Abstract
The Ca2+ coupling between endoplasmic reticulum (ER) and mitochondria is central to multiple cell survival and cell death mechanisms. Cytoplasmic [Ca2+] ([Ca2+]c) spikes and oscillations produced by ER Ca2+ release are effectively delivered to the mitochondria. Propagation of [Ca2+]c signals to the mitochondria requires the passage of Ca2+ across 3 membranes, namely the ER membrane, the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM). Strategic positioning of the mitochondria by cytoskeletal transport and interorganellar tethers provides a means to promote the local transfer of Ca2+ between the ER membrane and OMM. In this setting, even >100µM [Ca2+] may be attained to activate the low affinity mitochondrial Ca2+ uptake. However, a mitochondrial [Ca2+] rise has also been documented during submicromolar [Ca2+]c elevations. Evidence has been emerging that Ca2+ exerts allosteric control on the Ca2+ transport sites at each membrane, providing mechanisms that may facilitate the Ca2+ delivery to the mitochondria. Here we discuss the fundamental mechanisms of ER and mitochondrial Ca2+ transport, particularly the control of their activity by Ca2+ and evaluate both high and low [Ca2+] activated mitochondrial calcium signals in the context of cell physiology.
Keywords: Ca2+, endoplasmic reticulum, sarcoplasmic reticulum, mitochondria, IP3 receptor, ryanodine receptor, VDAC, uniporter, mitochondrial dynamics
Mechanisms of ER-mitochondrial Ca2+ transport: Ca2+-mediated feedforward and feedback pathways
Endoplasmic-and sarcoplasmic reticulum (ER/SR) Ca2+ release mediated by IP3R/RyR
The ER forms a luminally interconnected network of tubules and cysternae throughout the cytoplasm and shows inhomogeneous distribution of the Ca2+ uptake and release sites. Based on these properties, different cytoplasmic signals may converge in the ER to form spatio-temporally controlled Ca2+ release patterns [1;2].
ER Ca2+ uptake
The resting [Ca2+] in the lumen of the ER/SR ([Ca2+]ER) is in the range of 0.1–0.8 mM, ~4 orders of magnitude higher than [Ca2+]c. This steep [Ca2+] gradient, which is comparable to the one across the plasma membrane, is built up primarily by means of Ca2+ pumps of the SERCA family at the expense of ATP hydrolysis (Fig1A). SERCA pumps are encoded by 3 genes that yield more than 10 different isotypes at the protein level due to alternative splicing. The expression of the different isotypes is tissue and developmental stage specific. SERCA pumps are controlled by a host of Ca2+-dependent and independent cytosolic, intra-membrane as well as luminal factors [3]. Evidence has been presented that mitochondrial function e.g. ATP production and Ca2+ uptake may exert local control on SERCA activity in the adjacent ER [4–7].
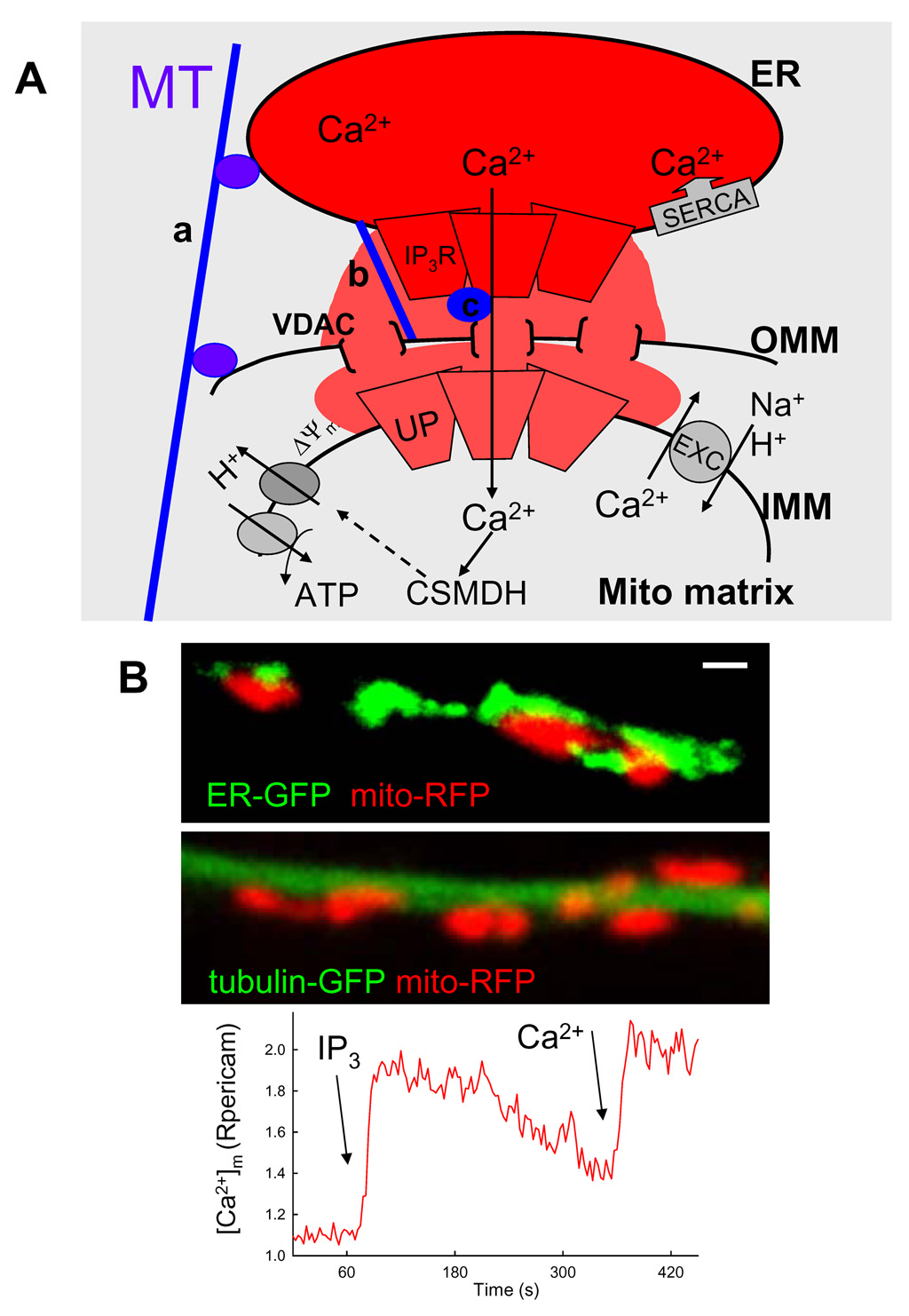
Fig1. Local coupling between ER and mitochondria.
