Abstract
The ischemic death of cardiomyocytes is associated in heart disease and heart failure. However, the molecular mechanism underlying ischemic cell death is not well defined. To examine the function of apoptosis repressor with a caspase recruitment domain (ARC) in the ischemic/hypoxic damage of cardiomyocytes, we generated cardio-specific ARC transgenic mice using a mouse α-myosin heavy chain promoter. Compared with the control, the hearts of ARC transgenic mice showed a 3-fold overexpression of ARC. Langendoff preparation showed that the hearts isolated from ARC transgenic mice exhibited improved recovery of contractile performance during reperfusion. The cardiomyocytes cultured from neonatal ARC transgenic mice were significantly resistant to hypoxic cell death. Furthermore, the ARC C-terminal calcium-binding domain was as potent to protect cardiomyocytes from hypoxic cell death as ARC. Genome-wide RNA expression profiling uncovered a list of genes whose expression was changed (>2-fold) in ARC transgenic mice. Among them, expressional regulation of developmentally regulated RNA-binding protein 1 (Drbp1) or the dimethylglycine dehydrogenase precursor (pMe2GlyDH) affected hypoxic death of cardiomyocytes. These results suggest that ARC may protect cardiomyocytes from hypoxic cell death by regulating its downstream, Drbp1 and pMe2GlyDH, shedding new insights into the protection of heart from hypoxic damages.
Programmed cell death, or apoptosis, is an evolutionarily conserved process that plays a critical role in embryonic development and adult tissue homeostasis. In humans and mice, dysregulated apoptosis has been implicated in the pathogenesis of cancer and in autoimmune, cardiovascular, and neurodegenerative diseases (1). Recently, apoptosis of cardiomyocytes has been recognized as a cellular mechanism of ischemic injury in the heart. Furthermore, a large body of research has focused on identifying the signaling molecules that might protect the myocardium from ischemic damage (2). For example, signaling molecules enhance apoptosis of cardiomyocytes, such as p38, c-Jun N-terminal kinase, tumor necrosis factor-α, p53, β-adrenergic receptors, and nitric oxide (2–4). In contrast, other signaling pathways have been demonstrated to protect the heart from apoptosis, such as cardiotrophin-1 through the gp130 receptor, p38β, insulin-like growth factor-1, Akt/protein kinase B, protein kinase C, and extracellular signal-regulated kinase 1/2 (2, 3, 5–9). Thus, an increased understanding of the signaling pathways that are regulated during ischemia/reperfusion is important for the development of effective therapies (10).
ARC3 (apoptosis repressor with CARD) is a caspase recruitment domain (CARD) protein that is expressed almost exclusively in long-lived tissues, such as heart, skeletal muscles, and brain. ARC selectively interacts with the initiator caspases-2 and -8, and significantly attenuates death receptor-induced apoptosis dependent on the activation of these caspases (11). In the H9c2 cell line, ectopic expression of ARC suppressed apoptosis, the protection being mediated, in total or in part, through the blockade of hypoxia-induced cytochrome c released from the mitochondria (12). Therefore, specific interference with both receptor and mitochondria death pathways, as well as its high cardiac expression, makes ARC a unique and central cardiac death repressor. This supposition is supported by the observation that viral gene transfer or TAT-mediated transduction of ARC reduces infarct size after the ischemia/reperfusion injury of isolated rat hearts and blocks the development of post-ischemic cardiomyopathy (13, 14). Recently, ARC knock-out mice also exhibited enhanced sensitivity to hypoxic injury in the heart (15). However, the detailed function of ARC as a suppressor of ischemic damage in the heart needs to be clarified. In this study, by generating cardio-specific ARC transgenic (Tg) mice and global gene expression analysis, we found that ARC protects cardiomyocytes from ischemic/hypoxic damages via its C-terminal Pro/Glu-rich region and expressional regulation of Drbp1 and pMe2GlyDH.
MATERIALS AND METHODS
Plasmid Construction and shRNA Analysis—The mouse ARC cDNA was subcloned into an expression vector under the control of a cardio-specific mouse α-myosin heavy chain (αMHC) promoter (16). ARC, NARC-(1–98), and CARC-(99–208) were subcloned into pcDNA3.1 (Invitrogen) and pEGFP (Clontech) (17). Drbp1 cDNA was kindly provided by Dr. H. Endo (Jichi Medical School, Japan) and Alas2 cDNA was subcloned into pcDNA3.1. For vector expressing shRNAs against pMe2GlyDH, oligonucleotides (number 1 forward, 5′-GATCC CCGGG ATAAA CTTGA AGAAG ATTCA AGAGA TCTTC TTCAA GTTTA TCCCT TTTTG GAAA-3′ and reverse, 5′-AGCTT TTCCA AAAAG GGATA AACTT GAAGA AGATC TCTTG AATCT TCTTC AAGTT TATCC CGGG-3′; number 2 forward, 5′-GATCC CCCTG AAAGG GTGGA CGAAT TTTCA AGAGA AATTC GTCCA CCCTT TCAGT TTTTG GAAA-3′ and reverse, 5′-AGCTT TTCCA AAAAC TGAAA GGGTG GACGA ATTTC TCTTG AAAAT TCGTC CACCC TTTCA GGGG-3′) containing the target sequence (Dharmacon) were synthesized, annealed, and cloned into BglII and HindIII sites of pSuper (OligoEngine).
Generation of ARC Tg Mice—The mouse ARC expression construct was injected into embryos and positive F0 mice were identified by PCR analysis using a synthetic oligonucleotide (forward, 5′-CCACA TTCTT CAGGA TTCTC-3′) corresponding to the MHC promoter and a oligonucleotide (reverse, 5′-CTTCT GGCGT CCAGT GG-3′) of ARC cDNA (Macrogen Inc., Korea). Three independent founder lines were identified and mated to FVB wild type (WT) mice to generate a pure FVB genetic background WT for the F2 generation, and then F3 Tg offspring were then backcrossed to Balb/c 3T3 mice under a 12:12 h light:dark cycle with access to food and water ad libitum. The animal protocols were approved by the Gwangju Institute of Science and Technology and Seoul National University Standing Committees on Animals.
