Abstract
In prion disease, the abnormal conformer of the cellular prion protein, PrPSc, deposits in fibrillar protein aggregates in brain and other organs. Limited exposure of PrPSc to proteolytic digestion in vitro generates a core fragment of 19–21 kDa, named PrP27–30, which is also found in vivo. Recent evidence indicates that abnormal truncated fragments other than PrP27–30 may form in prion disease either in vivo or in vitro. We characterized a novel protease-resistant PrP fragment migrating 2–3 kDa faster than PrP27–30 in Creutzfeldt-Jakob disease (CJD) brains. The fragment has a size of about 18.5 kDa when associated with PrP27–30 type 1 (21 kDa) and of 17 kDa when associated with type 2 (19 kDa). Molecular mass and epitope mapping showed that the two fragments share the primary N-terminal sequence with PrP27–30 types 1 and 2, respectively, but lack a few amino acids at the very end of C terminus together with the glycosylphosphatidylinositol anchor. The amounts of the 18.5- or 17-kDa fragments and the previously described 13-kDa PrPSc C-terminal fragment relatively to the PrP27–30 signal significantly differed among CJD subtypes. Furthermore, protease digestion of PrPSc or PrP27–30 in partially denaturing conditions generated an additional truncated fragment of about 16 kDa only in typical sporadic CJD (i.e. MM1). These results show that the physicochemical heterogeneity of PrPSc in CJD extends to abnormal truncated forms of the protein. The findings support the notion of distinct structural “conformers” of PrPSc and indicate that the characterization of truncated PrPSc forms may further improve molecular typing in CJD.
Transmissible spongiform encephalopathies (TSEs),2 or prion diseases, are a phenotypically heterogeneous group of neurodegenerative disorders of humans and animals, characterized by abnormal prion protein (PrP) tissue deposits (1). Most prion diseases are infectious. In the disease, the cellular form of PrP, PrPC, is thought to be converted into abnormal PrP, PrPSc, through a posttranslational event associated with an increase in β-sheet secondary structure and a greater aggregation of the protein (1–3). As a result, PrPSc becomes insoluble in nondenaturing detergent and, to large extent, a partial protease resistance, a distinctive property commonly used to distinguish PrPSc from PrPC. Incubation of a TSE-infected brain homogenate with proteinase K (PK) under conditions leading to a complete degradation of PrPC generates an N-terminal truncated form of PrPSc, commonly referred to as PrP27–30 (1, 4).
Creutzfeldt-Jakob disease (CJD), the most common human TSE, like scrapie, the prototype of animal TSEs, comprises a broad spectrum of clinico-pathological variants (5, 6). Phenotypic heterogeneity in TSEs has been attributed both to different strains of the infectious agent and to host genetic factors such as mutations or polymorphisms in the prion protein gene (PRNP) (7, 8). Disease susceptibility and phenotypic expression in humans are modulated by PRNP mutations and a polymorphism at codon 129 that encodes either methionine (M) or valine (V) (9, 10). Distinct agent strains have also been demonstrated (11–16) but have yet to be characterized in full.
Although uncertainties remain regarding the molecular basis of TSE strains and the relationship between strains and PrP, several lines of evidence indicate that PrPSc exists in a variety of molecular subtypes showing distinctive physico-chemical properties (Refs. 11, 17–24; for reviews about more recent studies, see Refs. 25 and 26). Most significantly, PrPSc molecules derived from different strains or disease variants with distinct pathology vary in their N-terminal site of PK cleavage. Based on differences in gel mobility and N-terminal sequence of the core fragments (i.e. PrP27–30) generated by PK digestion, Parchi et al. (19, 20) originally identified two major human PrPSc types: type 1 with a relative molecular mass of 21 kDa and the primary cleavage site at residue 82 and type 2 with a relative molecular mass of 19 kDa and the primary cleavage site at residue 97 (19–21). The two PrPSc types in conjunction with the codon 129 genotype largely explained CJD phenotypic variability and for the first time provided a molecular basis for disease classification (i.e. MM1, MM2, VV1, etc.) (27). More recently, the study of PrPSc under stringent pH conditions using a gel electrophoresis technique with increased resolution demonstrated that PrPSc types 1 and 2 are heterogeneous species, which can be further distinguished into six molecular subtypes that better fit the current histopathologic classification of sporadic CJD (sCJD) (28).
Although for many years full-length PrPSc and its truncated protease-resistant core, PrP27–30, were thought to be the only TSE-specific PrP components, it has been progressively recognized that abnormal PrP comprises additional truncated protein fragments. Unglycosylated PrPSc fragments of 7–8 kDa, truncated at both the N termini and the C termini, were first associated with Gerstmann-Sträussler-Scheinker disease (29–31), whereas either a 16-kDa or a 7-kDa C-terminal fragment was detected in scrapie-infected hamsters (24, 32). More recently, two novel C-terminal fragments of PrPSc (PrP-CTF) have been characterized in sCJD (33). These PrPSc fragments have a relative molecular mass of about 12 and 13 kDa (PrP-CTF 12/13), include glycosylated and unglycosylated forms and an intact C terminus, and originate from PrPSc cleavage at residues 162/167 and 154/156, respectively. In one study, the relative amount of PrP-CTF 12/13, although varying considerably from case to case, accounted for between 0 and 25% of the whole PrPSc signal and showed no apparent correlation with the disease subtype (33). At variance, another study (34) found the PrP-CTF 12/13 (named fPrP 11–12) in iatrogenic CJD without plaques but not in the dura mater-associated iatrogenic CJD with plaques. Two additional putative N-terminally truncated PrPSc fragments, possibly correlating with pathological features of the disease, include a 16–17-kDA unglycosylated fragment found in all PrPSc type 1 sCJD cases and the MM2C sCJD subtype and a fully glycosylated PrP fragment of about 17.5–18 kDa, linked to the sCJD subtypes VV2 and MV2 (35). Lastly, a similar truncated fragment of about 17 kDa was recently detected in sporadic CJDMM2 and variant CJD (vCJD) by another group (36).
To better characterize these fragments and the novel putative PrP abnormal fragments, we analyzed PrPSc by Western blotting and a panel of antibodies against various PrP epitopes in 40 CJD cases, including all sCJD subtypes and vCJD. We found that abnormal PrP aggregates in CJD include previously undetected PrPSc fragments sharing the primary N-terminal sequence with types 1 and 2 but lacking the very end of the C terminus together with the GPI anchor. Furthermore, we disclosed previously unreported differences in the amount of PrP-CTF 12/13 among CJD subtypes and describe a novel fragment specific to sCJDMM1 generated by PK digestion in partially denaturing conditions.
