Abstract
Although neutrophils are known to migrate in response to various chemokines and complement factors, the substances involved in the early stages of their transmigration and activation have been poorly characterized to date. Here we report the discovery of a peptide isolated from healthy porcine hearts that activated neutrophils. Its primary structure is H-Leu-Ser-Phe-Leu-Ile-Pro-Ala-Gly-Trp-Val-Leu-Ser-His-Leu-Asp-His-Tyr-Lys-Arg-Ser-Ser-Ala-Ala-OH, and it was indicated to originate from mitochondrial cytochrome c oxidase subunit VIII. This peptide caused chemotaxis at concentrations lower than that inducing β-hexosaminidase release. Such responses were observed in neutrophilic/granulocytic differentiated HL-60 cells but not in undifferentiated cells, and Gi2-type G proteins were suggested to be involved in the peptide signaling. Moreover the peptide activated human neutrophils to induce β-hexosaminidase secretion. A number of other amphipathic neutrophil-activating peptides presumably originating from mitochondrial proteins were also found. The present results suggest that neutrophils monitor such amphipathic peptides including the identified peptide as an initiation signal for inflammation at injury sites.
Neutrophils are a type of leukocyte involved in the innate defense system. Once tissue injury occurs because of an infection or toxic cell debris resulting from cell necrosis, these cells migrate from the bloodstream to the injury sites. They are then activated to produce superoxide and digestive enzymes and to phagocytose toxic cell debris and the infectious microorganisms (1, 2). Chemokines, such as interleukin 8, are produced at tissue injury sites where they induce the migration and activation of neutrophils (3). Most of these chemokines are synthesized after the inflammatory stimuli, suggesting that they might not be the substances promoting the initial migration and activation of neutrophils because the transmigration of these cells is often observed immediately after tissue injury. Thus, it is likely that there are neutrophil-activating substances inducing the initial phase of migration. Some of these substances may be present in mitochondria because the contents of disrupted mitochondria, which are thought to be released immediately after necrosis in damaged tissues, promote neutrophilic migration (4). The responsible proteins or peptides, however, have not yet been purified. In the present study, we purified and identified a novel class of neutrophil-activating peptides from healthy porcine hearts that activate primary human neutrophils and neutrophilic/granulocytic differentiated HL-60 cells (HL-60-derived neutrophilic/granulocytic cells).5
EXPERIMENTAL PROCEDURES
Peptides that activated HL-60-derived neutrophilic/granulocytic cells were purified from healthy porcine hearts (outlined in Fig. 1) by monitoring the activity of fractions that induce β-hexosaminidase release from the cells. This enzyme can be easily assayed and is known to be quantitatively released from HL-60-derived neutrophilic/granulocytic cells upon stimulation by well known neutrophil-activating factors such as complement 5a (C5a) and formyl-methionyl-leucyl-phenylalanine (fMLF) (5, 6).
FIGURE 1.
Purification procedures for the neutrophil-activating peptides.
Preparation of Crude Extract—Immediately after the animals were sacrificed, porcine hearts (about 2.0 kg for each treatment) were dissected, bled out, and washed quickly with ice-cold physiological saline. The hearts were then cut into ∼5-mm-thick slices, and pieces of the sliced tissue were boiled in 16 liters of ion-exchanged water at 100 °C for 10 min. The boiled tissue and supernatant were cooled down to room temperature and homogenized in 1 m acetic acid (AcOH) containing 20 mm HCl for 10 min using a whirling blender. After adding 4 liters of 1 m AcOH containing 20 mm HCl to the homogenate, peptides were extracted by stirring at 4 °C for 18 h. The homogenate was centrifuged at 20,000 × g for 10 min at 4 °C, and the supernatant containing various peptides was concentrated to 2.5 liters by evaporation. Then 3.75 liters of ice-cold acetone was added to the concentrated supernatant, and the mixture was stirred at 4 °C for 20 h. Following centrifugation (20,000 × g for 10 min at 4 °C), the supernatant was concentrated by evaporation. The concentrated supernatant was washed twice with diethyl ether, concentrated to 1 liter by evaporation, and recentrifuged (20,000 × g for 30 min at 4 °C) to remove insoluble materials. The supernatant was then concentrated and lyophilized, producing a crude extract powder.
Purification of Active Peptides—The crude extract powder was dissolved in 300 ml of 1 m AcOH and loaded onto a column of SP-Sephadex C-25 cation-exchange resin (7 × 37.5 cm; GE Healthcare), which separated the sample into three fractions. Fraction A was eluted with 1 m AcOH; fraction P was eluted with 2 m pyridine, and fraction PA was eluted with 2 m pyridine-AcOH (pH 5.0). These fractions were concentrated, lyophilized, and stored at -80 °C.
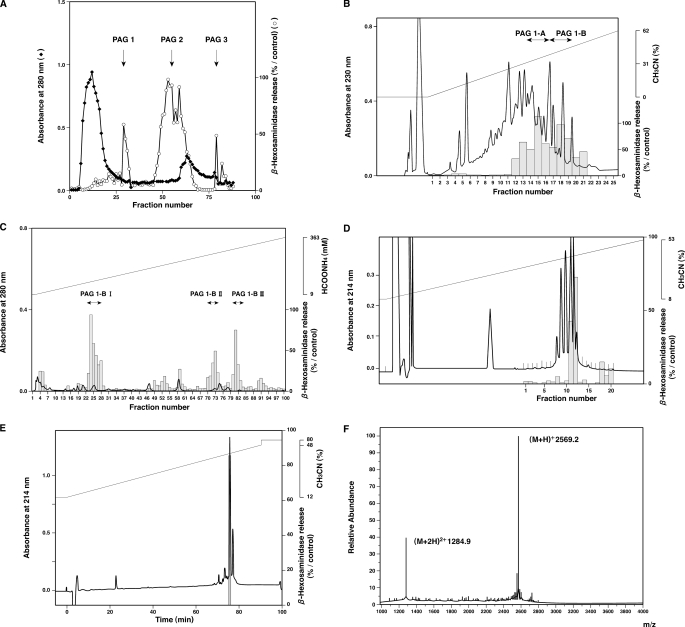
Among the three fractions, fraction PA was dissolved in 80 ml of 1 m AcOH, subjected to gel filtration chromatography on a Sephadex G-25 column (4 × 146 cm; GE Healthcare), and eluted with 1 m AcOH at a flow rate of 0.4 ml/min. Every 10 ml of eluate was collected using a fraction collector (GE Healthcare). The ability of each fraction to stimulate β-hexosaminidase secretion from the HL-60-derived neutrophilic/granulocytic cells was measured as described below. Thereafter the fractions were combined into three fractions according to the observed activity (PAG1, PAG2, and PAG3; Fig. 2A), and then the three fractions were lyophilized and stored at -80 °C. The procedures described above were repeated six times (30 hearts weighing ∼12 kg in total).
