Abstract
Respiratory proteins such as myoglobin and hemoglobin can, under oxidative conditions, form ferryl heme iron and protein-based free radicals. Ferryl myoglobin can safely be returned to the ferric oxidation state by electron donation from exogenous reductants via a mechanism that involves two distinct pathways. In addition to direct transfer between the electron donor and ferryl heme edge, there is a second pathway that involves “through-protein” electron transfer via a tyrosine residue (tyrosine 103, sperm whale myoglobin). Here we show that the heterogeneous subunits of human hemoglobin, the α and β chains, display significantly different kinetics for ferryl reduction by exogenous reductants. By using selected hemoglobin mutants, we show that the α chain possesses two electron transfer pathways, similar to myoglobin. Furthermore, tyrosine 42 is shown to be a critical component of the high affinity, through-protein electron transfer pathway. We also show that the β chain of hemoglobin, lacking the homologous tyrosine, does not possess this through-protein electron transfer pathway. However, such a pathway can be engineered into the protein by mutation of a specific phenylalanine residue to a tyrosine. High affinity through-protein electron transfer pathways, whether native or engineered, enhance the kinetics of ferryl removal by reductants, particularly at low reductant concentrations. Ferryl iron has been suggested to be a major cause of the oxidative toxicity of hemoglobin-based blood substitutes. Engineering hemoglobin with enhanced rates of ferryl removal, as we show here, is therefore likely to result in molecules better suited for in vivo oxygen delivery.
The physiological requirement that hemoglobin (Hb)3 and myoglobin (Mb) bind oxygen reversibly to a redox active metal (FeII) can give rise to undesired redox reactions. When such reactions involve peroxides, they lead to the formation of high oxidation states of the iron and to free radicals. The ferryl (Fe(IV)=O) derivatives of Mb and Hb are known to form whenever these heme proteins escape their normal cellular environments and the protective medium of the plasma and have been implicated in oxidative stress in a range of disease states, including rhabdomyolysis and subarachnoid hemorrhage (1–3). Ferryl Hb formation has also been implicated in apoptotic cell death (4), and the ferryl states of Mb and Hb can catalyze the formation of lipid oxidation products that are potent vasoactive compounds, such as the isoprostanes (2). Demonstrating that this redox chemistry occurs in vivo has proven difficult. Nevertheless, ferryl Mb has been identified in isolated ischemic rat hearts using addition of sodium sulfide that reacts with ferryl heme to give a distinctive optical band (5–7). Covalent modifications of Mb and Hb have been detected in vivo following Mb release from muscle cells (rhabdomyolysis) and hemolysis following subarachnoid hemorrhage (8). Formation of a heme to the protein covalent bond between the heme and the globin is a specific marker for oxidative stress in vivo, diagnostic of prior involvement of ferryl heme (9, 10), and implies participation of these highly oxidative species in the pathological complications that follow. Heme to protein cross-linking requires peroxide, and its formation is enhanced under conditions of acidosis (11). Cross-links have not been found in normal blood, probably because of the antioxidant defenses present and the regulation of blood pH (11). However, chlorin-type hemes, resulting from oxidative modifications to Hb, have been detected in blood and have been shown to increase with exercise (12). Similarly the tyrosine radical found on Hb following the reaction of the ferric Hb with H2O2, forming the ferryl species, has been detected in normal human blood (13, 14). Hb redox changes in vivo appear to be a fundamental part of the normal physiological, if unwanted, process. It is not unrealistic, therefore, to suggest that these redox processes occur when cell-free Hb is infused into patients as an artificial blood substitute and could add to their general cytotoxicity. Thus the fundamental mechanisms by which the protein handles and detoxifies the high ferryl oxidation state is a subject of medical and biotechnological interest.
Previously we have reported the mechanism by which ferryl Mb and Hb can be reduced to the ferric form by a wide range of organic compounds (15–17). Through the use of engineered Mb, we have shown the presence of two distinct pathways for electron transfer between the reductant and the ferryl heme iron (18). One, we suggest, involves direct electron transfer between the reductant and the heme edge (18). The affinity for reductants transferring electrons by this mechanism is low, dissociation constants being typically in the range of several millimolar. The other pathway transfers electrons to the ferryl heme iron via a tyrosine residue (Tyr-103, sperm whale, human, horse Mb). This tyrosine is close to the heme but is also surface-exposed, allowing an interface between the heme iron and bulk medium. This pathway of electron transfer exhibits high apparent affinity for reductants, generally in the low micromolar concentration range. As a consequence, at low concentrations of reductant, the rates of reduction of Mb(s) that possess this pathway is enhanced severalfold compared with proteins that lack this tyrosine pathway.
We have extended these studies and now report the results of experiments designed to elucidate the mechanism through which ferryl Hb is reduced by organic reductants. We have previously shown that the reduction of ferryl heme iron in Hb follows a double exponential time course. Here we demonstrate that the two kinetic phases arise from different rate constants for reduction of the α and β chains. The α chain behaves in a fashion similar to Mb, and at low reductant concentrations, reduction is much faster than the β chain. The dependence of the rate constant for reduction of the α chain on reductant concentration follows a double rectangular hyperbolic profile, as observed in Mb. The β chain does not exhibit this profile. We propose that the kinetic behavior of the chains is because of the presence of a tyrosine in the α chain (αTyr-42, C-7, analogous to Tyr-103 of sperm whale Mb) that is both close to the heme and is surface-exposed. This redox active residue is absent from the β chain, the equivalent position being occupied by a redox inactive phenylalanine (βPhe-41, C-7). To test this hypothesis we have generated a number of mutants of Hb that substitute the specific tyrosine in the α chain or introduce this residue at the homologous position in the β chain. Our results demonstrate that possession of a suitably located tyrosine provides a pathway for reduction of ferryl heme by organic reductants. We are therefore able to switch the electron transfer properties of the two chains or produce an Hb molecule in which both chains possess the pathway. This tyrosine-dependent pathway allows ferryl reduction to proceed much more rapidly at low reductant concentrations than can be achieved through direct donation to the heme edge, which occurs irrespective of the presence or absence of the specific tyrosine. The dependence of “through-protein” electron transfer on the presence of a tyrosine residue runs counter to expectations based on general outer sphere mechanisms (e.g. Marcus theory) and implies that the tyrosine residue is acting as true redox co-factor, presumably via a radical mechanism. We propose that the manipulation of such electron transfer pathways in engineered Hb-based blood substitutes could lead to a partial detoxification through attenuation of the oxidative activity of the high redox state of Hb in the presence of micromolar concentrations of reductant.