EXPERIMENTAL PROCEDURES
Patients and Tissues
We studied 40 sCJD cases and 4 vCJD cases phenotypically characterized in regard to clinical and histopathological features, pattern of PrP deposition, PRNP genotype, and Western blot profile of PrPSc. Sporadic CJD subtypes were classified according to Parchi et al. (27) by means of neurohistology, immunohistochemistry, Western blotting, and genetic analyses. Clinical data and relevant medical records were also examined. The sCJD cases included 9 MM1, 4 MV1, 4 VV1, 5 MV2 with kuru plaques, 10 VV2, 3 MM2-cortical (MM2-C), and 5 MM2-thalamic (MM2-T), also known as sporadic fatal insomnia. All the selected cases showed a single PrPSc type in their brain according to the method by Notari et al. (37). Autopsy brain tissues from three subjects free of neurological symptoms and signs and with a negative neuropathologic examination were used as negative controls. Brain tissues were obtained at autopsy and were kept frozen at –80 °C until use. Brain samples used were from the frontal cerebral cortex, striatum, thalamus, midbrain, and cerebellum. In addition, two white matter samples from the frontal lobe and the cerebellum were obtained from three cases of each sCJD group. The study was approved by the local Hospital Ethics Committee.
Antibodies
The following mouse monoclonal antibodies recognizing different human PrP epitopes were used at defined concentrations: 3F4 (residues 108–111) (38) obtained from Signet Laboratories, at 80 ng/ml, 6H4 (residues 144–152) obtained from Prionics AG, at 200 ng/ml, 12B2 (residues 89–93) (39) at 400 ng/ml, and Sha31 (residues 145–152), 12F10 (residues 144–152), SAF60 (residues 157–161), and Pri917 (residues 216–221) (40) at 400 ng/ml. In addition, two rabbit antiserums against either PrP N terminus (residues 23–40) or PrP C terminus (the 2301 antiserum, residues 220–231) were used.
Molecular Genetics
Genomic DNA was extracted from blood or frozen brain tissue. Genotyping of the PRNP coding region was performed as described (19).
Purification of PrPSc
Brain tissues (∼5 grams) were used for purification of PK-resistant PrPSc fragments according to a published method (41), as modified (42).
Analyses of PrPSc Truncated Forms after Removal of GPI Anchor
Aliquots of PK- and PNGase F-treated brain homogenates were methanol-precipitated, resuspended in 48% aqueous hydrofluoric acid, and incubated at 4 °C for 24 h, as described (43).
Sample Preparation and Western Blotting
Brain homogenates (10%, w/v) were prepared on ice in lysis buffer with high buffer capacity (LB 100) (100 mm Tris, 100 mm NaCl, 10 mm EDTA, 0.5% Nonidet P-40, 0.5% sodium deoxycholate), pH 6.9. Because the pH of Tris buffers changes significantly according to the buffer temperature, the lysis buffers were titrated to pH 6.9 at 37 °C (i.e. the temperature at which protease digestion is performed). Total protein concentration was estimated using a standard colorimetric method based on bicinchoninic acid (Pierce Biotechnology). All samples were diluted to 6 mg of protein/ml before protease digestion. Aliquots were treated for 1 h at 37 °C with PK (Roche Diagnostics, specific activity by certificate of analysis: 47.9 units/mg) at the concentration of 2 units/ml (1 unit corresponds to 50 μg/ml when PK specific activity is 20 units/mg). Protease digestion was terminated by the addition of 2 mm phenylmethylsulfonyl fluoride (PMSF). Samples were diluted in sample buffer (final concentration: 3% SDS, 4% β-mercaptoethanol, 10% glycerol, 2 mm EDTA, 62.5 mm Tris, pH 6.8) and boiled for 8 min before loading.
Protein samples (brain tissue equivalent to 0.2–1 mg of wet tissue) were separated in 13 or 15% SDS-polyacrylamide gels (37.5:1 acrylamide:bisacrylamide) using gel electrophoresis apparatus holding 7-cm running gels (Bio-Rad). Proteins were transferred to Immobilon P (Millipore) for 2 h at 60 V, blocked with 10% nonfat milk in Tween 20-Tris-buffered saline, pH 7.5, and probed with the appropriate antibody. The immunoreactivity was visualized by enhanced chemiluminescence (ECL standard or plus, GE Healthcare) on Kodak BioMax Light films (Eastman Kodak Co.).
PK Digestion in Partial Protein Denaturing Conditions
Temperature—Aliquots of brain homogenate (10%) in LB 100, pH 6.9, were treated at different temperatures (37, 50, 60, 70, 80 °C) with 10 units/ml PK for 1 h. Protease digestion was terminated by the addition of 2 mm PMSF. Then samples were resuspended in sample buffer (see above) and boiled for 8 min before loading.
SDS Plus Temperature (“PMSF–” Condition)—Brain homogenates (10%) in LB 100, pH 6.9, were treated at 37 °C with 10 units/ml PK for 1 h. Protease digestion was not terminated by the addition of 2 mm PMSF. The samples were resuspended in a β-mercaptoethanol-free sample buffer (final concentration: 3% SDS, 10% glycerol, 2 mm EDTA, 62.5 mm Tris, pH 6.8) and boiled for 8 min. β-mercaptoethanol was added at the final concentration of 4% just before loading.
GdnHCl—Aliquots of 50 μl from 10% brain homogenate in phosphate-buffered saline were mixed with 50 μl of GdnHCl stock solutions, with final GdnHCl 1 m. The solution was incubated for 1 h at 37 °C with 10 units/ml PK. The reaction was stopped with 2 mm PMSF. Proteins were precipitated with 8 volumes of methanol for 2 h at –20 °C. Samples were then centrifuged at 15,000 × g for 15 min at 4 °C, and the pellets were resuspended in sample buffer (see above) and boiled for 8 min before loading.