(A) Scheme depicting the mechanisms of local Ca2+ transport and physical linkage between an ER stack and an adjacent mitochondrion. Ca2+ stored in the ER lumen is released through the IP3 receptors (IP3R) giving rise to a high [Ca2+] microdomain that exposes an adjacent mitochondrion. Ca2+ traverses the outer mitochondrial membrane (OMM) through the voltage dependent anion-selective channels (VDAC) and the inner mitochondrial membrane (IMM) via the uniporter (UP). In the mitochondrial matrix Ca2+ binds to the Ca2+ sensitive mitochondrial dehydrogenases (CSMDH) to stimulate energy metabolism. Ca2+ exits mitochondria through the Na+/Ca2+ or H+/Ca2+ exchanger (EXC) and is taken back to the ER by the Ca2+ pumps (SERCA). [Ca2+] is indicated by shades of red.
Components of the physical coupling between ER and mitochondria: a, binding of both ER and mitochondria to a microtubule (MT) or to other cytoskeletal fibers; b, protein tethering ER membrane directly to the mitochondria and c, multimolecular complexes involving both the IP3Rs and VDACs.
(B) Visualization of mitochondria and ER (upper), mitochondria and a microtubule (middle) and the IP3-induced mitochondrial calcium signal (lower) in a projection of an RBL-2H3 cell. Confocal imaging was performed in cells expressing either an ER-targeted enhanced green fluorescent protein (ER-GFP) and a mitochondrial matrix targeted red fluorescent protein (mito-RFP) (upper) or tubulin-targeted enhanced green fluorescent protein (tubulin-GFP) and mito-RFP (middle). The length of the scalebar is 1µm. Fluorescence imaging was conducted in mitochondrial matrix targeted ratiometric pericam expressing permeabilized cell sequentially stimulated with IP3 (7.5µM) and Ca2+ (30µM). The graph shows the time course of the pericam ratio calculated for a mitochondrion located in a projection.
ER luminal Ca2+ buffering
The ER Ca2+ storage capacity is dependent on the expression of the intra-ER Ca2+ binding proteins [8;9] that may also have a role in chaperoning Ca2+ release channel proteins [10]. In pancreatic acinar cells, the Ca2+-binding capacity in the lumen of the ER was calculated to be ~100 times lower than in the cytosol (20 vs. ~1500–2000 [11]), suggesting greater mobility for the Ca2+ ions inside the store. The major luminal diffusible Ca2+ buffers are calreticulin (CRT) in the ER and calsequestrin (CSQ) in the SR. CRT may also bind to the SERCA [12] and CSQ forms a complex with the ryanodine receptor (RyR) [13] to effectively control [Ca2+]ER close to the transport sites and to regulate their activity. Changes in the ER Ca2+ loading in association with CRT deficiency or overexpression were not paralleled by similar changes in [Ca2+]ER [14;15], indicating that the steady-state [Ca2+]ER is maintained under a wide range of store Ca2+ content. Interestingly, in CRT-overexpressing cells the IP3-mediated [Ca2+]m rise was suppressed and was transient probably due to the rapid reuptake of Ca2+ mediated by the SERCA [5].
ER Ca2+ release
Two major and phylogenetically related families of Ca2+ release channels in the reticular Ca2+ stores are: the inositol 1,4,5-trisphosphate receptors (IP3Rs, types 1,2,3) that reside primarily in the ER (Fig1A) and the RyRs (types 1,2,3) predominantly in the SR of muscle cells. However, IP3Rs are also present in the SR of some muscle cells and RyR in the ER of certain non-muscle cells. Different cell types contain various combinations of the 3 IP3R isotypes, whereas in each tissue one RyR isotype is dominant (skeletal muscle, type 1; cardiac muscle, type 2; smooth muscle and brain, type 3). Recently, in a cell line that expresses all IP3R isoforms, type 3 IP3Rs were shown to preferentially transmit calcium signals to the mitochondria [16]. The IP3Rs and RyRs encounter dozens of molecular interactions to receive input from different signalling systems (see [17–19] for recent reviews). One of the principal factors that determine the Ca2+ release signal output is the regulation of the release channels by Ca2+ itself. Both, IP3R and RyR have been reported to be regulated by luminal and cytosolic Ca2+ as well. The control of Ca2+ release by luminal and cytosolic Ca2+ and other factors is expanded below.