EXPERIMENTAL PROCEDURES
Materials—Equine heart Mb, catalase, and desferrioxamine mesylate were purchased from Sigma. High pressure liquid chromatography grade acetonitrile and trifluoroacetic acid were from Fisher. CP20 (deferriprone, 1,2-dimethyl-3-hydroxypyrid-4-one) was given by Prof. Robert Hider, Department of Pharmacy, Kings College, London, UK.
Cloning and Expression of Human Hemoglobin—The genes for human Hb α and β chains were optimized for expression in Escherichia coli and cloned into the vector pETDuet resulting in HbpETDuet (19). HbpETDuet was a kind gift from Prof. Andrea Bellelli, Universitá di Roma “La Sapienza,” Italy. Mutant variants of the α and β genes were created using site-directed mutagenesis. Primers were purchased from MWG Biotec (Ebersberg, Germany). Primer sequences can be found in Table 1. A high fidelity enzyme, either Phusion (Finnzymes Oy, Espoo, Finland) or Pfu Ultra (Stratagene, La Jolla), was used according to the suppliers' specifications in PCRs with the following program: 95 °C for 2 min, 95 °C for 30 s, 55 °C for 1 min, 72 °C for 5 min, 16 cycles; 72 °C for 10 min. The template DNA was then digested using DpnI (Fermentas, Helsingborg, Sweden) and mutated plasmid transformed into E. coli BL21 DE3 using standard procedures (20). Resulting clones were sequenced with BigDye terminator version 3.1 (Applied Biosystems, Warrington, UK) to confirm correct sequence.
TABLE 1.
Primer sequences used for site-directed mutagenesis with relevant codons in boldface
| Primer | Sequence (5′–3′) |
|---|---|
| αY42V forward | CCTTCCCAACCACCAAAACCGTGTTCCCACACTTTGATCTG |
| αY42V reverse | CAGATCAAAGTGTGGGAACACGGTTTTGGTGGTTGGGAAGG |
| αY42 random forward | CCTTCCCAACCACCAAAACCNNKTTCCCACACTTTGATCTG |
| αY42 random reverse | CAGATCAAAGTGTGGGAAMNNGGTTTTGGTGGTTGGGAAGG |
| βF41Y forward | CCGTGGACCCAGCGTTACTTTGAATCCTTCGGTG |
| βF41Y reverse | CACCGAAGGATTCAAAGTAACGCTGGGTCCACGG |
E. coli BL21 DE3 harboring the plasmid HbpETDuet was grown in 2-liter Erlenmeyer flasks containing 1 liter of TB medium with 100 μg/ml carbenicillin at 37 °C and 120 rpm until A620 ≥1. Expression of Hb was then induced by adding 0.1 mm isopropyl 1-thio-β-d-galactopyranoside, 0.3 mm δ-aminolevulinic acid, and CO gas. Culture conditions after induction were 22 °C and 60 rpm. Cells were harvested and resuspended in 10 mm sodium phosphate buffer, pH 6.0, before sonication. Following centrifugation for 1 h at 20,000 rpm, the supernatant was adjusted to pH 6.2 and filtrated using a 0.45-μm Minisart filter (Sartorius). The Hb was purified using ion exchange chromatography with CM-Sepharose FF (GE Healthcare). After sample application, the column was washed with 10 mm sodium phosphate buffer, pH 6.0, until the absorbance returned to base line. The Hb was eluted with 70 mm sodium phosphate buffer, pH 7.2, and concentrated using Viva-Spin columns (Vivascience, 30-kDa molecular mass cutoff). The concentrated sample was then applied to a Sephacryl S-200 gel filtration column (GE Healthcare) using elution buffer on an ÄKTA purifier system. Globin-containing fractions were concentrated as above, flash-frozen in liquid nitrogen, and stored at -80 °C.
Prior to experimentation, the Hb was oxidized to the ferric form by the addition of a 1.5 m excess potassium ferricyanide following CO removal by shining light on the sample with gentle oxygenation using a stream of oxygen gas. Ferri-ferrocyanide was removed by filtration through a Sephadex G-25 column (10 × 1 cm). Concentration of Hb was determined from reduction of an aliquot of the ferric Hb using sodium dithionite to the deoxy form (ε430 nm = 133 mm-1 cm-1 (21)).
Measurement of Rate Constant for Ferryl Decay—Optical spectra of samples were measured using an Agilent 8453 spectrophotometer. Ferric Hb (20 μm) in 5 mm sodium phosphate buffer, pH 7.4, was reacted with H2O2 (100 μm) at 25°C for 9 min. At this time conversion of ferric Hb to ferryl Hb was greater than 95% as measured optically. Catalase (10 nm) was added to remove excess H2O2 as this was left to react for a further 1 min. Reductant was then added in 0.1 m sodium phosphate buffer, pH 7.4, in a 1:1 volume ratio so that final concentration of Hb was 10 μm. The optical spectrum was followed until reaction was complete. The time course (425–406 nm) was fitted to a double exponential function minimizing the least squares using the Microsoft Excel Solver program. Each set of rate constants resulting from reduction of α-Hb or β-Hb was then plotted as a function of reductant concentration, and this profile was fitted (least squares method) to a double rectangular hyperbola (17).
