Abstract
Previous studies showed that valyl-tRNA synthetase of Saccharomyces cerevisiae contains an N-terminal polypeptide extension of 97 residues, which is absent from its bacterial relatives, but is conserved in its mammalian homologues. We showed herein that this appended domain and its human counterpart are both nonspecific tRNA-binding domains (Kd ∼ 0.5 μm). Deletion of the appended domain from the yeast enzyme severely impaired its tRNA binding, aminoacylation, and complementation activities. This N-domain-deleted yeast valyl-tRNA synthetase mutant could be rescued by fusion of the equivalent domain from its human homologue. Moreover, fusion of the N-domain of the yeast enzyme or its human counterpart to Escherichia coli glutaminyl-tRNA synthetase enabled the otherwise “inactive” prokaryotic enzyme to function as a yeast enzyme in vivo. Different from the native yeast enzyme, which showed different affinities toward mixed tRNA populations, the fusion enzyme exhibited similar binding affinities for all yeast tRNAs. These results not only underscore the significance of nonspecific tRNA binding in aminoacylation, but also provide insights into the mechanism of the formation of aminoacyl-tRNAs.
Aminoacyl-tRNA synthetases are a group of ancient enzymes, each of which catalyzes the attachment of a specific amino acid to its cognate tRNAs. Aminoacyl-tRNAs are then delivered by elongation factor-1 (EF-1)3 to ribosomes for protein translation. In prokaryotes, there are typically 20 aminoacyl-tRNA synthetases, one for each amino acid (1–4). In eukaryotes, protein synthesis occurs not only in the cytoplasm, but also in organelles, such as mitochondria and chloroplasts (5). Thus, eukaryotes, such as yeast, commonly have two genes that encode distinct sets of proteins for each aminoacylation activity, one localized to the cytoplasm and the other to the mitochondria. Each set aminoacylates the isoaccepting tRNAs within its respective cell compartment and is sequestered from the isoacceptors confined in other compartments. In most cases, cytoplasmic and mitochondrial synthetase activities are encoded by two distinct nuclear genes, regardless of the cell compartments to which they are confined. However, in some cases, cytoplasmic and mitochondrial forms of a tRNA synthetase with a given amino acid specificity are encoded by the same nuclear gene through alternative initiation of translation, examples of which include ALA1 (coding for alanyl-tRNA synthetase) (6, 7), GRS1 (coding for glycyl-tRNA synthetase) (8), HTS1 (coding for histidyl-tRNA synthetase) (9), and VAS1 (coding for valyl-tRNA synthetase (ValRS)) (10). Because the isozymes are targeted to different compartments, the two isoforms of ValRS, for example, cannot be substituted for each other in vivo. A similar scenario has been observed for genes encoding mitochondrial and cytoplasmic forms of Arabidopsis thaliana alanyl-tRNA synthetase, threonyl-tRNA synthetase, and ValRS (11).
Many yeast cytoplasmic tRNA synthetases contain an N- or C-terminal polypeptide extension, which is absent from their bacterial homologs (12). A well-studied example is the appended domain (Ad) of yeast glutaminyl-tRNA synthetase (GlnRS), which binds crude yeast tRNAs, single-stranded RNA, and pseudoknot RNA with comparable affinities; the Kd values are ∼0.6 μm (13, 14). Similar examples have been identified in tRNA synthetases of higher eukaryotes, such as the EMAPII-like domain of plant methionyl-tRNA synthetase (15), the repeat domain of human methionyl-tRNA synthetase (16), and the N-terminal domain of mammalian lysyl-tRNA synthetase (17, 18). In addition to serving as a cis-acting tRNA-binding domain, the Ads of some yeast tRNA synthetases were found to participate in protein-protein interactions, such as those of yeast glutamyl-, methionyl-, (19), and seryl-tRNA synthetases (20). These interactions were shown to enhance tRNA binding and aminoacylation of the associated synthetases (19, 20).
Interestingly, many of the Ads of yeast tRNA synthetases contain one or several canonical nuclear localization signals (21), which are thought to play a role in the nuclear import of these otherwise “cytoplasmic” proteins. Nuclear aminoacylation of tRNAs may serve as a functional checkpoint for the maturity of tRNAs before they are exported from the nucleus (22, 23). By contrast, in higher eukaryotes, nine aminoacyl-tRNA synthetases and three auxiliary proteins (p43, p38, and p18) form a multi-enzyme complex through interactions of their hydrophobic Ads (24). In addition, ValRS from mammalian cells is exclusively isolated as a high molecular mass complex with the elongation factor, EF-1H (25–27). Recently, several tRNA synthetases from prokaryotes as well as eukaryotes have been shown to take part in functions beyond aminoacylation, including roles in mitochondrial RNA splicing, transcriptional and translational regulation, cytokine-like activity, and amino acid biosynthesis (28, 29).
In this report, we focused on the tRNA binding affinity and specificity of the Ad of yeast ValRS. Our results showed that the Ad of yeast ValRS is a cis-acting nonspecific tRNA-binding domain that significantly contributes to the tRNA binding and aminoacylation activity of the native enzyme. Fusion of this N-domain or its human counterpart (a positively charged portion of the Ad of human ValRS) to Escherichia coli GlnRS enabled the prokaryotic enzyme to efficiently bind yeast tRNAs and rescue the growth defect of a yeast GLN4 (encoding GlnRS) knock-out strain. Gel mobility shift assays further showed that this fusion enzyme bound to crude yeast tRNAs without specificity. These results suggest that while the Ad of yeast or human ValRS confers a nonspecific tRNA binding activity to the enzyme, the specificity of tRNA aminoacylation (kcat discrimination) was still maintained by the catalytic core of the enzyme.