Genomic DNA PCR and Reverse Transcription-PCR (RT-PCR)—Genomic DNA PCR from the tails of Tg mice and RT-PCR from the whole tissues of Tg mice were carried out as described previously (18, 19). Gene-specific oligonucleotides were synthesized: Alas2 forward, 5′-GTATT GGACG CTGCC CCATC C-3′ and reverse, 5′-CTTCA GGGTC TCCTC TATGG C-3′; Drbp1 forward, 5′-GTGAT CAGCA AGCAC ACATC C-3′ and reverse 5′-CGCAC GTAGC CCAAG CCTTT A-3′; pMe2GlyDH forward, 5′-GACAG AGCAG AGACT GTGAT-3′ and reverse, 5′-CATCT CCTGG GTTAT ACAGT C-3′; ARC forward, 5′-ATGGG CAACG TGCAG GAG-3′ and reverse, 5′-CTTCT GGCGT CCAGT GG-3′; and β-actin forward, 5′-GAGCT GCCTG ACGGC CAGG-3′ and reverse, 5′-CATCT GCTGG AAGGT GGAC-3′; atrial natriuretic factor forward, 5′-CCATA TTGGA GCAAA TCCTG-3′ and reverse, 5′-CGGCA TCTTC TCCTC CAGG-3′; β-MHC forward, 5′-TGCAA AGGCT CCAGG TCTGA GGGC-3′ and reverse, 5′-GCCAA CACCA ACCTG TCCAA GTTC-3′; MMP2 forward, 5′-GATAC CCTCA AGAAG ATGCA GAAGT-3′ and reverse, 5′-ATCTT GGCTT CCGCA TGGT-3′ (20).
Western Blot Analysis—Samples were homogenized in lysis buffer (120 mm NaCl, 1 mm EGTA, 1 mm EDTA, 1 mm MgCl2, 1 mm Na3VO3, 10 mm Na4P2O7, 10 mm sodium fluoride, 1% Triton, 10% glycerol, and 50 mm Tris-HCl, pH 8.0) and the amounts of protein were determined using the method of Bradford (Bio-Rad). Total 30–50 μg of cell extracts in a buffer (60 mm Tris-HCl, pH 6.8, 1% SDS, 10% glycerol, and 0.5% β-mercaptoethanol) were subjected to SDS-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes using the Semi-Dry Transfer system (Bio-Rad). The membranes were blocked with TBST buffer (20 mm Tris-Cl, pH 7.5, 150 mm NaCl, and 0.2% Tween 20) containing 3% bovine serum albumin. Proteins were then visualized using Enhanced Chemiluminescence. Anti-ARC antibody was purified by ARC affinity chromatography. Mouse anti-FADD and anti-poly(ADP-ribose)polymerase antibody were purchased from Pharmingen.
Histological Analysis and Immunohistochemistry—After perfusion, the hearts were fixed with 4% formaldehyde, embedded, and thin-sectioned, followed by deparaffinization and rehydration. The paraffin sections were blocked in 5% rabbit serum and then incubated overnight at 4 °C with anti-ARC antibody. For the preparation of frozen sections, hearts were isolated after perfusion and immediately frozen in liquid nitrogen-cooled OCT embedding medium (Tissue-Tek). The frozen sections were cut to a 5-μm thickness and mounted on silane-coated slides.
Langendoff Preparation of Isolated Mouse Hearts—The mice were anesthetized with pentobarbital (50 mg/kg, intraperitoneal injection) and their hearts were excised and perfused with oxygenated buffer (21, 22). The hearts were retrogradely perfused on a Langendoff apparatus with Krebs-Henseleit solution (118 mm NaCl, 4.7 mm KCl, 1.2 mm MgSO4, 1.2 mm KH2PO4, 25 mm NaHCO3, 11 mm glucose, 1 mm CaCl2, and 10 mm HEPES) with a gas mixture of 95% O2 and 5% CO2 at 37 °C. Through a left atrial incision, a latex balloon connected to a pressure transducer was inserted into the left ventricular (LV) cavity for the measurement of the LV isovolumic pressure. The LVEDp was identified as the lowest value from the LV pressure curve. The LVDp and heart rate were continuously monitored using a polygraph and a computer analysis system (PolyView, GRASS Co.).
Induction of Myocardial Infarction—After 1 h of ischemia and 24 h of reperfusion, the left anterior descending coronary artery was reoccluded, and 1 ml of 1.0% Evans blue was injected into the apex of each heart to stain nonischemic tissue. The hearts were then excised, washed with phosphate-buffered saline, embedded in agarose, and cut into five transverse slices for 15 min of incubation at room temperature with 1.5% 2,3,5-triphenyltetrazolium chloride to measure viable myocardium (red staining). Slices were photographed (each side) under a microscope and the left ventricular area, the area at risk, and the infarct area were determined by digital planimetry (14).
TUNEL Assay—Accumulated internucleosomal DNA fragments (apoptosis) were detected using an in situ apoptosis detection kit (Roche Applied Science). Percentages of positively stained cells were determined by counting the numbers of labeled cells and total cells in cross-sections (15).
Isolation of Neonatal Cardiomyocytes and DNA Transfection—ARC homozygous Tg mice for this study were produced by mating heterozygous ARC Tg mice in the Balb/c 3T3 background to heterozygous ARC Tg mice and used for primary culture. A procedure for culturing ventricular cardiomyocytes from neonatal mice was modified (23). For transfection, cardiomyocytes isolated from neonatal ARC Tg mice were allowed to stabilize for 3 days and then transfected with plasmids using PolyFectamine reagent according to the manufacturer's instruction (Qiagen).
In Vitro Hypoxia of Cultured Cardiomyocytes and Cell Death Assays—Cardiomyocytes were cultured in fetal bovine serum/minimal essential medium. Hypoxia was simulated by incubating cells in a hypoxic buffer (125 mm NaCl, 8 mm KCl, 1.2 mm KH2PO4, 1.25 mm MgSO4, 1.2 mm CaCl2, 6.25 mm NaHCO3, 20 mm 2-deoxyglucose, 5 mm sodium lactate, and 20 mm HEPES, pH 6.6) and by placing the cells under hypoxic pouches (GasPak™ EZ, BD Biosciences) at 37 °C (10). Cell death assays were performed using the Live/Dead cell viability kit (Molecular Probes).
Microarray Using GenePix 4000B Gene Expression Profiling— Total RNA samples were prepared from the hearts of 3- and 30-week-old mice by using an RNeasy Mini Kit (Qiagen). RNA samples (30 μg) were labeled with cyanine (Cy3) or cyanine (Cy5)-conjugated dCTP (Amersham) by a reverse transcription reaction using SuperScript II (Invitrogen). The labeled cDNA mixture was resuspended and mixed in 10 μl of hybridization solution (GenoCheck, Korea) and placed on an OpArray mouse genome 35K (OPMMV4, Operon Biotechnologies, GmbH). The hybridized slides were washed in a buffer (2× SSC, 0.1% SDS for 2 min, 1× SSC for 3 min, and then 0.2× SSC for 2 min) at room temperature.