FIGURE 2.
Purification and identification of MCT-1. The substances that induced β-hexosaminidase release from HL-60-derived neutrophilic/granulocytic cells were fractionated and purified by gel filtration chromatography using a Sephadex G-25 column (A), preparative RP-HPLC using an ODS column (B), ion-exchange HPLC using a cation-exchange column (C), and analytical RP-HPLC using a C18 column (D). The ability of each fraction to induce β-hexosaminidase release from the differentiated HL-60 cells was tested, and the activity in each fraction is denoted with a gray column. Fractions containing the activity were combined as indicated in each panel. In E, the active 12th fraction in D was further purified and analyzed by micro-RP-HPLC using a C2/C18 column, and each peak fraction was collected separately. Only one of the fractions was able to induce enzyme release. The substances in this peak fraction (PAG1-BII(F12) peak) were subjected to fast atom bombardment mass spectrometry, which demonstrated a [M + H]+ of 2569.2 (F).
The active PAG1 fraction was subsequently purified by preparative reverse-phase high performance liquid chromatography (RP-HPLC) on an octadecylsilane (ODS) column (20 × 250 mm; yMc, Kyoto, Japan) using a linear gradient of acetonitrile at a flow rate of 5 ml/min in the presence of 0.1% trifluoroacetic acid; the elution profile of the PAG1 fraction was monitored by measuring the absorbance at 230 nm (Fig. 2B). The fractions exhibiting the activity were eluted from the preparative ODS column at acetonitrile concentrations of 40–46% and were then combined as the PAG1-B fraction and concentrated. PAG1-B was fractionated using HPLC on a TSK-CM2SW cation-exchange column (4.6 × 250 mm; Tosoh, Tokyo, Japan) with a linear gradient of ammonium formate (pH 6.6) in the presence of 10% acetonitrile at a flow rate of 1 ml/min, and the elution profile was monitored using the absorbance at 280 nm (Fig. 2C). One-milliliter fractions were collected, and the fractions exhibiting the activity were eluted with 254–271 mm ammonium formate and combined as the PAG1-BII fraction. Then the PAG1-BII fraction was purified using RP-HPLC on an analytical C18 column (4.6 × 250 mm; Vydac, Columbia, MD) with a linear gradient of acetonitrile in the presence of 0.1% trifluoroacetic acid. The peptides were eluted at a flow rate of 1 ml/min, and the elution profile was monitored using the absorbance at 214 nm (Fig. 2D). Each fraction (1 ml) was collected and lyophilized, and the 12th fraction (PAG1-BII(F12)), which exhibited the activity and was eluted at an acetonitrile concentration of 33%, was dissolved in 200 μl of distilled water. This fraction was further purified using micro-RP-HPLC on a C2/C18 column (2.1 × 100 mm; μRPC C2/C18 SC 2.1/10, GE Healthcare) with a linear gradient of acetonitrile in the presence of 0.1% trifluoroacetic acid. The peptides were eluted at a flow rate of 100 μl/min, and the main peak fraction (PAG1-BII(F12) peak) eluted at an acetonitrile concentration of 40% was collected and lyophilized (Fig. 2E). The PAG1-BII(F12) peak fraction, which was confirmed to have the ability to induce β-hexosaminidase release from the HL-60-derived neutrophilic/granulocytic cells, was subjected to structural analyses.
Structural Analyses—The molecular weight of the substance in the purified active PAG1-BII(F12) peak fraction was measured using fast atom bombardment mass spectrometry (Jeol JMX-HX/HX10A, Jeol, Tokyo, Japan) (7). The amino acid sequence was determined by the Edman method using a primary amino acid sequence analyzer (PPSQ-10, Shimadzu, Kyoto, Japan) (8).
Peptide Synthesis—To confirm the identity of the purified peptide and to investigate its effects on HL-60 cells and neutrophils, the peptide and its human homologue were chemically synthesized using the t-butyloxycarbonyl method as described previously (9, 10). The homogeneity and purity of these synthetic peptides were proven to be >95% by analytical RP-HPLC using a C18 column (Develosil ODS-HG5, 4.6 × 150 mm, Nomura Chemicals, Aichi, Japan) and matrix-assisted laser desorption ionization time-of-flight mass spectrometry.
Preparation of Neutrophilic/Granulocytic Cells—HL-60 cells, a cell line derived from human acute leukemia cells (11), were obtained from the Riken Cell Bank (Tsukuba, Japan). Cells were cultured in RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum (HyClone, Logan, UT) at 5% CO2 and 37 °C in a humidified atmosphere. To differentiate HL-60 cells into neutrophilic/granulocytic cells, they were treated with 500 μm dbcAMP (Sigma-Aldrich) for 72 h unless otherwise specified (5).
Preparation of Human Neutrophils—Human neutrophils were obtained from healthy volunteers as described previously (12). Briefly 10 ml of peripheral blood containing 500 units of heparin sodium was divided into two aliquots, and each of the 5-ml blood samples was layered onto 5 ml of Polymorphprep (Axis-Shield Poc AS, Oslo, Norway) in a 15-ml conical tube. The sample tubes were then centrifuged at 500 × g for 30 min at 4 °C. After centrifugation, neutrophils were harvested and transferred to a fresh 15-ml conical tube. The purity of the neutrophil preparation was >98% as determined using Randolph's stain.