EXPERIMENTAL PROCEDURES
Construction of Various VAS1 Plasmids—Cloning of a wild-type (Wt) VAS1 gene into the low-copy-number yeast shuttle vector, pRS315, was previously described (30). A short DNA duplex coding for a FLAG tag was inserted in-frame at the 3′-end of the VAS1 gene. To facilitate cloning of various internal deletion constructs of VAS1, an NdeI site (CATATG) was first introduced by site-directed mutagenesis at the initiator codon of the cytoplasmic form of ValRS. (For convenience, the first amino acid residue of the cytoplasmic form of yeast ValRS is referred to as residue 1.) Subsequently, the DNA sequence coding for amino acid residues 98∼1058 of ValRS was PCR-amplified as an NdeI-XhoI fragment and substituted for the NdeI-XhoI fragment of the VAS1 construct that had an NdeI site inserted at the initiator codon, yielding Δ1–97. To construct Δ32–71, the DNA sequence coding for residues 72∼1058 of ValRS was PCR-amplified as a Bsu36I-XhoI fragment and substituted for the Bsu36I-XhoI fragment of the Wt VAS1 construct. Note that the Wt VAS1 gene contains a unique Bsu36I cleavage site at nucleotides 91∼97 (amino acid residues 31∼32) relative to the ATG initiator of the cytoplasmic form.
For fusion of the positively charged portion of the Ad of human ValRS (residues 200∼298) to Δ1–97, DNA sequence coding for the portion was PCR-amplified as an NdeI-NdeI fragment and inserted into the NdeI site of Δ1–97, yielding Ad(HsValRS)-Δ1–97. For fusion of the Ad of yeast ValRS (residues 1∼97) to E. coli GlnRS, DNA sequence coding for the Ad was PCR-amplified as an NdeI-NdeI fragment and inserted into the NdeI site at the 5′-end of the open reading frame of E. coli glnS cloned in pADH (13). To purify the Wt or truncated versions of yeast ValRS, DNA sequences coding for these enzymes were individually amplified as an NdeI-XhoI fragment and cloned into pADH (with a short DNA sequence coding for a His6 tag following the XhoI site). The expression of these constructs was under the control of a constitutive ADH promoter.
Complementation Assays for Cytoplasmic Function—The yeast VAS1 knock-out strain, CW1, was previously described (30). This strain is maintained by a plasmid containing the Wt VAS1 gene and a URA3 marker. Complementation assays for cytoplasmic ValRS activity were carried out by introducing a test plasmid carrying the gene of interest and a LEU2 marker into CW1 and determined by the ability of the transformants to grow in the presence of 5-fluoroorotate (5-FOA). Starting from a cell density of 4.0 A600, cell cultures were 3-fold serially diluted, and 10-μl aliquots of each dilution were spotted onto the designated plates containing 5-FOA. The plates were incubated at 30 °C for 3∼5 days. The transformants evicted the maintenance plasmid with a URA3 marker in the presence of 5-FOA, and thus could not grow on the selection medium unless a functional cytoplasmic ValRS was encoded by the test plasmid. Complementation assays for E. coli glnS fusion constructs essentially followed a similar strategy, except that the GLN4 knock-out strain, EFW6, was used instead (31).
Complementation Assays for Mitochondrial Function—CW1 was co-transformed with a test plasmid (carrying a LEU2 marker) and a second maintenance plasmid (carrying a HIS3 marker) that expresses only the cytoplasmic form of ValRS (due to a mutation in its ATG1 initiator codon). In the presence of 5-FOA, the first maintenance plasmid (carrying a URA3 marker) was evicted from the co-transformants, while the second maintenance plasmid was retained. Thus, all co-transformants survived 5-FOA selections, due to the presence of the cytoplasmic ValRS derived from the second maintenance plasmid. The mitochondrial phenotypes of co-transformants were further tested on YPG plates at 30 °C, with results documented on day 3 following plating. Because a yeast cell cannot survive on glycerol without functional mitochondria, the co-transformants did not grow on the YPG plates unless a functional mitochondrial ValRS was generated from the test plasmid.
Western Blot Analysis—The protein expression patterns of VAS1 fusions were determined by a chemiluminescence-based Western blot analysis. INVSc1 (Invitrogen, Carlsbad, CA) was first transformed with the fusion constructs, and total protein extracts were prepared from each transformant. Aliquots of the protein extracts (40 μg) were loaded onto a mini gel containing 10% polyacrylamide (size: 8 × 10 cm) and electrophoresed at 100 V for 1∼2 h. Following electrophoresis, the resolved proteins were transferred using a semi-dry transfer device to a polyvinylidene fluoride membrane in buffer containing 30 mm glycine, 48 mm Tris base, pH 8.3, 0.037% sodium dodecylsulfate, and 20% methanol. The membrane was probed with a mouse anti-FLAG tag antibody (Sigma) followed by a horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-mouse IgG antibody), and then exposed to x-ray film following the addition of the appropriate substrates. If the proteins of interest were His6-tagged, then an HRP-conjugated anti-His6 tag antibody (Invitrogen) was used instead.
Purification of His6-tagged Proteins—Purification of the His6-tagged proteins followed the protocols provided by the manufacturer of the nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen, Hilden, Germany) with minor modifications. After breaking the cell, the high-speed supernatant (1 h, 30,000 × g) was loaded onto a Ni-NTA column equilibrated with 0.1 m sodium phosphate buffer (pH 7.5) containing 300 mm NaCl, 10 mm imidazole, and 10% glycerol (buffer A). The column was washed with ten column volumes of buffer A containing 30 mm imidazole. The protein of interest was then eluted with 0.1 m Tris-HCl, pH 7.5 containing 300 mm NaCl, 10% glycerol, and 300 mm imidazole. Fractions containing the His6-tagged protein were pooled, concentrated with polyethylene glycol, and dialyzed against two changes of 0.1 m Tris-HCl, pH 7.5, containing 50 mm NaCl and 40% glycerol.
Aminoacylation Assay—Aminoacylation reactions were carried out at ambient temperature in a buffer containing 10 mm Tris-HCl, pH 7.9, 50 mm NaCl, 10 mm MgCl2, 1 mm dithiothreitol, 4 mm ATP, 0.1 mg/ml bovine serum albumin, 0.1 mm brewer's yeast tRNA (F. Hoffmann-La Roche, Basel, Switzerland), and 30 μm valine (5 μm [3H]valine; Moravek Biochemicals, Brea, CA). The specific activity of [3H]valine used was 35.0 Ci/mmol.