RESULTS
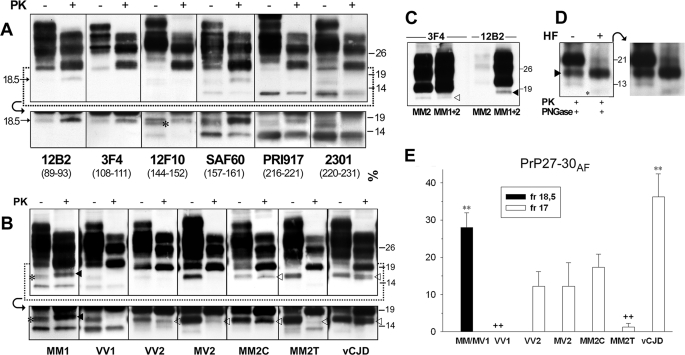
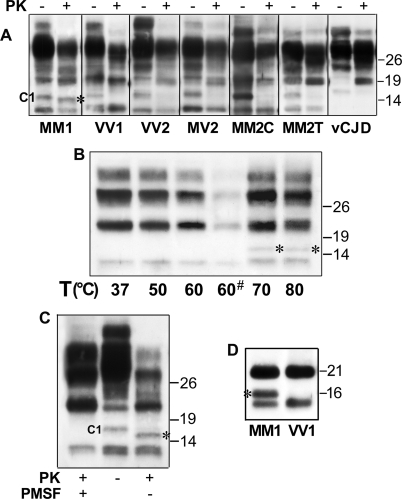
Analysis of Truncated PrPSc Forms in CJD, Evidence for Two Novel Fragments—To detect and characterize the full spectrum of truncated PrPSc fragments in CJD, we performed immunoblots on total brain homogenates using a panel of antibodies recognizing different PrP epitopes. In addition to known abnormal PrP truncated species such as PrP27–30 type 1 and PrP-CTF 12/13, the analyses of samples from sCJDMM1, the most common CJD subtype, showed a novel PK-resistant PrP truncated form with an apparent relative molecular mass of 18.5 kDa (Fig. 1A). The fragment was detected by all antibodies tested with epitopes located between PrP residues 89 and 221 (Fig. 1A) except 6H4 (data not shown), which likely recognize a conformational epitope that is not accessible in this fragment. By contrast, the fragment was not seen by antibodies raised against the PrP N terminus (residues 1–70, data not shown) and virtually undetected by the 2301 antiserum, which recognizes an epitope located at the very end (residues 220–231) of the C-terminal moiety (Fig. 1A).
FIGURE 1.
Detection and characterization of a novel PrPSc truncated fragment in CJD. A, WB profiles of PrPSc from sCJDMM1: comparison among seven antibodies. A sample from the frontal cortex was treated (+) or untreated (–) with PK and probed with antibodies recognizing different epitopes (as indicated). The lower panels show the results obtained in the 12–20-kDa range at a longer film exposure. The C1 fragment (44), which is detected by all antibodies except 12B2 and 3F4 in all PK-untreated samples, is marked with an asterisk in the 12F10 lower panel. The 12B2 and 3F4 panels, due to the absence of the C1 fragment, better show the 18.5-kDa fragment in both PK-treated and PK-untreated conditions. B, WB profiles of PrPSc from different CJD subtypes as revealed by SAF60. Frontal cortex homogenates from all sCJD subtypes and vCJD were treated (+) or untreated (–) with PK and probed with SAF60. The lower panels show the results obtained in the 12–20-kDa range at a longer film exposure. The 18.5- and 17-kDa fragments are marked with filled and empty arrowheads, respectively. The C1 fragment (44), visible in all PK-untreated samples, is marked with an asterisk in the MM1 panel. C, WB profiles of PrPSc from MM2C and MM1+MM2C: comparison between 3F4 and 12B2. Frontal cortex homogenates from MM2C and MM1+MM2C were digested by PK and probed either with 3F4 or with 12B2. In MM2C, the 12B2 antibody shows only very weak bands migrating at the lower edge of the type 1 band, representing partially cleaved fragments generated by an incomplete PK digestion of PrPSc type 2 (37). The 18.5- and 17-kDa fragments are marked with filled and empty arrowheads, respectively. D, the effect of aqueous hydrofluoric acid (HF) treatment on the 18.5-kDa fragment. Immunoblot analyses of a frontal cortex homogenate from sCJDMM1 are shown. A PK- and PNGase F-digested sample was untreated or treated with aqueous hydrofluoric acid. The 18.5-kDa fragment and a weak band migrating with an apparent molecular mass of about 10 kDa, which likely represents the PrP CTF12–13 after the GPI loss, are marked with a filled arrowhead and an asterisk, respectively. The panel on the right shows the result obtained at a longer film exposure. The results shown in panels A–D were reproduced twice with samples from at least three subjects. Approximate molecular masses in panels A–D are in kilodaltons. E, quantification of 18.5- and 17-kDa fragments (fr) in the CJD subtypes. Quantification was performed by densitometric analyses of chemiluminescence Western blot signals generated by PNGase F-treated samples probed with SAF60. The amount is expressed as the percentage of PrP27–30. Each bar represents the mean ± S.E. Statistical analysis was performed by one-way analysis of variance followed by Fisher least squares difference comparison test. **, p < 0.001 versus each different group but MM1/MV1 or vCJD, p < 0.05 versus MM2C; ++, p < 0.001 versus MM1/MV1 and vCJD, p < 0.05 versus VV2, MV2, and MM2C. The number of cases analyzed for each group is reported under “Experimental Procedures.”
The analyses of other sCJD subtypes and vCJD revealed that the 18.5-kDa fragment was only detectable in the MM1/MV1 subtype (Fig. 1B), but a similar faster migrating 17-kDa band was seen in all subtypes associated with PrPSc type 2. The 17-kDa fragment, like the 18.5-kDa band linked to the MM1/MV1 subtype, was readily detected by antibodies with epitopes scattered along PrP residues 99–221 but undetected by the 2301 antiserum (data not shown). At variance with the 18.5-kDa peptide, however, the 17-kDa band was not recognized by 12B2 (Fig. 1C), a monoclonal antibody raised against an epitope (residues 89–93) located between PrPSc types 1 and 2 primary cleavage sites. These results indicate that the 18.5- and 17-kDa fragments have different N-terminal ends, matching those of the PrP27–30 type to which they are associated. In addition, they likely share the C-terminal portion and lack the GPI anchor. To further prove that these fragments lack the GPI anchor, we analyzed in parallel samples treated or not with aqueous hydrofluoric acid, which removes the GPI modification (43). As expected, after the GPI loss, PrPSc types 1 and 2 exactly matched the migration of the 18.5- and 17-kDa fragments, whereas no further truncated forms appeared in the 12–16-kDa range (Fig. 1D and data not shown).
The intensity of the 17-kDa fragment varied significantly among the type 2-associated CJD subtypes (Fig. 1, B and E). The 17-kDa band showed the highest amounts in vCJD and the lowest in MM2-T. In addition, as a distinctive feature, the 17-kDa band often resolved as a doublet in vCJD (Fig. 1B).
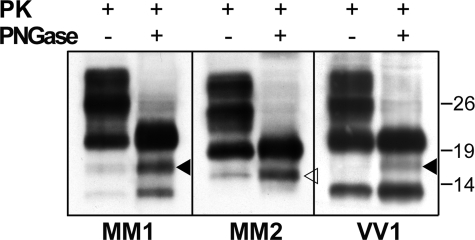
As expected from their predicted amino acid sequences based on antibody mapping, indicating that the fragments include the glycosylation sites, the amount of both the 18.5-kDa fragment and the 17-kDa fragment significantly increased after PNGase F treatment (Fig. 2). In this condition, weak traces of the 18.5-kDa band were also seen in the VV1 samples (Fig. 2), indicating that either one of the two fragments actually forms in all CJD subtypes, although in significantly different amounts.
FIGURE 2.