The IP3 sensitivity of the IP3R is increased by luminal Ca2+ [20;21] but it was claimed that this co-agonistic effect had been established via a cytosolic Ca2+ binding site by Ca2+ exiting through the IP3R [22]. On the other hand, single channel studies conducted at optimal [IP3] levels indicated the presence of a luminal Ca2+ inhibitory site [23;24]. The inhibition attributed to this mechanism could be relieved by cytosolic ATP (500 µM) [25]. Recently, ERp44, an ER luminal protein has been shown to interact with the IP3R1 in a Ca2+, redox and pH dependent manner and to inhibit the Ca2+ release when the Ca2+ load falls [26]. This mechanism does not apply to other IP3R isoforms and the luminal Ca2+ regulation of the IP3R2&3s has not yet been studied. Similarly to the IP3R, the activation of RyR is also regulated by the luminal [Ca2+]. Multiple reports showed that increasing luminal [Ca2+] causes a net increase in the channel activity [27–29]. A functionally relevant Ca2+ binding site has been localized on the RyR [30] but recent data shows that the stimulatory Ca2+ effect is mediated by the luminal CSQ that forms a regulatory complex with triadin and junction [13].
Overexpression of ER-targeted anti-apoptotic Bcl-2 protein caused increased Ca2+-leak from the ER, suppressed the IP3R-linked [Ca2+]m responses and gave protection against ceramide-induced apoptosis [31;32]. Binding of Bcl-2 to the IP3R may also be relevant for these effects [33;34]. To directly test the ER Ca2+ load dependence of the IP3R-mediated Ca2+ release and the corresponding mitochondrial Ca2+ uptake, we preloaded permeabilized RBL-2H3 cells with small pulses of Ca2+. As the ER Ca2+ load was augmented the Ca2+ release induced by optimal IP3 increased in a linear fashion. Interestingly, the mitochondrial Ca2+ uptake associated with the IP3-induced Ca2+ release displayed a supralinear relationship with increasing Ca2+ loading of the ER. This was also apparent when the [Ca2+]c was clamped by EGTA at resting levels (Csordás& Hajnóczky, unpublished). Thus, the local Ca2+ signal propagation from IP3R to the mitochondria is effectively controlled by the ER Ca2+ load.
IP3R/RyRs show a bell-shaped [Ca2+]c dependence for the activation of both receptors although some subtypes (RyR2 and in a study, IP3R3) lack inactivation at physiologically relevant [Ca2+]c ranges [reviewed in [17;18;35;36]]. Activation of the Ca2+ release channels by [Ca2+]c is one of the principal mechanisms for coordination of individual release events with each other giving rise to regenerative propagation of the Ca2+ release [reviewed e.g. in [37]]. On the other hand, the bell-shaped [Ca2+]c dependence of activation offers a mechanism for inhibiting release events by high [Ca2+] microdomains built at the mouth of the activated receptors. Besides the low-affinity inhibitory Ca2+-binding site responsible for the inhibition by high [Ca2+] independent of [IP3], there is also a high-affinity site that inhibits the channel in the absence of IP3 but with increasing ligand concentrations it gets converted to an activation site [17]. This latter mechanism may be important in increasing the fidelity of ligand-activated channel openings in groups of channels (see later).
Multiple lines of evidence have been presented that the local Ca2+ control of the IP3R/RyR can be modulated by the Ca2+ uptake and metabolic activity of the mitochondria located in the immediate vicinity of the release channels at the close contacts of ER/SR and mitochondria. By this mechanism, mitochondria can contribute to the shaping of the global [Ca2+]c signal. In permeabilized hepatocytes, IP3-induced [Ca2+]ER decreases were larger in low mitochondrial density regions of the cells as well as after pharmacological inhibition of the mitochondrial Ca2+ uptake [38], while enhancement of the mitochondrial Ca2+ accumulation after blocking the permeability transition pore suppressed the release activity [39]. On the other hand, [Ca2+]c spikes generated by microinjection of a non-hydrolizable IP3 analogue into Xenopus oocytes (IP3R1) became enhanced and more synchronized after boosting mitochondrial Ca2+ uptake by the energizing substrate succinate [40]. Similarly, pharmacological stimulation of the mitochondrial Ca2+ uptake sites enhanced the histamine-induced [Ca2+]c signals in HeLa and human fibroblast cells [41]. Besides the local Ca2+ clearance, mitochondria may also participate in the development of regenerative/oscillatory Ca2+ release patterns by returning Ca2+ in the inter-spike period via the mitochondrial Ca2+ extrusion pathways (Na+/Ca2+ and H+/Ca2+ exchangers) to ‘prime’ the next release event [42]. However, in H295R cells uncoupling of the mitochondria enhanced the oscillatory pattern of the [Ca2+]c signal and eliminated the amplitude difference between the [Ca2+]c rise recorded in the perinuclear and in the subplasmalemmal regions [43]. The wide range of the mitochondrial feedback effects on the IP3R-mediated [Ca2+]c signal reflects the contribution of several factors, including the spatial relationship between ER and mitochondria and the amount of Ca2+ available for mobilization.
