Abstract
The AppA BLUF (blue light sensing using FAD) domain from Rhodobacter
sphaeroides serves as a blue light-sensing photoreceptor. The charge
separation process between Tyr-21 and flavin plays an important role in the
light signaling state by transforming the dark state conformation to the light
state one. By solving the linearized Poisson-Boltzmann equation, I calculated
Em for Tyr-21, flavin, and redox-active Trp-104 and
revealed the electron transfer (ET) driving energy. Rotation of the Gln-63
side chain that converts protein conformation from the dark state to the light
state is responsible for the decrease of 150 mV in Em for
Tyr-21, leading to the significantly larger ET driving energy in the light
state conformation. The pKa values of protonation for
flavin anions are essentially the same in both dark and light state crystal
structures. In contrast to the ET via Tyr-21, formation of the
W state results in generation
of only the dark state conformation (even if the initial conformation is in
the light state); this could explain why Trp-104-mediated ET deactivates the
light-sensing yield and why the activity of W104A mutant is similar to that of
the light-adapted native BLUF.
state results in generation
of only the dark state conformation (even if the initial conformation is in
the light state); this could explain why Trp-104-mediated ET deactivates the
light-sensing yield and why the activity of W104A mutant is similar to that of
the light-adapted native BLUF.
Sensing blue light is a prerequisite for organisms to maintain their
functions. BLUF2
domains serve as a photoreceptor by regulating the activity of covalently
attached effector domains (1).
The photoactivation of the BLUF domain from Rhodobactor sphaeroides
is mediated by a red shift intermediate state that mainly consists of FAD and
Tyr-21 (2). The electronic
excited FAD* state decays to the charge-separated
Y -W-
-W- state. Via proton transfer (PT) from
Y
state. Via proton transfer (PT) from
Y to
to
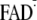 , the
Y
, the
Y -W-
-W-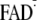 state transforms into the charge-neutral Y·-W-FADH· state and
finally decays to the ground state
(3). Recent spectroscopic
studies suggested that not only Tyr-21 but also Trp-104 act as electron donors
in the charge separation process, where
Tyr-21
state transforms into the charge-neutral Y·-W-FADH· state and
finally decays to the ground state
(3). Recent spectroscopic
studies suggested that not only Tyr-21 but also Trp-104 act as electron donors
in the charge separation process, where
Tyr-21 is the functionally
relevant charge state (i.e. the productive pathway in Ref.
4), whereas formation of
Trp-104
is the functionally
relevant charge state (i.e. the productive pathway in Ref.
4), whereas formation of
Trp-104 (i.e. the
nonproductive pathway in Ref.
4) does not contribute to light
sensing of AppA BLUF (4). Note
that although FAD is the cofactor in the native BLUF domain
(5), the BLUF fragment contains
a mixture of flavins (i.e. FMN (majority), FAD, and riboflavin) when
expressed in Escherichia coli
(6). In fact, FMN is identified
in the BLUF crystal structures from R. sphaeroides
(7,
8). Currently, there are
several points that need to be clarified for understanding the BLUF domain as
described below.
(i.e. the
nonproductive pathway in Ref.
4) does not contribute to light
sensing of AppA BLUF (4). Note
that although FAD is the cofactor in the native BLUF domain
(5), the BLUF fragment contains
a mixture of flavins (i.e. FMN (majority), FAD, and riboflavin) when
expressed in Escherichia coli
(6). In fact, FMN is identified
in the BLUF crystal structures from R. sphaeroides
(7,
8). Currently, there are
several points that need to be clarified for understanding the BLUF domain as
described below.
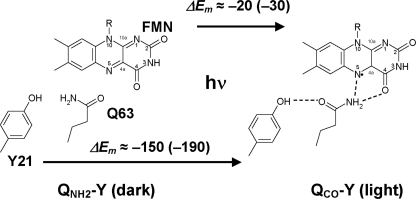
In the protein environment of the BLUF domain, the orientation of the key residue Gln-63 with respect to Tyr-21 is a matter of debate. From observations of the native BLUF crystal structure, Anderson et al. (7) proposed that the –NH2 group of the Gln-63 side chain forms a hydrogen bond with Tyr-21 in the dark state and that the –CO group of the Gln-63 side chain forms a hydrogen bond with Tyr-21 in the light state (Fig. 1). In contrast, from observations of the C20S mutant crystal structure, Jung et al. (8) assigned “dark” and “light” states that were opposite to those assigned by Anderson et al. (7). The majority of spectroscopic and mutational studies (9–12) suggested the same assignment of the dark and light structures as that proposed by Anderson et al. (7). In the present study, I tentatively follow the definition of Anderson et al. (7) if not otherwise specified (see Fig. 1 for the definition used in the present study).
FIGURE 1.
Changes in the orientation of the Gln-63 side chain from the dark state
structure to the light state structure. Associated changes in the ET
driving energy for the charge neutral [Y-W-FMN] (charge-separated
[Y -W-
-W-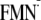 ]
state are indicated in meV units.
]
state are indicated in meV units.
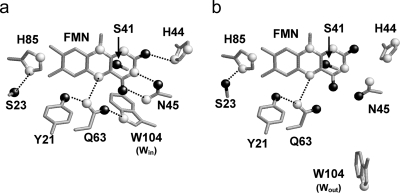
Another open question is the functionally relevant location of Trp-104 with
respect to the FMN binding site. In the crystal structure of the native BLUF
domain refined by Anderson et al.
(7), the indole nitrogen atom
of the Trp-104 side chain is at a hydrogen-bonding distance from the carbonyl
oxygen atom of the Gln-63 side chain (i.e. Win
conformation; see Fig.
2a). On the other hand, in the crystal structures of the
C20S mutant by Jung et al.
(8), Trp-104 is located on the
protein surface and exposed to the bulk solvent (i.e. Wout
conformation; see Fig.
2b). The Wout conformation is also identified
in the BLUF domain from Thermosynechococcus elongatus
(10). Jung et al.
(8) argued that the
Win conformation reported by Anderson et al.