Electron Paramagnetic Resonance—Hb (80 μm) was reacted with 0.8 mm H2O2 and transferred (250 μl) to Wilmad SQ EPR tubes (Wilmad Glass, Buena, NJ). Tubes were flash-frozen in dry ice-cooled methanol. Frozen samples were transferred to liquid nitrogen (77 K) where they were stored prior to measurements. All EPR spectra were measured using a Bruker EMX EPR spectrometer (X-band) equipped with a spherical high quality resonator SP9703 and an Oxford Instruments liquid helium system. The modulation frequency was 100 kHz. Accurate g values were obtained using the built-in microwave frequency counter and a 2,2-diphenyl-1-picrylhydrazyl powder standard, the g value for which is g = 2.0037 ± 0.0002 (22). Other instrumental settings are in the figure legends and have the following abbreviations: microwave frequency ν (GHz); microwave power P (mW); modulation amplitude Am (G); spectra sweep rate V (G/s); time constant τ (ms); number of scans per spectrum NS. The EPR spectra of the blank samples (frozen water) were subtracted from the EPR spectra of the protein samples to eliminate the base line caused by the walls of the resonator, quartz insert, or quartz EPR tube. The g = 2 component of the high spin met heme signal has been subtracted from the EPR spectra of the protein radicals.
RESULTS
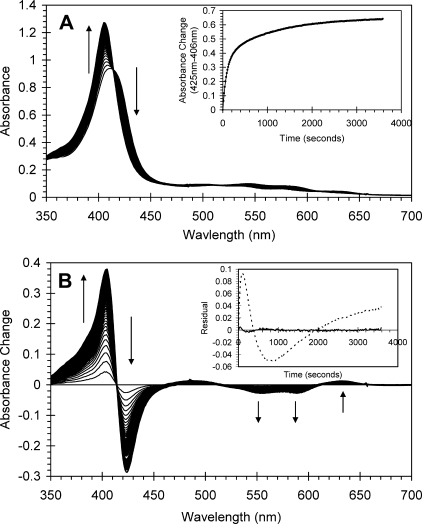
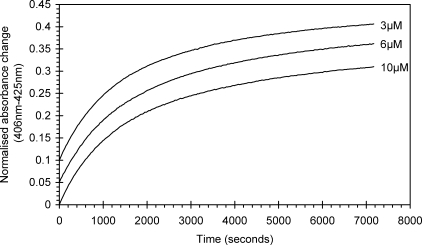
The optical changes following the addition of ascorbate (100 μm) to the ferryl derivative of authentic Hb (10 μm) are shown in Fig. 1. These are typical of the reduction of ferryl to ferric Hb, i.e. a decrease in the absorbance shoulder at 425 nm and a concurrent increase at 406 nm. Changes in the visible region are also observed, namely a decrease in the ferryl bands at 545 and 580 nm and an increase in absorbance at 630 nm, the latter indicative of the high spin ferric protein. All mutants studied showed optical spectra and changes in these that were essentially identical to those described for the wild type protein (data not shown). The time course for the reduction of wild type ferryl Hb is also shown in Fig. 1. This may best be fitted to the sum of two exponentials rather than the single exponential that is typical for Mb as illustrated by the residuals to the fits given in Fig. 1B. Fig. 1A, inset, shows an example time course for the reaction of ascorbate with ferryl Hb. The biphasic reduction kinetics of ferryl Hb does not arise from different and distinct reduction kinetics of Hb tetramers and αβ dimers that are together in solution in equilibrium because the relative amplitudes of the two phases are independent of protein concentration, the fast phase comprising 48.8% (±0.5%, n = 7). The dimer-tetramer equilibrium is concentration-dependent, with a dissociation constant (K4,2) of ∼2 μm for met-HbA (23). We therefore expect that 14% of the heme would be in tetramers for 3 μm Hb as opposed to 64% for 10 μm Hb. Thus the time courses for reduction of ferryl Hb at these two concentrations would be discernibly different in their relative phase amplitudes were these phases to be due to different kinetics for the dimer and tetramer (Fig. 2).
FIGURE 1.
Optical transitions following addition of ascorbate to ferryl human hemoglobin. Ferryl hemoglobin (10 μm) was generated by addition of hydrogen peroxide (30 μm) to ferric hemoglobin at pH 7.4, and excess peroxide was removed by the addition of catalase (10 nm). Ascorbate was added to a final concentration of 100 μm. A, absorbance spectra were taken every 15 s subsequent to ascorbate addition. Inset, time course (425–406 nm). B, difference spectra taken from A where initial ferryl spectrum has been set to zero. Inset, residuals from the fit of the time course to a single exponential (dotted line) or a double exponential (solid line).
FIGURE 2.
Normalized time courses for auto-reduction of ferryl hemoglobin. Auto-reduction of ferryl hemoglobin (3, 6, and 10 μm) was monitored optically at 406 and 425 nm. All time courses were normalized, and all can be described by a double exponential function. Time courses are offset for clarity.