The final concentration of ValRS used in the reaction was either 5 or 200 nm. Reactions were quenched by spotting 10-μl aliquots of the reaction mixture onto Whatman filters soaked in 5% trichloroacetic acid and 1 mm valine. The filters were washed three times, for 15 min each, in ice-cold 5% trichloroacetic acid before liquid scintillation counting. Data were obtained from at least three independent experiments and averaged. Kinetic parameters for the Wt and mutant enzymes were determined by directly fitting the data points to the Michaelis-Menten equation. Initial rates of aminoacylation were determined at 25 °C with tRNAVal concentrations ranging 0.5∼20 μm and enzyme concentrations ranging 15∼300 nm. To prepare yeast tRNAVal for determination of the kinetic parameters, yeast tRNAVal was transcribed using a DNA template constructed from complementary synthetic oligonucleotides containing a 12-bp overlapping region, which were then extended using the Klenow fragment of E. coli DNA polymerase I. Concentrations of tRNAs were determined by absorbance at 260 nm using calculated extinction coefficients based on base compositions.
Affinity Co-electrophoresis—Protein-tRNA interactions were assayed using affinity co-electrophoresis as previously described (13, 32). Crude yeast tRNA or in vitro-transcribed yeast tRNAVal was labeled with 32P using polynucleotide kinase (New England Biolabs, Beverly, MA), after dephosphorylation with calf intestine phosphatase. Proteins to be tested were 2-fold serially diluted and mixed with a 5% polyacrylamide solution, forming a mini-gel matrix with a protein gradient of 0.09∼24 or 0.003∼0.8 μm. Labeled tRNA was loaded into each well at an estimated concentration of 1 nm in 2-μl aliquots. Gels were run in a buffer containing 1× TBE (90 mm Tris borate and 2 mm EDTA) and 50 mm NaCl at 20 °C at 50 V for 1 h. After electrophoresis, gels were vacuum-dried and analyzed with a PhosphorImager (Bio-Rad). Dissociation constants (Kd) of the proteins and tRNA were determined as previously described (33).
RESULTS
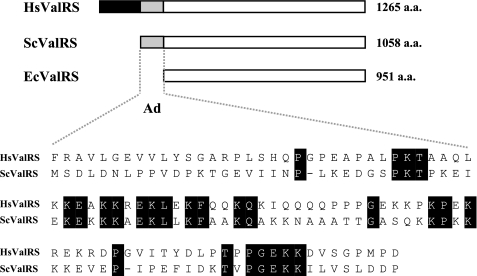
The Ad of Yeast ValRS Is Important for in Vivo Complementation Activity—Previous studies showed that a single VAS1 gene specifies both the mitochondrial and cytoplasmic forms of ValRS through alternative initiation of translation from two in-frame AUG codons 138 nucleotides apart (10, 30). Hence, the mitochondrial precursor form carries the same polypeptide sequence as its cytoplasmic counterpart, except for an N-terminal mitochondrial targeting sequence that is subsequently cleaved away upon being imported into mitochondria by a matrix-processing peptidase. (For convenience, the first amino acid residue of the cytoplasmic form of yeast ValRS is referred to as residue 1.) Comparison of E. coli, yeast (the cytoplasmic form), and human ValRSs showed that the catalytic cores of these enzymes are significantly homologous to one another (∼30% identity), but their N termini vary (Fig. 1). The yeast enzyme has a polypeptide extension of ∼97 residues, which is absent from its E. coli counterpart. Embedded inside this appended domain (Ad) is a lysine-rich cluster of 40 residues (residues 32∼71) that contains several canonical nuclear localization signals (21). These signals are thought to take part in the nuclear localization of this otherwise “cytoplasmic” enzyme.
FIGURE 1.
Ads of yeast and human ValRSs. A, comparison of E. coli, yeast, and human ValRSs. Although the catalytic cores of ValRSs from human, yeast, and E. coli are significantly homologous to one another, their N termini vary. The yeast protein contains an N-terminal domain of ∼97 residues, which is absent from its E. coli counterpart, but is conserved in its human homologue. The Ad of human ValRS can be roughly divided into two parts: a hydrophobic portion (residues 1–199) and a positively charged portion (residues 200–298). B, sequence alignment between the Ad of yeast ValRS (residues 1–97) and the positively charged portion of the Ad of human ValRS (residues 200–298). Hs, Homo sapiens; Sc, S. cerevisiae; Ec, E. coli.
In contrast to the yeast enzyme, human ValRS has acquired an additional hydrophobic domain (residues 1∼199) that is responsible for interacting with the elongation factor, EF-1H (25, 26, 34), while conserving the positively charged domain (residues 200∼298) that distinguishes the yeast enzyme from its bacterial counterparts (Fig. 1). The primary sequences of the catalytic cores of the yeast and human enzymes share ∼50% identity in primary sequence. It is worth mentioning that while the Ad of yeast ValRS and the equivalent domain in human ValRS are both rich in lysine residues (16 and 22%, respectively) and have similar molecular sizes (97 and 99 residues, respectively), the primary sequences share only 26% identity. So far, little is known about the biological function of the Ad of yeast ValRS or its counterpart in the human enzyme.
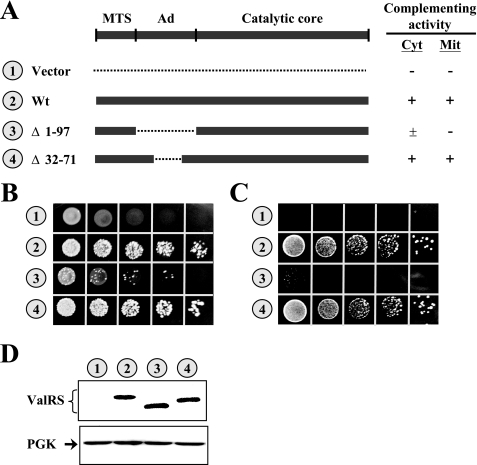
To investigate whether the Ad is essential for the in vivo function of yeast ValRS, various segments were deleted from the Ad of the Wt VAS1 sequence, and the ability of the resultant constructs to restore the growth phenotype of a vas1- yeast strain on 5-FOA and YPG was tested. As summarized in Fig. 2A, deletion of the lysine-rich insert (residues 32∼71) from the Ad had little effect on complementation activity; the truncated enzyme, Δ32–71, effectively rescued the growth defect of the knock-out strain on 5-FOA and YPG (Fig. 2, B and C, number 4). However, deletion of the entire Ad (residues 1∼97) severely impaired the complementation activity; the truncated enzyme, Δ1–97, showed poor cytoplasmic activity and little mitochondrial activity (Fig. 2, B and C, number 3). To examine whether the defective phenotype of Δ1–97 was caused by severe protein degradation, we next compared the protein expression levels of these constructs by Western blotting using an anti-FLAG tag antibody. As shown in Fig. 2D, a similar level of protein was detected in Wt, Δ32–71, and Δ1–97, suggesting that the defective phenotype of Δ1–97 was not caused by lower levels of protein expression.