(7) may be induced by the
presence of detergent molecules. However, the Win conformation can
be seen in most NMR solution structural models of the BLUF domain
(13). Recent spectroscopic
studies suggested that Q63L and W104A mutants are insensitive to blue light,
implying a functionally important role of a hydrogen bond between the two
residues (that should exist only in the Win conformation)
(14). It is known that
photoactivation of the BLUF domain induces the rotation of the Gln-63 side
chain (7). However, the driving
force of the Gln-63 rotation is yet unclear. It was proposed that proton
movements (namely the PT from
Y to the N5 atom of
to the N5 atom of
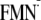 )
underlie the rotation from the observed kinetic isotope effects
(3).
)
underlie the rotation from the observed kinetic isotope effects
(3).
FIGURE 2.
Protein environment of the FMN binding site in the Win (7) (Protein Data Bank code 1YRX) (a) and Wout (8) (Protein Data Bank code 2IYI) (b) structures in the dark state. Nitrogen and oxygen atoms are depicted as white and black balls, respectively.
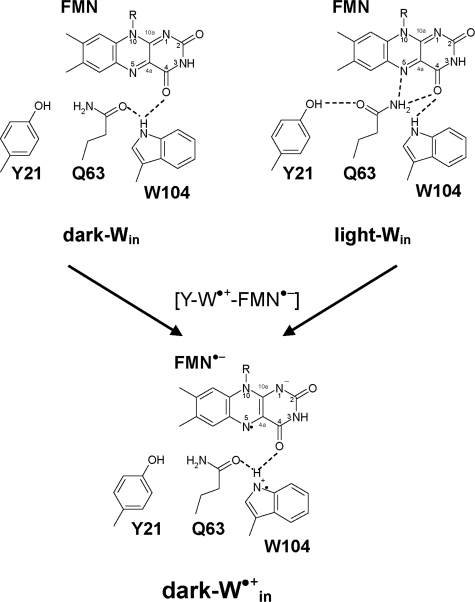
To clarify the functional relevance of these possible four conformers (i.e. dark-Wout and light-Wout (Protein Data Bank 2IYI and 2IYG, respectively) (Fig. 1) (8), dark-Win (Protein Data Bank 1YRX) (7), and light-Win (no corresponding Protein Data Bank entry; see Fig. 3)), the values of the ET driving energy for each protein conformer that are yet experimentally unavailable need to be clarified.
FIGURE 3.
Dark and light state conformations of Win
(7) in transition
from the charge-neutral [Y-W-FMN] state to the
[Y -W-
-W-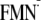 ]
state.
]
state.
Recently, I calculated the redox (midpoint) potential
(Em) of flavin
(15,
16) and redox-active tyrosine
(17) precisely by considering
the protonation states of all titratable sites in the proteins. In this study,
I present the (Em) of FMN for one-electron reduction
(Em(FMN/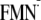 ),
protonated tyrosine for one-electron oxidation
(Em(Y/Y
),
protonated tyrosine for one-electron oxidation
(Em(Y/Y )),
and tryptophan for one-electron oxidation
(Em(W/W
)),
and tryptophan for one-electron oxidation
(Em(W/W ))
in the BLUF domain, by solving the linearized Poisson-Boltzmann equation for
all atoms in the crystal structures. From the calculated
Em, I obtain the driving energy of the ET in the charge
separation process (via Tyr-21 and Trp-104) of the known four BLUF conformers.
Then I evaluate (i) functional relevance of the conformers assigned by
Anderson et al. (7)
and Jung et al. (8),
(ii) difference of the ET energetics in the Win and Wout
structures, and (iii) why the charge separation process involving Trp-104 does
not lead to the light signaling state.
))
in the BLUF domain, by solving the linearized Poisson-Boltzmann equation for
all atoms in the crystal structures. From the calculated
Em, I obtain the driving energy of the ET in the charge
separation process (via Tyr-21 and Trp-104) of the known four BLUF conformers.
Then I evaluate (i) functional relevance of the conformers assigned by
Anderson et al. (7)
and Jung et al. (8),
(ii) difference of the ET energetics in the Win and Wout
structures, and (iii) why the charge separation process involving Trp-104 does
not lead to the light signaling state.
Since the dark and light state structures are originally available only for the Wout structure (8) (i.e. without performing the modeling of protein atomic coordinates), the computational results of the Wout structure (8) are mainly presented if not otherwise specified. In particular, the results of Wout and Win were essentially the same in the ET via Tyr-21 but not in the ET via Trp-104. In the present study, I used the same conditions sufficiently evaluated in previous studies on proteins that contain flavin (15, 16) and redox-active tyrosine (17).
THEORY
Atomic Coordinates and Charges—For performing computations of the BLUF domain, the crystal structures of the native BLUF domains by Anderson et al. (7) (Protein Data Bank code 1YRX) and the C20S mutant BLUF domains by Jung et al. (8) (Protein Data Bank codes 2IYI and 2IYG) were used. For the Wout crystal structure by Jung et al. (8), I replaced the original Sγ atom of the C20 side chain with an oxygen atom and used it as the native BLUF structure. In this study, the crystal structures with Protein Data Bank code 2IYI and 2IYG were regarded as dark and light state structures (i.e. dark-Wout and light-Wout), respectively (Fig. 1). This definition of the dark and light structures is the same as that proposed Anderson et al. (7, 9–12).
For the Win conformations, I used the crystal structure refined by Anderson et al. (7) as the dark state structure (i.e. dark-Win). I modeled the light-Win structure by rotating the side chain –NH2 and –CO groups of Gln-63 by 180° along the Cγ-Cδ axis (Fig. 3). The obtained atomic coordinates of the Gln-63 side chain for the light-Win structure in the chargeneutral [Y-W-FMN] state are shown in Table S1.
The positions of the hydrogen atoms were energetically optimized with CHARMM (18) by using the CHARMM22 force field. During this procedure, the positions of all nonhydrogen atoms were fixed, and the standard charge states of all of the titratable groups were maintained (i.e. basic and acidic groups were considered to be protonated and deprotonated, respectively). All of the other atoms whose coordinates were available in the crystal structure were not geometrically optimized. Note that the protein atomic coordinates obtained after the energy optimization process revealed that Tyr-21 is a hydrogen bond donor to the –NH2 and –CO groups of the Gln-63 side chain in the dark and light structures, respectively, in agreement with what was observed in recent density functional theory studies (19).
Atomic partial charges of the amino acids were adopted from the all atom
CHARMM22 (18) parameter set.