The [Ca2+]c signal generated by the activation of IP3R/RyR ranges from greatly confined elementary release events produced by a single or a few channels through propagating repetitive waves of release to concerted simultaneous activation of the entire release channel population throughout the ER. The IP3R/RyR channels are not distributed homogenously on the ER/SR surface but they form clusters where the inter-channel signalling is enhanced. These clustered channels may work as autonomous elementary release modules that can be activated independently of the rest of the channel population giving rise to isolated and spatially confined [Ca2+]c transients called ‘puffs’ (IP3R) or ‘sparks’ (RyR) [reviewed in [37]]. Depending on the channel density, the functional IP3R release units may have different IP3 sensitivities [44;45]. The release units formed by RyR in skeletal/cardiac muscle tissue show a regular pattern and subcellular distribution [46]. Dynamic reorganization of the IP3R clusterization is induced in certain cell systems by sustained increases in [Ca2+]c [47] or by prolonged openings of the IP3R channels [48].
In muscle cell lines and myotubes, mitochondria can pick up Ca2+ mobilized from a single RyR release unit ([Ca2+]c sparks) leading to the generation of single mitochondrial miniature [Ca2+]m signals (Ca2+ marks, [49]) and to feedback control on the Ca2+ release [49;50]. The effect of [Ca2+]c puffs on [Ca2+]m has not been directly measured. However, in Xenopus oocytes [Ca2+]c puffs were frequently colocalized with mitochondria but showed reduced frequency and intensities and never served as wave initiation sites, further indicating that mitochondria are involved in the control of local [Ca2+]c signalling events [51]. Furthermore, recent modeling studies estimated that only 25–35 out of 40–70 IP3Rs would contribute actively to the elementary release event Ca2+ puffs in Xenopus oocyte [45], which is likely due to local Ca2+-inhibition of clusterized IP3R-mediated release activity [52]. However, in response to optimal IP3 every channel synchronously opens in the cluster. The need for synchronized opening of the neighboring IP3Rs for activation of the mitochondrial Ca2+ uptake may be a reason why suboptimal IP3-induced Ca2+ release is not very efficient in raising [Ca2+]m [53].
Several factors originating from the mitochondria e.g. ATP, cytochrome c and reactive oxygen species (ROS) are important for the control of IP3R/RyR activity. The mitochondrial output of these factors is commonly induced by a [Ca2+]m signal. ATP (free of Mg2+) has been reported to potentiate Ca2+-activation of IP3R1&3 and may be present at particularly high concentrations in the close appositions of mitochondria and ER, establishing progressive sensitization of IP3R during oscillatory release activity [54–57]. Cytochrome c (cyto c) is normally confined to the mitochondrial intermembrane space and cristae, however pro-apoptotic conditions may cause permeabilization of the OMM and let cyto c escape to the cytosol. Boehning et al. found that cyto c specifically interacted in vitro and in vivo with IP3R, enhanced its activity by relieving its Ca2+-inhibition and was necessary for apoptosis in some paradigms based on the effects of a peptide specifically interfering with the cyto c binding to the IP3R [58;59]. ROS are produced by the mitochondria under physiological, and in larger amounts under pathophysiological conditions. Both superoxide anion and hydrogen peroxide stimulate IP3R/RyR-mediated [Ca2+]c signalling [60–62].
ER-mitochondrial interface
Ca2+ uptake via the ruthenium-sensitive Ca2+ uniporter is driven by the 150–180 mV (inside negative) mitochondrial membrane potential, its half-maximal transport rate, as measured in mitochondrial suspensions, is attained in the range of 10−6–10−4 M Ca2+ [63]. In an electrophysiological study on mitoplasts (mitochondria without the OMM) [64] the ruthenium red-sensitive Ca2+ transport was found to occur through highly selective ion channels. The inward rectifying Ca2+ current had half-saturation of the Ca2+ carrying capacity at 19 mM [Ca2+]c, orders of magnitude higher than that of Ca2+ uptake in mitochondrial suspension. (This difference may be attributed to the rapid dissipation of the membrane potential during Ca2+ entry, causing flux saturation at much lower [Ca2+]c in mitochondrial suspension, as opposed to the voltage-clamped mitoplasts). In view of these transport characteristics it has been assumed that [Ca2+]c far exceeding the micromolar range is required for net Ca2+ uptake, however, such [Ca2+]c values have not been observed experimentally in over the whole (so-called global) cytoplasm.
Protein binding significantly limits Ca2+ diffusion in the cytoplasm [65]. Therefore Ca2+, entering a cytosolic domain confined by neighbouring membranes, can generate local Ca2+ transients with amplitudes far exceeding those measured over the global cytoplasm. Mitochondria, forming a complex cytoplasmic tubulovesicular system [66;67], are frequently apposed to the smooth as well as the rough ER (Fig1B). The apposition, often termed as contact point, has been observed in several cell types by means of electron microscopy or tomography (e.g. [68]). Microdomains are thus formed and Ca2+ released through IP3R into such a confined space, may induce supramicromolar, or even submillimolar Ca2+ signal (Fig1A). The possibility of such an event was indicated in 1993 by the experiments of Rizzuto and his coworkers in Pozzan’s laboratory [69]. The mitochondrial response to a mean 600 nM elevation of [Ca2+]c in histamine-stimulated HeLa cells could be reproduced by superfusing the cells with a cytosolic medium containing at least 5 µM Ca2+ after cell permeabilization. Similar results were obtained in cardiac (H9c2) cells where the RyR-mediated elevation of global [Ca2+]c increases were in the submicromolar range but the rate of [Ca2+]m increases was as large as that induced with 30 µM Ca2+ in permeabilized cardiomyocytes [70]. Also in cardiac and skeletal muscle, EGTA/BAPTA antagonized the RyR-mediated increase in [Ca2+]c but had substantially less impact on [Ca2+]m [70–72]. In permeabilized RBL-2H3 cells IP3-induced Ca2+ elevation reached values more than 20-fold higher in the vicinity of mitochondria than those measured for the global cytosol [53]. In hepatocytes the long lag between [Ca2+]c and NAD(P)H increases induced by Ca2+ influx, as opposed to IP3 –induced Ca2+ release, was due to a delayed uptake of Ca2+ into the mitochondrial matrix [73]. The dependence of mitochondrial Ca2+ uptake on the distance between ER and mitochondria was examined in H295R adrenocortical cells. Following stimulation with angiotensin II both mitochondrial Ca2+ uptake rate and [Ca2+]m peak showed a close correlation with the vicinity of ER vesicles, where the distance of ER from the mitochondria was assessed on basis of the fluorescence intensity of a GFP-tagged protein targeted to the ER [74].