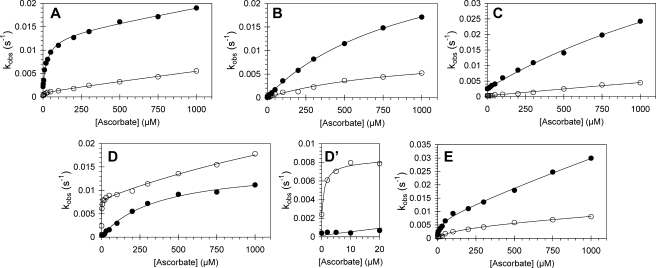
We have previously shown that a specific tyrosine residue in Mb (Tyr-103) dramatically alters the kinetics of ferryl reduction. A homologous residue (Tyr-42) is present in the α chain of Hb but not in the β chain. Therefore, to test the hypothesis that the biphasic time courses result from differences in the behavior of the α and β chains, we have constructed mutants in which the homologous tyrosine residues in Hb are altered. (i) Tyr-42 was substituted in the α chain (αY42V; αY42W); (ii) Tyr-42 was removed from the α chain and a tyrosine residue inserted into the β chain at position 41 (αY42V,βF41Y), thus switching the chains in this regard; and (iii) the α chain remained wild type, whereas a tyrosine was introduced into the β chain (βF41Y), thus producing an Hb molecule with tyrosine residues equivalent to Mb Tyr-103 in both chains. Fig. 3 shows the kinetics of ferryl Hb reduction as a function of ascorbate concentration for recombinant wild type human Hb (WTHb) and the mutant proteins. In Fig. 3A the results for reduction of ferryl WTHb by ascorbate are presented; note that the non-zero rate constant in the absence of ascorbate for both the fast and slow exponential time courses is because of auto-reduction of the ferryl protein. The faster rate constant increases considerably, from 2.23 × 10-3 to 1.10 × 10-2 s-1, as the concentration of ascorbate is increased from 0 to 100 μm. The faster rate constant displays a double rectangular hyperbolic dependence on ascorbate concentration. This profile has been extensively analyzed elsewhere and is typical of that reported previously for horse and sperm whale Mb (17, 18). The slower rate constant, however, shows a smaller increase over the same ascorbate concentration range with no sign of the double rectangular hyperbolic concentration dependence. From our previous work on ferryl Mb reduction, we have proposed that Tyr-103 of Mb acts as an electron conduit from the reductant to the heme iron, interfacing the heme with the protein surface; this is most clearly discerned at low ascorbate concentrations. Fig. 3B shows the rate constant profile for the reduction of αY42V by ascorbate. The striking difference between the profile for the wild type protein and this mutant is the lack of the “high affinity” section of the profile for the faster rate constants for ferryl decay. As such, the rate constant for ferryl decay at 100 μm ascorbate is only one-third that observed with WTHb. The differences between the slow rate constants for ferryl reduction between the wild type and mutant are negligible. Fig. 3C shows the rate constant profile for the reduction by ascorbate of αY42W. Tryptophan, like tyrosine, has the capacity to harbor free radicals. In this case, however, the tryptophan does not appear to act as a conduit between the reductant and heme moiety, the rate constants for ferryl Hb being closely similar to those of the αY42V mutant. Comparison of Fig. 3A with Fig. 3, B and C, strongly suggests that the biphasic time course seen with the WTHb includes a component that may be assigned to the α chain that displays the double hyperbolic behavior and a component, assigned to the β chain, that has a single hyperbolic dependence on ascorbate concentration. On substitution of αTyr-42, the α chain loses one pathway and hence the double hyperbolic dependence on ascorbate concentration. One may therefore predict that by introducing a correctly positioned tyrosine residue into the β chain the wild type behavior might be restored. The kinetic profile of this double mutant (αY42V,βF41Y) is shown in Fig. 3D. The data in this figure closely resemble that collected for the wild type protein, displaying a double rectangular hyperbola rate constant profile for the faster rate of reduction and a slower rate of ferryl reduction that can be fitted to a single rectangular hyperbola. The only differences are that high affinity pathway for the double rectangular hyperbola has a lower KD value (1.0 μm) than the WT (KD = 30 μm), and the rate constant for ferryl decay for the slow phase (1.1 × 10-2 s-1) is higher than the slow phase observed for the wild type (5.6 × 10-3 s-1).
FIGURE 3.
Dependence of the rate constants for ferryl hemoglobin reduction on ascorbate concentration for wild type and mutant hemoglobins. Ferryl hemoglobin (10 μm) was reacted with ascorbate in 25 mm sodium phosphate buffer, pH 7.4, and rate constants for the two phases of ferryl reduction were determined by fitting to a double exponential function. A, wild type hemoglobin; B, αY42V hemoglobin; C, αY42W hemoglobin; D, αY42V,βF41Y hemoglobin (D′ is an expanded view of D with low ascorbate concentrations); and E, βF41Y hemoglobin. Double rectangular hyperbolic functions are fitted to the rate constants (solid lines). Closed symbols represent hemoglobin α subunit, and open symbols represent hemoglobin β subunit.
In Fig. 3E the corresponding data for the βF41Y mutant are given. Despite technical difficulties resulting in high rates of precipitation following purification of this mutant, particularly when in the ferric oxidation state, the β subunit does appear to display the double rectangular hyperbola, but with lower rate constants than the α chain. The rate constant for auto-reduction (no reductant present) of the α chain in the αY42W mutant is 10-fold higher (2.53 × 10-3 s-1) (Fig. 3C) than for the αY42V mutant (2.37 × 10-4 s-1) (Fig. 3B) but comparable with that of wild type Hb (2.23 × 10-3 s-1) (Fig. 3A). This may indicate that tryptophan, like tyrosine, facilitates ferryl auto-reduction (24). Additionally, the rate constants for the α chains that process a redox active residue at position 42 are higher than those that do not have the a redox active residue and hence no through-protein electron transfer pathway, i.e. β chains (ranging from 2.23 to 2.61 × 10-4s-1) that are comparable with those of the αY42V mutant.
