Abstract
The rate, polarity, and symmetry of the flow of the plant hormone auxin are determined by the polar cellular localization of PIN-FORMED (PIN) auxin efflux carriers. Flavonoids, a class of secondary plant metabolites, have been suspected to modulate auxin transport and tropic responses. Nevertheless, the identity of specific flavonoid compounds involved and their molecular function and targets in vivo are essentially unknown. Here we show that the root elongation zone of agravitropic pin2/eir1/wav6/agr1 has an altered pattern and amount of flavonol glycosides. Application of nanomolar concentrations of flavonols to pin2 roots is sufficient to partially restore root gravitropism. By employing a quantitative cell biological approach, we demonstrate that flavonoids partially restore the formation of lateral auxin gradients in the absence of PIN2. Chemical complementation by flavonoids correlates with an asymmetric distribution of the PIN1 protein. pin2 complementation probably does not result from inhibition of auxin efflux, as supply of the auxin transport inhibitor N-1-naphthylphthalamic acid failed to restore pin2 gravitropism. We propose that flavonoids promote asymmetric PIN shifts during gravity stimulation, thus redirecting basipetal auxin streams necessary for root bending.
The plant hormone auxin (3-indolyl acetic acid, IAA)5 controls virtually all plant developmental and physiological processes. In roots, the differential growth response associated with gravity stimulation (gravitropism) occurs in the elongation zone (1, 2) and is a result of the asymmetric distribution of auxin to the lower side of epidermal cells (3). In these tissues accumulating auxin, cell elongation is inhibited and the root tip bends downwards. This cell-to-cell or polar auxin transport (PAT) is determined by the asymmetric cellular localization of auxin in- and efflux components of the ABCB/PGP/MDR, AUX1/LAX, and PIN-FORMED (PIN) family (4-7). Although ABCBs are apparently involved in long-range auxin transport and movements of auxin out of apical regions (8-10), AUX1 and PIN2/EIR1/WAV6AGR1 have been demonstrated to channel auxin from the lateral root cap basipetally to the expanding epidermal cells (11-13).
The regulation of auxin transport during root gravitropic responses is still largely unclear. Among various possible mechanisms, the localized synthesis and directed transport of flavonoids, plant-specific phenylpropanoid compounds, have been shown to modulate the rate of the gravity response (14, 15). A number of lines of experimentation have suggested that flavonoids may act as non-essential auxin transport inhibitors (16-20). This is mainly based on the finding that flavonoids displace binding of synthetic auxin transport inhibitors, like N-1-naphthylphthalamic acid (NPA), a herbicide (Naptalam®), in vitro (31, 50-52). Moreover, roots of transparent testa (tt) Arabidopsis mutant with manipulated flavonoid levels exhibit altered gravitropic curvature and auxin transport, which are restored to the wild-type level by exogenous application of flavonoids (16, 21). Nonetheless, the identity of the specific flavonoid compounds involved, their molecular targets as well as their mode of action in vivo are essentially unknown. Several lines of evidence suggest that ABCBs are directly (8-10, 22, 23) or indirectly (24) regulated by aglycone flavonols. High NPA concentrations cause inhibition of auxin efflux catalyzed by ABCB1/PGP1, ABCB19/PGP19/MDR1 (22, 23), and ABCB4/PGP4/MDR4 (9) (hereafter referred to as ABCBs), most probably by binding to the transporter itself (25). This is in analogy to flavonoids, functioning as inhibitors of plant (22, 23, 26) and mammalian ABCBs (27), probably by mimicking ATP and competing for ABCB nucleotide-binding domains (28). In contrast, the expression and subcellular location of PIN auxin efflux carriers is thought to be a consequence of flavonoid-mediated alteration of auxin concentrations (18, 19). In a pioneer study evidence that flavonoids are functioning as endocrine effectors that specifically determine individual PIN gene expression and protein localization was provided (18).
Here, we report that agravitropic loss-of-function mutant pin2/eir1/wav6/agr1 has impaired patterns of flavonol glycosides. We found that nanomolar concentrations of exogenous flavonols, which have apparently only a mild inhibitory effect on root elongation and gravitropic response in wild-type plants, can partially rescue the agravitropic phenotype of pin2 roots by promoting asymmetric PIN1 shifts, re-establishing polar auxin fluxes.
EXPERIMENTAL PROCEDURES
Chemicals—The following substances were obtained as indicated MeCN (HPLC Supra grade, Scharlau, E-Barcelona), HCOOH (Fluka, Puriss, Switzerland), methanol (MEOH, Fisher Scientific, UK), HPLC-grade acetonitrile (Fisher Scientific, UK), and H3PO4 (Applichem, Germany). Water was purified with a MilliQ Gradient apparatus (<5 ppb, Millipore, Milford, MA). Diphenylboric acid 2-aminoethyl ester (DPBA, Sigma, Germany), NPA (Fluka, Germany), kaempferol (Calbiochem, La Jolla, CA), and quercetin (Fluka, Germany) were dissolved in 100% dimethyl sulfoxide.
Growth Conditions and Plant Material—Seeds were surface sterilized for 5 h in a chamber containing vaporous HCl and sodium hypochlorite and stratified in a 0.1% agar solution for 2 days at 4 °C. Subsequently, the seeds were plated on sterile half-strength MS medium at pH 5.7 containing 2% sucrose solidified with 0.6% phytagel (Sigma), and vertically grown at 22 °C with a 16-h/8-h light/dark cycle. The mutant alleles used in this study were pin2-1 (29) and eir1-4 (30).
Flavonoid Fluorescence Staining—Flavonoid compound locations were visualized in vivo by the fluorescence of flavonoid-conjugated DPBA compounds after excitation with blue light. Plants were grown for 5 days before staining. Fluorescent staining of whole seedlings was performed according to Buer and Muday (14). Fluorescence was achieved by excitation with fluorescein isothiocyanate filters (450-490 nm, suppression long pass 515 nm) on a Leica DMR fluorescence microscope and ×10 or 20 objectives. Digital images were captured with a Leica DC300 F charge coupled device camera.
Extraction of Phenolic Compounds and HPLC Analysis—Excised roots were incubated overnight in the dark at 4 °C in 0.5 ml of 80% (v/v) methanol (MeOH), extracted, and centrifuged at 18,000 × g for 10 min. The supernatant was concentrated to dryness and resuspended in 0.1 ml of 80% MeOH. Aliquots (50 μl) were analyzed by a reverse-phase HPLC (Gynkotek, Germany). Absorbance spectra were recorded with a UVD340S diode array detector (Dionex, Switzerland). Data integration analysis was conducted using the Chromeoleon software (version 6.4, Dionex, Switzerland). The peak height was quantified at 330 nm. A calibration curve for kaempferol was used as reference for single peak quantification. All analyses were performed with at least three independent replicates, each representing 100 roots. Chromatographic conditions were Nucleosil 100-5 C18 column (5 μm, 2 × 250 mm, Macherey-Nagel, Düren, Germany); flow rate 1.00 ml min-1, gradient (step, time, %B over A) 1, 25 min, 10-25%; 2, 10 min, 25-70%). Solvent A was H2O, 0.1% (v/v) H3PO4 and solvent B was MeCN.