Measurement of the mitochondrial redox state in adrenal glomerulosa cells [75] and hepatocytes [73] showed that despite comparable [Ca2+]c increases, voltage and store-operated Ca2+ influx were much less effective than Ca2+ release from the IP3 –sensitive store in activating Ca2+ -sensitive mitochondrial dehydrogenases and increasing mitochondrial NAD(P)H levels. These observations also supported the notion that mitochondrial Ca2+ uptake occurs preferentially from high-Ca2+ microdomains formed between the IP3R and the mitochondrion.
Targeting aequorin to the outer surface of the IMM in HeLa cells made the measurement of [Ca2+] in the mitochondrial intermembrane space possible. After stimulation with histamine [Ca2+] rose in the intermembrane space to significantly higher values than in the global cytosol [76]. This observation has given a strong support to the concept that net mitochondrial Ca2+ uptake occurs from high-Ca2+ perimitochondrial microdomains.
The issue was raised whether the vicinity between ER and mitochondria is a stochastic event due to the dense packing of the organelles within the cell or specific interactions support their apposition. In HeLa cells repeatedly exposed to histamine, the comparison of nuclear and mitochondrial Ca2+ responses suggested the existence of specific interactions rather than stochastic approach of the two particles [77]. The existence of physical support for the ER-mitochondrial interface has been indicated by co-sedimentation of ER particles with mitochondria and electron microscopic observations of close associations between mitochondria and ER vesicles [68;78;79]. At these sites the shortest ER-OMM distance varies from 10nm to 100nM. Based on transmission electron micrographs of fixed H9c2, RBL-2H3 and DT40 cells, approx. 10% of the entire mitochondrial surface is at <100nm distance from the ER (Csordás& Hajnóczky, unpublished). In cells exposed to ER stress (serum starvation, tunicamycin) an increase in the ER-mitochondrial interface and enhanced ER-mitochondrial calcium signalling have been observed. Also, coupling of the two organelles with a fusion protein increased the ER-mitochondria interface area, reduced the ER-mitochondrial distance to about 6 nm and greatly facilitated the transfer of cytosolic Ca2+ signal into the mitochondria of RBL-2H3 cells [80].
Evidence has been presented for the contribution of three different components to the ER-mitochondrial scaffold (Fig1A): (a) anchorage of both ER and mitochondria to cytoskeletal structures; (b) tethers, forming direct links between ER membrane and OMM and (c) multimolecular complexes involving both IP3Rs and voltage-dependent anion selective channels (VDACs).
(a) Anchorage of both ER and mitochondria to cytoskeletal structures: Both ER and mitochondria are anchored to microtubules and microfilaments [81;82]. Organization of ER and mitochondria along the microtubules is apparent in cell processes (see Fig1B). Actin and tubulin [83;84] as well as microtubule and microfilament associated proteins e.g. gelsolin, a Ca2+-dependent actin regulatory protein and microtubule associated protein 2 (MAP2) [85;86] have been shown to interact with OMM structures. In addition, the microtubular and microfilamental motor proteins are primarily involved in dynamic positioning of ER and mitochondrial domains.
(b) Tethers, forming direct links between ER membrane and OMM: Recently, electron tomography analysis revealed the presence of tethers directly linking the ER to the OMM and showing similar morphology in both isolated organelles and in intact cells and tissue [80;87]. The tethers appear as narrow linear densities ending on both smooth ER and rough ER, in the latter case on or near ER-bound ribosomes. The inter-membrane distances spanned by the tethers range from 6 to 15 nm for the smooth ER and 19–30 nm for the rough ER. Since the ion channels protrude from the membrane surface [88], the vicinity of the tethers may also allow close association and in turn, communication between ER and OMM Ca2+ channels.
Based on the sensitivity to proteases the tethers are made of proteins [80]. The knock-out of all IP3R subtypes in DT40 cells still shows ER – mitochondrion tethers, indicating that IP3R-independent linkage exists between ER and mitochondria [80]. Recently, many mitochondria- or ER-associated proteins have been shown to be important for maintaining the spatial relationship between ER and mitochondria and hence, have also been implicated as possible ER-mitochondrial linking elements (DLP-1/DRP1-1[89;90], tumor autocrine motility factor receptor (AMFR)[91], PACS-2 and BAP-31 [92]). A complication is that a change in the position of mitochondria relative to the ER can be caused by ER-independent mechanisms e.g. an impairment in the cytoskeletal transport of the mitochondria. Furthermore, sustained IP3R-mitochondrial Ca2+ transfer has been observed even after experimentally induced redistribution of mitochondria [93]. However, it cannot be excluded that under these conditions the ER-mitochondrial associations may be sustained or reformed since the ER is present throughout the cell. A Ca2+-regulated connection between ER and mitochondria seems to be mediated by AMFR in MDCK cells [91;94].