Stimulation of Cells—To stimulate the cells with the purified fractions or synthetic peptides, HL-60 cells (undifferentiated or differentiated) or human neutrophils were washed three times with ice-cold HEPES-buffered Hanks' solution containing 0.1% bovine serum albumin (HBHS; 10 mm HEPES, 136.9 mm NaCl, 5.4 mm KCl, 1.2 mm CaCl2, 0.44 mm KH2PO4, 0.49 mm MgCl2, 0.41 mm MgSO4, 0.34 mm Na2HPO4, 5.5 mm glucose, and 4.2 mm NaHCO3 at pH 7.4) and suspended in HBHS at a density of 5.5 × 106 cells/ml. Cytochalasin B and DNase I were then added, each to a final concentration of 5 μg/ml. Each cell suspension (90 μl) was transferred to a tube (5 × 105 cells/tube) and placed on ice until stimulation. Each tube was preincubated at 37 °C for 10 min, and the fractions from the purification procedures or the synthetic peptide solutions (10 μl) were added to each tube. The samples were incubated at 37 °C for 10 min to stimulate the cells. Ten microliters of 0.5% Triton X-100 solution (final concentration, 0.05%) or distilled water was also added to the cell suspensions to estimate the total enzyme content or to prepare the negative control, respectively. Immediately after the 10-min incubation, 200 μl of ice-cold reaction quenching buffer (25 mm Tris, 123 mm NaCl, and 2.7 mm KCl at pH 7.4) was added to the cell suspensions to stop the stimulation. Then the tubes were centrifuged for 60 s at 4 °C at 5000 rpm, and the activities of β-hexosaminidase and lactate dehydrogenase in the supernatant were measured as described below.
Measurement of β-Hexosaminidase Activity—β-Hexosaminidase activity was measured as described previously (13). In brief, 90 μl of the cell-free supernatant obtained as described above was transferred to a 96-well Nunc-Immuno plate (Nunc, Roskilde, Denmark), and 60 μl of a β-hexosaminidase substrate solution (10 mm p-nitrophenyl-N-acetyl-β-d-glucosaminide (Sigma-Aldrich) in 40 mm citrate and 70 mm NaHPO4 at pH 4.5) was added to initiate the enzymatic reaction. After incubating the plate at 37 °C for 1 h, 100 μl of reaction quencher (400 mm glycine at pH 10.7) was added to stop the reaction, and the absorbance for each well was determined at 415 (sample) and 490 nm (reference). The β-hexosaminidase enzyme activity in the cell supernatant was expressed either as the percentage of the total enzyme activity released upon lysis of the cells with 0.05% Triton X-100 (percentage of total) or as the percentage of the secretion activity induced by treatment with 10 μm fMLF (percentage of positive control).
Measurement of Lactate Dehydrogenase Activity—To examine possible cell damage induced by the purified fractions or synthetic peptides, lactate dehydrogenase activity that leaked from the cells was also measured as described previously (13). In brief, the reaction was initiated by adding 400 μl of a substrate buffer solution (125 mm 2-amino-2-methyl-1-propanol, 125 mm lithium lactate, and 6.25 mm NAD at pH 9.5) to a tube containing 100 μl of the cell supernatant. Following a 1-h incubation at 37 °C, the reaction was quenched by cooling on ice, and the absorbance at 340 nm was measured. The enzyme activity of lactate dehydrogenase that leaked from the cells was calculated as the percentage of the total enzyme activity in the cells released upon disruption of the cells with 0.05% Triton X-100.
Measurement of Chemotactic Activity—Differentiated or undifferentiated HL-60 cells were washed three times with ice-cold HBHS, and a cell suspension was prepared at a density of 4 × 106 cells/ml. The cell suspension was preincubated at 37 °C for 10 min, and 500 μl was transferred to a Chemotaxicell chamber (2 × 106 cells/chamber; pore size, 3 μm; Kurabo, Osaka, Japan). The chambers were placed on a 24-well microplate filled with 1 ml of preheated (37 °C) HBHS containing the peptides and incubated further for 1 h at 37 °C. The Chemotaxicell chambers were then removed from the wells, and the number of cells migrating into each of the lower chambers was counted. Migration activity was expressed using a chemotaxis index, the number of cells migrating upon stimulation divided by the number of cells migrating without stimulation.
Measurement of Concentration of Intracellular Free Ca2+—To measure changes in the concentration of intracellular free Ca2+ ([Ca2+]i), differentiated or undifferentiated HL-60 cells were washed twice with a HEPES-Na solution (5 mm HEPES, 140 mm NaCl, 4 mm KCl, 1 mm NaH2PO4, 1 mm MgCl2, 1.25 mm CaCl2, 11 mm glucose, and 0.2% bovine serum albumin at pH 7.4). Then the Ca2+-sensitive fluorescence reagent fura-2/AM (Molecular Probes) was added to the cell suspension (4 ml to a final concentration of 4 μm). The reaction mixture was then shielded from light and shaken gently at room temperature for 60 min to load fura-2 into the HL-60 cells. Subsequently the cells were washed twice with the HEPES-Na solution, and a cell suspension was diluted to a final density of 1.0 × 106 cells/ml. One milliliter of the cell suspension was placed into a cuvette and stimulated with various samples with stirring at 30 °C. Using excitation wavelengths of 340 and 380 nm, the ratio of fluorescence intensity at 500 nm was measured with a fluorometer (CAF-100, Japan Spectroscopic Co., Tokyo, Japan). [Ca2+]i was then calculated as described previously (13, 14).
Treatment of Cells with Pertussis Toxin—To examine the inhibitory effects of pertussis toxin (PTX), cells were preincubated in 50 ng/ml PTX (List Biological Laboratories, Campbell, CA) at 37 °C for 16 h, and β-hexosaminidase release, migration activity, and changes in [Ca2+]i were measured in the PTX-treated or control cells.
Western Blot Analysis—The presence of Gαq/11- or Gαi2-type GTP-binding regulatory proteins (G proteins) in the cell lysates of differentiated or undifferentiated HL-60 cells was analyzed on Western blots using specific antibodies. Differentiated or undifferentiated HL-60 cells were washed twice with phosphate-buffered saline and suspended in a lysis buffer (50 mm HEPES, 150 mm NaCl, 5 mm MgCl2, 0.1% SDS, 0.5% deoxycholate, and 1% Nonidet P-40 at pH 7.5) containing protease inhibitors (1 μg/ml pepstatin A, 1 μg/ml phosphoramidon, and 0.2 mm phenylmethanesulfonyl fluoride) at a cell density of 1 × 108 cells/ml. The cell suspension was then placed on ice for 30 min and centrifuged (20,000 × g for 10 min at 4 °C). The resultant supernatant was transferred to a fresh tube and centrifuged under the same conditions. The obtained supernatant was then subjected to Western blot analysis after SDS gel electrophoresis. The Gαq/11- and Gαi2-type G proteins were visualized using anti-Gαq/11 antibodies (SA-232, BioMol, Plymouth Meeting, PA) and anti-Gαi2 antibodies (MS-244, LabVision, Fremont, CA), respectively.