Effect of deglycosylation on the 18.5- and 17-kDa PrPSc fragments. Immunoblot analyses of frontal cortex homogenates from MM1, MM2C, and VV1 subtypes are shown. PK-treated samples were either untreated or treated with PNGase F and probed with SAF60. The 18.5-and 17-kDa fragments are marked with filled and empty arrowheads, respectively. The same result was reproduced twice with samples from at least three subjects for each group. Approximate molecular masses are in kilodaltons.
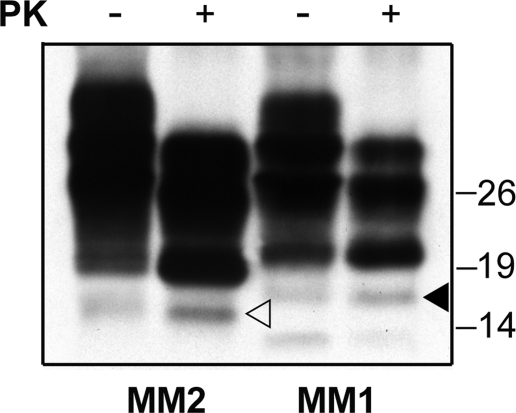
The analyses of partially purified (P3) PrP preparations in sarkosyl showed that the novel fragments copurify with PrPSc and PrP27–30 in the detergent insoluble fraction after extraction in sarkosyl (Fig. 3). The fragment was already visible in the PK-untreated preparations, although it increased in amount after PK treatment.
FIGURE 3.
The 18.5- and 17-kDa fragments are recovered in the P3 fraction. An immunoblot analysis of Sarkosyl extracted PrPSc (P3 fraction) from MM1 and MM2C subjects is shown. The samples were either untreated or treated with PK. Membrane was probed by SAF60. The 18.5- and 17-kDa fragments are marked with filled and empty arrowheads, respectively. The same result was reproduced twice with samples from at least three subjects for each group. Approximate molecular masses are in kilodaltons.
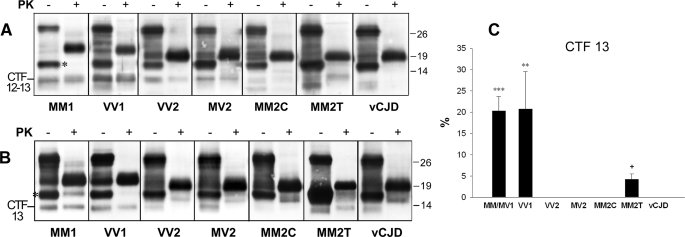
Analysis of PrP-CTF 12/13 in sCJD Subtypes MV1, VV1, VV2, MV2, MM2-C, and vCJD—In the publication by Zou et al. (33), where the PrP-CTF 12/13 were originally described in sCJD, these fragments accounted for between 0 and 25% of the whole PrPSc signal and showed no apparent correlation with the disease subtype. Satoh et al. (34), however, later showed that PrP-CTF 12/13 (named fPrP 11–12 in Ref. 34) distinguishes between two pathologically distinct subtypes of iatrogenic CJD. In view of these findings, we looked in more detail at the presence and amount of PrP-CTF 12/13 and extended the analyses to all CJD subtypes. We found that sCJDVV1 had the highest amount of these fragments and MM2-C had the lowest, whereas in vCJD, the two fragments were virtually undetectable (Fig. 4A). Accurate quantification of the relative amount of each of the two fragments was difficult due to the close migration of the two bands. To accomplish this task, we took advantage of monoclonal antibody SAF 60 (157–161), which binds the 13-kDa fragment (N terminus at residues 154–156) but not the 12-kDa fragment (N terminus at residues 162–167). Such an approach revealed that the 13-kDa CTF is virtually undetectable in vCJD and in all sCJD subtypes associated with PrPSc type 2 except the MM2-T (Fig. 4, B and C).
FIGURE 4.
WB analysis of PrP-CTF 12/13 in different sCJD subtypes and vCJD. A, frontal cortex homogenates from all sCJD subtypes and vCJD were untreated or treated with PK. All samples were then digested with PNGase F. Membrane was probed with the 2301 antiserum. The C1 fragment, which is seen in all untreated samples, is marked with asterisk in the MM1 panel. Approximate molecular masses are in kilodaltons. B, frontal cortex homogenates from all sCJD subtypes and vCJD were either untreated or treated with PK. All samples were then digested with PNGase F. Membrane was probed by SAF60. The C1 fragment, visible in all PK-untreated samples, is marked with an asterisk in the MM1 panel. Approximate molecular masses are in kilodaltons. C, relative amounts of 13 CTF in different CJD subtypes. Quantification was performed relative to the amount of PrP27–30 (in percentages) on WB stained with antibody SAF60. Each bar represents the mean ± S.E. Statistical analysis was performed by one-way analysis of variance followed by Fisher least squares difference comparison test (***, p < 0.001 versus each other group but VV1; **, p < 0.005 versus each other group but MM1/MV1) or the type 2 subtypes only (+, p < 0.001 versus each different group). The number of cases analyzed for each group is reported under “Experimental Procedures.”
Protease Digestion of PrPSc in Partial Denaturing Conditions—During our routine Western blot analyses for CJD diagnostic purposes, we occasionally observed an unexpected PK-resistant band of about 16 kDa. Given its inconsistency, we reasoned that this PrPSc fragment may result from an inefficient inactivation of PK before protein denaturation in SDS and heating. To verify whether pathological PrP aggregates assume structural features in partial denaturing conditions, allowing a further possibly subtype-specific cleavage of the protein, we performed PK digestion of brain homogenates in three different denaturing conditions (see “Experimental Procedures”). In all these conditions, PK digestion of PrPSc extracted from MM1 or MV1 sCJD generated a novel, N-terminal truncated fragment of about 16 kDa (Fig. 5). Epitope mapping indicated that the fragment has an intact C-terminal end and is truncated in the region between residue 112 and residue 144. The peptide is generated from PrP27–30 rather than from full-length PrPSc because it also forms when PK digestion in denaturing conditions is performed in a brain homogenate that has already been digested by PK, (i.e. “PMSF–” experimental conditions.) Interestingly, the 16-kDa fragment appeared in all sCJD MM1/MV1 samples, whereas, at least in the tested condition, it was not seen in any samples from the other CJD subtypes.
FIGURE 5.
WB analysis of PrPSc digestion in different partially denaturing conditions. A, immunoblot analysis of PrPSc from frontal cortex of all sCJD subtypes and vCJD. Samples were incubated in GdnHCl 1 m for 1 h and untreated or treated with PK. B, immunoblot analysis of PrPSc from frontal cortex of an MM1 case digested with PK at different temperatures. The incubation time was 30 min for all samples but one (marked with an asterisk), which was incubated for 1 h. C, immunoblot analysis of PrPSc from frontal cortex of an MM1 case. Standard (i.e. “PMSF+”) and “PMSF–” conditions, as indicated under “Experimental Procedures,” are compared. Membranes were probed by the 2301 antiserum. D, immunoblot analysis of PrPSc from frontal cortex of an MM1 and a VV1 subject. All samples are in PMSF–conditions and deglycosylated. Membranes were probed by the 2301 antiserum. The 16-kDa fragment generated in denaturing conditions (DCF 16) is marked with an asterisk. All results were reproduced twice with samples from nine sCJDMM1/MV1 and at least three subjects for each of the other groups. Approximate molecular masses are in kilodaltons.