Microarray Data Analysis—Microarray data analysis was carried out as described previously (24).
Statistical Analyses—All statistical analyses were performed using a two-tailed Student's t test or one-way analysis of variance followed by SigmaStat software.
RESULTS
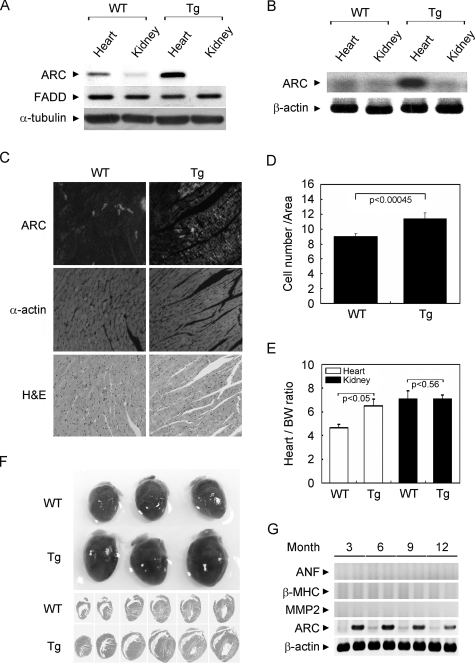
Generation of Tg Mice Overexpressing ARC in the Heart—To examine the molecular function of ARC in the hypoxic injury of heart, we generated cardio-specific Tg mice overexpressing mouse ARC using the promoter of mouse MHC gene (Fig. 1). ARC Tg mice were born normal and grew to adulthood without any abnormalities in their health and appearance. Compared with WT, an analysis of tissue extracts using Western blotting (Fig. 1A) and polymerase chain reaction (Fig. 1B) demonstrated an increased level of ARC protein and RNA with about 3-fold in the whole heart tissues of ARC Tg mice, but not in the kidney as well as in liver and brain.4 Similar expression levels of ARC were observed in the other two lines of ARC Tg mice we generated.4
FIGURE 1.
Generation of cardio-specific ARC Tg mice. A, Western blot analysis of ARC in the heart of ARC Tg mice. Tissue extracts (30 μg) were prepared from the heart and kidney isolated from 8–12-week-old ARC Tg and age-matched WT mice, and proved with the indicated antibodies after separation on SDS-polyacrylamide gel. FADD and α-tubulin were used as internal controls. B, RT-PCR analysis. Total RNA was extracted from the hearts and ARC was amplified by RT-PCR using mouse ARC-specific oligonucleotides. C, immunohistochemistry of ARC. Heart frozen sections were prepared and left ventricle (LV) sections of the hearts were probed with anti-ARC or anti-α-actin antibodies. Cardiac histological sections proved with anti-ARC antibodies were stained with hematoxylin and eosin (H & E). D, determination of cell numbers in the hearts. Cells were counted from each of 10 ARC-positive areas in paraffin sections of ARC Tg mice or the same areas of WT mice. Bars indicate mean ± S.E. E, heart/body and kidney/body weight ratios of WT (n = 10) and age-matched ARC Tg mice (n = 9). Bars depict mean ± S.E. F, gross morphology of the hearts in the 8–12-week-old WT and ARC Tg mice (upper panel). Hematoxylin and eosin (H & E) staining of serial longitudinal sections (lower panel). G, expression analysis of hypertrophy-associated genes in ARC Tg mice. Total RNAs were obtained from the hearts of ARC Tg mice at the indicated months and examined for the expression of atrial natriuretic factor (ANF), β-MHC, and MMP-2 by RT-PCR analysis.
Immunostaining analysis also revealed the increased expression of ARC in the left ventricle of the heart of ARC Tg mice (Fig. 1C). In ARC Tg mice, strong immunoreactivity against ARC was restricted to ∼40% of individual cardiomyocytes, which is consistent with the previous report demonstrating the expression pattern of heme oxygenase-1 under the control of MHC promoter (21). Although histological examination with hematoxylin and eosin (H & E) staining revealed no severe cardiac morphological defects, cardiac remodeling was evident in the hearts of ARC Tg mice with the relative increase in cell numbers (Fig. 1D). Comparison of heart sizes revealed that under resting conditions, the hearts of ARC Tg mice were apparently larger than those of age-matched WT mice (Fig. 1F). There was a 20% difference in the ratios of heart/body weights between 8-week-old ARC Tg and WT mice, whereas no detectible difference was observed with respect to kidney weight (Fig. 1E). In addition, examination of the expression of hypertrophic markers, such as atrial natriuretic factor and β-MHC, and MMP2, the sign of fibrosis, with RT-PCR analysis revealed no obvious increase in the hearts of ARC Tg mice (Fig. 1G). These findings suggest that heart enlargement observed in ARC Tg mice may result from the principal cause of the increased cell numbers.
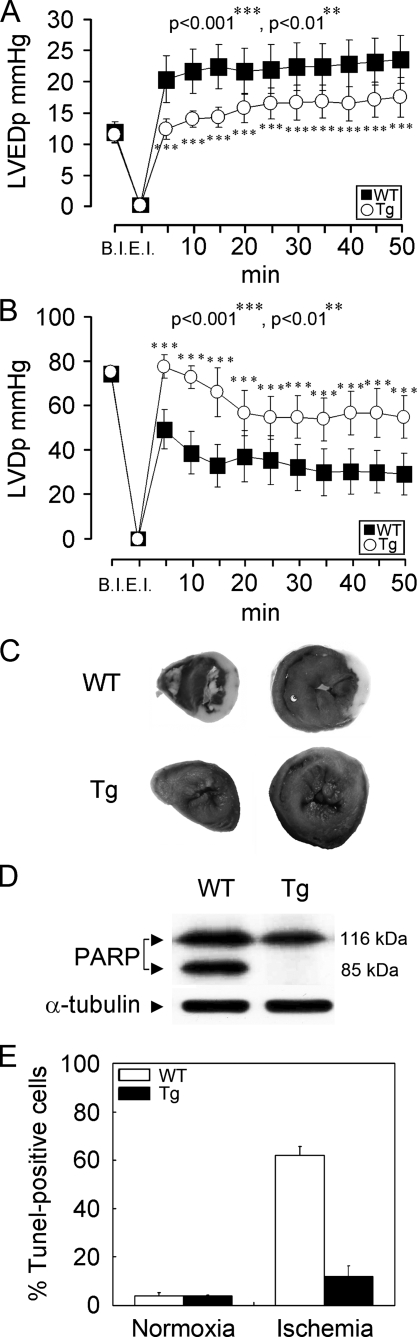
Hemodynamic Function of the Isolated Hearts of ARC Tg Mice—Under normal living conditions, ARC Tg mice displayed no apparent failure of the heart after a 12-month follow-up. To investigate the cardiac function of ARC, the hemodynamic parameters were recorded for 50 min of reperfusion after global ischemia. The baseline heart rates were similar between two groups, WT and ARC Tg mice.4 Compared with WT mice, however, the left ventricular development pressure (LVDp) and the left ventricular end-diastolic pressure (LVEDp) were significantly improved in the hearts of ARC Tg mice (Fig. 2, A and B). These findings indicate that ARC functions to suppress ischemia/reperfusion-induced deterioration of the Langendoff preparation exhibiting cardiac dysfunction and overt heart failure.