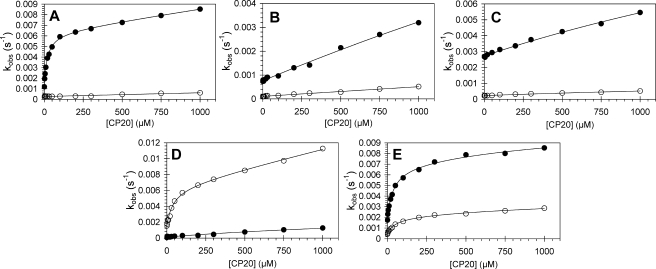
Fig. 4 shows the kinetics of ferryl Hb reduction for a different exogenous reductant (CP20). The data obtained confirm the observations made using ascorbate as reductant. The wild type protein (Fig. 4A) shows that the α chain exhibits a double rectangular hyperbolic concentration dependence, whereas the β chain does not. The double rectangular hyperbola collapses to a single hyperbola where the αTyr-42 has been mutated to Trp or Val (Fig. 4, B and C, respectively), appearing as almost straight lines (estimated KD by fitting >5 mm). The rate constants for ferryl reduction of the tryptophan mutant are again enhanced by an increased rate of auto-reduction compared with the valine mutation. The αY42V,βF41Y double mutant has a kinetic profile remarkably similar to that of wild type Hb. The βF41Y mutation results in double rectangular hyperbolic profiles when rate constants are plotted as a function of CP20 concentration (Fig. 4D).
FIGURE 4.
Dependence of the rate constants for ferryl hemoglobin reduction on CP20 concentration for wild type and mutant hemoglobins. Ferryl hemoglobin (10 μm) was reacted with CP20 in 25 mm sodium phosphate buffer, pH 7.4, and rate constants for ferryl reduction were calculated by fitting as described in the text. A, wild type hemoglobin; B, αY42V hemoglobin; C, αY42W hemoglobin; D, αY42V,βF41Y hemoglobin and E, βF41Y hemoglobin. Double rectangular hyperbolic functions are fitted to the rate constants (solid lines). Closed symbols represent hemoglobin α subunit; open symbols represent hemoglobin β subunit.
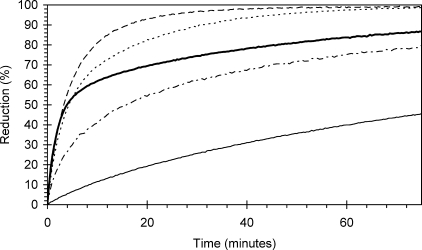
The kinetic profiles of the WTHb and four mutants at a single reductant concentration (100 μm CP20) are presented in Fig. 5. At this concentration of reductant, the differences between the kinetic profiles for each protein can easily be observed. The α subunit mutations that are designed to remove the through-protein electron transfer pathway exhibit much slower reduction kinetics compared with WTHb. Mutation of the β subunit to introduce a tyrosine at position 41 enhances the kinetics of ferryl decay at this reductant concentration, most easily seen at times greater than 5 min.
FIGURE 5.
Comparison of time courses for wild type and mutant hemoglobin ferryl reduction at a single reductant (CP20) concentration. Ferryl hemoglobin (10 μm) was reacted with CP20 (100 μm), and the time course for reduction followed optically. Thick solid line, wild type hemoglobin (αTyr-42, βPhe-41); dot-dashed line, αY42W hemoglobin; thin solid line, αY42V hemoglobin; dashed line, βF41Y hemoglobin; dotted line, αY42V,βF41Y double hemoglobin mutant.
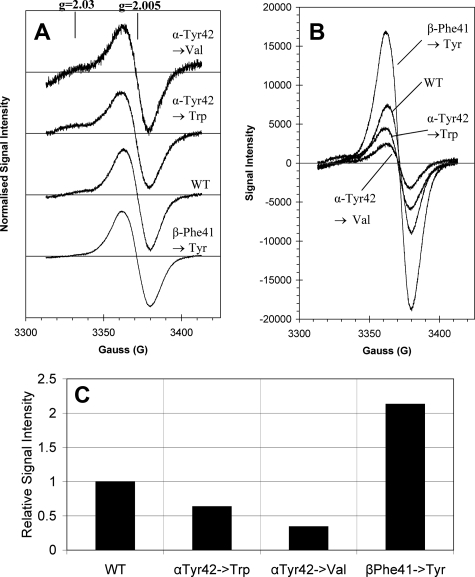
The EPR spectrum of the globin radical generated by reaction of the ferric Hb with excess H2O2 is shown in Fig. 6. Reaction of wild type Hb with peroxide yields signals that are typical for human Hb, exhibiting a large singlet feature at g = 2.005. This signal has been assigned to a tyrosine radical as is seen in Mb (14). Unlike Mb, however, there is no evidence of a signal with gz component at g = 2.03 assigned to a tryptophan peroxyl radical. All three mutants of Hb show virtually identical signal line shapes (Fig. 6A), each showing a main singlet radical species at g = 2.005 with a peak-to-trough width of about 18 G. Significant differences can be observed in the amplitude of the singlet feature in the different mutations. Both α tyrosine mutations significantly lower the intensity of the singlet species (Fig. 6, B and C), the αY42W exhibiting 64% and αY42Val showing only 34% signal intensity compared with wild type Hb. The βF41Y mutant, however, shows a much enhanced radical signal intensity.
FIGURE 6.
EPR spectra of the peroxide-generated globin radical on wild type and mutant hemoglobins. Hemoglobin (80 μm) was reacted with hydrogen peroxide (800 μm) in 50 mm sodium phosphate buffer, pH 7.4, for 30 s before flash-freezing in dry ice-cooled methanol. A, radical generated on wild type hemoglobin (wt), α mutants (αY42V and αY42W), and β mutant (βF41Y) are normalized, showing very similar characteristics, all centered close to g = 2.005 with slight variations in the amount of tryptophan peroxyl radical observed at g = 2.03. B, globin radical spectra absolute values. C, relative concentration of globin radicals with wild type normalized to 1.00. α mutants were 0.64 and 0.34 for αY42W and αY42V, respectively. β mutant radical concentration was 2.13 times that of the wild type protein.