Structural Elucidation: HPLC-ESI-MS/MS Analysis—HPLC-MS analyses were performed on an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA) fitted with a HTS PAL autosampler (CTC Analytics, Zwingen, Switzerland), an Agilent 1100 binary pump, and an Agilent 1100 photodiode array detector. Chromatographic conditions were Nucleosil 100-3 C18 column (3 μm, 2 × 250 mm, Macherey-Nagel, Hoerdt, France); flow rate 0.170 ml min-1, gradient (step, time, %B over A) 1, 25 min, 10-25%; 2, 10 min, 25-70%). Solvent A was H2O, 0.1% (v/v) HCOOH and solvent B was MeCN, 0.1% (v/v) HCOOH. The HPLC was connected to a Bruker ESQUIRE-LC quadrupole ion trap instrument (Bruker Daltonik GmbH, Bremen, Germany), equipped with a combined Hewlett-Packard Atmospheric Pressure Ion source (Hewlett-Packard Co., Palo Alto, CA). The HPLC output was directly interfaced to the ESI ion source. The MS conditions were: nebulizer gas (N2) 40 p.s.i., dry gas (N2) 9 liters/min, dry temperature 300 °C, HV capillary 4000 V, HV EndPlate offset -500 V, capillary exit -100 V, skimmer1-28.9 V, and trap drive 53.4. The MS acquisitions were performed in the negative electrospray ionization mode, at normal resolution (0.6 unit at half-peak height), under ion charge control conditions (10,000) in the mass range from m/z 100 to 1000.
The MS2 acquisitions were obtained in the auto-MS/MS mode. The isolation width was 4 units, the fragmentation cut-off set by “fast calc,” and the fragmentation amplitude set at 0.9 V in the “SmartFrag” mode. The total amounts of flavonoid compounds were calculated as the sum of the areas (×106 arbitrary unit) of the mass signals identified during HPLC-ESI-MS analysis. Each extraction consists of a pull of 100 different roots. Quantification of RT-EZ flavonoid compounds is the result of two independent extractions in which each time 150 5-mm long root apices from 12 different agar plates were pulled together.
Gravitropic Assays—4-Day light-grown seedlings were transferred from control plates to plates containing nutrient media optionally supplemented with quercetin or kaempferol (100 or 200 nm) or NPA (100 nm to 5 μm). After 24 h of adaptation and growth in the new media, plates were turned 90°.
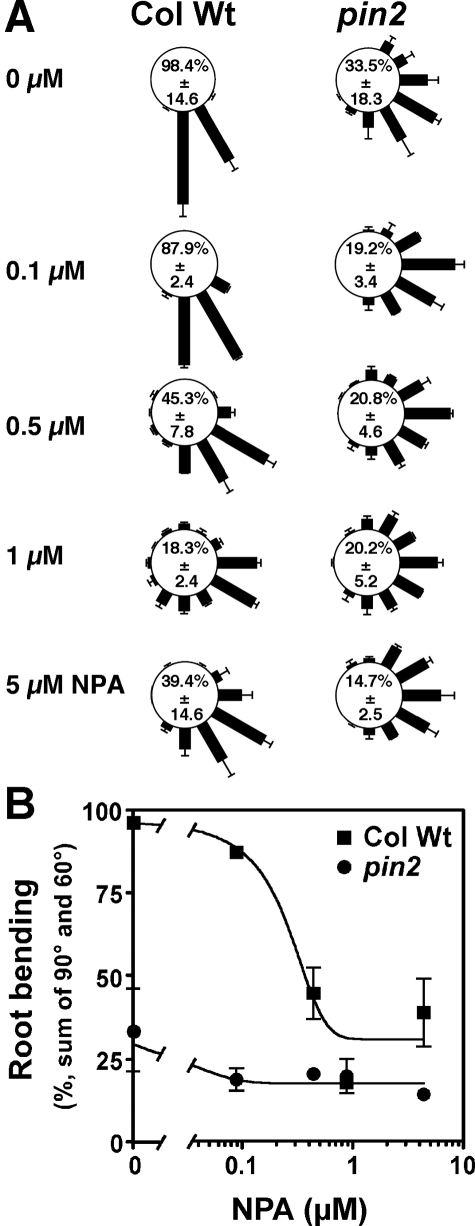
In one type of gravitropic assay, after 24 h of growth under gravistimulation, seedlings were scanned using Epson Perfection photo 2450 and angles of gravitropic curvature were measured from digital pictures using the tool “Image Manager” of the Leica IM1000 software (Leica, Heerbrug, CH). Each gravity stimulated root was assigned to one of 12, 30° sectors; the length of each bar represents the percentage of seedlings showing the same direction of root tip growth. To enable direct comparison of root bending, percentual occurrence of 60 and 90° bending (sum of 60 and 90° sectors), the dominant bending sectors of wild-type roots under control conditions (98.4%), was defined as relative root bending.
Short pulses of gravity stimulation were achieved by turning the plates 90° for 2 h, which corresponds to the peak of gravity-induced flavonoid accumulation (14). After 2 h of gravity stimulation, roots were excised and flavonoids extracted as described, or expression of DR5rev-GFP reporter protein analyzed on a Leica TCS SP2 CLSM.
Kinetics of root bending were performed and analyzed as described in Ref. 31. All gravitropic assays were performed in the dark to prevent phototropic responses. In some cases (Table 2 and supplemental Figs. S1 and S2), angles of gravitropic curvature were measured in a blind assay, to reduce possible unbiased calculations.
TABLE 2.
The majority of quercetin-treated pin2 roots form IAA gradients upon gravity stimulation
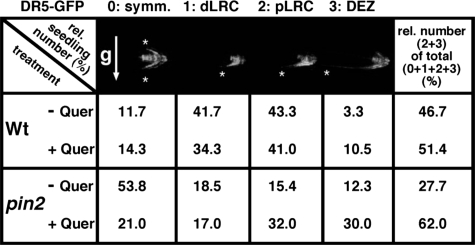
Relative numbers of wild type (Wt) and pin2 seedling showing IAA gradients. Definition of IAA gradients is based on the strength of IAA movement from the LRC to the EZ, as assessed by confocal imaging of DR5-GFP fluorescent signals upon 2-h gravity stimulation. Gradient symmetry is quantified in arbitrary units (0, symmetric signal; 1, weak signal asymmetry, up to distal lateral root cap (dLRC); 2, intermediate signal asymmetry, up to proximal lateral root cap (pLRC); 3, strong signal asymmetry, up to elongation zone (DEZ)). Number of analysed wild-type (pin2) seedlings was 60 (65) on solvent control and 105 (100) on 100 nm quercetin plates, respectively. Gravity vector (g) relative to the root is indicated by an arrow; symmetry of DR5-GFP signals by asterisks. For more details and absolute numbers, see supplemental Table S1.