Atomic charges of the 5′-phosphate group of FMN quinone
(–H3PO3,
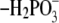 , and
, and
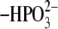 ) were adopted from those of
methylphosphate. The charges of FMN,
) were adopted from those of
methylphosphate. The charges of FMN,
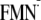 and
FMNH· were from a previous report on flavodoxin
(15). The atomic charges for
the redox-active tyrosine were adopted from Ref.
20 (protonated tyrosine with
neutral charge (Y) and protonated tyrosine radical with positive charge
Y
and
FMNH· were from a previous report on flavodoxin
(15). The atomic charges for
the redox-active tyrosine were adopted from Ref.
20 (protonated tyrosine with
neutral charge (Y) and protonated tyrosine radical with positive charge
Y in the redox pair
Y/Y
in the redox pair
Y/Y ). The atomic charges for
the redox-active tryptophan were adopted from Ref.
20 (protonated tryptophan with
neutral charge (W) and protonated tryptophan radical with positive charge
W
). The atomic charges for
the redox-active tryptophan were adopted from Ref.
20 (protonated tryptophan with
neutral charge (W) and protonated tryptophan radical with positive charge
W in the redox pair
W/W
in the redox pair
W/W ).
).
Protonation Pattern, Redox Potential, and pKa—The present computation is based on the electrostatic continuum model created by solving the linear Poisson-Boltzmann equation with the MEAD program (21). To facilitate a direct comparison with previous computational results, I uniformly used identical computational conditions and parameters, such as atomic partial charges and dielectric constants (e.g. see Refs. 15, 17, and 22). To obtain the absolute Em values of the protein, we calculated the electrostatic energy difference between the two redox states in a reference model system using a known experimental Em value. The difference in the Em value of the protein relative to the reference system was added to the known Em value (see below). All of the other titratable sites, including the 5′-phosphate group, were fully equilibrated to the redox state of FMN during the titration. The ensemble of the protonation patterns was sampled by the Monte Carlo method with Karlsberg.3 The dielectric constants were set to εp = 4 inside the protein and εw = 80 for water. All computations were performed at 300 K, pH 7.0, and an ionic strength of 100 mm. The linear Poisson-Boltzmann equation was solved using a three-step grid-focusing procedure at resolutions of 2.5, 1.0, and 0.3 Å. The Monte Carlo sampling yielded the probabilities [Aox] and [Ared] of the two redox states of molecule A. Em was evaluated using the Nernst equation. A bias potential was applied to obtain an equal amount of both redox states ([Aox] = [Ared]), thereby yielding the redox midpoint potential Em as the resulting bias potential. From this analogy, using the Henderson-Hasselbalch equation, pKa can be calculated as the pH at which the concentrations of the protonated and deprotonated residue species are equal (Henderson-Hasselbalch pKa). For convenience, the computed Em value was given with mV accuracy, without implying that the last digit was significant. In general, an Em value of ∼10 mV is a sufficiently reproducible range for the computational method used (e.g. see Refs. 15, 17, and 22).
pKa and Em Values in the Reference Model
System—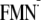 forms FMNH· upon protonation at the N5 nitrogen in FMN quinone
(Fig. 1). The value of 8.6
(23) was taken as the
pKa(N5) value in the reference model system of
forms FMNH· upon protonation at the N5 nitrogen in FMN quinone
(Fig. 1). The value of 8.6
(23) was taken as the
pKa(N5) value in the reference model system of
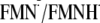 equilibrium in aqueous solution. The value of 6.4
(24) was considered as the
pKa value of the 5′-phosphate group of
equilibrium in aqueous solution. The value of 6.4
(24) was considered as the
pKa value of the 5′-phosphate group of
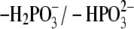 .
Note that the 5′-phosphate group was permanently deprotonated in the
.
Note that the 5′-phosphate group was permanently deprotonated in the
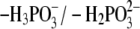 equilibrium (pKa = 1.4
(24)) in all of the crystal
structures that were investigated. Thus, in the present study, the
equilibrium (pKa = 1.4
(24)) in all of the crystal
structures that were investigated. Thus, in the present study, the
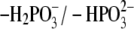 equilibrium (pKa = 6.4
(24)) was investigated, unless
otherwise specified. As a reference model system, the following values for
Em versus the normal hydrogen electrode were used:
Em(Y/Y
equilibrium (pKa = 6.4
(24)) was investigated, unless
otherwise specified. As a reference model system, the following values for
Em versus the normal hydrogen electrode were used:
Em(Y/Y ) =
+1380 mV (25) and
Em(W/W
) =
+1380 mV (25) and
Em(W/W ) =
+1070 mV (26) for one-electron
oxidation in an aqueous solution.
) =
+1070 mV (26) for one-electron
oxidation in an aqueous solution.
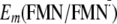 = -333 mV (15) was used as the
reference model system of
= -333 mV (15) was used as the
reference model system of
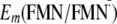 in an aqueous solution (see further discussion in the supplemental
materials).
in an aqueous solution (see further discussion in the supplemental
materials).
RESULTS AND DISCUSSION
Driving Energy in the ET Involving Tyr-21—By calculating
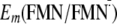 and
Em(Y/Y
and
Em(Y/Y ),
I obtained the driving energy (ΔG) of the ET between FMN and Y
to be -156 and -317 meV for the dark and light state structures in the
charge-separated
Y
),
I obtained the driving energy (ΔG) of the ET between FMN and Y
to be -156 and -317 meV for the dark and light state structures in the
charge-separated
Y -W-
-W-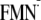 state, respectively (Table 1).
I found that the calculated ΔG in the light state structure
(-317 meV) is greater by 160 meV than that in the dark state structure (-156
meV; Table 1). This tendency
essentially holds true for the chargeneutral [Y-W-FMN] state. Since the light
state structure is energetically much favorable for the ET than the dark state
structure in the present study, the charge separation process via Tyr-21
([Y-W-FMN →
Y
state, respectively (Table 1).
I found that the calculated ΔG in the light state structure
(-317 meV) is greater by 160 meV than that in the dark state structure (-156
meV; Table 1). This tendency
essentially holds true for the chargeneutral [Y-W-FMN] state. Since the light
state structure is energetically much favorable for the ET than the dark state
structure in the present study, the charge separation process via Tyr-21
([Y-W-FMN →
Y -W-
-W-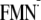 should complete with the light state structure
(Fig. 1).
should complete with the light state structure
(Fig. 1).
