Riewald, M., Petrovan, R. J., Donner, A., Mueller, B. M., and Ruf, W. (2002) Science 296 1880–1882 [DOI] [PubMed] [Google Scholar]
- 8.Kerschen, E. J., Fernandez, J. A., Cooley, B. C., Yang, X. V., Sood, R., Mosnier, L. O., Castellino, F. J., Mackman, N., Griffin, J. H., and Weiler, H. (2007) J. Exp. Med. 204 2439–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce, D. E., Gelbert, L., Ciaccia, A., DeHoff, B., and Grinnell, B. W. (2001) J. Biol. Chem. 276 11199–11203 [DOI] [PubMed] [Google Scholar]
- 10.Riewald, M., and Ruf, W. (2005) J. Biol. Chem. 280 19808–19814 [DOI] [PubMed] [Google Scholar]
- 11.White, B., Schmidt, M., Murphy, C., Livingstone, W., O'Toole, D., Lawler, M., O'Neill, L., Kelleher, D., Schwarz, H. P., and Smith, O. P. (2000) Br. J. Haematol. 110 130–134 [DOI] [PubMed] [Google Scholar]
- 12.Feistritzer, C., Lenta, R., and Riewald, M. (2005) J. Thromb. Haemost. 3 2798–2805 [DOI] [PubMed] [Google Scholar]
- 13.Feistritzer, C., and Riewald, M. (2005) Blood 105 3178–3184 [DOI] [PubMed] [Google Scholar]
- 14.Domotor, E., Benzakour, O., Griffin, J. H., Yule, D., Fukudome, K., and Zlokovic, B. V. (2003) Blood 101 4797–4801 [DOI] [PubMed] [Google Scholar]
- 15.Cheng, T., Liu, D., Griffin, J. H., Fernandez, J. A., Castellino, F., Rosen, E. D., Fukudome, K., and Zlokovic, B. V. (2003) Nat. Med. 9 338–342 [DOI] [PubMed] [Google Scholar]
- 16.Griffin, J. H., Zlokovic, B., and Fernandez, J. A. (2002) Semin. Hematol. 39 197–205 [DOI] [PubMed] [Google Scholar]
- 17.Han, M. H., Hwang, S. I., Roy, D. B., Lundgren, D. H., Price, J. V., Ousman, S. S., Fernald, G. H., Gerlitz, B., Robinson, W. H., Baranzini, S. E., Grinnell, B. W., Raine, C. S., Sobel, R. A., Han, D. K., and Steinman, L. (2008) Nature 451 1076–1081 [DOI] [PubMed] [Google Scholar]
- 18.Isermann, B., Vinnikov, I. A., Madhusudhan, T., Herzog, S., Kashif, M., Blautzik, J., Corat, M. A., Zeier, M., Blessing, E., Oh, J., Gerlitz, B., Berg, D. T., Grinnell, B. W., Chavakis, T., Esmon, C. T., Weiler, H., Bierhaus, A., and Nawroth, P. P. (2007) Nat. Med. 13 1349–1358 [DOI] [PubMed] [Google Scholar]
- 19.Bernard, G. R., Vincent, J. L., Laterre, P. F., LaRosa, S. P., Dhainaut, J. F., Lopez-Rodriguez, A., Steingrub, J. S., Garber, G. E., Helterbrand, J. D., Ely, E. W., and Fisher, C. J., Jr. (2001) N. Engl. J. Med. 344 699–709 [DOI] [PubMed] [Google Scholar]
- 20.Warren, B. L., Eid, A., Singer, P., Pillay, S. S., Carl, P., Novak, I., Chalupa, P., Atherstone, A., Penzes, I., Kubler, A., Knaub, S., Keinecke, H. O., Heinrichs, H., Schindel, F., Juers, M., Bone, R. C., and Opal, S. M. (2001) J. Am. Med. Assoc. 286 1869–1878 [DOI] [PubMed] [Google Scholar]
- 21.Abraham, E., Reinhart, K., Opal, S., Demeyer, I., Doig, C., Rodriguez, A. L., Beale, R., Svoboda, P., Laterre, P. F., Simon, S., Light, B., Spapen, H., Stone, J., Seibert, A., Peckelsen, C., De Deyne, C., Postier, R., Pettila, V., Artigas, A., Percell, S. R., Shu, V., Zwingelstein, C., Tobias, J., Poole, L., Stolzenbach, J. C., and Creasey, A. A. (2003) J. Am. Med. Assoc. 290 238–247 [Google Scholar]
- 22.Bertolini, G., Rossi, C., Anghileri, A., Livigni, S., Addis, A., and Poole, D. (2007) Intensive Care Med. 33 426–434 [DOI] [PubMed] [Google Scholar]