FIGURE 2.
Complementation assays for Wt and mutant yeast VAS1 constructs. CW1, a yeast VAS1 knock-out strain, was transformed with Wt and mutant VAS1 constructs, and the ability of the transformants to rescue the growth defect of the knock-out strain was tested. A, schematic summary of VAS1 constructs and their complementation activities. Shaded boxes and dashed lines denote sequences coding for yeast ValRS and deletions, respectively. The symbols “+” and “-” indicate positive and negative complementation, respectively. Mit, mitochondrial; Cyt, cytoplasmic. B, complementation assays for cytoplasmic ValRS activity on a 5-FOA plate. C, complementation assays for mitochondrial ValRS activity on a YPG plate. D, assay of protein expression by Western blotting. Upper panel, ValRS; lower panel, PGK (as a loading control). Numbers 1–4 (circled) in B and C represent the constructs shown in A.
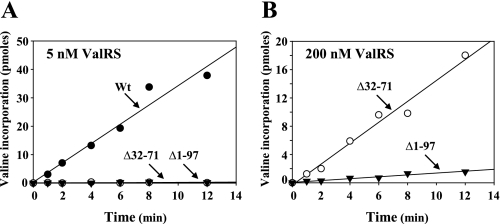
The Ad of Yeast ValRS Is Critical for in Vitro Aminoacylation Activity—To investigate whether these deletion constructs retain aminoacylation activity in vitro, His6-tagged ValRS proteins were purified to homogeneity using Ni-NTA column chromatography and were assayed using unfractionated yeast tRNA as the substrate. Aminoacylation was carried out at a final concentration of 5 nm ValRS protein. As shown in Fig. 3A, Δ32–71 and Δ1–97 had little aminoacylation activity in vitro compared with the Wt under the conditions used. To get a better idea of the possible difference in aminoacylation activity between Δ32–71 and Δ1–97, the protein concentration used for the assay was increased by as much as 40-fold. As shown in Fig. 3B, Δ32–71 showed significantly higher aminoacylation activity than did Δ1–97, which might explain why Δ32–71 retained a near Wt growth phenotype in vivo and Δ1–97 did not (Fig. 2). Kinetic parameters for these enzymes were subsequently determined using in vitro-transcribed yeast tRNAVal as the substrate. As shown in Table 1, the Km and kcat values for the Wt were 0.2 μm and 0.2 s-1, respectively, while the Km and kcat values for Δ32–71 were 10 μm and 0.06 s-1, respectively, under the conditions described under “Experimental Procedures.” So, deletion of the lysine-rich insert drastically reduced the enzyme affinity for tRNAVal (a 50-fold reduction), but only slightly reduced its turnover number (a 3-fold reduction). Because Δ1–97 was essentially inactive in aminoacylation, we could not successfully determine its Km and kcat values. Taken together, these results suggested that the lysine-rich insert and the Ad is critical for the tRNA binding of the enzyme.
FIGURE 3.
Aminoacylation assays for Wt and mutant yeast ValRSs. A, aminoacylation assay. The aminoacylation activity of the Wt and mutant ValRSs was tested at a final concentration of 5 nm. The relative amounts of [3H]valine that were incorporated into tRNA were subsequently determined by a liquid scintillation counter. B, aminoacylation assay. The aminoacylation activity of the mutant ValRSs was tested at a final concentration of 200 nm.
TABLE 1.
Kinetic parameters for aminoacylation of tRNAVal by wild-type and mutant ValRS
| ValRS variant | Km | kcat | kcat/Km | Relative efficiency |
|---|---|---|---|---|
| μm | s–1 | m–1 s–1 | ||
| Wild-type | 0.2 ± 0.02a | 0.2 ± 0.04 | 1 × 106 | 1 |
| Δ32–71 | 10 ± 0.08 | 0.06 ± 0.02 | 6 × 103 | 0.006 |
| Δ1–97 | NDb | ND | ND | ND |
Each value is determined from a hyperbolic fit of two independent data sets
ND, not determined
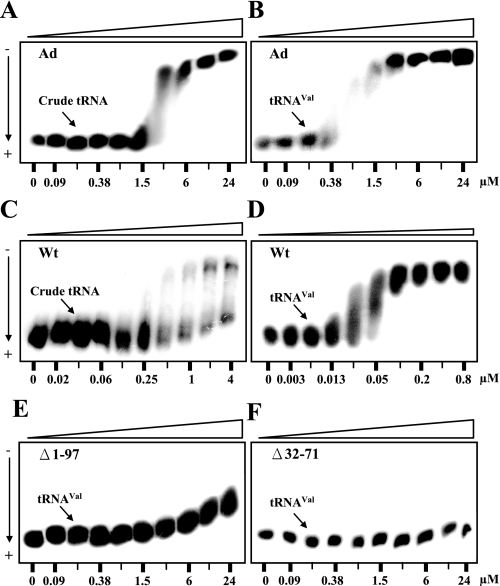
The Ad per se Is a Nonspecific tRNA-binding Domain—Because the Ad is rich in lysine residues (∼22%) and important for tRNA binding of the enzyme (Figs. 1 and 2), we wondered whether the Ad itself can bind tRNA and with what affinity. Pursuant to this objective, purified recombinant Ad was subjected to a gel mobility-shift assay, also known as polyacrylamide gel coelectrophoresis. An aliquot of 32P-labeled in vitro-transcribed yeast tRNAVal or unfractionated yeast tRNA (∼1 nm) was loaded into each well of a 5% polyacrylamide gel, where 2-fold dilutions of the purified Ad had been incorporated into the gel, forming a protein gradient of 0.09∼24 μm. No protein was incorporated into the left-most lane as a control. As shown in Fig. 4, the Ad had a Kd of 2.0 and 0.5 μm toward unfractionated yeast tRNA and in vitro-transcribed yeast tRNAVal, respectively (Fig. 4, A and B), suggesting that the Ad per se is a nonspecific tRNA-binding domain.