TABLE 1.
Redox potentials and ET driving energies in mV and meV units, respectively
| Structure | Darka-Woutb(2IYIc) | Lighta-Woutb(2IYGc) | Darka-Winb(1YRXd) | Lighta-Winb(1YRXd(modeled)e) |
|---|---|---|---|---|
Em(FMN/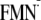 ) )
|
||||
| [Y-W-FMN]f | –564 | –587 | –556 | –549 |
[Y -W- -W-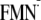 ]g ]g
|
–470 | –496 | –479 | –425 |
[Y-W - -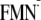 ]h ]h
|
–473 | –477 | –454 | NDi |
Em(Y/Y ) )
|
||||
| [Y-W-FMN]f | 1799 | 1645 | 1745 | 1683 |
[Y -W- -W-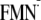 ]g ]g
|
1724 | 1537 | 1702 | 1549 |
Em(W/W ) )
|
||||
| [Y-W-FMN]f | 1145 | 1123 | 1334 | 1451 |
[Y-W - -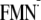 ]h ]h
|
1133 | 1116 | 1330 | ND |
| ΔG (ET via Tyr-21)j | ||||
| [Y-W-FMN]f | 13 | –118 | –49 | –118 |
[Y -W- -W-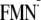 ]g ]g
|
–156 | –317 | –169 | –376 |
| ΔG (ET via Trp-104)j | ||||
| [Y-W-FMN]f | –641 | –640 | –460 | –350 |
[Y-W - -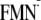 ]h ]h
|
–744 | –757 | –566 | ND |
Dark (light) state structure where the –NH2 (–CO) group of the Gln-63 side chain is a hydrogen-bonding partner of Tyr-21
Wout (Win) conformation where Trp-104 is in (out of) the FMN binding pocket
See Ref. 8
See Ref. 7
The –NH2 and –CO groups of Gln-63 were rotated by 180° along the Cγ-Cδ axis, and their positions were energetically optimized with CHARMM (18)
Em(FMN/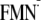 ),
Em(Y/Y
),
Em(Y/Y ),
and
Em(W/W
),
and
Em(W/W )
were calculated in the charge-neutral (Y-W and W-FMN) states, respectively, by
using the atomic coordinates of the ground [Y-W-FMN] conformer
)
were calculated in the charge-neutral (Y-W and W-FMN) states, respectively, by
using the atomic coordinates of the ground [Y-W-FMN] conformer
Em(FMN/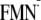 )
and
Em(Y/Y
)
and
Em(Y/Y )
were calculated in the charge-neutral (Y-W and W-FMN) states, respectively, by
using the atomic coordinates of the
[Y-W
)
were calculated in the charge-neutral (Y-W and W-FMN) states, respectively, by
using the atomic coordinates of the
[Y-W -
-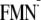 ]
conformer. Hydrogen atom positions are energetically optimized in the presence
of the
[Y-W
]
conformer. Hydrogen atom positions are energetically optimized in the presence
of the
[Y-W -
-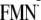 ]
charge state
]
charge state
Em(FMN/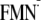 )
and
Em(W/W
)
and
Em(W/W )
were calculated in the charge-neutral Y-W and Y-FMN states, respectively, by
using the atomic coordinates of the
[Y-W
)
were calculated in the charge-neutral Y-W and Y-FMN states, respectively, by
using the atomic coordinates of the
[Y-W -
-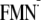 ]
conformer. Hydrogen atom positions are energetically optimized in the presence
of the
[Y-W
]
conformer. Hydrogen atom positions are energetically optimized in the presence
of the
[Y-W -
-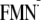 ]
charge state
]
charge state
ND, not determined
ΔG (ET via Tyr-21) =
Em(Y/Y )
–
Em(FMN/
)
–
Em(FMN/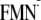 )
– 2350 (meV), and ΔG (ET via Trp-104) =
Em(W/W
)
– 2350 (meV), and ΔG (ET via Trp-104) =
Em(W/W )
–
Em(FMN/
)
–
Em(FMN/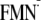 )
– 2350 (meV) (see, for instance, Ref.
3)
)
– 2350 (meV) (see, for instance, Ref.
3)
The revealed larger ET driving energy (i.e. energetically more exergonic) in the light state structure in the present study supports the validity of the assignment of the dark and light state conformations by Anderson et al. (7) and those suggested in spectroscopic and mutational studies (9–12).
Influence of Gln-63 on
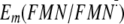 and
Em(Y/Y
and
Em(Y/Y )—I
investigated the influence of the BLUF protein environment on
)—I
investigated the influence of the BLUF protein environment on
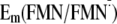 and
Em(Y/Y
and
Em(Y/Y ).
The different orientations of the –NH2 and –CO groups
of Gln-63 in the dark and light structures alter
Em(Y/Y
).
The different orientations of the –NH2 and –CO groups
of Gln-63 in the dark and light structures alter
Em(Y/Y )
by 230 mV (Table 2). Note that
this influence is partially compensated by associated changes in the
protonation states of titratable residues and the hydrogen atom coordinates of
some residues, resulting in the net Em shift of 154 mV
(see Table 1). The lower
Em(Y/Y
)
by 230 mV (Table 2). Note that
this influence is partially compensated by associated changes in the
protonation states of titratable residues and the hydrogen atom coordinates of
some residues, resulting in the net Em shift of 154 mV
(see Table 1). The lower
Em(Y/Y )
in the light state structure implies that obviously, the proximity of the
oxygen atom of the –CO group can stabilize the
Y
)
in the light state structure implies that obviously, the proximity of the
oxygen atom of the –CO group can stabilize the
Y state effectively. In the
dark state structure, the –CO group, in turn, destabilizes the
Y
state effectively. In the
dark state structure, the –CO group, in turn, destabilizes the
Y state by 67 mV because of
the proximity of its polar carbon atom to Tyr-21. On the other hand, the side
chain orientation of Gln-63 does not essentially affect
state by 67 mV because of
the proximity of its polar carbon atom to Tyr-21. On the other hand, the side
chain orientation of Gln-63 does not essentially affect
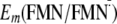 (Tables 1 and
3). Thus, the significant
change in ΔG by switching from the dark state conformation to
the light state conformation can be attributed predominantly to the change in
Em(Y/Y
(Tables 1 and
3). Thus, the significant
change in ΔG by switching from the dark state conformation to
the light state conformation can be attributed predominantly to the change in
Em(Y/Y )
and not that in
)
and not that in
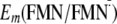 .