(c) Role of multimolecular complexes involving both IP3Rs and VDAC: Studies of the IP3R-interacting proteins did not reveal direct interaction between IP3R and the VDAC. However, both IP3R and VDAC1 serves as a scaffold for many cytoplasmic proteins and both of these proteins may reside in a common multimolecular complex (Fig1A). Grp-75, a molecular chaperone is candidate for bringing together and for regulating the Ca2+ transfer between IP3R and VDAC1 [95]. The IP3R-VDAC complex is not likely to form the tethers described above since the tethers were also demonstrated in cells lacking any IP3Rs [80]. The IP3R-VDAC complex has multiple Ca2+ binding sites and is exposed to fluctuations in [Ca2+], providing a hint that this form of linkage may be controlled by [Ca2+].
Early immuno-electronmicroscopy studies indicated the concentration of IP3Rs at the ER-mitochondrial interface [96;97]. In RBL-2H3 cells, the ER-mitochondrial coupling displays a “quasi-synaptic” organization that would involve enrichment of both IP3Rs and the mitochondrial Ca2+ uptake sites at the interface [53]. While the IP3R has not been visualized directly with transmission electron microscopy, the distribution of native RyRs has been established in muscle. RyRs decorate predominantly the SR surface of the terminal cysternae facing toward the T tubules, opposite to the mitochondria [66]. The shortest distance between RyR and the OMM is ~37nm in cardiac muscle [71] and more variable and usually longer in skeletal muscle, up to ~130 nm in mouse leg muscles [66]. Since microdomain formation requires the vicinity of membranes and not all the mitochondria are in juxtaposition to ER or the plasma membrane, only a fraction of the mitochondria is exposed to Ca2+ hotspots, therefore the Ca2+ signal of single mitochondria within the same cell is heterogenous. The fraction of mitochondria showing great responsiveness to [Ca2+]c elevation [98–101] may reflect the ultrastructure of a given cell type.
Several recent results suggest that the ER-mitochondrial interface is dynamically controlled. In tracheal smooth muscle cells acetylcholine or depolarization-induced Ca2+ signal is followed by significant reduction of the proportion of mitochondria that was enveloped by sarcoplasmic reticulum [102]. In contrast, in permeabilized MDCK cells, the addition of rat liver cytosol stimulates the dissociation of smooth ER and mitochondria under conditions of low [Ca2+] but [Ca2+] above 1 µM favors their close association [91]. Narrowing of the ER-mitochondrial gap occurs in intact cells exposed to ER stress agents (serum starvation, tunicamycin treatment) and may be an important step in the execution of apoptosis [80]. Stability of the ER-mitochondrial association is also supported by the inhibition of mitochondrial movements in the presence of increased [Ca2+]c [81]. The ER-mitochondrial interface may also be modified by ROS. ROS are well known to induce cytosolic and mitochondrial Ca2+ signal, followed by the deterioration of mitochondrial function [103]. In adrenal glomerulosa cells high-power UV light induces a high rate of superoxide formation and evokes Ca2+ signalling whereas low-power light induces a low rate of O2.- formation without eliciting Ca2+ signalling. Nevertheless, the latter impairs the transfer of Ca2+ signal from the cytosol into the mitochondrial matrix only in angiotensin-stimulated cells but not when [Ca2+]c is elevated by voltage or store-operated Ca2+ influx [104], suggesting that the local ER-mitochondrial Ca2+ coupling has been specifically affected by the UV-treatment.
Ca2+ permeation through OMM
Early studies of isolated mitochondria and purified VDAC reconstituted in artificial membranes provided considerable support for the view that the OMM is freely permeable for Ca2+ due to the presence of VDAC. However, a series of recent experiments conducted in permeabilized or in intact cells indicate that the OMM permeability may limit the transport of ions and small molecules between cytoplasm and mitochondria e.g. ADP, H+ [105–107] or Ca2+ [108;109]. It is possible that the OMM transport barrier is less apparent in isolated mitochondria because the preparation procedure led to some rearrangement or damage in the OMM structure. Indeed an increase in the OMM permeability of isolated cardiac mitochondria for 3nm nanoparticles has been documented [110]. Also, the studies of the purified VDAC were usually conducted at supraphysiological ionic strength and [Ca2+], which could have affected the channel function. Evaluation of this possibility by incubation of purified VDAC and isolated OMM incorporated to lipid bilayers or liposomes in physiological intracellular medium showed small subconductances of the VDAC and low Ca2+ permeability [111]. Higher subconductances and sustained opening of VDAC to maximal conductance occurred when [Ca2+] was elevated, indicating that Ca2+ affects the gating of VDAC. Importantly, to attain an increase in VDAC Ca2+ permeability it was sufficient to increase [Ca2+] from 20nM to 2µM [111], indicating a possible physiological relevance of the Ca2+ dependence of the VDAC. Most recently, Tan and Colombini have reported a lack of effect of Ca2+ on VDAC conductance but again the measurement was done in the presence of 1M KCl and [Ca2+] was not tested in the low [Ca2+] buffer (Figure 1 in [112]). A putative Ca2+ binding site of the VDAC has been identified by Shoshan-Barmatz and coworkers [113;114] but the Ca2+ effect can also be conferred to VDAC by an associated protein that is differently accessible in physiological and higher ionic strengths. Since the Ca2+ effect was documented in ATP free conditions, Ca2+-dependent protein phosphorylation does not seem to be involved.