FIGURE 4.
Gel mobility-shift assays for the tRNA binding activities of the Ad and Wt and mutant yeast ValRSs. Affinities of the Ad and Wt and mutant ValRSs toward 32P-labeled unfractionated yeast tRNA or in vitro-transcribed yeast tRNAVal were determined by polyacrylamide gel coelectrophoresis. At the bottom of each gel are the concentrations of the protein used. As a control, the left-most lane in each gel contained no protein. A, Ad binding unfractionated yeast tRNA. B, Ad binding yeast tRNAVal. C, Wt binding unfractionated yeast tRNA; D, Wt binding yeast tRNAVal. E, Δ1–97 binding yeast tRNAVal; F, Δ32–71 binding yeast tRNAVal.
To advance understanding of the functional role of the Ad in the tRNA binding of the native enzyme, the Kd values of the Wt and truncated enzymes (Δ32–71 and Δ1–97) for tRNAVal were also examined using a similar approach. As shown in Fig. 4, Δ1–97 and Δ32–71 lost much of their tRNA binding activity (with a Kd > 24 μm in both cases) compared with the Wt (with a Kd of ∼0.05 μm for tRNAVal) (Fig. 4, D–F). This result suggests that the lysine-rich insert and the Ad significantly contribute to the tRNA binding activity of the native enzyme, which is consistent with the kinetic parameters shown in Table 1. In addition, it was interesting to find that the native enzyme has an apparent affinity for tRNAVal, which was ∼10-fold higher than that of the Ad (compare Fig. 4, B and D) and more than 50-fold higher than that of the catalytic core (compare Fig. 4, D and E), suggesting that the Ad may act synergistically with the catalytic core to form a high-affinity binding site for tRNAVal. Furthermore, different from the tRNA binding characteristics of the Ad, which showed similar binding affinities for mixed tRNA populations, the native enzyme exhibited heterogeneous binding curves toward crude yeast tRNA (compare Fig. 4, A and C). Because crude yeast tRNA contains many different tRNA species, it is not clear whether the tRNA population that bound the yeast enzyme with higher affinity represents only the cognate tRNAs (Fig. 4C).
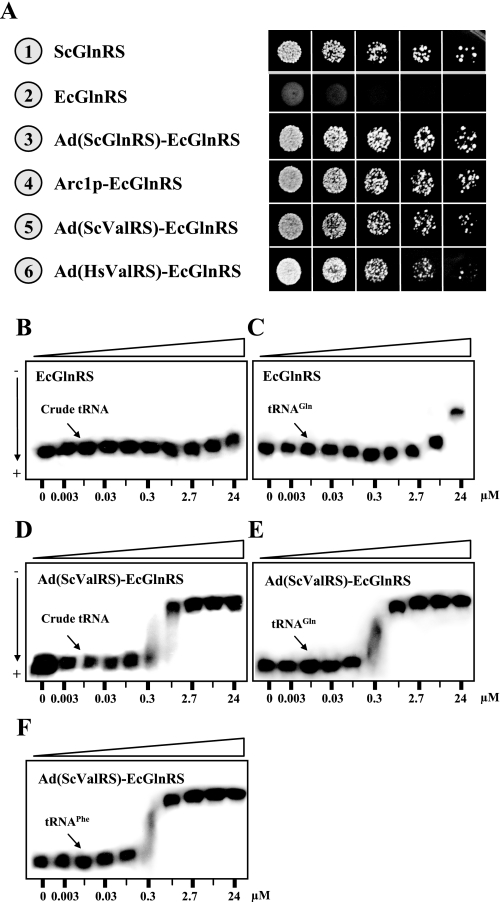
The Ad of Yeast ValRS Converted E. coli GlnRS into a Functional Yeast Enzyme—We previously showed that E. coli GlnRS could not charge yeast tRNA and therefore was unable to rescue the growth defect of EFW6, a yeast GLN4 (encoding GlnRS) knock-out strain, but fusion of Arc1p or the Ad of yeast GlnRS to the E. coli enzyme enabled the fusion enzyme to charge yeast tRNA and restore the growth phenotype of the knock-out strain (13, 31) (Fig. 5). Because the Ad of yeast ValRS possesses a similar nonspecific tRNA binding activity, we wondered whether this domain could also rescue the E. coli enzyme. To test this possibility, the Ad of yeast ValRS was fused in-frame to the N terminus of E. coli GlnRS (resulting in Ad(ScValRS)-EcGlnRS), and the complementation activity of the fusion construct was tested. As shown in Fig. 5, Ad(ScValRS)-EcGlnRS successfully restored the growth phenotype of EFW6 on 5-FOA (Fig. 5A, number 5). Given that aminoacylation generally takes place under conditions wherein an active synthetase/tRNA complex has been formed, this effect of reviving an “inactive” enzyme with a nonspecific tRNA-binding domain was particularly remarkable. Perhaps, the Ad can increase the affinity of the fusion enzyme for all tRNAs, including yeast tRNAGln, and this elevated affinity for yeast tRNAGln might be sufficient for the fusion enzyme to confer the necessary aminoacylation activity to the knock-out strain.
FIGURE 5.
Converting E. coli GlnRS into a functional yeast enzyme. Arc1p and the Ads of yeast GlnRS, yeast ValRS, and human ValRS were independently fused at the N terminus of E. coli GlnRS, and the ability of the fusion enzymes to rescue the growth defect of a yeast GLN4 knock-out strain was tested. A, complementation assays for cytoplasmic GlnRS activity on a 5-FOA plate. B, E. coli GlnRS binding unfractionated yeast tRNA. C, E. coli GlnRS binding yeast tRNAGln. D, Ad(ScValRS)-EcGlnRS binding unfractionated yeast tRNA. E, Ad(ScValRS)-EcGlnRS binding yeast tRNAGln. F, Ad(ScValRS)-EcGlnRS binding yeast tRNAPhe.