RESULTS
Purification and Identification of Active Peptides—Previous reports have shown that proteins are fragmented into peptides during various extraction steps and that such fragmentation can be prevented by the immediate boiling of the tissues to inactivate various proteases (15). In fact, various physiologically functional peptides including atrial natriuretic polypeptide and ghrelin were successfully purified and identified by utilizing this methodology (16, 17). In addition, most peptides, particularly linear ones, are quite stable during the boiling treatment in distilled water.6 Thus, we boiled the porcine hearts immediately before the peptidergic substances were extracted.
Peptides activating HL-60-derived neutrophilic/granulocytic cells were purified from healthy porcine hearts (Figs. 1 and 2) by monitoring the activity of the fractions inducing β-hexosaminidase release from the cells. The structure of the active substance purified from porcine hearts (PAG1-BII(F12) peak fraction; Fig. 2E) was analyzed by fast atom bombardment mass spectrometry and automated Edman degradation (7, 8). The determined amino acid sequence (Leu-Ser-Phe-Leu-Ile-Pro-Ala-Gly-Trp-Val-Leu-Ser-His-Leu-Asp-His-Tyr-Lys-Arg-Ser-Ser-Ala-Ala; Fig. 3) coincided with that of the C-terminal 23 residues of porcine cytochrome c oxidase subunit VIII (GenomeNet Database, COX8H protein (Sus scrofa), accession number NP001090969). Its molecular mass of 2568.2 Da ([M + H]+, 2569.2; Fig. 2F) indicated that the peptide had a free carboxyl group at its C terminus. We chemically synthesized this tricosapeptide and compared its retention on analytical RP-HPLC using a C18 column with that of the purified one. Because the two peptide preparations exhibited identical retention times, the major content of the purified fraction was confirmed to be the peptide having the determined structure. We named this peptide “mitocryptide-1 (MCT-1)” because it was considered to be a peptide hidden within mitochondrial protein cytochrome c oxidase subunit VIII, and such cryptic peptides hidden in protein sequences were previously named as “cryptides” by us (18), and other groups also used the same word after our designation (19, 20). The porcine MCT-1 was 57% homologous to the C-terminal portion of human cytochrome c oxidase subunit VIII (12 of the 21 residues were identical; Fig. 3).
FIGURE 3.
Primary structures of MCT-1 and its human homologue hMCT-1.
Biological Functions of MCT-1—To confirm that MCT-1 is the active compound that induced β-hexosaminidase release from the HL-60-derived neutrophilic/granulocytic cells, we chemically synthesized MCT-1 (purity, >95%) and compared its ability to induce β-hexosaminidase release with that of the purified MCT-1. Purified MCT-1 at ∼4 × 10-7 m (estimated from the amount of the first amino acid Leu recovered in the Edman analysis) induced 84% secretion relative to the positive control (Fig. 2E). Comparably synthetic MCT-1 at 1 × 10-6 m caused 84.8 ± 0.5% secretion of the positive control. Because the peptide concentration estimated from Edman analysis is often lower than that obtained from amino acid analysis after acid hydrolysis (usually less than half in our experience), these results indicate that the purified peptide is equipotent as the synthesized peptide. Moreover synthetic MCT-1 induced β-hexosaminidase release from the cells in a concentration-dependent manner (EC50, 2.7 × 10-7 m). The β-hexosaminidase release induced by MCT-1, however, may have been caused by the disruption or lysis of the cells. To confirm this possibility, the leakage of the cytosolic protein lactate dehydrogenase from the cells was measured as an indicator of cell disruption (13, 21). MCT-1 did not induce a significant level of lactate dehydrogenase release from the differentiated or undifferentiated HL-60 cells at 3 × 10-4 m, demonstrating that MCT-1 is the molecule in the PAG1-BII(F12) peak fraction that induced cell activation.
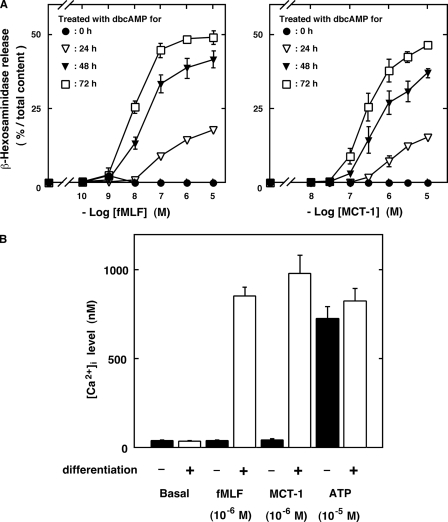
To further characterize the biological actions of MCT-1 and to determine whether it induces neutrophil-specific functions, we analyzed its effects on differentiated and undifferentiated HL-60 cells and compared the effects with those of fMLF, a bacterial model peptide known to induce various effects on neutrophils (5, 6). As shown in Fig. 4, fMLF induced not only β-hexosaminidase release (Fig. 4A, left) but also an increase in [Ca2+]i (Fig. 4B) in the differentiated HL-60 cells but not in the undifferentiated cells. This phenomenon is in sharp contrast to that where ATP induced [Ca2+]i elevation both in the differentiated and undifferentiated HL-60 cells (Fig. 4B). Chemically synthesized MCT-1 also induced β-hexosaminidase release (Fig. 4A, right) and an increase in [Ca2+]i (Fig. 4B) in the differentiated HL-60 cells but not in the undifferentiated cells as did fMLF. The effects of fMLF are reported to be affected by the degree of differentiation; the activities were enhanced when the cells were treated for longer periods of time with dbcAMP (5). Such differentiation degree-dependent effects, confirmed for fMLF (Fig. 4A, left), were also observed for MCT-1 (Fig. 4A, right). These observations suggest that MCT-1 induces a specific set of functions in neutrophilic/granulocytic cells.
FIGURE 4.
Effects of MCT-1 on undifferentiated or differentiated HL-60 cells. A, β-hexosaminidase release upon stimulation with various concentrations of fMLF (left) or MCT-1 (right) from the neutrophilic/granulocytic differentiated HL-60 cells following treatment with 500 μm dbcAMP for 24 (open triangles), 48 (closed triangles), or 72 h (open squares). The effects of the peptides on the undifferentiated cells were also measured (filled circles). B, [Ca2+]i increase in undifferentiated (filled bars) and differentiated (treated with 500 μm dbcAMP for 72 h; open bars) HL-60 cells after stimulation with vehicle, fMLF, or MCT-1. The effect of ATP was also determined as the control known to be unaffected by differentiation. All data are expressed as mean ± S.E. of six independent experiments.