A Pitfall in the Analyses of PrPSc Truncated Forms Related to the Protease Resistance of the C1 Fragment—During our study, we noticed that PK-treated samples obtained from different structures of the same brain often contained variable amounts of a 18-kDa peptide, matching the previously described (44) C1 PrPC fragment by epitope mapping and electrophoretic mobility (Fig. 6A). Given that the samples showing the highest amount of this form were from subcortical structures, such as the thalamus and brainstem, in which the gray matter is notoriously more difficult to dissect from the white matter, we thought that the relative amount of white matter present in the different samples could be the critical variable responsible for this phenomenon (i.e. the relative abundance of the 18-kDa PK-resistant band). To address this question, we analyzed PK-treated homogenates of pure white matter with 3F4, SAF60, and the 2301 antiserum. Unexpectedly, we found the 18-kDa PK-resistant band, matching the C1 PrP fragment, in white matter samples from both CJD samples and controls (Fig. 6, B and C). Furthermore, the fragment showed a similar degree of PK resistance in both sCJD and control cases, indicating that it does not represent a truncated PrPSc form. Since we could not disclose any significant difference in the relative amount of the C1 fragment between white and gray matter samples, the relative persistence of the 18-kDa band in PK-treated brain homogenates samples suggests either a relatively high degree of protease resistance of the fragment generated by the white matter cells or a relative inhibition of PK activity by the lipid-rich biochemical environment of the white matter.
FIGURE 6.
Analysis of C1-resistant fragment. A–C, immunoblot analysis of PrP in two different samples from an MM2-T subject (lanes 1–2 versus lane 3, respectively) (A); white matter from an MM1 subject (B); and white matter from a CJD-negative case (C). The samples were either untreated or treated with PK at the indicated concentrations. All samples were digested with PNGase F. Membrane was probed by SAF60. The C1 fragment is indicated with an arrow. Approximate molecular masses are in kilodaltons.
DISCUSSION
We have identified a novel PrP fragment in brains of sCJD- and vCJD-affected subjects migrating 2–3 kDa faster than PrP27–30. The fragment has an apparent molecular mass of about 18.5 kDa when associated with PrP27–30 type 1 and of ∼17 kDa when associated with type 2. Epitope mapping, relative molecular mass estimation, and GPI anchor removal from PrP27–30 by aqueous hydrofluoric acid indicated that the peptide represents an “anchorless” fragment of PrP27–30 (PrP27–30AF) types 1 and 2, respectively, which lacks a few amino acids at the very end of the C terminus together with the GPI anchor. Like PrP27–30, PrP27–30AF was detected in both PK-untreated and PK-treated samples. In addition, PK digestion increased the amount of the fragment. Thus, the peptide is partially formed in vivo and partially generated by in vitro limited proteolysis. Evidence showing that anchorless forms of PrP are associated with both pathological and physiological conditions is accumulating. In 1990, Stahl et al. (45) first reported that ∼15% of PrPSc molecules purified from infected hamsters are truncated at glycine-228. Cell culture studies (43, 46) later showed that PrP is not only released by phospholipase digestion of GPI but also shed from the membrane by protease cleavage at its extreme C-terminal portion. Interestingly, these two different routes of GPI loss produce two different PrP anchorless forms well distinguished by their difference in gel migration (43, 46). In fact, although PrP digested with phospholipase, which only cleaves the hydrophobic portion of the GPI, migrates more slowly than full-length PrP, the GPI loss generated by protease digestion of the PrP C terminus produces a faster migrating fragment corresponding to an apparent molecular mass loss of about 2–3 kDa, i.e. the molecular mass of the GPI anchor. The same 2–3-kDa loss in apparent molecular mass is detectable when PrP is treated with aqueous hydrofluoric acid, which leads to a complete loss of GPI (43, 45), or when the anchorless PrP is derived from a genetically engineered GPI-minus PrP (47).
Our data indicate that there is a significant subtype-specific heterogeneity in the amount of PrP27–30AF, particularly evident in sCJD VV1 and MM2-T, where the fragment is only detectable in traces. Given that both these CJD subtypes show a significantly lower accumulation of pathological PrPSc than the others, there could be a relation between this property and the reduced formation of PrPSc anchorless fragment. The pathogenetic role of the anchorless form of PrP in prion disease is unclear. Although cell-free experiments in vitro have shown that anchorless PrP can be converted to PrPSc (48, 49), the absence of the GPI anchor reduces conversion in scrapie-infected cells (50), which led to the general belief that conversion in prion disease involves membrane-bound GPI-linked PrP. Interestingly, an anchorless version of PrP has recently been shown to sustain PrPSc replication in vivo, although it did not generate any characteristic symptoms in GPI-negative transgenic mice (47). Nevertheless, scrapie is accelerated in mice coexpressing wild-type and GPI-negative PrP (47). By demonstrating the existence of types 1 and 2 PrP27–30AF, our findings suggest that the anchorless form of PrP could also be important for CJD pathogenesis.
Pursuing the report of Satoh et al. (34), who reported the absence of PrP-CTF 12/13 in dura mater-related iatrogenic CJD associated with amyloid plaques, we reanalyzed CJD-associated PrP-CTF 12/13 more closely and found a significant subtype-specific difference both in the total amount and in the relative ratio of these fragments. To the second aim, we took advantage of SAF60, which binds the 13-kDa fragment but not the 12-kDa fragment. We demonstrated that all type 2 CJD except the MM2-T show only traces of the 13-kDa fragment, whereas in vCJD, both fragments are virtually undetectable. In contrast, the VV1 sCJD subtype shows the highest relative amounts of these fragments. These data demonstrate a significant heterogeneity in composition and relative amount of differently truncated abnormal PrPSc fragments in CJD and suggest a possible role in the determinism of strain-specific features. Interestingly, a PrP fragment of comparable size with human PrP-CTF12–13 was recently observed in H-type BSE cases from cattle but not in classic BSE using the C terminus-specific (group C) antibodies (51). In addition to the presence and relative amount of abnormal PrPSc truncated fragments formed in vivo, distinct PrPSc subtypes may differ in the pattern of truncated peptides generated in vitro after partial disruption of the native protein structure. In this study, we have shown that PK digestion of PrPSc type 1 from MM and MV sCJD under partially denaturing conditions generates an additional fragment of about 16 kDa, which is not seen in the same treatment conditions in the other CJD subtypes. This finding has at least three implications, each deserving comment. First of all, the data confirm that at a molecular level, sCJD MM1/MV1 represents a phenotypically homogenous group, likely related to a single agent strain. Secondly, the specificity of the finding, limited to one sCJD subtype, supports the concept of TSE strains being related to the PrPSc structure. Lastly, the data have practical implications for the current molecular typing and classification of CJD cases since the specific search for the 16-kDa fragment in denaturing conditions may help to identify the sCJDMM1/MV1 subtype at molecular level.