To gain further insights into the tRNA binding properties of the fusion enzyme, the apparent binding affinities of the fusion enzyme for yeast tRNAGln and unfractionated yeast tRNA were determined. As shown in Fig. 5, E. coli GlnRS did not significantly bind unfractionated yeast tRNA or yeast tRNAGln (with a Kd > 24 μm in both cases) (Fig. 5, B and C). However, upon fusion of the E. coli enzyme with the Ad of yeast ValRS, the affinities of the fusion enzyme for yeast tRNAGln, tRNAPhe, and unfractionated yeast tRNA were drastically increased. The fusion enzyme bound these tRNA ligands with comparable affinities, at Kd values of ∼0.5 μm (Fig. 5, D–F). Thus, the Ad enhanced the binding affinity of the prokaryotic enzyme for cognate and noncognate tRNAs. While our previous reports demonstrated that the Ad of yeast GlnRS and Arc1p increased the binding affinity of E. coli GlnRS for yeast tRNAGln (13, 14), this appears to be the first piece of experimental evidence showing that the Ad increases the binding affinity of the prokaryotic enzyme not only to tRNAGln, but also to noncognate tRNAs.
In contrast to the yeast enzyme, human ValRS has a rather large Ad (residues 1∼298). The N-terminal part of the Ad (residues 1∼199) is hydrophobic in nature and is responsible for interacting with the elongation factor, EF-1H, while the C-terminal part (residues 200∼298) is positively charged and is thought to be homologous to the Ad of yeast ValRS. It should be mentioned, however, that the primary sequence of the positively charged portion of the Ad of human ValRS actually shares only 26% identity with that of the Ad of yeast ValRS (Fig. 1). Remarkably, fusion of the lysine-rich domain of human ValRS (residues 200∼298) to E. coli GlnRS also rescued the prokaryotic enzyme; the fusion enzyme Ad(HsValRS)-EcGlnRS successfully restored the growth phenotype of the GLN4 knock-out strain on 5-FOA (Fig. 5A, number 6), reinforcing the notion that this portion of human ValRS could be a nonspecific tRNA-binding domain.
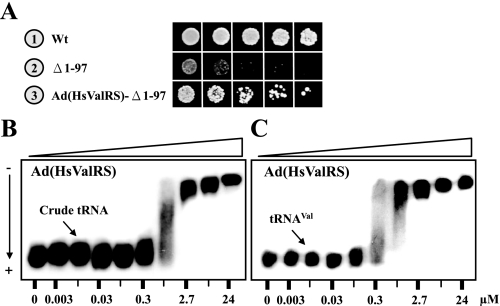
The Defective Yeast ValRS Mutant, Δ1–97, Was Rescued by the Ad of Human ValRS—To further elucidate the tRNA binding property of the lysine-rich domain of human ValRS (residues 200∼298), we next fused this domain to the yeast ValRS mutant Δ1–97, which was shown to be defective in tRNA binding, aminoacylation, and complementation (Figs. 2, 3, 4), and the complementation activity of the resultant construct was tested. As shown in Fig. 6A, fusion of the lysine-rich domain of human ValRS to Δ1–97, resulting in Ad(HsValRS)-Δ1–97, significantly improved the complementation activity of the truncated yeast enzyme; Ad(HsValRS)-Δ1–97 successfully rescued the growth defect of the VAS1 knock-out strain on 5-FOA. Since the hydrophobic portion of the Ad of human ValRS was previously assigned a role of interacting with EF-1H, the notion that the adjacent domain possesses nonspecific tRNA binding activity was particularly noteworthy in terms of tRNA turnover. To directly test the tRNA binding activity of the lysine-rich domain, the DNA sequence coding for residues 200∼298 of human ValRS was cloned and expressed, and the recombinant protein was purified to homogeneity using Ni-NTA column chromatography. Fig. 6 shows that this domain bound yeast tRNAVal and unfractionated yeast tRNA with comparable affinity, the Kd values of which were ∼0.5 μm (Fig. 6, B and C). So, the nonspecific tRNA binding activity has been conserved in the Ads of yeast and human ValRSs during evolution.
FIGURE 6.
Rescuing a defective yeast ValRS mutant with the Ad of human ValRS. The positively charged portion of the Ad of human ValRS (residues 200–298) was fused at the N terminus of the truncated yeast ValRS mutant, Δ1–97, and the ability of the fusion protein to rescue the growth defect of a yeast VAS1 knock-out strain was tested. A, positively charged portion of the Ad of human ValRS (residues 200–298) was fused at the N terminus of the truncated yeast ValRS mutant, Δ1–97, and the ability of the fusion protein to rescue the growth defect of a yeast VAS1 knock-out strain was tested. B, Ad of human ValRS (residues 200–298) binding unfractionated yeast tRNA. C, Ad of human ValRS (residues 200–298) binding yeast tRNAVal.
DISCUSSION
A general feature regarding the Ads of yeast tRNA synthetases is their intrinsic capability to nonspecifically bind to RNA (12). Such activity is thought to enhance the overall efficiency of aminoacylation by attracting tRNA to the vicinity of the catalytic core. The binding affinity of a tRNA synthetase toward its cognate tRNAs is generally characterized by dissociation constants of the order of 0.1∼1 μm under physiological conditions (35). The relatively low affinity ensures that the synthetases (or tRNAs) turn over rapidly during aminoacylation and translation. Interestingly, the binding affinities of the Ads of yeast and human ValRSs toward tRNAVal also fall into this range (Figs. 4, 6), making them useful as a tRNA-binding cofactor during aminoacylation.