Immunocytochemistry—PIN1 immunolocalization was performed as previously described (32) with PIN1 specific antibody (33) at 1:1000 dilution and anti-rabbit Cy3-conjugated secondary antibodies. Confocal imaging of Cy3 and DR5rev::GFP was carried out on a Leica SP2 AOBS microscope. In some cases (Fig. 4C and supplemental Fig. S3), symmetries of DR5-GFP gradients and PIN1 distribution were measured in a blind assay, to reduce possible unbiased calculations.
FIGURE 4.
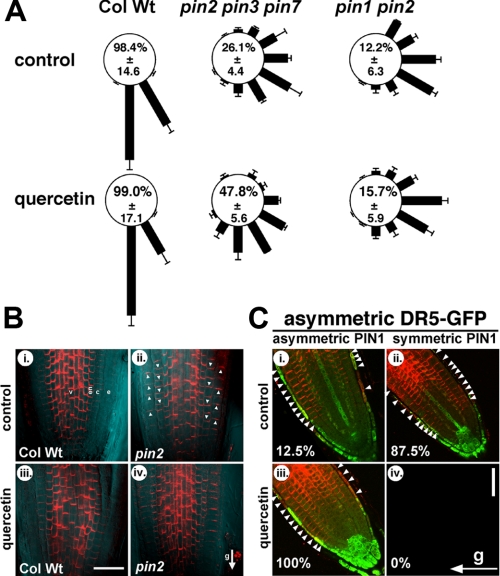
Flavonoid-dependent rescue of pin2 agravitropic phenotype requires PIN1 and is correlated with asymmetric PIN1 distribution across gravity-stimulated tissues. A, gravity responses of pin2 pin3 pin7 and pin1 pin2 (pin1 eir1) roots after 24 h in the presence of quercetin. The length of each bar represents the mean percentages ± S.D. of seedlings showing the same direction of root growth of at least three independent experiments; numbers correspond to the mean (±S.D.) percent occurrence of 60 and 90° bending (sum of 60 and 90° sectors). B, whole-mount in situ immunolocalization of PIN1 protein (red) in 5-day pin2 (eir1-4; ii and iv) and wild-type (Col Wt; i and iii) vertical seedlings transferred on media supplemented with 100 nm quercetin. Gravity vector is indicated by an arrow. White arrows indicate PIN1 protein apical localization in the epidermis and basal localization in the cortex cells of pin2 root tip. Note that the appearance of slightly different PIN1 signals in the pin2 epidermis and cortex (ii-iv) do not reflect unequal expression but are the result of unequal background intensities due to scattered light. v, vascular bundle; en, endodermis; c, cortex; e, epidermis. Bar, 30 μm. C, whole mount in situ immunolocalization of PIN1 protein in pin2 after 2 h of gravity stimulation; gravity vector is indicated by an arrow. 4-Day pin2 seedlings were transferred on media supplemented with 100 nm quercetin or the solvent (control). Red, PIN1; green, DR5rev-GFP expression. White arrows indicate more pronounced PIN1 proteins levels at the lower or upper side of the gravity-stimulated root tip. Bar, 30 μm. Percentages indicate relative occurrence of asymmetric or symmetric PIN1 distributions with asymmetric DR5-GFP signals; the total number of analyzed roots that showed simultaneous clear DR5-GFP and PIN1 signals was 47.
Data Analyses—Statistical analysis was performed using SPSS 11.0 (SPSS Inc., Chicago, IL).
RESULTS
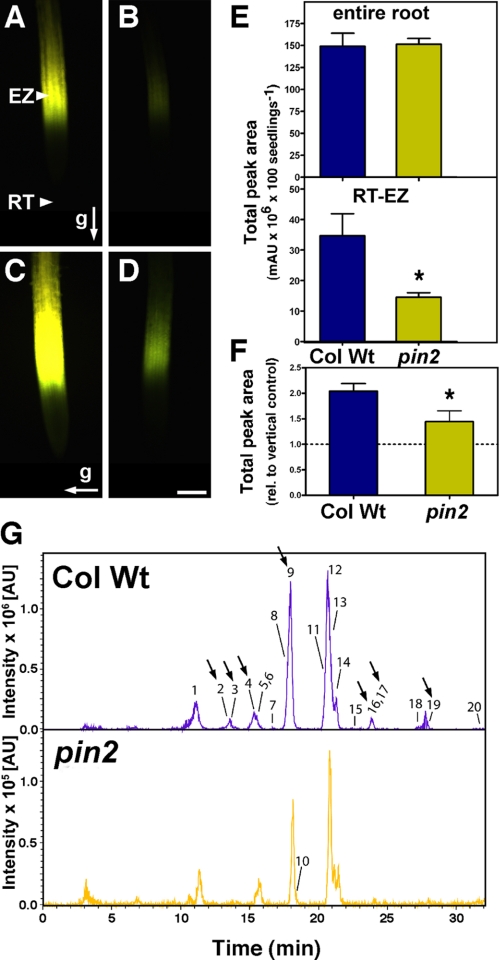
pin2 Roots Have an Altered Flavonoid Pattern—pin2/eir1/wav6/agr1 Arabidopsis mutant (referred to as pin2 hereafter), one of the best characterized auxin transport mutants, exhibits reduced basipetal auxin transport and agravitropic root growth (2, 29, 30, 34). As a starting point of this work, we investigated whether defects in basipetal auxin transport in pin2, which result in agravitropic responses (35), are linked to an altered accumulation of specific endogenous flavonoids and whether flavonoids could be directly implicated in the control of the gravitropic responses. DPBA, a fluorescent dye that specifically interacts with flavonoids, allows in situ flavonoid staining and localization in Arabidopsis seedlings (18, 20, 36). In wild-type seedlings, flavonoid DPBA staining is restricted to the shoot apex and cotyledons, the root-shoot junction, along the primary root, and most intensely to the root elongation zone (Fig. 1A and supplemental Fig. S1, A-C) (36). In contrast, flavonoid-DPBA fluorescence in the pin2 mutant was clearly lower at the root tip-elongation zone (RT-EZ) (Fig. 1B and supplemental Fig. S1, E-G). Manipulation of endogenous auxin levels by addition of 100 nm IAA increased DPBA fluorescence in the wild type (14) and, although to a lesser extend, also in the pin2 RT-EZ (supplemental Fig. S1, D and H), suggesting that auxin and flavonoid levels in planta are interconnected (18, 36).
FIGURE 1.