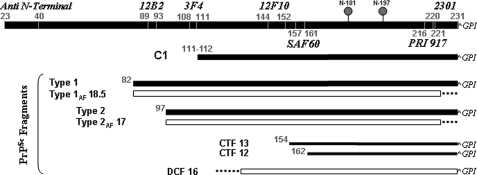
In this regard, it is increasingly clear that there is a PrPSc “signature” in CJD, which is the result of the combination between PrP27–30 and additional fragments, which are specifically or “quantitatively” related to either type 1 or type 2 (Table 1, Fig. 7). The first, and perhaps strongest, implication in this respect concerns the sCJD subtypes associated with PrPSc type 1, which, accordingly to the current CJD classification, include two pathological phenotypes correlating with MM1/MV1 and VV1, respectively. Similarly, the PrPSc signature given by the relative ratio of PrP27–30, PrP27–30AF, and PrP-CTF 12 or 13 may help to distinguish among the different CJD subtypes associated with PrPSc type 2 (Table 1).
TABLE 1.
Summary and relative amounts of detected PrPSc truncated fragments in the CJD subtypes
Quantification was performed by densitometric analyses of chemiluminescence Western blot signals generated by PNGase F-treated samples probed either with SAF60 or 2301 antibodies for detection of PrP27–30AF, CTF 13 or with CTF 12/13, DCF 16, respectively. The amounts are expressed as a percentage of PrP27–30. Each value represents the mean ± S.E. The number of cases analyzed for each group is reported under “Experimental Procedures.”
| PrP27-30 | PrP27-30AF | DCF 16a | CTF 13 | CTF 12/13 | |
|---|---|---|---|---|---|
| MM/MV1 | 100 | 28.0 ± 3.9 | 31.3 ± 6.0 | 20.3 ± 3.3 | 22.3 ± 2.5 |
| VV1 | 100 | <1b | NDc | 20.8 ± 8.7 | 72.6 ± 13.6 |
| VV2 | 100 | 12.2 ± 4.0 | ND | ND | 19.2 ± 9.0 |
| MV2 | 100 | 12.2 ± 6.4 | ND | ND | 21.7 ± 16.6 |
| MM2-C | 100 | 17.4 ± 3.5 | ND | ND | 2.6 ± 1.7 |
| MM2-T | 100 | 1.3 ± 1.0 | ND | 4.2 ± 1.3 | 16.9 ± 7.3 |
| vCJD | 100 | 36.2 ± 6.2 | ND | ND | ND |
DCF 16 = the 16-kDa PrPSc fragment generated by PK digestion performed in partially denaturing conditions.
Only traces were detectable.
ND, not detectable.
FIGURE 7.
Diagram of CJD-associated PrPSc fragments. The C1 PrPC fragment and the epitope location of the PrP antibodies used in this study are also shown. DCF 16 = the 16-kDa PrPSc fragment generated by PK digestion in partially denaturing conditions.
In this study, we also found that the previously described 18-kDa C1 PrPC fragment (44) shows a relatively high degree of protease resistance in white matter control samples. The observation that the C1 fragment has a higher PK resistance when compared with full-length PrPC has been already underlined in previous studies (52–54). We extended these observations and found that in white matter samples, the fragment is still detectable after PK digestion at an enzyme concentration of 20 units/ml, which is associated with a complete degradation of full-length PrPC. Although we could not exclude that a relative inefficiency of PK digestion in white matter homogenates plays a role in determining the apparent high PK resistance of C1, the finding has, nevertheless, a significant implication for PrPSc typing studies. Indeed the C1 fragment may be easily misinterpreted as an abnormal truncated PrPSc form such as PrP27–30AF when antibodies recognizing both fragments (i.e. C1 and PrP27–30) are used. Thus, it is important that, whenever possible, the characterization of such fragments by Western blotting is performed on gray matter samples containing the least possible white matter. Furthermore, 3F4 is recommended for detection of PrP27–30AF associated with both type 1 and type 2 (37) despite its low affinity for these fragments, and 12B2 is recommended for specific detection of the PrP27–30AF associated with type 1.
A previous study by Zanusso et al. (35) identified protease-resistant PrP fragments in the 16–18.5-kDa range in CJD. However, the PrP27–30AF we have described here migrates more slowly in CJD type 1 than in CJD type 2, irrespective of the codon 129 genotype, whereas the fragments described by Zanusso et al. (35) showed the opposite behavior (i.e. 16–17 kDa in sCJDMM1 and 17.5–18 in VV2/MV2). Moreover, PrP27–30AF are not recognized by antibodies against the very end of the C terminus, whereas the two truncated forms described by Zanusso et al. (35) were only N-terminally truncated. Conversely, it seems that the 16–17- and 17.5–18-kDa PrP fragments described by Zanusso et al. (35) show significant similarities with the 18-kDa-resistant form we interpreted as the C1 PrPC fragment, which is especially seen in white matter-enriched samples, and with the 16-kDa PrPSc form we detected in MM1 cases in partially denaturing conditions. Whether this is only a coincidence or whether there is indeed at least a partial correspondence between the two pairs of fragments remains to be seen.
In conclusion, by showing that PrP heterogeneity in CJD not only involves PrP27–30 but also extends to other abnormal truncated PrP fragments, our findings raise the issue of the pathogenetic role of these peptides, including neurotoxic potential or involvement in the determinism of prion infectivity. The use of PK digestion in partial denaturing conditions and antibodies against the central and C-terminal PrP regions provide additional tools for PrPSc typing in CJD and possibly other TSEs. In addition, our data further support the concept of TSE strains and pathological features being related to PrPSc structure. We believe that studies such as the present one will help not only to refine strain typing in CJD and other prion diseases but also to probe strain-related PrPSc structure, which is still poorly understood. Finally, detailed knowledge of strain-related PrPSc fragment profiles determined by protease digestion either in vivo or in vitro will provide an essential reference for all scientists generating PrPSc-like aggregates in vitro from recombinant PrP to gain information on the biological relevance of their models.
Acknowledgments
Prof. James Ironside at the National CJD Surveillance Unit in the UK kindly provided the tissues of variant CJD used for this study. We also thank Dr. Barbara Polischi for technical support.