.
TABLE 2.
Influence of the protein environment on
Em(Y/Y )
for the [Y-W-FMN]
([Y
)
for the [Y-W-FMN]
([Y -W-
-W-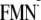 ])
conformation in mV
])
conformation in mV
Em(Y/Y ) )
|
||
|---|---|---|
| Darka-Woutb(2IYI)c | Lighta-Woutb(2IYG)c | |
| Redox potential | ||
| Em(p)d | 1799 | 1645 |
| Em(w)e | 1380 | 1380 |
| ΔEm(w→p)f | 419 | 265 |
| Protein element | ||
| Protein volumeg | 341 | 339 |
| Protein charge | 78 | –74 |
| ΔPhosphateh | –14 | –24 |
| ΔBackbone | 16 | 15 |
| ΔSide chain | 76 | –65 |
| Gln-63 | 102 (63) | –126 (–127) |
| –NH2 | 28 (–10) | –24 (–25) |
| –CO | 67 (66) | –110 (–110) |
Dark (light) state structure where the –NH2 (–CO) group of the Gln-63 side chain is a hydrogen-bonding partner of Tyr-21
Conformation where Trp-104 is out of the FMN binding pocket
See Ref. 8
Em(Y/Y )
in the BLUF domain
)
in the BLUF domain
Em(Y/Y )
in water (25)
)
in water (25)
Shift in
Em(Y/Y )
from water to protein
)
from water to protein
Influence of uncharged protein dielectric volume (i.e. the space covered by the merged van der Waals volumes of protein atoms)
Influence of the FMN 5′-phosphate group. Note that this group is absent in FAD
TABLE 3.
Influence of the protein environment on
Em(FMN/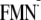 )
for the [Y-W-FMN]
([Y
)
for the [Y-W-FMN]
([Y -W-
-W-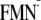 ])
conformation
])
conformation
Em(FMN/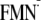 ) )
|
||
|---|---|---|
| Darka-Woutb(2IYI)c | Lighta-Woutb(2IYG)c | |
| Redox potential | ||
| Em(p)d | –564 | –587 |
| Em(w)e | –333 | –333 |
| ΔEm(w→p)f | –231 | –254 |
| Protein element | ||
| Protein volumeg | –207 | –209 |
| Protein charge | –24 | –45 |
| ΔPhosphateh | –56 | –101 |
| ΔBackbone | –20 | –41 |
| ΔSide chain | 52 | 97 |
| Tyr-21 | 8 | 2 |
| Ser-23 | –12 (–10) | –14 (–19) |
| Ser-41 | –72 (–19) | –74 (–4) |
| Asn-45 | –27 | –20 |
| –NH2 | 0 | 16 |
| –CO | –25 | –32 |
| Gln-63 | –2 | 0 |
| –NH2 | 26 | 28 |
| –CO | –27 | –26 |
Dark (light) state structure where the –NH2 (–CO) group of the Gln-63 side chain is a hydrogen-bonding partner of Tyr-21
Conformation where Trp-104 is out of the FMN binding pocket
See Ref. 8
Em(FMN/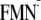 )
in the BLUF domain
)
in the BLUF domain
Em(FMN/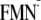 )
in water (15)
)
in water (15)
Shift in
Em(FMN/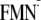 )
from water to protein
)
from water to protein
Influence of uncharged protein dielectric volume (i.e. the space covered by the merged van der Waals volumes of protein atoms)
Influence of the FMN 5′-phosphate group. Note that this group is absent in FAD
Driving Force of Side Chain Rotation of Gln-63—Regardless of
the difference in the hydrogen bond pattern with regard to the Gln-63 and FMN
pair, the calculated pKa(N5) values of FMN are essentially
the same in both dark and light state structures (pKa(N5)
= 12.2 and 12.3 in the [Y-W-FMN] state and 14.0 and 13.9 in the
[Y -W-
-W-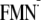 ]
state, respectively). In other words, ΔG for the PT process
from Y
]
state, respectively). In other words, ΔG for the PT process
from Y to
to
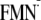 is
essentially the same in the dark and light state structures. On the other
hand, as mentioned above, ΔG for the ET [Y-W-FMN →
Y
is
essentially the same in the dark and light state structures. On the other
hand, as mentioned above, ΔG for the ET [Y-W-FMN →
Y -W-
-W-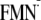 ]
is 160 meV greater in the light state structure than that in the dark state
structure
([Y
]
is 160 meV greater in the light state structure than that in the dark state
structure
([Y -W-
-W-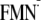 ]
state; Table 1). It is unlikely
that the BLUF must maintain the dark state conformation during the charge
separation process at the expense of this available 160-meV energy of the ET.
Instead, it appears that the driving force of the Gln-63 side chain rotation
is a result of the light-induced charge
[Y
]
state; Table 1). It is unlikely
that the BLUF must maintain the dark state conformation during the charge
separation process at the expense of this available 160-meV energy of the ET.
Instead, it appears that the driving force of the Gln-63 side chain rotation
is a result of the light-induced charge
[Y -W-
-W-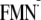 ]
(rather than the different ΔG for the PT from
Y
]
(rather than the different ΔG for the PT from
Y to the N5 atom of
to the N5 atom of
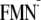 .
.
Indeed, the functional relationship between the rotation of a –CO-containing group and the light-induced charge can be seen in other photoassociated systems. In bacterial photosynthetic reaction centers from R. sphaeroides, light-induced charge separation leads to electron transfer from a monomeric bacteriochlorophyll to a bacteriopheophytin. It has been reported from spectroscopic studies that there exist two distinct conformations of the bacteriopheophytin in the reduced state with regard to the orientation of the acetyl group (27). The rotation of the acetyl group of the electron acceptor bacteriopheophytin takes place in coupling with the charge separation process after the excitation of chromophores (completed in 4 ps) (27). As a consequence, the rotation of the acetyl group contributes to a stabilization of the reduced state of the bacteriopheophytin by upshifting its Em (28). Notably, this “light-induced acetyl conformational switch” can be activated by induced anionic charges on the bacteriopheophytin upon its photoreduction, leading to the stabilization of the charge-separated state in the photosynthetic reaction centers (27).