FIGURE 2.
Cardiac tolerance of ARC Tg mice to ischemic damage. A and B, cardiac functional analysis. Time course of left ventricular-developed pressure (LVDp) (A) and left ventricular end diastolic pressure (LVEDp) (B). Hearts were isolated from 8–12-week-old WT (filled square) and ARC Tg (open circle) mice. After stabilization for 20 min, the hearts were subjected to global ischemia for 30 min and then reperfused for the indicated times. Pressure gradients are representative for n = 7 per group. B.I., before ischemia; E.I., end ischemia. p values denote statistic significance with Student's t test. ***, p < 0.0001; **, p < 0.01. C–E, ischemic damage analysis. Representative infarct-injured hearts of WT and ARC Tg mice. After 1 h of ischemia followed by 24 h of reperfusion, the hearts were isolated and stained with 2,3,5-triphenyltetrazolium chloride (C). D, Western blot analysis of poly(ADP-ribose)polymerase (PARP) cleavage. The lysates were prepared from infarct-injured hearts as described in C. Frozen sections of the injured heats were stained with TUNEL assay and TUNEL-positive cells were quantitated. Bars depict mean ± S.E. (E).
We then assessed the myocardial infarct after occluding left anterior descending coronary artery followed by reperfusion for 24 h. Total left ventricular area, the area at risk, and the infarct area were measured. Large infarcts were observed in the hearts of WT mice (Fig. 2C). Infarct size (infarct/risk area) was significantly reduced in Tg mice compared with WT mice (about 40%).4 This was accompanied by a significant decrease in apoptosis as measured with proteolytic cleavage of poly-(ADP-ribose)polymerase, a hallmark of apoptosis (Fig. 2D). Similarly, an evaluation of death rates using the TUNEL assay revealed reduced cell death (5.2-fold decrease) in the hearts of ARC Tg mice compared with WT mice during ischemic damages (Fig. 2E).
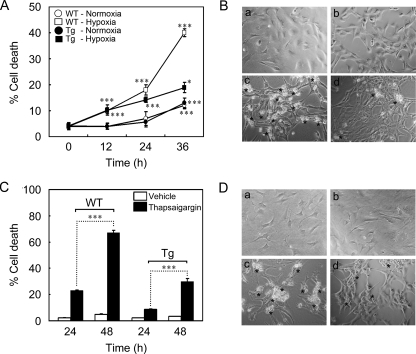
Suppression of Hypoxia-induced Cell Death by ARC in Cardiomyocytes—We then addressed sensitivity of the primary cardiomyocytes isolated from ARC Tg mice to hypoxia-induced cell death. Although the incubation of control cardiomyocytes in the hypoxic conditions induced 42% of cell death at 36 h, the hypoxic death rate in the same condition decreased to 20% in the cardiomyocytes prepared from ARC Tg mice (Fig. 3, A and B). These results show that the primary cardiomyocytes expressing ARC derived from ARC Tg mice are resistant to hypoxic cell death in vitro.
FIGURE 3.
Increased resistance of the primary cardiomyocytes cultured from ARC Tg mice to hypoxic damages. A and B, hypoxic cell death of primary cardiomyocytes. Cardiomyocytes were prepared from neonatal WT and ARC homozygous Tg mice, cultured for 3 days in vitro, and then left untreated or exposed to the hypoxic condition for the indicated times. Asterisks indicate dying cells under hypoxia at 36 h (B). Cell death assays were performed using Live/Dead viability kit as described under “Materials and Methods.” C and D, tolerance of ARC cardiomyocytes to thapsigargin-induced cell death. Cardiomyocytes cultured from neonatal WT and ARC Tg mice were exposed to thapsigargin (3 μm) for the indicated times and death rates were determined (C) as described in A. Asterisks indicate dying cells at 48 h (D). Bars represent mean ± S.E. of three independent experiments. *, p < 0.01; **, p < 0.001; ***, p < 0.0001 versus control.