- 23.Kanji, S., Perreault, M. M., Chant, C., Williamson, D., and Burry, L. (2007) Intensive Care Med. 33 517–523 [DOI] [PubMed] [Google Scholar]
- 24.Bae, J. S., Yang, L., Manithody, C., and Rezaie, A. R. (2007) J. Biol. Chem. 282 9251–9259 [DOI] [PubMed] [Google Scholar]
- 25.Mosnier, L. O., Gale, A. J., Yegneswaran, S., and Griffin, J. H. (2004) Blood 104 1740–1744 [DOI] [PubMed] [Google Scholar]
- 26.Mosnier, L. O., Yang, X. V., and Griffin, J. H. (2007) J. Biol. Chem. 282 33022–33033 [DOI] [PubMed] [Google Scholar]
- 27.Walker, F. J. (1984) Semin. Thromb. Hemostasis 10 131–138 [DOI] [PubMed] [Google Scholar]
- 28.Yegneswaran, S., Smirnov, M. D., Safa, O., Esmon, N. L., Esmon, C. T., and Johnson, A. E. (1999) J. Biol. Chem. 274 5462–5468 [DOI] [PubMed] [Google Scholar]
- 29.Nicolaes, G. A., Tans, G., Thomassen, M. C., Hemker, H. C., Pabinger, I., Varadi, K., Schwarz, H. P., and Rosing, J. (1995) J. Biol. Chem. 270 21158–21166 [DOI] [PubMed] [Google Scholar]
- 30.Preston, R. J., Ajzner, E., Razzari, C., Karageorgi, S., Dua, S., Dahlback, B., and Lane, D. A. (2006) J. Biol. Chem. 281 28850–28857 [DOI] [PubMed] [Google Scholar]
- 31.Preston, R. J., Villegas-Mendez, A., Sun, Y. H., Hermida, J., Simioni, P., Philippou, H., Dahlback, B., and Lane, D. A. (2005) FEBS J. 272 97–108 [DOI] [PubMed] [Google Scholar]
- 32.Berg, D. T., Gerlitz, B., Shang, J., Smith, T., Santa, P., Richardson, M. A., Kurz, K. D., Grinnell, B. W., Mace, K., and Jones, B. E. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 4423–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hackeng, T. M., Sere, K. M., Tans, G., and Rosing, J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 3106–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahlback, B., Hildebrand, B., and Malm, J. (1990) J. Biol. Chem. 265 8127–8135 [PubMed] [Google Scholar]
- 35.Giri, T. K., de Frutos, P. G., Yamazaki, T., Villoutreix, B. O., and Dahlback, B. (1999) Thromb. Haemostasis 82 1627–1633 [PubMed] [Google Scholar]
- 36.He, X., Shen, L., and Dahlback, B. (1995) Eur. J. Biochem. 227 433–440 [DOI] [PubMed] [Google Scholar]
- 37.He, X., Shen, L., Villoutreix, B. O., and Dahlback, B. (1998) J. Biol. Chem. 273 27449–27458 [DOI] [PubMed] [Google Scholar]
- 38.Mille-Baker, B., Rezende, S. M., Simmonds, R. E., Mason, P. J., Lane, D. A., and Laffan, M. A. (2003) Blood 101 1416–1418 [DOI] [PubMed] [Google Scholar]
- 39.Saller, F., Kaabache, T., Aiach, M., Gandrille, S., and Borgel, D. (2006) J. Thromb. Haemost. 4 704–706 [DOI] [PubMed] [Google Scholar]
- 40.Saller, F., Villoutreix, B. O., Amelot, A., Kaabache, T., Le Bonniec, B. F., Aiach, M., Gandrille, S., and Borgel, D. (2005) Blood 105 122–130 [DOI] [PubMed] [Google Scholar]
- 41.Smirnov, M. D., Safa, O., Regan, L., Mather, T., Stearns-Kurosawa, D. J., Kurosawa, S., Rezaie, A. R., Esmon, N. L., and Esmon, C. T. (1998) J. Biol. Chem. 273 9031–9040 [DOI] [PubMed] [Google Scholar]
- 42.Scaldaferri, F., Sans, M., Vetrano, S., Graziani, C., De Cristofaro, R., Gerlitz, B., Repici, A., Arena, V., Malesci, A., Panes, J., Grinnell, B. W., and Danese, S. (2007) J. Clin. Investig. 117 1951–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]