As with the Ad of yeast GlnRS, for which the tRNA binding activity is mainly attributed to two lysine-rich clusters located at both ends of the Ad (14), the tRNA binding activity of the Ad of yeast ValRS appears to be largely attributable to a positively charged sequence between residues 32 and 71. However, unlike the Ad of yeast GlnRS, where large deletions in the Ad had little effect on the in vitro aminoacylation activity (36) or in vivo complementation activity (37), the Ad of yeast ValRS played a critical role in the biochemical activity of the enzyme. Deletion of just the lysine-rich cluster from the valine enzyme severely impaired its tRNA binding and aminoacylation activities (Δ32–71 in Figs. 3 and 4). Kinetic studies further showed that the tRNA binding affinity (Km) and catalytic efficiency (kcat/Km) of this truncated enzyme were reduced by more than 50-fold compared with those of the Wt (Table 1). However, despite the drastic impairment in activity, this truncated enzyme still retained its complementing activity in vivo (Δ32–71 in Fig. 2). Therefore, it appears that a mutant enzyme with as low as 1%∼2% aminoacylation activity relative to the native enzyme is sufficient to retain a near Wt growth phenotype for the knock-out strain. Perhaps for a similar reason, fusion of the Ads of yeast and human ValRSs to E. coli GlnRS enabled this prokaryotic enzyme to rescue the growth defect of the yeast knock-out strain EFW6 (Fig. 5). This finding was particularly inspiring in view of the fact that while these Ads possess only nonspecific tRNA binding activity, they can promote the formation by the prokaryotic enzyme of an active complex with yeast tRNAGln and not an abortive complex that is often seen with non-cognate or mutant tRNAs. This also reinforces the notion that kcat discrimination plays a key role in the catalysis and formation of Gln-tRNAGln.
In contrast to the nonspecific tRNA binding activity of the Ads and the fusion protein, Ad(ScValRS)-EcGlnRS, native yeast ValRS appeared to possess certain tRNA specificity (Figs. 4, 5, 6). It is likely that the Ad and the catalytic core of the yeast enzyme have coevolved during evolution to form a high-affinity binding site for tRNAVal. Therefore, the native yeast enzyme had higher affinity for tRNAVal than did the Ad or the catalytic core alone (Fig. 4, B, D, E). So far, it is not clear whether the yeast enzyme only binds cognate tRNAs with high affinity. A comparative binding assay with various purified isoacceptors is currently underway to resolve this issue. Although the Ads of GluRS and MetRS are also rich in positively charged residues and important for aminoacylation, they do not function as tRNA-binding domains. Instead, these Ads specifically interact with a tRNA-binding cofactor, Arc1p, which, in turn, recruits tRNA to the associated enzymes for aminoacylation (38). A functionally similar tRNA-recruiting domain was identified in an auxiliary protein associated with the mammalian multi-synthetase complex (39).
ValRS from mammalian cells is exclusively isolated as a high molecular mass complex with the elongation factor, EF-1H (25–27). Like yeast ValRS, the mammalian enzyme also contains a strong affinity for the polyanionic carrier heparin-Ultrogel. However, the mammalian enzyme exhibits additional hydrophobic properties (34). Sequence analysis revealed that mammalian ValRS has conserved the positively charged N-terminal extension (residues 200–298) that distinguishes yeast ValRS from its bacterial counterparts, while acquiring an additional hydrophobic domain (residues 1–199) that is responsible for interacting with the four subunits of elongation factor EF-1H (α, β, γ, and δ subunits) (25–27, 40) (Fig. 1). Excision of the hydrophobic extension (residues 1–200) by the enzyme, elastase, severely impaired association with EF-1H, but had little effect on the in vitro catalytic activity of the enzyme (40). A recent report further showed that association of mammalian ValRS with EF-1H is required for stimulation of its aminoacylation activity in-trans by EF-1α (41). This observation provides a structural basis for the functional interaction between ValRS and EF-1α, which may play a role in tRNA channeling by directly transferring valyl-tRNA from the synthetase to ribosomes. In contrast, yeast appears to lack a similar mechanism for tRNAVal channeling.
As for the positively charged portion of the Ad of human ValRS (residues 200–298), our results suggested that it possesses a tRNA binding property similar to that of the Ad of yeast ValRS and may act as a tRNA-binding cofactor in cis to the catalytic core of the synthetase (Fig. 6). If that is the case, the Ad of human ValRS can concurrently interact with EF-1H (using its hydrophobic sequence) and tRNA (using its positively charged sequence), and further enhance the efficiency of tRNAVal channeling. To our knowledge, mammalian ValRS is the only tRNA synthetase that carries such a unique sequence feature. In addition, ValRS may be one of the few examples wherein nonspecific tRNA binding activity is conserved in the Ads of homologous yeast and human tRNA synthetases. However, regardless of the detailed interpretation, the most significant findings reported here are the faithful transfer of nonspecific tRNA binding activity from an Ad to a fusion enzyme and the capability of a nonspecific tRNA-binding domain to enhance the formation and catalysis of an active synthetase/tRNA complex. Thus, acquiring a nonspecific tRNA-binding domain might be one of the feasible mechanisms that enables a bacterial tRNA synthetase to gain extra affinity toward yeast tRNA and consequently evolve into a functional yeast enzyme.
Acknowledgments
We thank Rebecca Alexander (Wake Forest University) and Shen-Liang Chen (National Central University, Taiwan) for helpful suggestions and critical reading of the manuscript.
This work was supported in part by Grant NSC96-2311-B-008-002 (to C. C. W.) from the National Science Council (Taipei, Taiwan) and Grant 96-2001-INER-0034 (to C. C. W.) from the Institute of Nuclear Energy Research (Taoyuan, Taiwan). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: EF-1, elongation factor-1; Ad, appended domain; 5-FOA, 5-fluoroorotic acid; GlnRS, glutaminyl-tRNA synthetase; ValRS, valyl-tRNA synthetase; Wt, wild type; NTA, nitrilotriacetic acid.
References
- 1.Carter, C. W., Jr. (1993) Annu. Rev. Biochem. 62 715-748 [DOI] [PubMed] [Google Scholar]
- 2.Martinis, S. A., and Schimmel, P. (1996) in Escherichia coli and Salmonella Cellular and Molecular Biology (Neidhardt, F. C., ed) pp. 887-901, 2nd Ed., Am. Soc. Microbiol., Washington, D. C.