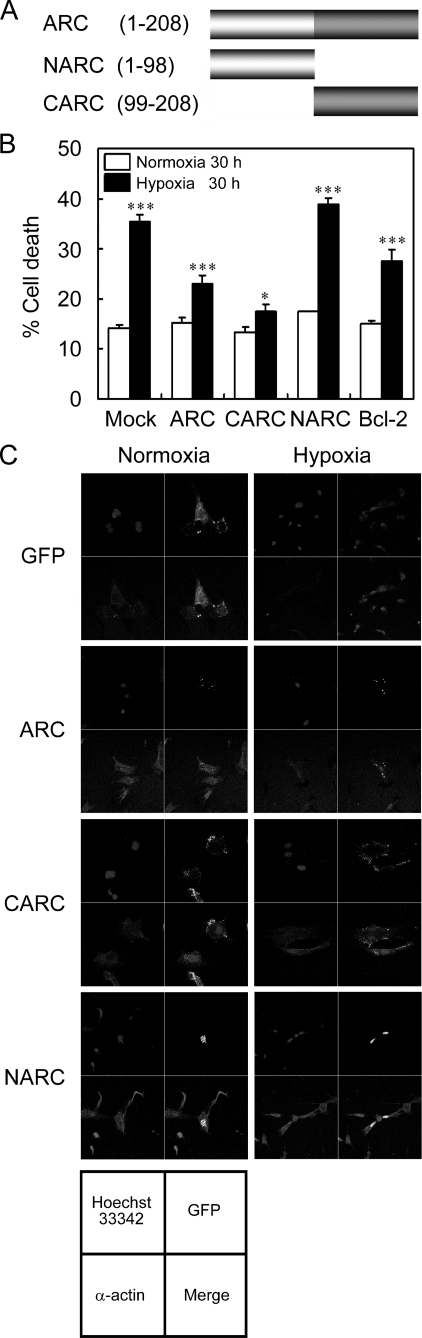
Previously, we found that ARC may be a calcium-binding protein (25). Thus, we addressed whether ARC affects calcium overload-induced cell death of the primary cardiomyocytes. Incubation with thapsigargin, an inhibitor of Ca2+-ATPase in the endoplasmic reticulum, induced 67% of cell death in WT cardiomyocytes but 30% in the cardiomyocytes cultured from the neonatal ARC Tg mice, indicating that ARC suppresses calcium overload-induced death of cardiomyocytes (Fig. 3, C and D). We then analyzed the domain(s) of ARC responsible for the anti-hypoxic cell death activity using its deletion mutants (Fig. 4). The primary cardiomyocytes transiently transfected with ARC or ARC C terminus rich in proline and glutamate (Pro/Glu-rich domain) (CARC, amino acid residues, 99–208) were resistant to hypoxic cell death to a degree comparable with that of Bcl-2, an anti-apoptotic protein, whereas the ARC N terminus (CARD domain) (NARC, amino acid residues, 1–98) failed to show such inhibitory effects on hypoxic cell death (Fig. 4, B and C). These results indicate that ARC exhibits anti-hypoxic cell death activity in cardiomyocytes through its C-terminal Pro/Glu-rich region, a calcium-binding region.
FIGURE 4.
Domain mapping of ARC regulating hypoxia-induced death of cardiomyocytes. A, schematic representation of ARC and its deletion mutants (NARC and CARC). CARD and Pro/Glu-rich domain of ARC are illustrated and the numbers indicate amino acid residues. B, suppression of hypoxia-induced death of cardiomyocytes by ARC Pro/Glu-rich domain. Cardiomyocytes were cultured for 3 days in vitro and then transfected with GFP (Mock) and GFP-fused ARC, CARC, NARC, or Bcl-2 for 24 h. Cells were then exposed to the hypoxic condition for 30 h and examined for cell viability based on the morphology of GFP-positive cells under fluorescence microscope. Bars represent mean ± S.E. of three independent experiments (B). *, p < 0.01; **, p < 0.001; ***, p < 0.0001 versus control. C, expression pattern of GFP-fused ARC and its deletion mutants. Cardiomyocytes were transfected with either GFP or GFP-fused ARC, CARC, and NARC for 24 h and then left untreated or exposed to the hypoxic condition for 30 h. Cells were immunostained with anti-α-actin antibodies, a positive control of cardiac myocytes, and Hoechst 33342 (nuclei). The samples were examined under a confocal laser-scanning microscope. Green fluorescent protein, green; α-actin; red, nuclei, blue.
Altered Profiles of Gene Expression in the Heart of ARC Tg Mice—Given the vast number of effectors that might potentially influence the survival versus the apoptotic decision of myocardial cells after ischemia-reperfusion injury, we performed a large-scale genomic screening for altered gene expression. Specifically, hearts harvested from 3- and 30-week-old control and ARC Tg mice were analyzed for gene expression profiling using the GenePix 4000B array. This array contains all of the genes in the murine Unigene data base that have been functionally characterized (∼40,000). Heart samples were cross-compared between WT and ARC Tg mice, resulting in 170 genes being significantly detected in one or more groups after internal normalization. Of these genes, 39 genes exhibited significantly altered expression between the hearts of ARC Tg and WT mice (Table 1).
TABLE 1.
Relative expression ratios of the selected groups of genes assessed by RNA microarray analysis Cardiac RNA was collected from two wild type and two ARC Tg mice (Tg 1, 3-week-old; Tg 2, 30-week-old) and subjected to expression profiling using the GenePix 4000B array. –Fold change filters include the requirement that the genes be present in at least 200% of the controls for up-regulated genes and in less than 50% of the controls for down-regulated genes. Absolute normalized expression data are shown for each sample in the right-hand columns along with the GenBank accession number.
|
Relative gene expression (ratio)
|
|||
|---|---|---|---|
| Tg 1 | Tg 2 | GenBank™ | |
| Enzyme activity | |||
| N-Acylsphingosine amidohydrolase (acid ceramidase)-like (Asahl) | 0.212107421 | 0.178740707 | NM_025972 |
| Carbonic anhydrase 3 | 2.805291579 | 2.709155955 | NM_007606 |
| Methionine aminopeptidase-like 1 | 2.21796869 | 1.83970689 | NM_025633 |
| Dimethylglycine dehydrogenase precursor (pMe2GlyDH) | 0.048853625 | 0.052353731 | NM_028772 |
| Adipsin | 2.954059736 | 1.977203728 | NM_013459 |
| Aminolevulinic acid synthase 2, erythroid (Alas2) | 2.160885548 | 2.036045855 | NM_009653 |
| 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (Pfkfb3), transcript variant 1 | 0.412229347 | 0.450426963 | NM_133232 |
| Unknown | |||
| Mus musculus RIKEN cDNA 4930550L24 gene | 0.252136945 | 0.466819994 | XM_621012 |
| Bromodomain and WD repeat domain containing 3 | 0.0485131 | 0.056798788 | XM_356350 |
| Bardet-Biedl syndrome 4 homolog (human) | 0.331905826 | 0.359647179 | NM_175325 |
| Protein binding | |||
| Neurofibromatosis 2 | 2.433380622 | 1.551598644 | NM_010898 |
| PREDICTED: DMRT-like family B with proline-rich C-terminal | 0.365815777 | 0.433494475 | XM_205469 |
| WW domain binding protein 1 | 0.302490345 | 0.373305013 | NM_016757 |
| Fras1-related extracellular matrix 2 | 2.064505125 | 1.519267093 | NM_172862 |
| Protocadherin β4 (Pcdhb4) | 2.004928795 | 1.566438906 | NM_053129 |