Defects in basipetal auxin transport are associated with altered root flavonoid accumulation. A-D, flavonoid accumulation in the entire root and root elongation zone of wild type (Col Wt; A and C) and pin2 (B and D). Accumulation of flavonoids in control and 2-h gravity-stimulated roots visualized in situ using DPBA (yellow fluorescence) as described under “Experimental Procedures.” The arrows indicate the direction of the gravity vector relative to the root. Bar, 100 μm. E, total amount of flavonoid derivatives detected in the entire root and RT-EZ of wild type and pin2. F, gravity-induced root phenolic compound accumulation normalized to phenolic compound accumulation in vertical control. Values represent mean ± S.E. (n = 2-5 replicates); *, significantly different from the wild type (Student's t test, p < 0.05). G, representative sum of extracted ion chromatograms [M - H]- of flavonoid derivatives found in wild type and pin2 RT-EZ analyzed by HPLC-ESI-MS. Significantly altered compounds are indicated by arrows. Peak numbers correspond to flavonoid derivates listed in Table 1. Note the 10 times lower intensity scale for pin2 root elongation zone in comparison to wild type.
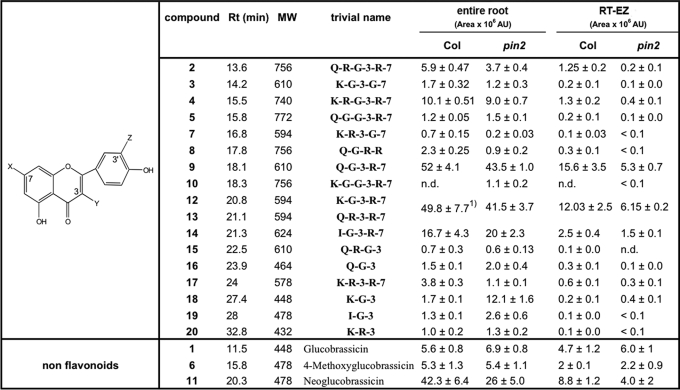
To determine how flavonoid distribution was affected by PAT alterations, we qualitatively and quantitatively investigated endogenous flavonoid derivatives present in wild-type and pin2 RT-EZ and entire roots using HPLC-UV-(-)-ESI-MS and HPLC-ESI-MS/MS, respectively (Table 1). Consistent with DPBA staining profiles (Figs. 1, A and B and supplemental S1), we found that the total amount of flavonoids was significantly reduced in the RT-EZ of pin2 mutant (Fig. 1, E and G), whereas no significant difference was observed over the entire root (Figs. 1E and supplemental S2). pin2 roots showed altered accumulation of specific flavonol glycosides both in the RT-EZ and in the entire root (Table 1 and arrows in the extraction ion chromatograms of the masses of interest in Figs. 1G and supplemental S2). In pin2 entire roots and RT-EZ, a shift from di- and triglycosylated flavonols to monoglycosylated flavonols, such as K-G-3 (compound 18), was observed (Table 1), which suggests that auxin levels may have an effect on the expression or activity of corresponding glycosyltransferases. This is supported by in silico (www.genevestigator.ethz.ch) expression analysis of two glycosyltransferase genes that are involved in flavonoid biosynthesis in Arabidopsis (37). At5g17050, which encodes for a flavonoid 3-O-glucosyltransferase, is induced by 1-naphtylacetic acid and 2,4-dichlorophenoxyacetic acid treatment, whereas At4g14090, anthocyanin 5-O-glucosyltransferase, is down-regulated by auxin transport inhibitor treatments. Conversely, those peaks, whose accumulation is affected in pin2 roots, may be functionally important for the regulation of auxin transport during root gravitropism.
TABLE 1.
Flavonoid derivatives detected in the MeOH extracts of entire root and RT-EZ of wild type and pin2
The identification of the flavonoid derivatives was achieved by HPLC-UV(−)ESI-MS/MS, comparison to reference compounds was according to Refs. 46-49. Each compound was quantified by integration of the corresponding signal area (×106 arbitrary units (AU)) presented in the extract ion chromatogram (EIC, [M-H]−) after HPLC-MS experiment. Values of entire roots represent mean ± S.E. of at least three independent experiments (Student's t test, p< 0.05, n≤ 2-4). Each extraction consists of a pool of 100 different roots. Quantification of RT-EZ flavonoid compounds is the result of at least two independent extractions in which each time 150 5-mm long root apices from 12 different agar plates were pooled. The natural flavonoid derivates described have abbreviations as follows: G, glucose; K, kaempferol; I, isorhammetin; Q, quercetin; R, rhamnose. Numbers indicate the position of glycosylation relative to the flavonol core. All flavonols identified are O-glycosylated.
1 Compounds 12 are 13 are co-eluting in the HPLC-(−)-ESI-MS chromatogram and show the same quasi-molecular ions (m/z 593); therefore, they were integrated together.
As previously reported (14, 38), a 2-h gravity stimulation increased the DBPA fluorescence in wild-type RT-EZ by nearly 2-fold, with a maximum at 1.5 to 2.5 h after stimulation. A smaller but significant increase in DPBA fluorescence was observed also in pin2 mutant (Fig. 1, C-D). Flavonoid quantification by HPLC-UV (Fig. 1F) was consistent with the DPBA staining. Collectively, our results demonstrate that the synthesis and transient accumulation of specific flavonoid glycosides in the root tip-elongation zone, but not over the entire root or in the shoot, are impaired quantitatively and qualitatively in pin2 (Figs. 1, supplemental S1 and S2).
Flavonoids Partially Rescue the Agravitropic Response of pin2 Roots—To test whether flavonoid concentrations play a critical role in the response to gravity stimuli, we searched for conditions in which flavonoids could be supplied without negatively affecting root growth and gravitropism most probably by acting as auxin transport inhibitors (16, 18). Concentrations up to 100 nm kaempferol or quercetin did not significantly influence wild-type gravitropic responses after 24 h (95.5 and 99.0% instead of 98.4% (relative root bending (= sum of 90 and 60° sectors, respectively); see “Experimental Procedures” (Fig. 2A)). Moreover, root-bending kinetics demonstrated that treatment with 100 nm quercetin did not significantly alter the bending performance of wild-type roots over 24 h compared with the solvent control (Fig. 2B). Importantly, bending of wild-type roots in the presence and absence of quercetin is virtually identical after 2 and 24 h, time points used in this study), however, small but not significant differences are found between 2 and 24 h.
FIGURE 2.