In analogy, the light-induced charge of the
[Y -W-
-W-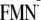 ]
state (rather than protonation process) may be the driving force for the
rotation of the Gln-63 side chain; the Gln-63 reorientation is probably
synchronized with the completion of the charge separation process [Y-W-FMN
→
Y
]
state (rather than protonation process) may be the driving force for the
rotation of the Gln-63 side chain; the Gln-63 reorientation is probably
synchronized with the completion of the charge separation process [Y-W-FMN
→
Y -W-
-W-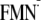 ].
].
Driving Energy in the ET Involving Trp-104—In Ref. 3, the driving energy for the ET via Tyr-21 and Trp-104 was formerly estimated to be -620 and -400 meV, respectively, and it was concluded that Tyr-21 had a better signal overlap with flavin than Trp-104 due to the larger driving energy (3). On the other hand, the present study shows that the driving energy for the ET involving Trp-104 is significantly larger than that involving Tyr-21 (Table 1). Hence, the magnitude of the driving energy for the ET (via Tyr-21 and Trp-104) appears not to be a main factor that determines the ability of the light-signaling state formation of the BLUF domain (see below).
ET Driving Energy and Coupling in the Win and Wout Conformations—There have been two different conformations identified in the crystal structures with regard to the location of Trp-104 (i.e. Win (7) and Wout (8)) (Fig. 2). Their functional relevance is yet unclear experimentally (see discussions in Refs. 8 and 13). I found that the driving energy for the ET via Trp-104 in the dark-Wout (8) structure is 180 meV greater than in the dark-Win (7) structure (Table 1). In contrast, the donor-acceptor distances (e.g. NTrp-104-O4FMN) are 3.8 and 14.5 Å for the Win (7) and Wout (8) structures, respectively (see also Fig. 2). Obviously, the ET coupling of the Win structure is significantly larger than that of the Wout structure (see Refs 30 and 31). Note that the corresponding distance for the ET via Tyr-21 (i.e. OTyr-21-N5FMN) is 4.4–4.6 Å (7, 8), at the same level as the donor-acceptor distance for the ET via Trp-104 in the Win structure.
Recent studies on Y21F and W104F mutants suggested that ET via Tyr-21 and Trp-104 compete in the charge separation process (4). Hence, if the dark-Wout structure is the only functionally relevant conformation, ET via Trp-104 may not be able to compete with ET via Tyr-21 due to its very low ET coupling. Thus, the Win structure is probably necessary to explain the competition of the two ET processes (see below for further discussion).
Transformation of the Light-Win Conformation to the
Dark-Win Conformation—In the Win crystal
structure, only the atomic coordinates of the dark state conformation (where
the –NH2 group of Gln-63 forms a hydrogen bond with the
Tyr-21) are available (7).
Therefore, to investigate the light-Win conformation, I modeled the
atomic coordinates by rotating the side chain –NH2 and
–CO groups of the Gln-63 side chain by 180° along the
Cγ-Cδ axis and energetically optimizing the
atomic coordinates of the Gln-63 side chain. In the [Y-W-FMN] state,
Em(W/W )
of the dark-Win structure was lower by ∼120 mV than that of the
modeled light-Win structure
(Table 1), indicating that
W
)
of the dark-Win structure was lower by ∼120 mV than that of the
modeled light-Win structure
(Table 1), indicating that
W can be stabilized
effectively in the dark state structure. Note that the significantly
destabilized W
can be stabilized
effectively in the dark state structure. Note that the significantly
destabilized W state in the
modeled light-Win structure is a result of the loss of the hydrogen
bond between the indole nitrogen atom of Trp-104 with the –CO group of
Gln-63 (Table 4).
state in the
modeled light-Win structure is a result of the loss of the hydrogen
bond between the indole nitrogen atom of Trp-104 with the –CO group of
Gln-63 (Table 4).
TABLE 4.
Influence of the protein environment on
Em(W/W )
for the [Y-W-FMN]
([Y-W
)
for the [Y-W-FMN]
([Y-W -
-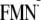 ])
state conformation in mV
])
state conformation in mV
Em(W/W ) )
|
||
|---|---|---|
| Darka-Winb(1YRX)c | Lighta-Winb(1YRX)c(modeled)d | |
| Redox potential | ||
| Em(p)e | 1334 | 1451 |
| Em(w)f | 1070 | 1070 |
| ΔEm(w→p)g | 264 | 381 |
| Protein element | ||
| Protein volumeh | 289 | 287 |
| Protein charge | –25 | 94 |
| ΔPhosphatei | –12 | –12 |
| ΔBackbone | 18 | 15 |
| ΔSide chain | ||
| Asn-45 | 102 (89) | 99 (NDj) |
| –NH2 | 49 (37) | 47 (ND) |
| –CO | 49 (49) | 48 (ND) |
| Gln-63 | –74 (–75) | –12 (ND) |
| –NH2 | –13 (–14) | 0 (ND) |
| –CO | –61 (–61) | 13 (ND) |
Dark (light) state structure where the –NH2 (–CO) group of the Gln-63 side chain is a hydrogen-bonding partner of Tyr-21
Conformation where Trp-104 is in the FMN binding pocket
See Ref. 7
The –NH2 and –CO groups of Gln-63 were rotated by 180° along the Cγ-Cδ axis, and their positions were energetically optimized with CHARMM (18)
Em(W/W )
in the BLUF domain
)
in the BLUF domain
Em(W/W )
in water (26)
)
in water (26)
Shift in
Em(W/W )
from water to protein
)
from water to protein
Influence of uncharged protein dielectric volume (i.e. the space covered by the merged van der Waals volumes of protein atoms)
Influence of the FMN 5′-phosphate group. Note that this group is absent in FAD
ND, not determined (due to the irrelevant energetics of the protein structure)
By using the same procedure, I tried to generate the modeled
light-Win structure for the charge-separated
[Y-W -
-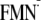 ]
state. Unexpectedly, I could obtain only the dark state conformation for the
[Y-W
]
state. Unexpectedly, I could obtain only the dark state conformation for the
[Y-W -
-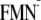 ]
state and not the light state conformation
(Fig. 3). In other words, when
I apply the
[Y-W
]
state and not the light state conformation
(Fig. 3). In other words, when
I apply the
[Y-W -
-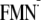 ]
charge state to the light state structure and then perform energy optimization
of the atomic coordinates, the Gln-63 side chain undergoes a 180° rotation
along the Cγ-Cδ axis, resulting in the
atomic coordinates of the dark state structure during the energy optimization
process (Fig. 3).