Importantly, the limitation of Ca2+ transport by the OMM appeared only when the response of mitochondria to short-lasting IP3R-mediated [Ca2+]c spikes was studied [108],[109]. Therefore, the Ca2+ permeability of the OMM seems to become a limiting factor when subregions of the mitochondrial surface have to allow the rapid equilibration of the intermembrane space [Ca2+] with the cytosol. VDAC expression and distribution in the OMM is relevant for this process [109]. Furthermore, regulation of VDAC gating by Ca2+ may provide a supplementary mechanism to enhance the permeation of ions and solutes across the OMM during repetitive [Ca2+]c elevations [111].
Ca2+ permeation through the IMM
Ca2+ influx occurs through a channel termed the uniporter (UP) and the rate of influx depends upon the driving force (Fig1A), the main component of which is the highly negative transmembrane potentials across the IMM generated by the electron transport in normally respiring mitochondria. Ca2+ efflux is mediated by separate pathways that are also coupled to the proton motive force developed by the respiratory chain. The IMM has a Ca2+/2H+ exchanger and/or a Ca2+/3Na+ exchanger analogous to that found in the plasma membrane (Fig1A). Ca2+ efflux along the concentration gradient may also occur through a mechanism referred as the permeability transition pore (PTP). The molecular structure of each of these IMM Ca2+ flux pathways remains to be solved. Therefore it is of significance that Graier and coworkers have shown that overexpression and silencing of uncoupling proteins 2 and 3 (UCP2 and UCP3) effectively increased and suppressed mitochondrial Ca2+ uptake, respectively. Furthermore, liver mitochondria isolated from UCP2−/− mice showed lack of the ruthenium red sensitive mitochondrial Ca2+ sequestration [115]. These data would indicate that UCP2 is either part of the UP or is an important controller of it. Since in UCP2 and UCP3 expression in hepatocytes has been under the limit of detection, a minuscule amount of UCP would need to be sufficient to support the robust mitochondrial Ca2+ uptake in these cells [116;117]. It is also relevant that a change in UCP2 and UCP3 expression may affect mitochondrial metabolism for example the production of ROS [118;119] that may also exert an effect on the UP activity. Furthermore, recent studies of UCP2 knockout and overexpression paradigms have also indicated that UCP2 is not required for mitochondrial Ca2+ uptake and ATP production in pancreatic β cells [119;120].
Another candidate for the UP activity in heart mitochondria has been the RyR [121]. Follow-up studies have indicated that the biochemical and pharmacological properties of the protein isolated from the mitochondrial preparation is similar to RyR1 [122;123], whereas electrophysiological studies indicate both common and distinctive properties of the SR and mitochondrial preparation derived channels [123]. The RyR1 knockout mice are expected to provide a powerful model to establish the role of RyR1 in Ca2+ transport by cardiac mitochondria.
Evidence has been accumulating that numerous signalling molecules and drugs affect the calcium signal propagation to the mitochondria. In many cases, no distinction has been made between targeting of the OMM and IMM components of the Ca2+ uptake and is unclear whether the driving force of the Ca2+ uptake was affected. Pinton et al. showed that various protein kinase C (PKC) isozymes exert differential control on the [Ca2+]c signal delivery to the mitochondria [124]. Szanda et al. have presented both pharmacological and genetic evidence that p38 MAPK serves as a negative modulator of the mitochondrial Ca2+ uptake [125]. p38 MAPK seems to act in concert with PKC epsilon, in inhibition of the [Ca2+]m signal [125]. Notably, p38 MAPK phosphorylates Bcl-2 family proteins that may confer an effect to the UP [126]. Also, PKC epsilon may target the mitochondrial K(ATP) channels [124] inducing a change in matrix volume which may alter the mitochondrial Ca2+ uptake. A p38 MAPK inhibitor, SB202190 also caused augmented mitochondrial Ca2+ uptake in ATP-free conditions [127;128], indicating that interfering with protein phosphorylation is not the sole mechanism of the drug’s effect.
Allosteric regulation of the mitochondrial Ca2+ uptake by Ca2+ has been established first in studies of isolated mitochondria [129]. In cell paradigms, both Ca2+-induced desensitization [130–132] and Ca2+-induced potentiation of the mitochondrial Ca2+ uptake [133;134] have been observed (for a recent review see [135]). A mechanism of the Ca2+ induced facilitation seems to be mediated through calmodulin to the UP [131;134] but other Ca2+ sensing molecules and multiple signalling pathways may also be involved. The regulatory pathways to the UP allow tuning of mitochondrial Ca2+ uptake activity to the changing needs of the stimulated cells.
Ca2+ buffers and effectors in the mitochondrial matrix
Calcium ions entering the mitochondrial matrix increase [Ca2+]m but over 99.9% of the total matrix calcium content is in bound form. The calcium binding species includes cardiolipin and other anionic phospholipids that bind Ca2+ with high affinity. Another important Ca2+ binding species are the carboxy-anion-containing metabolites of the Krebs cycle (citrate, oxalo-acetate) and inorganic phosphate, which can also form poorly soluble salts with Ca2+. There is no evidence for the presence of a specialized Ca2+ buffering protein in the mitochondrial matrix. Buffering of gradually accumulated Ca2+ is more efficient than that of a bolus of Ca2+ [136]. Ca2+ binding affinity of the anionic metabolites of the Krebs cycle is sensitive to matrix pH. Furthermore, the IMM pH gradient also affects the distribution of the carboxylated anions and phosphate between the mitochondria and cytosol. Thus, matrix Ca2+ buffering is dynamically regulated by mitochondrial metabolism primarily, through the changes in matrix pH.