DISCUSSION
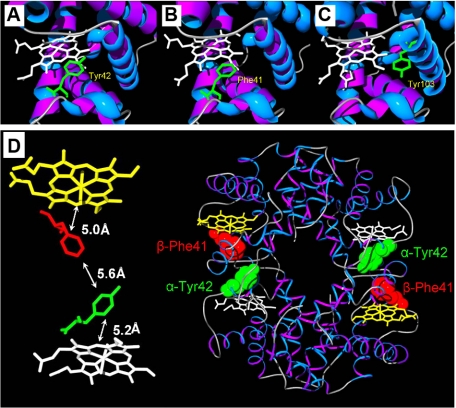
The data in this paper reveal a double exponential time course for the reduction of ferryl Hb. This represents the separate reduction of the α and β chains, rather than the dimers and tetramers, as the time courses are independent of protein concentration. Previously we have shown that the double rectangular hyperbolic profile for ferryl reduction results from a mechanism in which the ferryl heme is reduced by two independent pathways for electron transfer. One pathway is typically low affinity, showing KD values in the range of several millimolar reductant concentration and involves direct reduction of the ferryl heme by the reductant gaining access to the heme pocket. This pathway is highly dependent on the size of the reductant, with large molecules such as desferrioxamine having a smaller concentration dependence than small reductants such as p-Cresol and phenol (17). In addition, the charge and hydrophobicity of the reductant also affect the interaction with the heme pocket. The second pathway typically shows high affinity with a dissociation constant in the low micromolar range. This pathway involves a through-protein electron “hopping” mechanism that transmits electrons from the reductant to the heme iron via one or more amino acids. By comparing the properties of a number of native and engineered Mbs, we have proposed that Tyr-103 acts as an electron conduit from the reductant to the heme iron (18). We have also postulated that only the α chain of Hb should show a double rectangular hyperbolic rate constant profile as a function of reductant concentration as only the α chain possesses a tyrosine at the C-7 position (Tyr-42) in an equivalent position to Tyr-103 of Mb, Fig. 7, A–C. The aromatic ring of tyrosine is ∼5 Å from the heme edge (methylene carbon). The β chain, on the other hand, does not have a tyrosine but a phenylalanine residue in the homologous position. One may therefore expect that the rate constant for β chain reduction should not show the double rectangular hyperbolic concentration seen for the α chain. The data presented in this study support these suppositions. Replacement of the α chain Tyr-42 residue by a valine completely removes the high affinity, through-protein, electron transfer pathway, simplifying the rate constant profile to a single rectangular hyperbola (Figs. 3 and 4). Replacement with a tryptophan residue also completely removes the high affinity pathway but increases the rate of ferryl auto-reduction (Figs. 3 and 4). Given the kinetic assignments above, one may reason that mutation of a tyrosine residue at the appropriate location in the β chain to form the double mutant αY42V,βF41Y should regenerate the behavior of the WT protein, one chain expressing both pathways (β) and the other (α) only one, the roles of the chains being reversed compared with the WT protein. This prediction is borne out by the data presented in Figs. 3 and 4.
FIGURE 7.
Position of residues selected for mutation in α human hemoglobin (A), β human hemoglobin (B), and comparison with position of Tyr-103 of horse myoglobin (C). Position and distances between α hemoglobin Tyr-42 and β hemoglobin Phe-41 in human hemoglobin tetramer (D). Tyr-103 of horse Mb is close to the heme and is surface-exposed making it ideal to act as an electron conduit from exogenous reductants to the ferryl heme iron (C). Human hemoglobin α chain has a tyrosine in approximately the same position (Tyr-42) (A); however, this residue in human hemoglobin β chain is a phenylalanine (B). In the tetramer of human hemoglobin, the α chain Tyr-42 and the β chain Phe-41 are very close together (<6Å) (D). Both residues are close to their respective heme groups. Mutation of β phenylalanine at position 41 (redox inactive) to a redox active tyrosine may allow electron transfer between the hemes.
The behavior of Hb bearing a single mutation, βF41Y is, unexpectedly, rather complex. It was engineered to have a maximal rate of ferryl reduction, and indeed Fig. 5 shows that this mutation is the most rapidly reducible of all those studied. Again, as expected, the mutant showed evidence of enhanced rates of ferryl reduction (Fig. 3D), and the best fit was to a double rectangular hyperbola. However, the extent of the enhancement of β chain ferryl reduction by the through-protein electron pathway was not as dramatic as in the double mutant discussed above, which had the same tyrosine introduced into the β chain, but also had the homologous α chain tyrosine replaced (αY42V,βF41Y). There are two possible explanations for this apparent anomaly. First, the position of the mutation, namely position 41 in the β chain, lies very close to the α-β interface (Fig. 7D). As Tyr-42 on the α chain is also close to this interface, this may result in a single binding site for electron transfer to both the α and β chains. If the α chain pathway is kinetically preferred, then the β chain may not receive electrons through this route. Second, with two redox active tyrosines close to each other in the β mutant, an electron transfer pathway may be created that would allow electron cross-talk between the hemes of the two chains. Thus the enhanced rate of ferryl reduction observed with the α chain in the β mutant compared with WTHb could have resulted from electron transfer from the α heme to the β chain. The αY42V,βF41Y double mutant should eliminate both the one site problem of α-β ferryl reduction and any electron cross-talk between the hemes. These possibilities may be explored further through other mutations. For example, mutation on the other side of the heme group on the β subunit (e.g. K66Y or F71Y) may allow electron transfer between the reductant and heme from a different site to the α chain heme reduction. The C-7 tyrosine of the α chain is very highly conserved in vertebrates (see supplemental material). The phenylalanine in β Hb is also largely conserved in homeotherms (mammals and birds). In animals such as amphibians, fish, and some reptiles, however, a tyrosine occurs in the C-7 position of both α and β chains. From this we predict that the presence of the C-7 tyrosine in the β chain would engender the through-protein electron pathways in both chains. At present the evolutionary advantage or disadvantage of possessing tyrosine on both Hb chains, in terms of their potential cytotoxicity when dealing with high redox states, is unclear. However, stabilization of the subunit interface must be an important factor.