| Vitamin D receptor interacting protein (Vdrip) | 0.230963855 | 0.145157177 | NM_026119 |
| FYVE, RhoGEF, and PH domain containing 1 | 0.180371462 | 0.239611358 | NM_008001 |
| Ligand of numb-protein X 1 (Lnx1) | 0.389637699 | 0.367762517 | NM_010727 |
| Synuclein, α (Snca) | 2.01965798 | 1.90034372 | NM_009221 |
| Signal transducer | |||
| Period homolog 3 (Drosophila) (Per3) | 2.586591092 | 1.821002595 | NM_011067 |
| Regulator of G protein signaling 7 (Rgs7) | 2.1900124 | 1.738080612 | NM_011880 |
| Other | |||
| RIKEN cDNA 1600012F09 gene | 2.617133021 | 2.601587207 | NM_025904 |
| Ribosomal protein S6 kinase polypeptide 3 | 2.388833873 | 1.727797544 | NM_148945 |
| UDP-GlcNAc:βGal β-1,3-N-acetylglucosaminyltransferase 1 | 2.197476071 | 1.886600148 | NM_175383 |
| Signal recognition particle 54 | 2.070249964 | 1.75692098 | NM_028527 |
| PREDICTED: M. musculus similar to surface sperm protein P26h | 0.414372494 | 0.475688281 | CK137444 |
| RIKEN cDNA 1810008A14 gene | 0.412743742 | 0.499033244 | NM_025457 |
| Sad1 and UNC84 domain containing 1 | 0.395520299 | 0.300412406 | NM_177576 |
| Hypothetical protein 4931417A20 | 0.356462299 | 0.458295539 | NM_145380 |
| Par-3 (partitioning defective 3) homolog (Caenorhabditis elegans) | 0.327191914 | 0.354896792 | NM_031235 |
| Similar to transmembrane protein induced by tumor necrosis factor α | 0.237574687 | 0.283793964 | AK_132283 |
| RIKEN cDNA 2310056P07 gene | 0.234790079 | 0.175357973 | NM_027342 |
| RIKEN cDNA 4930451I11 gene | 0.233646847 | 0.2583342 | NM_183131 |
| RIKEN cDNA 4930563C04 gene, mRNA | 0.106973251 | 0.160551176 | NM_029231 |
| Transcription activity | |||
| Hairy and enhancer of split 7 (Drosophila) | 0.331072736 | 0.428150795 | NM_033041 |
| Ankyrin repeat and SOCS box-containing protein 12 | 0.327740074 | 0.367856146 | NM_080858 |
| Nucleic acid binding | |||
| Developmentally regulated RNA binding protein 1 (Drbp1) | 4.843207045 | 4.596722765 | NM_153405 |
| G patch domain containing 3, mRNA | 0.230790527 | 0.329046637 | NM_172876 |
| Receptor activity | |||
| Leucine-rich repeat-containing G protein-coupled receptor 7 (Lgr7) | 2.405636205 | 2.106995516 | NM_212452 |
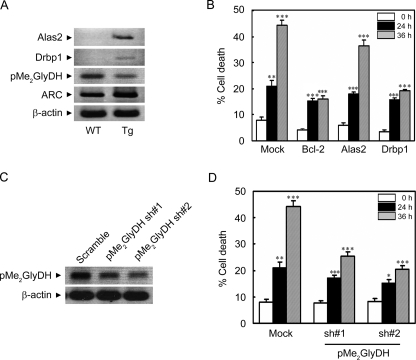
The raw data shown in Table 1 represent the 2n relationship of expression between 3- and 30-week-old ARC Tg mice hearts compared with those of WT mice. The expressions of 23 genes, including the dimethylglycine dehydrogenase precursor (pMe2GlyDH), bromodomain, WD repeat domain containing 3, RIKEN cDNA 4930563C04, and FYVE RhoGEF were significantly down-regulated in the hearts of ARC Tg mice. On the contrary, 16 genes, including the developmentally regulated RNA-binding protein 1 (Drbp1), Alas2, and carbonic anhydrase 3, exhibited increased expression more than 2-fold in the hearts of ARC Tg mice. The data of the microarray analysis were confirmed by RT-PCR analysis (Fig. 5A). Compared with control, the level of Alas2 and Drbp1 mRNAs was increased in the hearts of ARC Tg mice, whereas the precursor form of pMe2GlyDH, the substrate for mitochondria-associated flavinylation reaction, was decreased.
FIGURE 5.
Regulation of ischemic/hypoxic cell death by Drbp1 and pMe2GlyDH. A, RT-PCR analysis showing expressional regulation of Alas2, Drbp1, and pMe2GlyDH in the hearts of ARC Tg mice. Total RNA was isolated from the hearts of 6-week-old WT and ARC Tg mice. RT-PCR analysis was performed using gene-specific synthetic oligonucleotides. β-Actin was used as an internal control. B, suppression of hypoxic death of the primary cardiomyocytes by the ectopic expression of Drbp1. Cardiomyocytes were cultivated from neonatal mice, cotransfected with pGFP, and pcDNA (Mock), pBcl-2, pAlas2, or pDrbp1 for 48 h, and then left untreated or exposed to the hypoxic condition for the indicated times. Determination of cell death was assessed based on the morphology of green fluorescent protein-positive cells under a fluorescence microscope. Bcl-2 was employed as a positive control. Bars represent mean ± S.E. of three independent experiments. C and D, reduced expression of pMe2GlyDH protects cardiomyocytes from hypoxic cell death. Neonatal cardiomyocytes were cultured from WT mice, cultivated for 3 days in vitro, and transfected with pMe2GlyDH-shRNA (sh) numbers 1 or 2 for 48 h. Total RNA was isolated and subjected to RT-PCR analysis to examine the expression of pMe2GlyDH (C). After transfection with pMe2GlyDH-shRNA, the cells were left untreated or exposed to the hypoxic condition for 24 or 36 h (D). Cell death were examined as described in B.*, p < 0.01; **, p < 0.001; ***, p < 0.0001 versus control.
We then examined the contribution of the expressional regulation of Alas2, Drbp1, and pMe2GlyDH to hypoxic cell death. Interestingly, ectopic expression of Drbp1, but not Alas2, protected the primary cardiomyocytes from hypoxic cell death as much as Bcl-2 especially at the later time point (36 h) (Fig. 5B). On the contrary, the reduction of pMe2GlyDH expression using shRNAs (numbers 1 and 2) effectively suppressed hypoxic death of cardiomyocytes, reducing death rates from 45 to 21% at 36 h (Fig. 5, C and D). These results suggest that the cardioprotective effects of ARC in the ARC Tg mice may be contributed by the expressional regulation of its downstream mediators, Drbp1 and pMe2GlyDH.