Exogenous flavonols partially rescue the agravitropic response of pin2 by restoring asymmetric auxin gradients. A, gravity responses of wild-type (Col Wt) and pin2 (eir1-4) roots 24 h after reorientation of 90° to horizontal in the presence of the indicated auxin transport inhibitors. Each gravity-stimulated root was assigned to one of 12, 30° sectors (see schematic). The length of each bar represents the mean percentages ± S.D. of seedlings showing the same direction of root growth of at least three independent experiments; numbers correspond to the mean (±S.D.) percent occurrence of 90 and 60° bending (sum of 90° and 60° sectors). B, time series of wild-type (Col Wt) and pin2 (eir1-4) root curvature after reorientation of 90° to horizontal in the presence and absence of 100 nm quercetin analyzed as described in Ref. 24). Data are mean ± S.E. (n = 4 with 50 seedlings per allele and treatment). C, expression of the auxin-reporter construct DR5rev-GFP in wild type (Col Wt) and pin2 (eir1-4) root tips was assessed prior to, and after 2 h gravity stimulation on control (top row), quercetin (middle row), and kaempferol-treated roots (bottom row). White asterisks indicate more pronounced DR5-GFP expression at the lower side of gravistimulated roots suggesting enhanced basipetal auxin reflux. The gravity vector relative to the root tip is indicated by an arrow. QC, quiescent center; QI, columella initials; col, mature columella cells; dLRC, distal lateral root cap; pLRC, proximal lateral root cap. Bar, 75 μm.
Roots of the eir1-4 mutant, a severe agravitropic allele of pin2 (30), were gravity stimulated for 24 h in the presence of 100 nm flavonoids. Intriguingly, pin2 gravitropic root bending (33.5%) was significantly restored by quercetin (50.0%) and kaempferol (52.1%, Fig. 2, A and B). Restoration of relative root bending by flavonols was roughly 25% and therefore only partial.
The same gravitropic assay was performed in the presence of the synthetic inhibitor of polar auxin efflux NPA, which blocks basipetal IAA movement from the root tip (39). In wild-type plants, treatment with 5 μm NPA, a concentration routinely used, resulted in an agravitropic phenotype (39.4%) (16, 35), similar to that found for pin2. However, NPA failed to restore but rather impaired pin2 root gravitropism (14.7%). To exclude that restoration of gravitropism in pin2 was only found at lower concentrations or at a particular NPA dose as reported for a phosphatase 2A mutant, rcn1 (40), we quantified root gravitropism of wild-type and pin2 after treatment with NPA from 5 μm down to the concentration used with the flavonols (100 nm). Unlike quercetin and kaempferol, over this concentration range NPA had a negative effect on gravitropism and was not able to restore root gravitropism in wild-type nor in pin2 (Fig. 3). Finally, we performed quercetin treatment in the presence of NPA, and found that in wild-type NPA, quercetin had an additive effect (28.6%), whereas in pin2 the quercetin action was prevented by NPA (13.0%), indicating that the rescue of pin2 agravitropic response by quercetin is NPA-sensitive (Fig. 2A).
FIGURE 3.
Exogenous NPA does not rescue the agravitropic response of pin2. A, gravity responses of wild-type (Col Wt) and pin2 (eir1-4) roots 24 h after reorientation of 90° to horizontal in the presence of the indicated concentrations of NPA. Each gravity-stimulated root was assigned to one of 12, 30° sectors (see Fig. 2). The length of each bar represents the mean percentages ± S.D. of seedlings showing the same direction of root growth of at least three independent experiments; numbers correspond to the mean (±S.D.) percent occurrence of 90 and 60° bending (sum of 90 and 60° sectors). B, mean percentages of plants showing 90 and 60° bending (sum of 90 and 60° sectors) ± S.D. of three independent experiments.
The cause of pin2 agravitropic root growth is a failure in the accumulation of auxin at the lower side of the root elongation zone (30). We therefore tested whether flavonoid treatment restored the asymmetric auxin distribution necessary for differential growth of epidermal cells during gravitropic responses. This was achieved by monitoring the DR5:GFP expression, which reflects relative auxin levels. As previously reported (41), in wild-type seedlings with vertically grown roots, the GFP fluorescence appeared in specific stele cell files and was localized in the quiescent center, in the columella initials, and in the mature columella cells (col) (Fig. 2C). Vertically oriented pin2 roots exhibited a strong signal in the distal lateral root cap with only weak extension toward the proximal lateral root cap, reflecting a defect in basipetal auxin distribution. In vertically oriented roots treatments with 100 nm quercetin or kaempferol resulted in fluorescence essentially similar to the control condition in both the wild-type and pin2 mutant (Fig. 2C). Upon a 2-h gravity stimulation, fluorescent signals in wild-type roots appeared in the lower sides, in the distal lateral root cap and EZ, both in solvent controls (control) and, although slightly reduced, in the presence of flavonoids (Fig. 2C, asterisks). In contrast, flavonoid treatment of gravity-stimulated pin2 roots resulted in a gain of a strong asymmetric signal in the entire lateral root cap with significantly increased fluorescence on the lower half of the root and decreased fluorescence on the upper half of the root (Fig. 2C, asterisks).
To statistically quantify asymmetric auxin accumulation after gravity stimulation, we determined root auxin gradient symmetry (in arbitrary units from 0 to 3) in relation to their tip orientations relative to the future gravity stimulation vector prior to gradient analysis (for details see legends of Table 2 and supplemental Figs. S1 and S2). Compared with wild type (11.7%, Table 2), the majority of pin2 seedlings tested exhibited a symmetrical auxin distribution (53.8% of seedlings showed auxin gradient symmetry “0”). Only occasionally strong asymmetric auxin gradients (defined as percentage of sum of gradient symmetry “2 + 3”) could be observed in pin2 upon gravity stimulation (27.7%, Table 2), which is in line with the results of the gravitropic assays (Fig. 2A). Interestingly, a similar frequency of clear auxin gradients in pin2 was also observed in vertically grown roots (31.7%) but was not found in the wild type (1.7%, supplemental Table S2). The gain of signal asymmetry between the lower and upper side in flavonoid-treated, gravity-stimulated pin2 roots continued in the elongation zone and was observed in the majority of the flavonoid-treated roots tested (62.0% compared with 27.7% without treatment, Table 2). In contrast, quercetin treatment had only a negligible effect on wild type (51.4 compared with 46.7% without treatment). Interestingly, also weak auxin gradients apparently result in gravitropic responses in wild-type roots, because inclusion of class 1 roots (Table 2; weak signal asymmetry, auxin gradient up to distal lateral root cap) enhances the relative number of roots showing an asymmetric gradient to 88.3%. This number corresponds to that of the roots exhibiting gravitropic responses (Fig. 2). These data show that exogenous flavonoids are able to reestablish the asymmetric DR5-GFP activity in the pin2 mutant roots building the prerequisite for restoration of gravitropic responses.