]
charge state to the light state structure and then perform energy optimization
of the atomic coordinates, the Gln-63 side chain undergoes a 180° rotation
along the Cγ-Cδ axis, resulting in the
atomic coordinates of the dark state structure during the energy optimization
process (Fig. 3).
Functional Relevance of the Win Conformations—The
present study demonstrates that the light-Win conformation of the
[Y-W -
-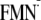 ]
state is unusually unstable due to its energetically unfavorable hydrogen bond
pattern. Thus, it is likely that the involvement of Trp-104 in the
Win charge separation process would lead to the elimination of the
light-Win conformation and, as a consequence, a decrease in the
yield of the light signaling state of the BLUF domain. Indeed, spectroscopic
studies on the W104F mutant indicate its 1.5-fold increase in the quantum
yield of signaling state formation with respect to the native BLUF
(32), implying that Trp-104 in
the native BLUF is likely to lower the quantum yield of signaling state
formation. This is in agreement with the present result.
]
state is unusually unstable due to its energetically unfavorable hydrogen bond
pattern. Thus, it is likely that the involvement of Trp-104 in the
Win charge separation process would lead to the elimination of the
light-Win conformation and, as a consequence, a decrease in the
yield of the light signaling state of the BLUF domain. Indeed, spectroscopic
studies on the W104F mutant indicate its 1.5-fold increase in the quantum
yield of signaling state formation with respect to the native BLUF
(32), implying that Trp-104 in
the native BLUF is likely to lower the quantum yield of signaling state
formation. This is in agreement with the present result.
In addition, recent mutational studies have suggested that there exist two
competing light-induced ET pathways in the BLUF domain: one ET pathway via
Tyr-21 that can form the signaling state and another ET pathway via Trp-104
that leads to the deactivation of signaling state formation
(4). From the present result,
only the Win conformation is able to yield the dark state structure
upon the formation of the
[Y-W -
-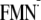 ]
state (Fig. 3). In the
Wout structure, there does not exist such a strict constraint on
the orientation of the Gln-63 side chain because of the isolation of Trp-104
from Gln-63 (14.5 Å). Thus, to explain competitions of ET via Tyr-21 and
Trp-104 (4,
32), the existence of the
Win conformation (7)
is functionally required, as implied in former studies
(7,
12–14).
]
state (Fig. 3). In the
Wout structure, there does not exist such a strict constraint on
the orientation of the Gln-63 side chain because of the isolation of Trp-104
from Gln-63 (14.5 Å). Thus, to explain competitions of ET via Tyr-21 and
Trp-104 (4,
32), the existence of the
Win conformation (7)
is functionally required, as implied in former studies
(7,
12–14).
Remarkably, spectroscopic studies by Masuda et al.
(14) demonstrated that (i) the
W104A mutant is insensitive to blue light and (ii) its activity is similar to
that of the light-adapted native BLUF. These two facts are indicative that the
loss of Trp-104 in the native Win structure does not result in the
transformation to the dark state structure and that the BLUF domain is always
adapted to the light state structure in the absence of Trp-104. Formation of
W in the Win
structure could reset the Gln-63 orientation to the dark (i.e.
ground) state so that the BLUF domain can again perform photosensing
effectively.
in the Win
structure could reset the Gln-63 orientation to the dark (i.e.
ground) state so that the BLUF domain can again perform photosensing
effectively.
CONCLUSION
The driving energy of the ET via Tyr-21 in the light state structure is greater by 160 meV than that in the dark state structure (Table 1 and Fig. 1); this suggests that assignments of the BLUF dark/light conformers by Anderson et al. (7) are more reasonable than those by Jung et al. (8) to explain the energetics of the photoinduced ET event.
The calculated pKa values indicate that the driving
energy for the PT from Y to
to
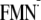 is
essentially the same in the dark and light state structures.
is
essentially the same in the dark and light state structures.
The driving energy of the ET via Trp-104 is larger than that via Tyr-21.
I observed that
Win transforms the
light state to the dark state (Fig.
3). This is in contrast to the case where
Y
transforms the
light state to the dark state (Fig.
3). This is in contrast to the case where
Y transforms the dark state
to the light state. Hence,
Win
transforms the dark state
to the light state. Hence,
Win may facilitate
to reset the Gln-63 orientation to the dark (i.e. ground) state so
that the BLUF domain can again perform photosensing effectively. These are
probably the reasons why the W104A mutant is insensitive to blue light and its
activity is similar to that of the light adapted native BLUF in spectroscopic
studies (14).
may facilitate
to reset the Gln-63 orientation to the dark (i.e. ground) state so
that the BLUF domain can again perform photosensing effectively. These are
probably the reasons why the W104A mutant is insensitive to blue light and its
activity is similar to that of the light adapted native BLUF in spectroscopic
studies (14).
Supplementary Material
Acknowledgments
I am grateful to Dr. Arieh Warshel and Dr. Ernst-Walter Knapp for useful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
Footnotes
The abbreviations used are: BLUF, blue light sensing using FAD; dark state
structure, protein conformation where the –NH2 group of the
Gln-63 side chain is a hydrogen-bonding partner of Tyr-21; ET, electron
transfer; light state structure, protein conformation where the –CO
group of the Gln-63 side chain is a hydrogen-bonding partner of Tyr-21; PT,
proton transfer; Y, tyrosine residue Tyr-21; [Y-W-FMN], charge neutral state;
[Y -W-
-W-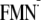 ],
charge-separated state involving Tyr-21;
[Y-W
],
charge-separated state involving Tyr-21;
[Y-W -
-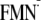 ],
charge-separated state involving Trp-104; W, tryptophan residue Trp-104;
Win, protein conformation where Trp-104 is located in the proximity
of FMN; Wout, protein conformation where Trp-104 is located on the
protein surface.
],
charge-separated state involving Trp-104; W, tryptophan residue Trp-104;
Win, protein conformation where Trp-104 is located in the proximity
of FMN; Wout, protein conformation where Trp-104 is located on the
protein surface.