DISCUSSION
Much progress has been made over the last decade in understanding the genetic and biochemical functions of ARC in vitro and in vivo (11, 13, 14, 15, 25). In the present study, we demonstrated that ARC, especially the C terminus calcium-binding domain, and its downstream mediators play an important role in heart by protecting cardiomyocytes from ischemia/hypoxia-induced cell death. Major findings from our analysis using ARC Tg mice and cultured cardiomyocytes support this conclusion. First, the hearts from the ARC Tg mice exhibited improved recovery of contractile performance during reperfusion after ischemia. Second, consistent with the reduced ischemic area and cell death in the hearts of ARC Tg mice, cultured cardiomyocytes from neonatal ARC Tg were more resistant to hypoxic injury compared with control. Third, ectopic expression of the C-terminal Pro/Glu-rich region of ARC protected the cardiomyocytes from hypoxic cell death. Fourth, the expressional regulation of Drbp1 and pMe2GlyDH in the heart of ARC Tg mice may be associated with the anti-ischemic/hypoxic cell death activity of ARC.
Ischemic/hypoxic cell death in neuronal cells is associated with an intracellular burst of calcium overload. However, a role of calcium in ischemic death of cardiomyocytes is not well established yet. Recently, we proposed that ARC binds to calcium in vitro and that it can lower the intracellular calcium transient and calcium accumulation triggered by treatments with ATP and thapsigargin, respectively (25). In particular, the C-terminal Pro/Glu-rich region of ARC binds to calcium, whereas the N-terminal CARD is believed to be involved in protein-protein interaction of the proteins containing CARD. Our observations that the C-terminal Pro/Glu-rich region of ARC suppressed cardiac cell death triggered by hypoxia suggest that the calcium binding ability of ARC may be responsible for its anti-ischemic/hypoxic activity in the cardiomyocytes. Similarly, the C terminus of ARC also exhibited neuroprotective activity against hypoxic injury (25). Also, our proposal may explain that Nop30, an alternative splicing variant of ARC containing the common N-terminal CARD but lacking the divergent C terminus, failed to protect cardiomyocytes from ischemic/hypoxic cell death.4
In an effort to find the downstream mediator(s) of ARC in hearts, we also found that ARC induced the alteration in gene expression profiles that may be associated with the increased resistance of ARC Tg cardiomyocytes to ischemic/hypoxic injury. Among them, Drbp1 was increased 4–5-fold in the heart of ARC Tg mice and was very potent to suppress ischemic/hypoxic death of the primary cardiomyocytes. Drbp1 is relatively abundant in heart and known to bind tightly to RNA stability elements (AU-rich elements) in the 3′-untranslated region of various mRNAs through four RRM-type RNA-binding domains (26). Therefore, this protein may be induced to stabilize some fragile mRNAs that are required for cell survival of the cardiomyocytes in ARC Tg mice during hypoxic damage. In addition, the increased expression of Alas2 in the heart of ARC Tg mice reflects its HIF1α-independent increase in the brain during hypoxia (27). Although Alas2 regulates mRNAs encoding the p45 subunit of NF-E2, β-globin, and enzymes in the heme biosynthetic pathway that shows resistance to cerebral and myocardial ischemia of Tg mice of neuroglobin (28, 29), it may not be relevant to support the tolerance of the cardiomyocytes of ARC Tg mice to ischemic/hypoxic cell death.
On the contrary, down-regulation of pMe2GlyDH was evident in the heart of ARC Tg mice and pMe2GlyDH-shRNA protected the primary cardiomyocytes from hypoxic damage. Interestingly, of patients with cardiomyopathy and myocarditis, 36 and 25%, respectively, were anti-flavoenzyme-positive by Western blot and enzyme-linked immunosorbent assay, but only 13% of patients with other heart diseases and none of healthy controls were positive (30). Thus, as a substrate for mitochondria-associated flavinylation reaction, pMe2GlyDH deserves more characterization as a possible risk factor of heart diseases. Furthermore, together with detailed characterization, such global regulation of gene expression in the heart will be valuable to understand the signaling pathway of cardiac protection against ischemic injury. In the cases of ARC, the cardioprotective activity of ARC against ischemic/hypoxic cell death may then converge into blocking the release of cytochrome c from mitochondria (15).
Taken together, our results suggest that the cardiac expression of ARC suppresses ischemic/hypoxic injury of cardiomyocytes through its C-terminal calcium-binding domain and expressional regulation of Drbp1 and pMe2GlyDH, adding valuable molecules to our understanding about the ischemic/hypoxic damages of cardiomyocytes.
Acknowledgments
We thank Dr. H. Endo (Jichi Medical School, Japan) for Drbp1.
This work was supported in part by the Brain Research Center of 21C Frontier, Ubiquitome, CRI of the Korea Science and Engineering Foundation, and a Basic Science grant of the Korean Research Foundation (to Y. K. J.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ARC, apoptosis repressor with a caspase recruitment domain; CARD, caspase recruitment domain;H&E, hematoxylin and eosin; LVDp, left ventricular development pressure; LVEDp, left ventricular end-diastolic pressure; MHC, myosin heavy chain; Tg, transgenic; shRNA, short hairpin RNA; WT, wild type; RT, reverse transcriptase; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling.
J.-O. Pyo, J. Nah, H.-J. Kim, J.-W. Chang, Y.-W. Song, D.-K. Yang, D.-G. Jo, H.-R. Kim, H.-J. Chae, S.-W. Chae, S.-Y. Hwang, S.-J. Kim, H.-J. Kim, C. Cho, C.-G. Oh, W. J. Park, and Y.-K. Jung, unpublished observations.