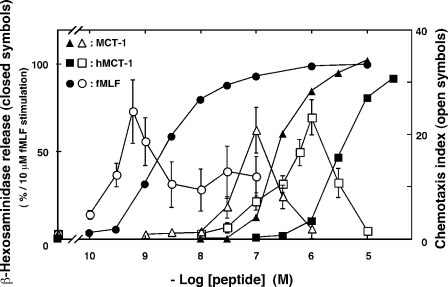
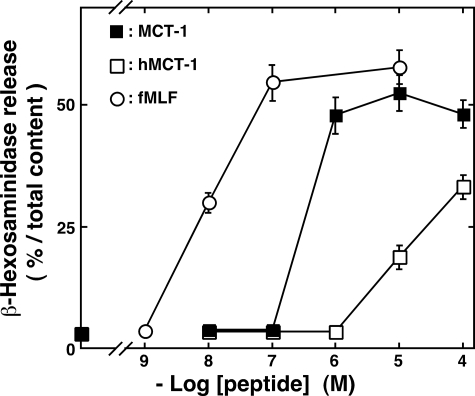
Similar to neutrophils, differentiated HL-60 cells are known to migrate in response to concentration gradients of chemoattractants, such as fMLF and C5a (1–3, 22). The concentration-dependent induction of β-hexosaminidase release and migration in differentiated HL-60 cells by MCT-1 was investigated and compared with the effects of stimulation with fMLF. MCT-1 induced the release of the enzyme and chemotaxis in concentration-dependent manners (Fig. 5; EC50 for β-hexosaminidase release, 2.7 × 10-7 m;EC50 for chemotaxis, 3.0 × 10-8 m) as was observed following stimulation with fMLF. Moreover a chemically synthesized human homologue of MCT-1 (hMCT-1), the primary structure of which is shown in Fig. 3, also induced β-hexosaminidase secretion and chemotaxis (EC50 for secretion, 3.5 × 10-6 m; EC50 for chemotaxis, 3.9 × 10-7 m). Notably these experiments showed that the peptide concentrations required to induce chemotaxis were about 3–10-fold lower than those needed to induce β-hexosaminidase release. These findings demonstrate that the differentiated HL-60 cells recognize the concentration of hMCT-1 as well as that of MCT-1 and that the different concentrations induce different responses.
FIGURE 5.
Effects of MCT-1 and its human homologue hMCT-1 on the stimulation of β-hexosaminidase release and chemotaxis on HL-60-derived neutrophilic/granulocytic cells. The abilities of fMLF (circles), MCT-1 (triangles), and hMCT-1 (squares) to induce β-hexosaminidase release (closed symbols) are expressed as the percentages of enzyme secretion induced by stimulation with 10 μm fMLF. Chemotactic activities (open symbols) stimulated by fMLF (circles), MCT-1 (triangles), and hMCT-1 (squares) are expressed using the chemotaxis index, which indicates the fold increase in the number of cells that migrated compared with the number obtained with the vehicle. All data are expressed as mean ± S.E. of six independent experiments.
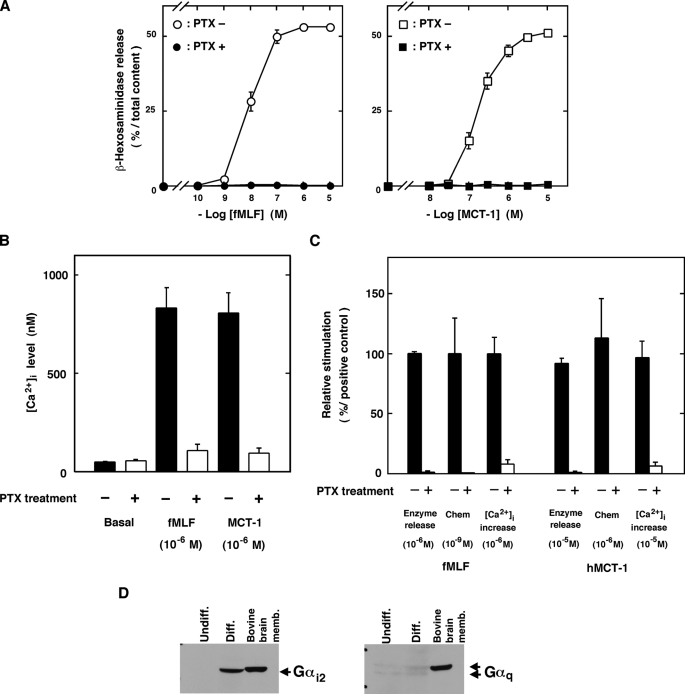
Mechanisms of Cell Activation— It is known that chemoattractant factors, such as fMLF, C5a, and interleukin 8, stimulate neutrophils via the activation of Gi-type G proteins because treatment of the cells with PTX, which induces ADP-ribosylation of the Gi proteins, specifically renders them insensitive to stimulations with these factors (22, 23). Therefore, we examined the effects of PTX on β-hexosaminidase release, chemotaxis, and increases in [Ca2+]i induced by MCT-1 or hMCT-1. As shown in Fig. 6, the effects of fMLF (Fig. 6, A, left, and B), MCT-1 (Fig. 6, A, right, and B), and hMCT-1 (Fig. 6C) were completely inhibited by the pretreatment with 50 ng/ml PTX for 16 h. These results indicate that MCT-1 exerts its effects in neutrophilic/granulocytic cells via the activation of Gi proteins.
FIGURE 6.
Involvement of Gi protein in MCT-1 signaling. Inhibitory effects of PTX on the stimulation with fMLF, MCT-1, and hMCT-1 (A–C) are shown. A, β-hexosaminidase release from cells treated with 50 ng/ml PTX for 16 h (closed symbols) or from control cells (open symbols) after stimulation with various concentration of fMLF (left, circles) or MCT-1 (right, squares). B, [Ca2+]i increase induced by fMLF or MCT-1 in cells treated with 50 ng/ml PTX for 16 h (open columns) or in control cells (filled columns). C, abilities of hMCT-1 and fMLF to induce β-hexosaminidase release, chemotaxis, and increases in [Ca2+]i were measured in cells treated with 50 ng/ml PTX for 16 h (open columns) or in control cells (filled columns). In C, the effects of fMLF and hMCT-1 on PTX-treated cells are expressed as percentages of those induced by fMLF in the control cells. All data are expressed as mean ± S.E. of five independent experiments. Chem, chemotaxis. D, the expression of Gαi2 (left) or Gαq (right) proteins in the undifferentiated (Undiff.) or differentiated (Diff.) cells was analyzed on Western blots using specific antibodies (see “Experimental Procedures”). As positive controls, Gαi2 and Gαq proteins in bovine brain membrane were also visualized using the same specific antibodies. memb., membrane.