The effect of mutation of the αTyr-42 to Phe on the normal function of human Hb is well known. As with most mutations oxygen affinity is increased, whereas the Bohr effect and Hill coefficient are lowered through destabilization of the deoxy-T (tense) state quaternary structure (25, 26). A natural occurrence of βPhe to Tyr mutation in a human β Hb chain of an adult has been reported in the medical literature (27). Known as hemoglobin M equon, this mutation resulted in increase of Heinz bodies during a severe hemolytic crisis following a viral illness treated with acetaminophen, but otherwise this mutation did not appear to significantly affect the health of the patient.
In the experiments described above, the ferryl heme species are equivalent to peroxidase compound II. This is a species containing ferryl heme iron alone. However, when hydrogen peroxide reacts with ferric Hb, an accompanying radical cation is formed with the ferryl species, yielding a transient species formally equivalent to peroxidase compound I. Subsequent radical migration rapidly converts the Hb from compound I-like to compound II-like. Radical cation migration away from the heme is better considered as electron transfer to the heme and therefore should be dependent on specific electron transfer pathways. In fact the cation radical migrates away from the heme within microseconds and is thus not observed as a cation radical by EPR. However, deprotonation of this radical on a tyrosyl residue, for instance, allows stabilization of the radical over several minutes and thus can be readily observed. The positions of the amino acids that harbor these radicals have been debated over many decades. However, the consensus is that the tyrosyl radical may be assigned to Tyr-146 on horse Mb or Tyr-151 on sperm whale and human Mb. The tyrosine closest to the heme (Tyr-103) does not seem to be populated by a radical species. However, removal of Tyr-103 dramatically decreases the tyrosyl radical signal at position 146/151. This has been interpreted as electron transfer between a short lived Tyr-103 radical and a Tyr-146/151 (either by intra- or intermolecular electron transfer) (14). Our observations with the Hb mutants are consistent with the findings on Mb. The tyrosyl signal observed on wild type Hb is dramatically decreased by removal of αTyr-42, yet the line shape of the signal (with the exception of the appearance of a tryptophan peroxyl radical signal) does not change. This indicates that the identity of the radical singlet species in all proteins is the same, but removal of αTyr-42 blocks the formation of this radical species. The βF41Y mutant dramatically enhances the yield of the tyrosyl radical observed by EPR implying that both tyrosine residues, operating in the electron transfer pathways, lead to stable neutral radical species that can be detected.
Mechanisms of electron transfer based on Marcus theory cannot readily account for the dramatic switching on or switching off of these pathways by substitution of tyrosine by another amino acid residue. The particular property of tyrosine that distinguishes it from most other residues (excluding tryptophan) is its ability to exist as a stable neutral radical. Therefore, we believe that in the electron transfer pathways discussed here tyrosine is acting as a true redox center, cycling between the tyrosine and the tyrosyl radical, ferrying electrons from the bulk medium to the ferryl heme. Tyrosine is performing a function in these proteins that is more usually undertaken by a redox co-factor.
The results described above demonstrate that the redox properties, especially the kinetics of ferryl iron reduction, can be modulated by mutation of specific tyrosine “co-factors.” The ferryl oxidation state of Mb and Hb has been implicated in the pathological responses observed in vivo when these hemoproteins gain access to compartments poorly protected by antioxidant defenses, e.g. the post-glomerular space or the cerebrospinal fluid (1–3). Recently a meta-analysis of clinical trials of second generation Hb-based blood substitutes has highlighted the toxicity of the formulations so far available for therapeutic use and has concluded that none are safe (28). This analysis draws attention to the toxic effects of NO scavenging by cell-free Hb, but this is unlikely to be the sole mechanism for toxicity given that Hb-based blood substitutes that have been engineered to have decreased NO affinity and do not elevate blood pressure have nevertheless failed to meet clinical goals (29). There is now an increasing awareness that the oxidative properties of Hb have played a major role in the toxicity of modified Hbs that have undergone clinical trials as blood substitutes (30, 31). We propose that the toxicity that arises from the redox activity of Hb can be ameliorated by modulating the electron transfer pathways that enhance removal of the toxic ferryl form of Hb as demonstrated above. Proteins that either hinder the leakage of the oxidative equivalent from the heme (by removal of the C-7 tyrosine residue) or, conversely, hasten ferryl heme reduction (inserting an appropriately located tyrosine residue) are likely to exhibit altered cellular toxicity. Such Hbs may be a sound starting point for the rational design of the next generation of Hb-based blood substitutes.
Supplementary Material
This work was supported by European Union Framework VI and Biotechnology and Biological Sciences Research Council. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: Hb, hemoglobin; Mb, myoglobin; CP20 deferriprone, 1, 2-dimethyl-3-hydroxypyrid-4-one; WT, wild type.