This study was supported by grants from the European Commission (Grant FOOD-CT-2004-506579) and the Italian Ministry of University, Research and Technology (Grant FIRB-2003-RBNE03FMCJ_006). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TSE, transmissible spongiform encephalopathies; PrP, prion protein; PK, proteinase K; CJD, Creutzfeldt-Jakob disease; sCJD, sporadic CJD; vCJD, variant CJD; CTF, C-terminal fragments; PMSF, phenylmethylsulfonyl fluoride; GPI, glycosylphosphatidylinositol; PNGase F, N-glycosidase F; WB, Western blot.
References
- 1.Prusiner, S. B. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caughey, B. W., Dong, A., Bhat, K. S., Ernst, D., Hayes, S. F., and Caughey, W. S. (1991) Biochemistry 30 7672–7680 [DOI] [PubMed] [Google Scholar]
- 3.Pan, K. M., Baldwin, M., Nguyen, J., Gasset, M., Serban, A., Groth, D., Mehlhorn, I., Huang, Z., Fletterick, R. J., Cohen, F. E., and Prusiner, S. B. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 10962–10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton, D. C., McKinley, M. P., and Prusiner, S. B. (1982) Science 218 1309–1311 [DOI] [PubMed] [Google Scholar]
- 5.Gambetti, P., Kong, Q., Zou, W., Parchi, P., and Chen, S. G. (2003) Br. Med. Bull. 66 213–239 [DOI] [PubMed] [Google Scholar]
- 6.Kretzschmar, H. A., and Parchi, P. (2007) in Prions in Humans and Animals (Hoernlimann, B., Riesner, D., and Kretzschmar, H. eds.) pp. 287–305, De Gruyter, Berlin-New York
- 7.Bruce, M. E., McConnell, I., Fraser, H., and Dickinson, A. G. (1991) J. Gen. Virol. 72 595–603 [DOI] [PubMed] [Google Scholar]
- 8.Goldmann, W., Hunter, N., Smith, G., Foster, J., and Hope, J. (1994) J. Gen. Virol. 75 989–995 [DOI] [PubMed] [Google Scholar]
- 9.Palmer, M. S., Dryden, A. J., Hughes, J. T., and Collinge, J. (1991) Nature 352 340–342 [DOI] [PubMed] [Google Scholar]
- 10.Barron, R. M., Thomson, V., Jamieson, E., Melton, D. W., Ironside, J., Will, R., and Manson, J. C. (2001) EMBO J. 20 5070–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telling, G. C., Parchi, P., DeArmond, S. J., Cortelli, P., Montagna, P., Gabizon, R., Mastrianni, J., Lugaresi, E., Gambetti, P., and Prusiner, S. B. (1996) Science 274 2079–2082 [DOI] [PubMed] [Google Scholar]
- 12.Bruce, M. E., Will, R. G., Ironside, J. W., McConnell, I., Drummond, D., Suttie, A., McCardle, L., Chree, A., Hope, J., Birkett, C., Cousens, S., Fraser, H., and Bostock, C. J. (1997) Nature 389 498–501 [DOI] [PubMed] [Google Scholar]
- 13.Parchi, P., Brown, P., Capellari, P., Gibbs, C. J., Jr., and Gambetti, P. (1999) in Alzheimer' s Disease and Related Disorders: Etiology, Pathogenesis, and Therapeutics (Iqbal, K., Swaab, D. F., Winblad, D., and Wisniewski, H. M., eds) pp. 561–567, John Wiley & Sons Inc., New York
- 14.Korth, C., Kaneko, K., Groth, D., Heye, N., Telling, G., Mastrianni, J., Parchi, P., Gambetti, P., Will, R., Ironside, J., Heinrich, C., Tremblay, P., De-Armond, S. J., and Prusiner, S. B. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 4784–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonno, R., Di Bari, M., Cardone, F., Vaccari, G., Fazzi, P., Dell'Omo, G., Cartoni, C., Ingrosso, L., Boyle, A., Galeno, R., Sbriccoli, M., Lipp, H. P., Bruce, M., Pocchiari, M., and Agrimi, U. (2005) PLoS Pathog. 2 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi, A., Asano, M., Mohri, S., and Kitamoto, T. (2007) J. Biol. Chem. 282 30022–30028 [DOI] [PubMed] [Google Scholar]
- 17.Kascsak, R. J., Rubenstein, R., Merz, P. A., Carp, R. I., Robakis, N. K., Wisniewski, H. M., and Diringer, H. (1986) J. Virol. 59 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessen, R. A., and Marsh, R. F. (1994) J. Virol. 68 7859–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parchi, P., Castellani, R., Capellari, S., Ghetti, B., Young, K., Chen, S. G., Farlow, M., Dickson, D. W., Sima, A. A. F., Trojanowski, J. Q., Petersen, R. B., and Gambetti, P. (1996) Ann. Neurol. 39 767–778 [DOI] [PubMed] [Google Scholar]
- 20.Parchi, P., Capellari, S., Chen, S. G., Petersen, R. B., Gambetti, P., Kopp, N., Brown, P., Kitamoto, T., Tateishi, J., Giese, A., and Kretzschmar, H. (1997) Nature 386 232–233 [DOI] [PubMed] [Google Scholar]
- 21.Parchi, P., Zou, W., Wang, W., Brown, P., Capellari, S., Ghetti, B., Kopp, N., Schulz-Schaeffer, W. J., Kretzschmar, H. A., Head, M. W., Ironside, J. W., Gambetti, P., and Chen, S. G. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 10168–10172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somerville, R. A., Chong, A., Mulqueen, O. U., Birkett, C. R., Wood, S. C., and Hope, J. (1997) Nature 386 564. [DOI] [PubMed] [Google Scholar]
- 23.Safar, J., Wille, H., Itri, V., Groth, D., Serban, H., Torchia, M., Cohen, F. E., and Prusiner, S. B. (1998) Nat. Med. 4 1157–1165 [DOI] [PubMed] [Google Scholar]
- 24.Caughey, B., Raymond, G. J., and Bessen, R. A. (1998) J. Biol. Chem. 273 32230–32235 [DOI] [PubMed] [Google Scholar]
- 25.Parchi, P., Notari, S., Stramiello, R., and Capellari, S. (2005) in Prions: Food and Drug Safety (Kitamoto, T., ed) pp. 77–96, Springer-Verlag, Tokyo
- 26.Baron, T., Biacabe, A. G., Arsac, J. N., Benestad, S., and Groschup, M. H. (2007) Vaccine 25 5625–5630 [DOI] [PubMed] [Google Scholar]