Gi proteins are heterotrimeric G proteins consisting of α and βγ subunits. The α subunits are categorized into three subfamilies: Gαi1, Gαi2, and Gαi3 (24, 25). Because Gi proteins have been indicated in the cell signaling induced by MCT-1, the expression of the Gi1, Gi2, and Gi3 α subunit proteins in differentiated and undifferentiated HL-60 cells was investigated using Western blot analysis. Notably the Gαi2 proteins, which were not detected or were detected at very low levels in the undifferentiated cells, were highly expressed in the differentiated cells (Fig. 6D, left). In contrast, Gαq/11 proteins, known to be responsible for ligand-stimulated increases in [Ca2+]i in various cell types (26), were detected at similar very low levels in the undifferentiated and differentiated cells (Fig. 6D, right). In addition, Gαi3 proteins were also expressed at similar levels in the undifferentiated and differentiated cells, whereas Gαi1 proteins were not detected in either the undifferentiated or differentiated cells (data not shown). These findings together with the results that the activation of the cells by MCT-1 was completely prevented by PTX shown in Fig. 6, A–C, indicate that Gαi2 proteins are involved in the MCT-1 signaling in HL-60-derived neutrophilic/granulocytic cells.
Effects of MCT-1 on Neutrophils—Because MCT-1 and hMCT-1 were confirmed to activate neutrophilic/granulocytic-differentiated HL-60 cells, we examined the effects of these peptides and fMLF on the secretion of β-hexosaminidase in human neutrophils obtained from five different healthy volunteers. Both MCT-1 and hMCT-1 stimulated neutrophils isolated from the peripheral blood of all volunteers and induced enzyme secretion at concentrations above 1 × 10-7 and 1 × 10-6 m, respectively, as did fMLF (Fig. 7). These results demonstrate that MCT-1 and hMCT-1 also activate neutrophils.
FIGURE 7.
Effects of MCT-1 and hMCT-1 on the stimulation of β-hexosaminidase release in neutrophils from healthy volunteers. The secretion of the enzyme stimulated by various concentrations of MCT-1 (closed squares) and hMCT-1 (open squares) as well as fMLF (open circles) is expressed as the percentages of the total enzyme content of the cells. All data are expressed as mean ± S.E. of five independent experiments.
DISCUSSION
Mitocryptide-1 as a Neutrophil-activating Factor—Neutrophils are involved in innate immunity by monitoring infections and tissue damage and by scavenging for toxic debris at inflammatory sites (1, 2). They immediately infiltrate into the damaged areas from the bloodstream to remove toxic materials, although the mechanisms underlying the immediate transmigration and activation of neutrophils have yet to be fully elucidated. In the present study, we purified and identified a peptidergic substance that induces the activation of neutrophils and neutrophilic/granulocytic differentiated HL-60 cells, which share a number of features with neutrophils (5, 6, 22, 27–29). These observations suggest that this peptide induces immediate activation of neutrophils in vivo.
As a source of the neutrophil-activating substances, we selected healthy porcine hearts because it is known that ischemia reperfusion injuries causing severe tissue damage lead to a dramatic infiltration of neutrophils into the heart (30–32). A preliminary screening study to identify biologically active peptides from hearts revealed that there were a number of peptidergic substances that could activate neutrophils.6 Therefore, neutrophil-activating peptides were extracted and purified from porcine hearts, and the complete structure of one peptide was determined (Figs. 1, 2, 3). This peptide was designated as mitocryptide-1 (MCT-1) because it was considered a C-terminal tricosapeptide hidden in mitochondrial cytochrome c oxidase subunit VIII, and the designation was also based on our recent use of cryptides for naming functional peptides hidden in a protein sequence (18). hMCT-1 (Fig. 3) and MCT-1 were found to induce not only β-hexosaminidase release but also chemotaxis and an increase in [Ca2+]i in HL-60-derived neutrophilic/granulocytic cells. These activities of the peptides were observed only after the differentiation of HL-60 cells into neutrophilic/granulocytic cells (Fig. 4). Moreover MCT-1 actually stimulated neutrophils isolated from human peripheral blood to induce β-hexosaminidase release (Fig. 7). These findings demonstrate that MCT-1 is a biologically active peptide acting on neutrophils and neutrophilic/granulocytic cells.
Is MCT-1 present in intact heart tissues, or is it an artifact produced during the extraction or purification steps? To answer this question, we attempted to demonstrate the presence of MCT-1 in porcine heart tissue by immunohistochemistry. Unfortunately the antibody raised against MCT-1 cross-reacted with its parent protein, and we have not been able to demonstrate the presence of the peptide to date in situ. We also tried to confirm the presence of this peptide in purified human mitochondria of HeLa cells using the recently developed mass spectrometry analysis. The presence of many peptides was suggested; however, we still have not succeeded in identifying the homologue of this peptide. Hence we could not rule out the possibility that MCT-1 was produced during the extraction or purification steps. Nevertheless it is likely that this peptide is present even in healthy tissues for the following reasons. (i) Proteases were inactivated immediately after sacrificing the animals by boiling the heart tissues in water. This treatment was previously used in the successful identification of various peptidergic hormones and neurotransmitters such as atrial natriuretic polypeptide and ghrelin (16, 17). (ii) MCT-1 was reproducibly purified in three independent experiments. These findings strongly suggest that MCT-1 represents a novel class of endogenous activating factors that exert their effects on neutrophils.
Is MCT-1 present in the heart tissue at a concentration sufficient to induce neutrophil migration? Because neutrophils are known to transmigrate from the bloodstream to the injury sites by recognizing the concentration gradients of chemoattractants (1–3), the local concentrations of MCT-1 around the injured cells would be important for the induction of neutrophil migration. However, the local concentrations of peptides in particular sites of tissues are generally quite difficult to determine in contrast to those in the bloodstream. The question regarding the concentration of MCT-1 will be answered in future analyses including the inhibitory effects of antagonists or antibodies against MCT-1 on neutrophil infiltration into damaged tissues.