- 3.Giege, R., Sissler, M., and Florentz, C. (1998) Nucleic Acids Res. 26 5017-5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelchat, M., and Lapointe, J. (1999) Biochem. Cell Biol. 77 343-347 [PubMed] [Google Scholar]
- 5.Dietrich, A., Weil, J. H., and Marechal-Drouard, L. (1992) Annu. Rev. Cell Biol. 8 115-131 [DOI] [PubMed] [Google Scholar]
- 6.Tang, H. L., Yeh, L. S., Chen, N. K., Ripmaster, T., Schimmel, P., and Wang, C. C. (2004) J. Biol. Chem. 279 49656-49663 [DOI] [PubMed] [Google Scholar]
- 7.Chang, K. J., Lin, G., Men, L. C., and Wang, C. C. (2006) J. Biol. Chem. 281 7775-7783 [DOI] [PubMed] [Google Scholar]
- 8.Chang, K. J., and Wang, C. C. (2004) J. Biol. Chem. 279 13778-13785 [DOI] [PubMed] [Google Scholar]
- 9.Natsoulis, G., Hilger, F., and Fink, G. R. (1986) Cell 46 235-243 [DOI] [PubMed] [Google Scholar]
- 10.Chatton, B., Walter, P., Ebel, J. P., Lacroute, F., and Fasiolo, F. (1988) J. Biol. Chem. 263 52-57 [PubMed] [Google Scholar]
- 11.Souciet, G., Menand, B., Ovesna, J., Cosset, A., Dietrich, A., and Wintz, H. (1999) Eur. J. Biochem. 266 848-854 [DOI] [PubMed] [Google Scholar]
- 12.Mirande, M. (1991) Prog. Nucleic Acids Res. Mol. Biol. 40 95-142 [DOI] [PubMed] [Google Scholar]
- 13.Wang, C. C., and Schimmel, P. (1999) J. Biol. Chem. 274 16508-16512 [DOI] [PubMed] [Google Scholar]
- 14.Wang, C. C., Morales, A. J., and Schimmel, P. (2000) J. Biol. Chem. 275 17180-17186 [DOI] [PubMed] [Google Scholar]
- 15.Kaminska, M., Deniziak, M., Kerjan, P., Barciszewski, J., and Mirande, M. (2000) EMBO J. 19 6908-6917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminska, M., Shalak, V., and Mirande, M. (2001) Biochemistry 40 14309-14316 [DOI] [PubMed] [Google Scholar]
- 17.Francin, M., Kaminska, M., Kerjan, P., and Mirande, M. (2002) J. Biol. Chem. 277 1762-1769 [DOI] [PubMed] [Google Scholar]
- 18.Francin, M., and Mirande, M. (2006) Biochemistry 45 10153-10160 [DOI] [PubMed] [Google Scholar]
- 19.Simos, G., Segref, A., Fasiolo, F., Hellmuth, K., Shevchenko, A., Mann, M., and Hurt, E. C. (1996) EMBO J. 15 5437-5448 [PMC free article] [PubMed] [Google Scholar]
- 20.Godinic, V., Mocibob, M., Rocak, S., Ibba, M., and Weygand-Durasevic, I. (2007) FEBS J 274 2788-2799 [DOI] [PubMed] [Google Scholar]
- 21.Schimmel, P., and Wang, C. C. (1999) Trends Biochem. Sci. 24 127-128 [DOI] [PubMed] [Google Scholar]
- 22.Lund, E., and Dahlberg, J. E. (1998) Science 282 2082-2085 [DOI] [PubMed] [Google Scholar]
- 23.Sarkar, S., Azad, A. K., and Hopper, A. K. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 14366-14371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirande, M., Lazard, M., Martinez, R., and Latreille, M. T. (1992) Eur. J. Biochem. 203 459-466 [DOI] [PubMed] [Google Scholar]
- 25.Bec, G., Kerjan, P., Zha, X. D., and Waller, J. P. (1989) J. Biol. Chem. 264 21131-21137 [PubMed] [Google Scholar]
- 26.Venema, R. C., Peters, H. I., and Traugh, J. A. (1991) J. Biol. Chem. 266 12574-12580 [PubMed] [Google Scholar]
- 27.Motorin, Y. A., Wolfson, A. D., Lohr, D., Orlovsky, A. F., and Gladilin, K. L. (1991) Eur. J. Biochem. 201 325-331 [DOI] [PubMed] [Google Scholar]
- 28.Martinis, S. A., Plateau, P., Cavarelli, J., and Florentz, C. (1999) EMBO J. 18 4591-4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francklyn, C., Perona, J. J., Puetz, J., and Hou, Y. M. (2002) RNA 8 1363-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, C. C., Chang, K. J., Tang, H. L., Hsieh, C. J., and Schimmel, P. (2003) Biochemistry 42 1646-1651 [DOI] [PubMed] [Google Scholar]
- 31.Whelihan, E. F., and Schimmel, P. (1997) EMBO J. 16 2968-2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cilley, C. D., and Williamson, J. R. (1997) RNA 3 57-67 [PMC free article] [PubMed] [Google Scholar]
- 33.Lim, W. A., Sauer, R. T., and Lander, A. D. (1991) Methods Enzymol. 208 196-210 [DOI] [PubMed] [Google Scholar]
- 34.Bec, G., and Waller, J. P. (1989) J. Biol. Chem. 264 21138-21143 [PubMed] [Google Scholar]
- 35.Schimmel, P. R., and Soll, D. (1979) Annu. Rev. Biochem. 48 601-648 [DOI] [PubMed] [Google Scholar]
- 36.Ludmerer, S. W., Wright, D. J., and Schimmel, P. (1993) J. Biol. Chem. 268 5519-5523 [PubMed] [Google Scholar]
- 37.Ludmerer, S. W., and Schimmel, P. (1987) J. Biol. Chem. 262 10807-10813 [PubMed] [Google Scholar]
- 38.Simos, G., Sauer, A., Fasiolo, F., and Hurt, E. C. (1998) Mol. Cell 1 235-242 [DOI] [PubMed] [Google Scholar]
- 39.Shalak, V., Kaminska, M., Mitnacht-Kraus, R., Vandenabeele, P., Clauss, M., and Mirande, M. (2001) J. Biol. Chem. 276 23769-23776 [DOI] [PubMed] [Google Scholar]
- 40.Bec, G., Kerjan, P., and Waller, J. P. (1994) J. Biol. Chem. 269 2086-2092 [PubMed] [Google Scholar]
- 41.Negrutskii, B. S., Shalak, V. F., Kerjan, P., El'skaya, A. V., and Mirande, M. (1999) J. Biol. Chem. 274 4545-4550 [DOI] [PubMed] [Google Scholar]