References
- 1.Thompson, C. B. (1995) Science 267 1456-1462 [DOI] [PubMed] [Google Scholar]
- 2.Bishopric, N. H., Andreka, P., Slepak, T., and Webster, K. A. (2001) Curr. Opin. Pharmacol. 1 141-150 [DOI] [PubMed] [Google Scholar]
- 3.Dawn, B., and Bolli, R. (2002) Ann. N. Y. Acad. Sci. 962 18-41 [DOI] [PubMed] [Google Scholar]
- 4.Aoki, H., Kang, P. M., Hampe, J., Yoshimura, K., Noma, T., Matsuzaki, M., and Izumo, S. (2002) J. Biol. Chem. 277 10244-10250 [DOI] [PubMed] [Google Scholar]
- 5.Bueno, O. F., and Molkentin, J. D. (2002) Circ. Res. 91 776-781 [DOI] [PubMed] [Google Scholar]
- 6.Matsui, T., Li, L., Wu, J. C., Cook, S. A., Nagoshi, T., Picard, M. H., Liao, R., and Rosenzweig, A. (2002) J. Biol. Chem. 277 22896-22901 [DOI] [PubMed] [Google Scholar]
- 7.Miao, W., Luo, Z., Kitsis, R. N., and Walsh, K. (2000) J. Mol. Cell. Cardiol. 32 23397-23402 [DOI] [PubMed] [Google Scholar]
- 8.Yamashita, K., Kajstura, J., Discher, D. J., Wasserlauf, B. J., Bishopric, N. H., Anversa, P., and Webster, K. A. (2001) Circ. Res. 88 609-614 [DOI] [PubMed] [Google Scholar]
- 9.Brar, B. K., Stephanou, A., Pennica, D., and Latchman, D. S. (2001) Cytokine 16 93-96 [DOI] [PubMed] [Google Scholar]
- 10.Hamacher-Brady, A., Brady, N. R., Logue, S. E., Sayen, M. R., Jinno, M., Kirshenbaum, L. A., Gottlieb, R. A., and Gustafsson, A. B. (2007) Cell Death Differ. 14 146-157 [DOI] [PubMed] [Google Scholar]
- 11.Koseki, T., Inohara, N., Chen, S., and Nunez, G. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 5156-5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekhterae, D., Lin, Z., Lundberg, M. S., Crow, M. T., Brosius, F. C., 3rd, and Nunez, G. (1999) Circ. Res. 85 e70-e77 [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee, S., Bish, L. T., Jayasankar, V., Stewart, A. S., Woo, Y. J., Crow, M. T., Gardner, T. J., and Sweeney, H. L. (2003) J. Thorac. Cardiovasc. Surg. 125 1461-1469 [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson, A. B., Sayen, M. R., Williams, S. D., Crow, M. T., and Gottlieb, R. A. (2002) Circulation 106 735-739 [DOI] [PubMed] [Google Scholar]
- 15.Donath, S., Li, P., Willenbockel, C., Al-Saadi, N., Gross, V., Willnow, T., Bader, M., Martin, U., Bauersachs, J., Wollert, K. C., Dietz, R., and von Harsdorf, R. (2006) Circulation 113 1203-1212 [DOI] [PubMed] [Google Scholar]
- 16.Gulick, J., Subramaniam, A., Neumann, J., and Robbins, J. (1991) J. Biol. Chem. 266 9180-9185 [PubMed] [Google Scholar]
- 17.Jo, D. G., Lee, J. Y., Hong, Y. M., Song, S., Mook-Jung, I., Koh, J. Y., and Jung, Y. K. (2004) J. Neurochem. 88 604-611 [DOI] [PubMed] [Google Scholar]
- 18.Tian, R., Miao, W., Spindler, M., Javadpour, M. M., McKinney, R., Bowman, J. C., Buttrick, P. M., and Ingwall, J. S. (1996) Proc. Natl. Acad. Sci. U. S. A. 96 13536-13541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, Z., He, Y., Tuteja, D., Xu, D., Timofeyev, V., Zhang, Q., Glatter, K. A., Xu, Y., Shin, H. S., Low, R., and Chiamvimonvat, N. (2005) Circulation 112 1936-1944 [DOI] [PubMed] [Google Scholar]
- 20.Abmayr, S., Crawford, R. W., and Chamberlain, J. S. (2004) Hum. Mol. Genet. 13 213-221 [DOI] [PubMed] [Google Scholar]
- 21.Yet, S. F., Tian, R., Layne, M. D., Wang, Z. Y., Maemura, K., Solovyeva, M., Ith, B., Melo, L. G., Zhang, L., Ingwall, J. S., Dzau, V. J., Lee, M. E., and Perrella, M. A. (2001) Circ. Res. 89 168-173 [DOI] [PubMed] [Google Scholar]
- 22.Serviddio, G., Di Venosa, N., Federici, A., D'Agostino, D., Rollo, T., Prigigallo, F., Altomare, E., Fiore, T., and Vendemiale, G. (2005) FASEB J. 19 354-361 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura, T. Y., Goda, K., Okamoto, T., Nakamura, T., and Goshima, K. (1993) Circ. Res. 73 758-770 [DOI] [PubMed] [Google Scholar]
- 24.Irizarry, R. A., Warren, D., Spencer, F., Kim, I. F., Biswal, S., Frank, B. C., Gabrielson, E., Garcia, J. G., Geoghegan, J., Germino, G., Griffin, C., Hilmer, S. C., Hoffman, E., Jedlicka, A. E., Kawasaki, E., Martinez-Murillo, F., Morsberger, L., Lee, H., Petersen, D., Quackenbush, J., Scott, A., Wilson, M., Yang, Y., Ye, S. Q., and Yu, W. (2005) Nat. Meth. 2 329-330 [DOI] [PubMed] [Google Scholar]
- 25.Jo, D. G., Jun, J. I., Chang, J. W., Hong, Y. M., Song, S., Cho, D. H., Shim, S. M., Lee, H. J., Cho, C., Kim, D. H., and Jung, Y. K. (2004) Mol. Cell. Biol. 22 9763-9770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamada, H., Sakashita, E., Shimazaki, K., Ueno, E., Hamamoto, T., Kagawa, Y., and Endo, H. (2002) Biochem. Biophys. Res. Commun. 297 96-104 [DOI] [PubMed] [Google Scholar]
- 27.Meguro, K., Igarashi, K., Yamamoto, M., Fujita, H., and Sassa, S. (1995) Blood 86 940-948 [PubMed] [Google Scholar]
- 28.Hofer, T., Wenger, R. H., Ferreira, G. C., and Gassmann, M. (2003) Blood 101 348-350 [DOI] [PubMed] [Google Scholar]
- 29.Khan, A. A., Wang, Y., Sun, Y., Mao, X. O., Xie, L., Miles, E., Graboski, J., Chen, S., Ellerby, L. M., Jin, K., and Greenberg, D. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 17944-17948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto, A., Stahle, I., Klein, R., Berg, P. A., Pankuweit, S., and Brandsch, R. (1998) Clin. Exp. Immunol. 111 541-547 [DOI] [PMC free article] [PubMed] [Google Scholar]