Flavonoids Promote Asymmetric PIN1 Shifts—Because some levels of functional redundancy between PIN proteins have been demonstrated (42, 43), we tested the root gravitropic responses of the triple pin2 pin3 pin7 mutant in the presence of 100 nm quercetin after 24 h. A strong agravitropic root phenotype was evident for pin2 pin3 pin7 in control conditions (Fig. 4A) (43), which could be partially rescued by the application of 100 nm quercetin (47.8 compared with 26.1% without treatment, Fig. 4A) or kaempferol (data not shown). From this result, we conclude that neither PIN3 nor PIN7 are required for flavonoid-dependent rescue of the agravitropic response of pin2, being in-line with their proposed role and expression in gravity perception tissues (43). Importantly, when we performed the same gravitropic assay with roots of the double mutant pin1 pin2, quercetin could not complement its agravitropic phenotype (15.7 compared with 12.2% without treatment, Fig. 4A). These data demonstrate that PIN1 is essential for flavonoid-dependent, partial complementation of pin2 gravitropism, which is in agreement with its expression in gravity transduction or response tissues.
To trace the behavior of PIN1 protein during pin2 gravitropic responses and to uncover a possible link between the action of flavonoids and PIN1 activity in vivo, we analyzed PIN1 localization in pin2 roots exposed to 100 nm quercetin prior to, and during a 2-h gravity stimulation. Consistent with previous reports (42, 43), in vertically oriented wild-type roots PIN1 was mainly found at the basal (lower) end of vascular and endodermis cells with occasional weak expression in the epidermis and cortex (Fig. 4B, i). In vertical pin2 roots, PIN1 was ectopically expressed in the endogenous PIN2 domain, showing symmetric apical (up) localization in the epidermis and basal (down) localization in cortex cells (arrows in Fig. 4B, ii) (42). This symmetric PIN1 location was only rarely altered by a gravity stimulus on solvent control (12.5% of roots showing this pattern, Fig. 4C, i). Treatments with 100 nm quercetin expanded the distribution of PIN1 and slightly its expression as previously reported (18) but did not affect its symmetry of expression neither in wild type nor in pin2 vertically grown roots (Figs. 4B, iii-iv). In contrast, gravity stimulation of pin2 roots in the presence of 100 nm quercetin resulted in asymmetric expression of PIN1 protein, with stronger PIN1-specific signals at the lower side of the root tip (Fig. 4C, iii). The establishment of flavonoid-mediated PIN1 gradients strictly correlated with the development of asymmetric DR5-GFP signals (100%, Fig. 4C, iii), whereas in the absence of quercetin asymmetric PIN1 patterns correlating with asymmetric DR5-GFP signals were only rarely found (12.5%, Fig. 4C, i). We never found asymmetric DR5-GFP gradients that correlated with symmetric PIN1 patterns (0%, Fig. 4C, iv) or symmetric DR5-GFP gradients correlating with asymmetric PIN1 expression (0%, supplemental Fig. S3, i). But for a few roots we found a weak correlation between asymmetric PIN1 patterns and symmetric DR5-GFP (22.2%, supplemental Fig. S3, iii).
DISCUSSION
The current paradigm in auxin research is that flavonoids act as non-essential, but important multifunctional, endogenous modulators of PAT by transiently accumulating in the epidermal cells of the root elongation zone (14, 16-19, 36). Our data demonstrate that defects in basipetal auxin transport are associated with altered root flavonoid accumulation. In conditions in which PIN2-dependent auxin transport is genetically blocked, synthesis and transient accumulation of specific flavonoid glycosides in the root tip elongation zone, but not over the entire root or in the shoot, are impaired quantitatively and qualitatively (Figs. 1, and supplementalS1 and S2). However, pin2 roots retain the ability to accumulate flavonoids in response to a gravity stimulus, but not as efficiently as the wild type.
Application of low concentrations of exogenous flavonoids partially restores gravitropic root tip bending in a genetic background defective in basipetal auxin transport and this mode of flavonoid action is apparently distinct from that of NPA. Moreover, our results showing that gravity stimulation of pin2 roots in the presence of a wide concentration range of NPA shown to inhibit PAT (24, 35, 40) did not complement pin2 gravitropism, suggest that in our experimental conditions and concentrations of flavonoids (Fig. 2B) predominantly do not act as PAT inhibitors. Quantitative cell biology analysis of DR5-GFP signals show that partial, chemical complementation of gravitropism by exogenous flavonoids is accompanied by re-establishment of asymmetric distribution of DR5-GFP in the pin2 mutant roots. This suggests that restoration of gravitropic responses is achieved via bypassing the requirement of an active PIN2 protein, thus implying the activation of a PIN2-independent mechanism for basipetal auxin transport.
Quantification of root gravitropic response of different pin mutant combinations suggests that PIN1 is essential for flavonoid-dependent complementation of pin2 gravitropism, which is in agreement with its expression in gravity transduction or response tissues. Using a quantitative cell biological approach, we also provide evidence that flavonoid-dependent rescue of pin2 agravitropism by PIN1 is correlated with asymmetric PIN1 distribution across gravity-stimulated tissues. In summary, our findings suggest that PIN1 is the auxin efflux complex component that facilitates basipetal auxin fluxes for gravitropic responses in flavonoid-treated pin2 roots. The observed basal-apical PIN1 shifts in pin2 roots (Fig. 4B, ii) are in line with the finding that PIN1 and PIN2 have redundant roles in the root meristem size control (43) and that PIN1 can functionally replace PIN2 when ectopically expressed and localized at the upper side of epidermal cells (5). Moreover, PIN1 showed a “PIN2-like” apical localization in epidermis and basal localization in cortex cells in roots of pin2 mutants (42). However, our data indicate that flavonoids are the native key effectors that promote asymmetric PIN1 shifts with stronger PIN1-specific signals at the lower side of the root tip in response to a gravity stimulus, thus redirecting basipetal auxin streams necessary for root tip bending.
The fact that flavonols can inhibit PAT and displace NPA from their membrane binding sites has led to the idea that flavonoids and NPA act on similar targets using identical mechanisms. Several lines of evidence suggest that plant ABCBs, like ABCB1, ABCB4, and ABCB19, are direct targets of flavonoid regulation (8, 22, 23) via protein phosphorylation, protein-protein interaction (31), or inhibition of ATPase activity or allosteric binding in analogy to mammalian ABCBs (28). This direct, modulatory role of flavonoids can be widely phenocopied by NPA. However, the effect of flavonoids on members of the PIN family has received less attention and seems to be indirect (transcriptional) and result in activation of auxin streams (18, 20) and cannot be mimicked by NPA.
In summary, with this work we provide two lines of evidence demonstrating that flavonoids, at least under our experimental conditions, do not solely inhibit efflux transportes, but are able to function as versatile modulators of polar auxin flows. First, flavonoid concentrations applied to the roots did not significantly alter over 24 h wild-type gravitropism and therefore most likely also not PAT. Second, NPA over a wide concentration range failed to restore pin2 root gravitropism, whereas partial rescue of pin2 agravitropic response by quercetin was NPA sensitive. Our data showing that flavonoids can promote PIN1 shifts in response to a gravity stimulus underline an involvement of flavonoids in cellular trafficking of auxin transport complex components as recently suggested (18, 20).