References
- 1.Holt, S., Reeder, B., Wilson, M., Harvey, S., Morrow, J. D., Roberts, L. J., II, and Moore, K. (1999) Lancet 353 1241. [DOI] [PubMed] [Google Scholar]
- 2.Moore, K. P., Holt, S. G., Patel, R. P., Svistunenko, D. A., Zackert, W., Goodier, D., Reeder, B. J., Clozel, M., Anand, R., Cooper, C. E., Morrow, J. D., Wilson, M. T., Darley-Usmar, V., and Roberts, L. J., II (1998) J. Biol. Chem. 273 31731-31737 [DOI] [PubMed] [Google Scholar]
- 3.Reeder, B. J., Sharpe, M. A., Kay, A. D., Kerr, M., Moore, K., and Wilson, M. T. (2002) Biochem. Soc. Trans. 30 745-748 [DOI] [PubMed] [Google Scholar]
- 4.D'Agnillo, F., and Alayash, A. I. (2002) Free Radic. Biol. Med. 33 1153-1164 [DOI] [PubMed] [Google Scholar]
- 5.Arduini, A., Eddy, L., and Hochstein, P. (1990) Free Radic. Biol. Med. 9 511-513 [DOI] [PubMed] [Google Scholar]
- 6.Walters, F. P., Kennedy, F. G., and Jones, D. P. (1983) FEBS Lett. 163 292-296 [DOI] [PubMed] [Google Scholar]
- 7.Giulivi, C., and Cadenas, E. (1993) FEBS Lett. 332 287-290 [DOI] [PubMed] [Google Scholar]
- 8.Reeder, B. J., and Wilson, M. T. (2005) Curr. Med. Chem. 12 2741-2751 [DOI] [PubMed] [Google Scholar]
- 9.Vuletich, J. L., Osawa, Y., and Aviram, M. (2000) Biochem. Biophys. Res. Commun. 269 647-651 [DOI] [PubMed] [Google Scholar]
- 10.Osawa, Y., and Korzekwa, K. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 7081-7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeder, B. J., Svistunenko, D. A., Sharpe, M. A., and Wilson, M. T. (2002) Biochemistry 41 367-375 [DOI] [PubMed] [Google Scholar]
- 12.Vollaard, N. B., Reeder, B. J., Shearman, J. P., Menu, P., Wilson, M. T., and Cooper, C. E. (2005) Free Radic. Biol. Med. 39 1216-1228 [DOI] [PubMed] [Google Scholar]
- 13.Svistunenko, D. A., Patel, R. P., Voloshchenko, S. V., and Wilson, M. T. (1997) J. Biol. Chem. 272 7114-7121 [DOI] [PubMed] [Google Scholar]
- 14.Svistunenko, D. A., Dunne, J., Fryer, M., Nicholls, P., Reeder, B. J., Wilson, M. T., Bigotti, M. G., Cutruzzola, F., and Cooper, C. E. (2002) Biophys. J. 83 2845-2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper, C. E., Green, E. S., Rice-Evans, C. A., Davies, M. J., and Wrigglesworth, J. M. (1994) Free Radic. Res. 20 219-227 [DOI] [PubMed] [Google Scholar]
- 16.Reeder, B. J., and Wilson, M. T. (2005) Chem. Res. Toxicol. 18 1004-1011 [DOI] [PubMed] [Google Scholar]
- 17.Reeder, B. J., Hider, R. C., and Wilson, M. T. (2008) Free Radic. Biol. Med. 44 264-273 [DOI] [PubMed] [Google Scholar]
- 18.Reeder, B. J., Cutruzzola, F., Bigotti, M. G., Hider, R. C., and Wilson, M. T. (2008) Free Radic. Biol. Med. 44 274-283 [DOI] [PubMed] [Google Scholar]
- 19.Panetta, G., Arcovito, A., Morea, V., Bellelli, A., and Miele, A. E. (2008) Biochim. Biophys. Acta, 1784 1462-1470 [DOI] [PubMed] [Google Scholar]
- 20.Sambrook, J., and Russell, D. W. (2001) Molecular Cloning: A Laboratory Manual, pp. 1.112-1.115, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 21.Antonini, E., and Brunori, M. (1971) in Frontiers in Biology (Neuberger, A., and Tatum, E. L., eds) pp. 13-52, North-Holland Publishing Co., Amsterdam
- 22.Weil, J. A., Bolton, J. R., and Wertz, J. E. (eds) (1994) Electron Paramagnetic Resonance: Elementary Theory and Practical Applications, pp. 510-513, Wiley Interscience, New York
- 23.Bull, C., and Hoffman, B. M. (1975) Proc. Natl. Acad. Sci. U. S. A. 72 3382-3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lardinois, O. M., and Ortiz de Montellano, P. R. (2004) Biochemistry 43 4601-4610 [DOI] [PubMed] [Google Scholar]
- 25.Imai, K., Fushitani, K., Miyazaki, G., Ishimori, K., Kitagawa, T., Wada, Y., Morimoto, H., Morishima, I., Shih, D. T., and Tame, J. (1991) J. Mol. Biol. 218 769-778 [DOI] [PubMed] [Google Scholar]
- 26.Kavanaugh, J. S., Rogers, P. H., Arnone, A., Hui, H. L., Wierzba, A., DeYoung, A., Kwiatkowski, L. D., Noble, R. W., Juszczak, L. J., Peterson, E. S., and Friedman, J. M. (2005) Biochemistry 44 3806-3820 [DOI] [PubMed] [Google Scholar]
- 27.Burkert, L. B., Sharma, V. S., Pisciotta, A. V., Ranney, H. M., and Bruckheimer, S. (1976) Blood 48 645-651 [PubMed] [Google Scholar]
- 28.Natanson, C., Kern, S. J., Lurie, P., Banks, S. M., and Wolfe, S. M. (2008) J. Am. Med. Assoc. 299 2304-2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maillett, D. H., and Olson, J. S. (2006) in Designing Recombinant Hemoglobin for Use as a Blood Substitute (Winslow, R., ed) 1st Ed., Academic Press, Amsterdam
- 30.Alayash, A. I., D'Agnillo, F., and Buehler, P. W. (2007) Exp. Opin. Biol. Ther. 7 665-675 [DOI] [PubMed] [Google Scholar]
- 31.Winslow, R. M. (2006) Vox Sang. 91 102-110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.