- 27.Parchi, P., Giese, A., Capellari, S., Brown, P., Schulz-Schaeffer, W., Windl, O., Zerr, I., Budka, H., Kopp, N., Piccardo, P., Poser, S., Rojiani, A., Streichemberger, N., Julien, J., Vital, C., Ghetti, B., Gambetti, P., and Kretzschmar, H. (1999) Ann. Neurol. 46 224–233 [PubMed] [Google Scholar]
- 28.Notari, S., Capellari, S., Giese, A., Westner, I., Baruzzi, A., Ghetti, B., Gambetti, P., Kretzschmar, H. A., and Parchi, P. (2004) J. Biol. Chem. 279 16797–16804 [DOI] [PubMed] [Google Scholar]
- 29.Tagliavini, F., Prelli, F., Ghiso, J., Bugiani, O., Serban, D., Prusiner, S. B., Farlow, M. R., Ghetti, B., and Frangione, B. (1991) EMBO J. 10 513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parchi, P., Chen, S. G., Brown, P., Zou, W., Capellari, S., Budka, H., Hainfellner, J., Reyes, P. F., Golden, G. T., Hauw, J. J., Gajdusek, D. C., and Gambetti, P. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 8322–8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piccardo, P., Dlouhy, S. R., Lievens, P. M., Young, K., Bird, T. D., Nochlin, D., Dickson, D. W., Vinters, H. V., Zimmerman, T. R., Mackenzie, I. R., Kish, S. J., Ang, L. C., De Carli, C., Pocchiari, M., Brown, P., Gibbs, C. J., Jr., Gajdusek, D. C., Bugiani, O., Ironside, J., Tagliavini, F., and Ghetti, B. (1998) J. Neuropathol. Exp. Neurol. 57 979–988 [DOI] [PubMed] [Google Scholar]
- 32.Kocisko, D. A., Lansbury, P. T., Jr., and Caughey, B. (1996) Biochemistry 35 13434–13442 [DOI] [PubMed] [Google Scholar]
- 33.Zou, W. Q., Capellari, S., Parchi, P., Sy, M. S., Gambetti, P., and Chen, S. G. (2003) J. Biol. Chem. 278 40429–40436 [DOI] [PubMed] [Google Scholar]
- 34.Satoh, K., Muramoto, T., Tanaka, T., Kitamoto, N., Ironside, J. W., Nagashima, K., Yamada, M., Sato, T., Mohri, S., and Kitamoto, T. (2003) J. Gen. Virol. 84 2885–2893 [DOI] [PubMed] [Google Scholar]
- 35.Zanusso, G., Farinazzo, A., Prelli, F., Fiorini, M., Gelati, M., Ferrari, S., Righetti, P. G., Rizzuto, N., Frangione, B., and Monaco, S. (2004) J. Biol. Chem. 279 38936–38942 [DOI] [PubMed] [Google Scholar]
- 36.Pan, T., Li, R., Kang, S. C., Pastore, M., Wong, B. S., Ironside, J., Gambetti, P., and Sy, M. S. (2005) J. Neurochem. 92 132–142 [DOI] [PubMed] [Google Scholar]
- 37.Notari, S., Capellari, S., Langeveld, J., Giese, A., Strammiello, R., Gambetti, P., Kretzschmar, H. A., and Parchi, P. (2007) Lab. Investig. 87 1103–1112 [DOI] [PubMed] [Google Scholar]
- 38.Kascsak, R. J., Rubenstein, R., Merz, P. A., Tonna-DeMasi, M., Fersko, R., Carp, R. I., Wisniewski, H. M., and Diringer, H. (1987) J. Virol. 61 3688–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langeveld J. P., Jacobs, J. G., Erkens, J. H., Bossers, A., van Zijderveld, F. G., and van Keulen, L. J. (2006) BMC Vet. Res. 2 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feraudet, C., Morel, N., Simon, S., Volland, H., Frobert, Y., Creminon, C., Villette, D., Lehmann, S., and Grassi, J. (2005) J. Biol. Chem. 280 11247–11268 [DOI] [PubMed] [Google Scholar]
- 41.Bolton, D. C., Bendheim, P. E., Marmorstein, A. D., and Potempska, A. (1987) Arch. Biochem. Biophys. 258 579–590 [DOI] [PubMed] [Google Scholar]
- 42.Zou, W. Q., Colucci, M., Gambetti, P., and Chen, S. G. (2002) in Neurogenetics: Methods and Protocols (Methods in Molecular Biology) (Potter, N. T., ed) pp. 305–314, Humana Press Inc., Totowa, NJ
- 43.Borchelt, D. R., Rogers, M., Stahl, N., Telling, G., and Prusiner, S. B. (1993) Glycobiology 3 319–329 [DOI] [PubMed] [Google Scholar]
- 44.Chen, S. G., Teplow, D. B., Parchi, P., Gambetti, P., and Autilio-Gambetti, L. (1995) J. Biol. Chem. 270 19173–19180 [DOI] [PubMed] [Google Scholar]
- 45.Stahl, N., Baldwin, M. A., Burlingame, A. L., and Prusiner, S. B. (1990) Biochemistry 29 8879–8884 [DOI] [PubMed] [Google Scholar]
- 46.Parkin, E. T., Watt, N. T., Turner, A. J., and Hooper, N. M. (2004) J. Biol. Chem. 279 11170–11178 [DOI] [PubMed] [Google Scholar]
- 47.Chesebro, B., Trifilo, M., Race, R., Meade-White, K., Teng, C., LaCasse, R., Raymond, L., Favara, C., Baron, G., Priola, S., Caughey, B., Masliah, E., and Oldstone, M. (2005) Science 308 1435–1439 [DOI] [PubMed] [Google Scholar]
- 48.Kocisko, D. A., Come, J. H., Priola, S. A., Chesebro, B., Raymond, G. J., Lansbury, P. T., and Caughey, B. (1994) Nature 370 471–474 [DOI] [PubMed] [Google Scholar]
- 49.Lawson, V. A., Priola, S. A., Wehrly, K., and Chesebro, B. (2001) J. Biol. Chem. 276 35265–35271 [DOI] [PubMed] [Google Scholar]
- 50.Caughey, B., and Raymond, G. J. (1991) J. Biol. Chem. 266 18217–18223 [PubMed] [Google Scholar]
- 51.Jacobs, J. G., Langeveld, J. P. M., Biacabe, A.-G., Acutis, P.-L., Polak, M. P., Gavier-Widen, D., Buschmann, A., Caramelli, M., Casalone, C., Mazza, M., Groschup, M., Erkens, J. H. F., Davidse, A., van Zijderveld, F. G., and Baron, T. (2007) J. Clin Microbiol. 45 1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buschmann, A., Kuczius, T., Bodemer, W., and Groschup, M. H. (1998) Biochem. Biophys. Res. Commun. 253 693–702 [DOI] [PubMed] [Google Scholar]
- 53.Capellari, S., Parchi, P., Russo, C. M., Sanford, J., Sy, M. S., Gambetti, P., and Petersen, R. P. (2000) Am. J. Pathol. 157 613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan, J., Xiao, X., McGeehan, J., Dong, Z., Cali, I., Fujioka, H., Kong, Q., Kneale, G., Gambetti, P., and Zou, W. Q. (2006) J. Biol. Chem. 28 34848–34858 [DOI] [PubMed] [Google Scholar]