The partial restoration of gravitropism in pin2 by flavonoid treatment parallels the previously published complete restoration of basipetal auxin transport (16, 17) and root gravitopism of tt4 mutants (14) by external application of flavonoids and are both consistent with a positive role of flavonoids in facilitating gravitropic curvature. tt4 mutants lack a functional chalcone synthase and produce therefore no flavonoids, whereas adding the flavonoid precursor naringenin restores flavonoid synthesis (16, 20). However, these and our studies reached despite the different tools used opposite conclusions on the negative or positive regulatory impact of flavonoids on auxin transport. This paradox might be at least partially explained by recent work from Peer et al. (18) that reports opposite regulation of PIN2 and PIN1 expression by flavonoids: whereas PIN2 expression is enhanced in the absence of flavonoids, PIN1 expression is depressed. Moreover, PIN1 is delocalized from the plasma membrane in the absence of flavonoids, whereas PIN2 is not (18). This suggests that flavonoids might act as negative regulators of PIN2, but as positive effectors of PIN1. The same study demonstrates that auxin reduces PIN1 expression in the root. One might speculate that this might be the underlying mechanism by which PIN1 is induced in the pin2 mutant, which owns presumably lower auxin levels in these cells that are relevant for basipetal auxin reflux. Finally, one should keep in mind that inverse modulation of root gravitropism by flavonoids may result from a combination of ABCB regulation and PIN trafficking that might be concentration-dependent and interconnected.
Although the cellular targets of flavonoid action are now known, the underlying mechanism remains elusive. Transport assays with PIN proteins suggest that flavonoids probably do not interact with PINs directly. However, flavonoids affect specific PIN expression, location, and cellular trafficking probably through interaction with regulatory proteins (18, 20). Recently, plasma membrane PIN shifts have been demonstrated to be caused by antagonistic PIN phosphorylation via protein kinase PINOID (PID) and protein phosphatase 2A (44, 45). Our data together with the fact that flavonols are routinely used as both protein phosphatase and kinase inhibitors make PID or PID-related WAG kinases (46) and/or protein phosphatase 2A-like phosphatases the most likely candidate targets for flavonol-mediated PIN-shifts.
Supplementary Material
Acknowledgments
We thank Dr. Heller (National Research Centre for Environment and Health (GSF), Munich) and Dr. Veit (Kaufering) for providing flavonol standards that were invaluable for the flavonol structure analysis, M. Lopes for help during the root bending assays, and V. Sovero and S. Peters for comments on the manuscript.
This work was supported by the Swiss National Foundation Grant 3100AO-111912 (to M. G.) and a grant to E. M. (within the NCCR Plant Survival), a grant from the Novartis foundation (to M. G.) and by a grant from the Ministry of Science and Technology of South Korea (to Y. L. and E. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S3 and Tables S1 and S2.
Footnotes
The abbreviations used are: IAA, 3-indolyl acetic acid; PAT, polar auxin transport; NPA, N-1-naphthylphthalamic acid; EZ, elongation zone; RT, root tip; HPLC, high performance liquid chromatography; DPBA, diphenylboric acid 2-aminoethyl ester; MS, mass spectrometry; GFP, green fluorescent protein.
References
- 1.Muday, G. K. (2001) J. Plant Growth Regul. 20 226-243 [DOI] [PubMed] [Google Scholar]
- 2.Chen, R., Hilson, P., Sedbrook, J., Rosen, E., Caspar, T., and Masson, P. H. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 15112-15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore, I. (2002) Curr. Biol. 12 R452-454 [DOI] [PubMed] [Google Scholar]
- 4.Vieten, A., Sauer, M., Brewer, P. B., and Friml, J. (2007) Trends Plant Sci. 12 160-168 [DOI] [PubMed] [Google Scholar]
- 5.Wisniewska, J., Xu, J., Seifertova, D., Brewer, P. B., Ruzicka, K., Blilou, I., Rouquie, D., Benkova, E., Scheres, B., and Friml, J. (2006) Science 312 883. [DOI] [PubMed] [Google Scholar]
- 6.Geisler, M., and Murphy, A. S. (2006) FEBS Lett. 580 1094-1102 [DOI] [PubMed] [Google Scholar]
- 7.Kerr, I. D., and Bennett, M. J. (2007) Biochem. J. 401 613-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blakeslee, J. J., Bandyopadhyay, A., Lee, O. R., Mravec, J., Titapiwatanakun, B., Sauer, M., Makam, S. N., Cheng, Y., Bouchard, R., Adamec, J., Geisler, M., Nagashima, A., Sakai, T., Martinoia, E., Friml, J., Peer, W. A., and Murphy, A. S. (2007) Plant Cell 19 131-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis, D. R., Miller, N. D., Splitt, B. L., Wu, G., and Spalding, E. P. (2007) Plant Cell 19 1838-1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu, G., Lewis, D. R., and Spalding, E. P. (2007) Plant Cell 19 1826-1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swarup, R., Friml, J., Marchant, A., Ljung, K., Sandberg, G., Palme, K., and Bennett, M. (2001) Genes Dev. 15 2648-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchant, A., Kargul, J., May, S. T., Muller, P., Delbarre, A., Perrot-Rechenmann, C., and Bennett, M. J. (1999) EMBO J. 18 2066-2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abas, L., Benjamins, R., Malenica, N., Paciorek, T., Wisniewska, J., Moulinier-Anzola, J. C., Sieberer, T., Friml, J., and Luschnig, C. (2006) Nat. Cell Biol. 8 249-256 [DOI] [PubMed] [Google Scholar]
- 14.Buer, C. S., and Muday, G. K. (2004) Plant Cell 16 1191-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buer, C. S., Muday, G. K., and Djordjevic, M. A. (2007) Plant Physiol. 145 478-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown, D. E., Rashotte, A. M., Murphy, A. S., Normanly, J., Tague, B. W., Peer, W. A., Taiz, L., and Muday, G. K. (2001) Plant Physiol. 126 524-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy, A., Peer, W. A., and Taiz, L. (2000) Planta 211 315-324 [DOI] [PubMed] [Google Scholar]