Presence of Various Unidentified Neutrophil-activating Peptides—In the present study, it has also been demonstrated that there were a number of peptidergic factors that can activate neutrophilic/granulocytic cells. There were also some fractions that induced β-hexosaminidase release from the differentiated HL-60 cells other than the fraction containing MCT-1 (Fig. 2). We are currently attempting to purify these peptidergic substances. At present, more than 10 peptides have been purified, and their partial structures have been determined. Although their complete structures are not yet known, we have found that most of the peptides are fragments of mitochondrial proteins with primary structures not related to MCT-1 but with common amphipathic characteristics. These observations suggest that mitochondria contain a number of functional peptides including MCT-1 capable of activating neutrophils. It has been reported that fractions containing disrupted mitochondria promote neutrophil migration in vitro, and synthetic N-terminal formylated fragments of mitochondrial NADH dehydrogenase induce β-hexosaminidase release (4, 33). The molecules from mitochondria inducing these activities, however, have not yet been isolated and identified. Mitochondrial peptides such as MCT-1 and other purified peptides may be the molecules responsible for the induction of these functions (4).
Signaling Mechanisms Induced by Mitocryptide-1—In the present study, Gi-type G proteins were found to participate in the signaling induced by MCT-1 because the stimulation of the cells was completely abolished by treatment with PTX, a toxin that selectively inactivates Gi-type G proteins (Fig. 6, A–C) (22, 23). Moreover we investigated the expression of subfamilies of Gi proteins in undifferentiated and differentiated HL-60 cells and found that differentiation into neutrophilic/granulocytic cells induced the expression of Gi2 proteins but not Gi1 or Gi3 proteins (Fig. 6D). Some chemoattractants, such as fMLF, C5a, and interleukin 8, have been shown to stimulate neutrophils via the activation of Gi proteins, although the responsible subfamilies have not yet been identified (22). The present results suggest that MCT-1 stimulates the cells by transmitting signals via the activation of Gi2 proteins.
What molecules serve as receptors for MCT-1 leading to the activation of Gi2 proteins? It has been demonstrated that Gi proteins can be directly activated by amphipathic peptides, such as mastoparan and substance P (34–39). MCT-1 also has amphipathic characteristics similar to those of mastoparan; i.e. they contain positively charged Lys and Arg residues and hydrophobic amino acid residues (e.g. Phe, Leu, and Trp) at positions that allow them to form amphiphilic structures. It is thus possible that the receptor molecules for MCT-1 are the Gi2 proteins themselves. We attempted to find the “receptor” of MCT-1 by cross-linking MCT-1 analogues having various cross-linker moieties with the proteins in HL-60-derived neutrophilic/granulocytic cells. To date, however, we have not succeeded in identifying the MCT-1 receptor. Further investigation is required to clarify the receptor molecules of MCT-1.
Probable Physiological Roles of Amphipathic Neutrophil-activating Peptides Including Mitocryptide-1—What are the physiological functions induced by MCT-1 in neutrophils? Neutrophils transmigrate from the bloodstream to inflammatory sites in response to concentration gradients of chemoattractant factors (1–3). The attracted neutrophils then start to perform various functions, such as phagocytosis, superoxide generation, and production of inflammatory cytokines. It has been reported that various adhesion molecules, such as integrins, participate in neutrophilic transmigration, whereas CXC chemokines (e.g. interleukin 8) and complement factors (e.g. C5a) induce both the migration and activation of neutrophils. The activation of neutrophils, however, is not completely inhibited by antagonists or neutralizing antibodies against chemokines (40, 41). Moreover neutrophilic transmigration is thought to occur rapidly. These observations suggest the presence of unknown neutrophil-activating substances. MCT-1 induced not only the migration of HL-60-derived neutrophilic/granulocytic cells but also β-hexosaminidase secretion from neutrophils, suggesting that MCT-1 may be one of these substances. Interestingly the migration activity promoted by MCT-1 was observed only at concentrations lower than those inducing β-hexosaminidase release (Fig. 5); this observation indicates that MCT-1 induces two different responses in neutrophilic/granulocytic cells depending on its concentration. Moreover the presence of various amphipathic neutrophil-activating peptides was indicated in the present study. These findings suggest the novel initiation mechanisms of inflammation in innate immunological mechanisms involving neutrophils and amphipathic neutrophil-activating peptides including MCT-1. It is expected that the comprehensive identification of such neutrophil-activating peptides will help elucidate the new roles of neutrophils. We are currently identifying such peptides comprehensively using a bioinformatics approach (18).
Acknowledgments
We thank K. Matsumoto, C. Obata, F. Saito, and J. Osuga for skillful and dedicated assistance in these experiments.
The conceptual idea of the present study was brought about through the discussion between the late Prof. T. Higashijima at the University of Texas Southwestern Medical Center at Dallas and H. M.; therefore, the authors are grateful to him and dedicate this article to him.
This study was supported by a research grant from the Special Research Project on the Circulation Biosystem, the University of Tsukuba, the Naito Foundation, Mitsubishi Chemical Corp., JT Inc., and Grant-in-aid for Scientific Research 06680605 from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HL-60-derived neutrophilic/granulocytic cells, neutrophilic/granulocytic differentiated HL-60 cells; C5a, complement 5a; fMLF, formyl-methionyl-leucyl-phenylalanine; AcOH, acetic acid; RP-HPLC, reverse-phase high performance liquid chromatography; ODS, octadecylsilane; dbcAMP, dibutyryl cyclic adenosine monophosphate; HBHS, HEPES-buffered Hanks' solution containing 0.1% bovine serum albumin; [Ca2+]i, intracellular concentration of free calcium; PTX, pertussis toxin; G protein, GTP-binding regulatory protein; MCT-1, mitocryptide-1; hMCT-1, human homologue of MCT-1.