Rabenstein, B., Ullmann, G. M., and Knapp, E.-W. (1998) Eur. Bophys. J. 27, 626–637.
References
- 1.Han, Y., Braatsch, S., Osterloh, L., and Klug, G. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 12306-12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraft, B. J., Masuda, S., Kikuchi, J., Dragnea, V., Tollin, G., Zaleski, J. M., and Bauer, C. E. (2003) Biochemistry 42 6726-6734 [DOI] [PubMed] [Google Scholar]
- 3.Gauden, M., van Stokkum, I. H. M., Key, J. M., Luhrs, D. C., van Grondelle, R., Hegemann, P., and Kennis, J. T. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 10895-10900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauden, M., Grinstead, J. S., Laan, W., van Stokkum, I. H. M., Avila-Perez, M., Toh, K. C., Boelens, R., Kaptein, R., van Grondelle, R., Hellingwerf, K. J., and Kennis, J. T. M. (2007) Biochemistry 46 7405-7415 [DOI] [PubMed] [Google Scholar]
- 5.Masuda, S., and Bauer, C. E. (2002) Cell 110 613-623 [DOI] [PubMed] [Google Scholar]
- 6.Laan, W., van der Horst, M. A., van Stokkum, I. H. M., and Hellingwerf, K. J. (2003) Photochem. Photobiol. 78 290-297 [DOI] [PubMed] [Google Scholar]
- 7.Anderson, S., Dragnea, V., Masuda, S., Ybe, J., Moffat, K., and Bauer, C. (2005) Biochemistry 44 7998-8005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung, A., Reinstein, J., Domratcheva, T., Shoeman, R. L., and Schlichting, I. (2006) J. Mol. Biol. 362 717-732 [DOI] [PubMed] [Google Scholar]
- 9.Masuda, S., Hasegawa, K., and Ono, T.-A. (2005) Biochemistry 44 1215-1224 [DOI] [PubMed] [Google Scholar]
- 10.Kita, A., Okajima, K., Morimoto, Y., Ikeuchi, M., and Miki, K. (2005) J. Mol. Biol. 349 1-9 [DOI] [PubMed] [Google Scholar]
- 11.Unno, M., Masuda, S., Ono, T.-A., and Yamauchi, S. (2006) J. Am. Chem. Soc. 128 5638-5639 [DOI] [PubMed] [Google Scholar]
- 12.Grinstead, J. S., Avila-Perez, M., Hellingwerf, K. J., Boelens, R., and Kaptein, R. (2006) J. Am. Chem. Soc. 128 15066-15067 [DOI] [PubMed] [Google Scholar]
- 13.Grinstead, J. S., Hsu, S.-T., Laan, W., Bonvin, A. M., Hellingwerf, K. J., Boelens, R., and Kaptein, R. (2006) ChemBioChem 7 187-193 [DOI] [PubMed] [Google Scholar]
- 14.Masuda, S., Tomida, Y., Ohta, H., and Takamiya, K. (2007) J. Mol. Biol. 368 1223-1230 [DOI] [PubMed] [Google Scholar]
- 15.Ishikita, H. (2007) J. Biol. Chem. 282 25240-25246 [DOI] [PubMed] [Google Scholar]
- 16.Ishikita, H. (2008) Biochemistry 47 4394-4402 [DOI] [PubMed] [Google Scholar]
- 17.Ishikita, H., and Knapp, E. W. (2006) Biophys. J. 90 3886-3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks, B. R., Bruccoleri, R. E., Olafson, B. D., States, D. J., Swaminathan, S., and Karplus, M. (1983) J. Comput. Chem. 4 187-217 [Google Scholar]
- 19.Takahashi, R., Okajima, K., Suzuki, H., Nakamura, H., Ikeuchi, M., and Noguchi, T. (2007) Biochemistry 46 6459-6467 [DOI] [PubMed] [Google Scholar]
- 20.Popovic, D. M., Zmiric, A., Zaric, S. D., and Knapp, E.-W. (2002) J. Am. Chem. Soc. 124 3775-3782 [DOI] [PubMed] [Google Scholar]
- 21.Bashford, D., and Karplus, M. (1990) Biochemistry 29 10219-10225 [DOI] [PubMed] [Google Scholar]
- 22.Ishikita, H., Saenger, W., Biesiadka, J., Loll, B., and Knapp, E.-W. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 9855-9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Draper, R. D., and Ingraham, L. L. (1968) Arch. Biochem. Biophys. 125 802-808 [DOI] [PubMed] [Google Scholar]
- 24.Kumler, W. D., and Eiler, J. J. (1943) J. Am. Chem. Soc. 65 2355-2361 [Google Scholar]
- 25.Tommos, C., and Babcock, G. T. (2000) Biochim. Biophys. Acta 1458 199-219 [DOI] [PubMed] [Google Scholar]
- 26.Tommos, C., Skalicky, J. J., Pilloud, D. L., Wand, A. J., and Dutton, P. L. (1999) Biochemistry 38 9495-9507 [DOI] [PubMed] [Google Scholar]
- 27.Müh, F., Williams, J. C., Allen, J. P., and Lubitz, W. (1998) Biochemistry 37 13066-13074 [DOI] [PubMed] [Google Scholar]
- 28.Ishikita, H., Loll, B., Biesiadka, J., Galstyan, A., Saenger, W., and Knapp, E.-W. (2005) FEBS Lett. 579 712-716 [DOI] [PubMed] [Google Scholar]
- 29.Harriman, A. (1987) J. Phys. Chem. 91 6102-6104 [Google Scholar]
- 30.Moser, C. C., Keske, J. M., Warncke, F., Farid, R. S., and Dutton, P. L. (1992) Nature 355 796-802 [DOI] [PubMed] [Google Scholar]
- 31.Page, C. C., Moser, C. C., Chen, X., and Dutton, P. L. (1999) Nature 402 47-52 [DOI] [PubMed] [Google Scholar]
- 32.Laan, W., Gauden, M., Yeremenko, S., van Grondelle, R., Kennis, J. T., and Hellingwerf, K. J. (2006) Biochemistry 45 51-60 [DOI] [PubMed] [Google Scholar]
- 33.Ishikita, H., Soudackov, A. V., and Hammes-Schiffer, S. (2007) J. Am. Chem. Soc. 129 11146-11152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.