- 18.Peer, W. A., Bandyopadhyay, A., Blakeslee, J. J., Makam, S. I., Chen, R. J., Masson, P. H., and Murphy, A. S. (2004) Plant Cell 16 1898-1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peer, W. A., and Murphy, A. S. (2006) in The Science of Flavonoids (Grotewold, E., ed) pp. 239-268, Springer, Berlin
- 20.Peer, W. A., and Murphy, A. S. (2007) Trends Plant Sci. 12 556-563 [DOI] [PubMed] [Google Scholar]
- 21.Taylor, L. P., and Grotewold, E. (2005) Curr. Opin. Plant Biol. 8 317-323 [DOI] [PubMed] [Google Scholar]
- 22.Bouchard, R., Bailly, A., Blakeslee, J. J., Oehring, S. C., Vincenzetti, V., Lee, O. R., Paponov, I., Palme, K., Mancuso, S., Murphy, A. S., Schulz, B., and Geisler, M. (2006) J. Biol. Chem. 281 30603-30612 [DOI] [PubMed] [Google Scholar]
- 23.Geisler, M., Blakeslee, J. J., Bouchard, R., Lee, O. R., Vincenzetti, V., Bandyopadhyay, A., Titapiwatanakun, B., Peer, W. A., Bailly, A., Richards, E. L., Ejendal, K. F. K., Smith, A. P., Baroux, C., Grossniklaus, U., Muller, A., Hrycyna, C. A., Dudler, R., Murphy, A. S., and Martinoia, E. (2005) Plant J. 44 179-194 [DOI] [PubMed] [Google Scholar]
- 24.Bailly, A., Sovero, V., Vincenzetti, V., Santelia, D., Bartnik, D., Koenig, B. W., Mancuso, S., Martinoia, E., and Geisler, M. (2008) J. Biol. Chem. 283 21817-21826 [DOI] [PubMed] [Google Scholar]
- 25.Noh, B., Murphy, A. S., and Spalding, E. P. (2001) Plant Cell 13 2441-2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terasaka, K., Blakeslee, J. J., Titapiwatanakun, B., Peer, W. A., Bandyopadhyay, A., Makam, S. N., Lee, O. R., Richards, E. L., Murphy, A. S., Sato, F., and Yazaki, K. (2005) Plant Cell 17 2922-2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris, M. E., and Zhang, S. (2006) Life Sci. 78 2116-2130 [DOI] [PubMed] [Google Scholar]
- 28.Conseil, G., Baubichon-Cortay, H., Dayan, G., Jault, J. M., Barron, D., and Di Pietro, A. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 9831-9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller, A., Guan, C. H., Galweiler, L., Tanzler, P., Huijser, P., Marchant, A., Parry, G., Bennett, M., Wisman, E., and Palme, K. (1998) EMBO J. 17 6903-6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luschnig, C., Gaxiola, R. A., Grisafi, P., and Fink, G. R. (1998) Genes Dev. 12 2175-2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs, M., and Rubery, P. H. (1988) Science 241 346-349 [DOI] [PubMed] [Google Scholar]
- 32.Friml, J., Benkova, E., Mayer, U., Palme, K., and Muster, G. (2003) Plant J. 34 115-124 [DOI] [PubMed] [Google Scholar]
- 33.Paciorek, T., Zazimalova, E., Ruthardt, N., Petrasek, J., Stierhof, Y. D., Kleine-Vehn, J., Morris, D. A., Emans, N., Jurgens, G., Geldner, N., and Friml, J. (2005) Nature 435 1251-1256 [DOI] [PubMed] [Google Scholar]
- 34.Utsuno, K., Shikanai, T., Yamada, Y., and Hashimoto, T. (1998) Plant Cell Physiol. 39 1111-1118 [DOI] [PubMed] [Google Scholar]
- 35.Rashotte, A. M., Brady, S. R., Reed, R. C., Ante, S. J., and Muday, G. K. (2000) Plant Physiol. 122 481-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peer, W. A., Brown, D. E., Tague, B. W., Muday, G. K., Taiz, L., and Murphy, A. S. (2001) Plant Physiol. 126 536-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tohge, T., Nishiyama, Y., Hirai, M. Y., Yano, M., Nakajima, J., Awazuhara, M., Inoue, E., Takahashi, H., Goodenowe, D. B., Kitayama, M., Noji, M., Yamazaki, M., and Saito, K. (2005) Plant J. 42 218-235 [DOI] [PubMed] [Google Scholar]
- 38.Buer, C. S., Sukumar, P., and Muday, G. K. (2006) Plant Physiol. 140 1384-1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casimiro, I., Marchant, A., Bhalerao, R. P., Beeckman, T., Dhooge, S., Swarup, R., Graham, N., Inze, D., Sandberg, G., Casero, P. J., and Bennett, M. (2001) Plant Cell 13 843-852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rashotte, A. M., DeLong, A., and Muday, G. K. (2001) Plant Cell 13 1683-1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ottenschlager, I., Wolff, P., Wolverton, C., Bhalerao, R. P., Sandberg, G., Ishikawa, H., Evans, M., and Palme, K. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2987-2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vieten, A., Vanneste, S., Wisniewska, J., Benkova, E., Benjamins, R., Beeckman, T., Luschnig, C., and Friml, J. (2005) Development 132 4521-4531 [DOI] [PubMed] [Google Scholar]
- 43.Blilou, I., Xu, J., Wildwater, M., Willemsen, V., Paponov, I., Friml, J., Heidstra, R., Aida, M., Palme, K., and Scheres, B. (2005) Nature 433 39-44 [DOI] [PubMed] [Google Scholar]
- 44.Friml, J., Yang, X., Michniewicz, M., Weijers, D., Quint, A., Tietz, O., Benjamins, R., Ouwerkerk, P. B., Ljung, K., Sandberg, G., Hooykaas, P. J., Palme, K., and Offringa, R. (2004) Science 306 862-865 [DOI] [PubMed] [Google Scholar]
- 45.Michniewicz, M., Zago, M. K., Abas, L., Weijers, D., Schweighofer, A., Meskiene, I., Heisler, M. G., Ohno, C., Zhang, J., Huang, F., Schwab, R., Weigel, D., Meyerowitz, E. M., Luschnig, C., Offringa, R., and Friml, J. (2007) Cell 130 1044-1056 [DOI] [PubMed] [Google Scholar]
- 46.Santner, A. A., and Watson, J. C. (2006) Plant J. 45 752-764 [DOI] [PubMed] [Google Scholar]
- 47.Kerhoas, L., Aouak, D., Cingöz, A., Routaboul, J. M., Lepiniec, L., Einhorn, J., and Birlirakis, N. (2006) J. Agric. Food Chem. 54 6603-6612 [DOI] [PubMed] [Google Scholar]
- 48.Routaboul, J. M., Kerhoas, L., Debeaujon, I., Pourcel, L., Caboche, M., Einhorn, J., and Lepiniec, L. (2006) Planta 224 96-107 [DOI] [PubMed] [Google Scholar]
- 49.Stobiecki, M., Malosse, C., Kerhoas, L., Wojlaszek, P., and Einhorn, J. (1999) Phytochem. Anal. 10 198-207 [Google Scholar]
- 50.Morris, D. A. (2000) Plant Growth Regul. 32 161-172 [DOI] [PubMed] [Google Scholar]
- 51.Lomax, T. L., Muday, G. K., and Rubery, P. H. (1995) in Plant Hormones: Physiology, Biochemistry and Molecular Biology (Davies, P. J., ed) pp. 509-530, Kluwer, Dordrecht, Netherlands
- 52.Luschnig, C. (2002) Trends Plant Sci. 7 329-332 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.