H. Mukai, unpublished observation.
References
- 1.Springer, T. A. (1994) Cell 76 301-314 [DOI] [PubMed] [Google Scholar]
- 2.Ley, K. (1996) Cardiovasc. Res. 32 733-742 [PubMed] [Google Scholar]
- 3.Baggiolini, M., Dewald, B., and Moser, B. (1994) Adv. Immunol. 55 97-179 [PubMed] [Google Scholar]
- 4.Carp, H. (1982) J. Exp. Med. 155 264-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaplinski, T. J., and Niedel, J. E. (1982) J. Clin. Investig. 70 953-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dougherty, R. W., Godfrey, P. P., Hoyle, P. C., Putney, J. W., Jr., and Freer, R. J. (1984) Biochem. J. 222 307-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takao, T., Gonzalez, J., Yoshidome, K., Sato, K., Asada, T., Kammei, Y., and Shimonishi, Y. (1993) Anal. Chem. 65 2394-2399 [DOI] [PubMed] [Google Scholar]
- 8.Reim, D. F., and Speicher, D. W. (1997) Current Protocols in Protein Science, pp. 11.10.1-11.10.38, John Wiley and Sons, New York
- 9.Mukai, H., Kawai, K., Suzuki, Y., Yamashita, K., and Munekata, E. (1987) Am. J. Physiol. 252 E765-E771 [DOI] [PubMed] [Google Scholar]
- 10.Mukai, H., Kawai, K., Suzuki, S., Ohmori, H., Yamashita, K., and Munekata, E. (1989) Am. J. Physiol. 257 E235-E240 [DOI] [PubMed] [Google Scholar]
- 11.Collins, S. J., Gallo, R. C., and Gallagher, R. E. (1977) Nature 270 347-349 [DOI] [PubMed] [Google Scholar]
- 12.Kato, M., Kimura, H., Motegi, Y., Tachibana, A., Minakami, H., Morikawa, A., and Kita, H. (2002) J. Immunol. 169 5252-5259 [DOI] [PubMed] [Google Scholar]
- 13.Nakajima, T., Wakamatsu, K., and Mukai, H. (2000) Methods and Tools in Biosciences and Medicine. Animal Toxins, pp. 116-126, Birkhauser, Basel, Switzerland
- 14.Fukuhara, S., Mukai, H., Kako, K., Nakayama, K., and Munekata, E. (1996) J. Neurochem. 67 1282-1292 [DOI] [PubMed] [Google Scholar]
- 15.Kangawa, K., Minamino, N., Fukuda, A., and Matsuo, H. (1983) Biochem. Biophys. Res. Commun. 114 533-540 [DOI] [PubMed] [Google Scholar]
- 16.Kangawa, K., Tawaragi, Y., Oikawa, S., Mizuno, A., Sakuragawa, Y., Nakazato, H., Fukuda, A., Minamino, N., and Matsuo, H. (1984) Nature 312 152-155 [DOI] [PubMed] [Google Scholar]
- 17.Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., and Kangawa, K. (1999) Nature 402 656-660 [DOI] [PubMed] [Google Scholar]
- 18.Ueki, N., Someya, K., Matsuo, Y., Wakamatsu, K., and Mukai, H. (2007) Biopolymers 88 190-198 [DOI] [PubMed] [Google Scholar]
- 19.Pimenta, D. C., and Lebrun, I. (2007) Peptides 28 2403-2410 [DOI] [PubMed] [Google Scholar]
- 20.Nice, E. C. (2007) Expert Rev. Proteomics 4 705-707 [DOI] [PubMed] [Google Scholar]
- 21.Lagunoff, D., and Rickard, A. (1987) In Vitro Methods for Studying Secretion, pp. 13-28, Elsevier, Amsterdam
- 22.Klinker, J. F., Wenzel-Seifert, K., and Seifert, R. (1996) Gen. Pharamacol. 27 33-54 [DOI] [PubMed] [Google Scholar]
- 23.Oinuma, M., Katada, T., and Ui, M. (1987) J. Biol. Chem. 262 8347-8353 [PubMed] [Google Scholar]
- 24.Gilman, A. G. (1987) Annu. Rev. Biochem. 56 615-649 [DOI] [PubMed] [Google Scholar]
- 25.Kajiro, Y., Itoh, H., Kozasa, T., Nakafuku, M., and Satoh, T. (1991) Annu. Rev. Biochem. 60 349-400 [DOI] [PubMed] [Google Scholar]
- 26.Blank, J. L., Ross, A. H., and Exton, J. H. (1991) J. Biol. Chem. 266 18206-18216 [PubMed] [Google Scholar]
- 27.Murphy, P. M., and McDermott, D. (1991) J. Biol. Chem. 266 12560-12567 [PubMed] [Google Scholar]
- 28.Krautwurst, D., Seifert, R., Hescheler, J., and Schultz, G. (1992) Biochem. J. 288 1025-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, J., Wang, F., Keymeulen, A. V., Rentel, M., and Bourne, H. R. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 6884-6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romson, J. L., Hook, B. G., Kunkel, S. L., Abrams, G. D., Schork, M. A., and Lucchesi, B. R. (1983) Circulation 67 1016-1023 [DOI] [PubMed] [Google Scholar]
- 31.Korthuis, R. J., Grisham, M. B., and Granger, D. N. (1988) Am. J. Physiol. 254 H823-H827 [DOI] [PubMed] [Google Scholar]
- 32.Vinten-Johansen, J. (2004) Cardiovasc. Res. 61 481-497 [DOI] [PubMed] [Google Scholar]
- 33.Shawar, S. M., Rich, R. R., and Becker, E. L. (1995) Biochem. Biophys. Res. Commun. 211 812-818 [DOI] [PubMed] [Google Scholar]
- 34.Higashijima, T., Uzu, S., Nakajima, T., and Ross, E. M. (1988) J. Biol. Chem. 263 6491-6494 [PubMed] [Google Scholar]
- 35.Higashijima, T., Burnier, J., and Ross, E. M. (1990) J. Biol. Chem. 265 14176-14186 [PubMed] [Google Scholar]
- 36.Mukai, H., Munekata, E., and Higashijima, T. (1992) J. Biol. Chem. 267 16237-16243 [PubMed] [Google Scholar]
- 37.Vitale, N., Mukai, H., Rouot, B., Thierse, D., Aunis, D., and Bader, M.-F. (1993) J. Biol. Chem. 268 14715-14723 [PubMed] [Google Scholar]
- 38.Mousli, M., Bueb, J.-L., Bronner, C., Rouot, B., and Landry, Y. (1990) Trends Pharmacol. Sci. 11 358-362 [DOI] [PubMed] [Google Scholar]
- 39.Tanaka, T., Kohno, T., Kinoshita, S., Mukai, H., Itoh, H., Ohya, M., Miyazawa, T., Higashijima, T., and Wakamatsu, K. (1998) J. Biol. Chem. 273 3247-3252 [DOI] [PubMed] [Google Scholar]
- 40.Zogorski, J., and Wahl, S. M. (1997) J. Immunol. 159 1059-1062 [PubMed] [Google Scholar]
- 41.McColl, S. R., and Clark-Lewis, I. (1999) J. Immunol. 163 2829-2835 [PubMed] [Google Scholar]