Despite the robust Ca2+ buffering capacity in the mitochondrial matrix, [Ca2+]c spikes yield rapid elevations in [Ca2+]m from 100nM to at least micromolar and in some cases over hundred micromolar [101]. Mitochondrial matrix Ca2+ effectors are several key enzymes that enhance ATP production, providing an important mechanism for synchronizing energy production with the energy demands of Ca2+-activated processes during cell stimulation (excitation–metabolism coupling). Another effector is the PTP that is activated during massive mitochondrial Ca2+ loading or by the combination of some forms of stress and Ca2+ loading and leads to mitochondrial membrane permeabilization and ensuing cell death, either apoptotic or necrotic.
Dynamic changes in ER/mitochondrial morphology
The amount of ER and mitochondria, the distribution and connectedness of the organelles in the cell may affect both the bulk cytoplasm-mediated and the local ER-mitochondrial Ca2+ signalling but only few data have become available. The ER and mitochondrial fraction of cell volume shows large, cell type specific differences and undergoes changes during cell development and differentiation. Overexpression of a the peroxisome-proliferator-activated-receptor-c-coactivator -1α (PGC-1α) that triggers mitochondrial biogenesis resulted in selective suppression of the [Ca2+]m signal by reducing the efficacy of mitochondrial Ca2+ uptake sites and increasing organelle volume [137]. The distribution of the ER and mitochondria are controlled by cytoskeletal transport and anchorage [82]. In certain cell types, subdomains of the ER and subsets of mitochondria have a stable position but the majority of organelles display permanent movement. Ca2+-dependent control of the motility allows a homeostatic distribution of the mitochondria to the sites of ATP and Ca2+ buffering demand [81;138–140]. Importantly, Ca2+-induced attenuation of mitochondrial motility occurs in the sub-micromolar range of [Ca2+]c (IC50 ≈ 400nM), indicating that mitochondrial motility is controlled in the physiological range of [Ca2+]c [79]. Ca2+ uptake leads to the formation of Ca2+ hot-spots in the mitochondrial matrix and Ca2+ diffusion is limited to short segments of the mitochondrial network [141]. Szabadkai et al. have provided evidence that fragmentation of the mitochondria leads to suppression of the spreading of Ca2+ in the matrix of the mitochondrial network [142]. Ca2+ is a potent inducer of fragmentation of both ER [143]; [47] and mitochondria [144]. Ca2+ engages calcineurin to dephosphorylate and in turn, activate the mitochondrial fission protein, Drp1 [145].
Transfer of submicromolar [Ca2+]c elevations into the mitochondrial matrix
There are reports on intact cells suggesting that Ca2+ is sequestered by mitochondria also when the organelle is exposed to Ca2+ at submicromolar concentration. In these cases Ca2+ enters the cytosol through the plasma membrane and it can be assumed that the formation of a high-Ca2+ microdomain between the plasma membrane and subplasmalemmal mitochondria was not responsible for the mitochondrial Ca2+ uptake. The limitation of these studies, similarly to those reporting on high-Ca2+ microdomains, is that perimitochondrial [Ca2+] cannot be directly measured but only assessed. In bullfrog sympathetic neurons increased mitochondrial calcium uptake could be detected when depolarization with K+ raised [Ca2+]c above 300–500 nM [146;147]. In a further study on the same cell type mitochondrial Ca2+ transport processes were analyzed on basis of the kinetics of depolarization-induced Ca2+ signal and the application of transport inhibitors. It was found that above 400 nM [Ca2+]c, net mitochondrial Ca2+ transport is dominated by uptake but net Ca2+ uptake occurs even at 200–300 nM [148]. Considering, however, that at least in one of these studies [147], the extent of Ca2+ accumulation depended on proximity of mitochondria to the plasma membrane, the formation of subplasmalemmal high-Ca2+ microdomains in these experiments may not be ruled out. In another excitable cell type, the arterial smooth muscle cells, [Ca2+]c was raised by brief activations of voltage-operated Ca2+ channels. Even when [Ca2+]c remained in the low submicromolar range, mitochondrial depolarisation slowed the rate of Ca2+ removal from the cytosol, indicating that polarized mitochondria can sequester Ca2+ also at low [Ca2+]c values [149]. In HeLa cells, a [Ca2+]c signal was evoked by histamine (IP3–induced Ca2+ release), thapsigargin (Ca2+ leak from the ER) or readdition of Ca2+ following store depletion. The efficiency of mitochondrial Ca2+ uptake from high-Ca2+ perimitochondrial microdomains was clearly shown by the observation that for equivalent [Ca2+]c increases, the rate of [Ca2+]m rise was greater with IP3 –induced signal than any other source. However, irrespective of the source of Ca2+, already in the range of 100 to 400 nM [Ca2+]c, Ca2+ uptake varied nearly linearly with [Ca2+]c. This indicates the ability of mitochondria to take up Ca2+ in the low submicromolar range under some situations. It is worth mentioning that the theshold value for Ca2+ uptake by individual mitochondria showed great variance [130].
There are observations also in endocrine cells indicating mitochondrial Ca2+ uptake in the low submicromolar [Ca2+]c range. Aldosterone secretion by adrenal glomerulosa cells is rapidly stimulated by angiotensin II. Mitochondrial Ca2+ response to angiotensin is generated through the formation of high-Ca2+ perimitochondrial microdomain [150]. Physiological elevation of extracellular [K+] induces long-lasting but small elevation of [Ca2+]c. Rat glomerulosa cells respond to an elevation of [K+] as small as 0.5 mM with a slowly developing [Ca2+]c signal that never attains 200 nM [151;151], yet this signal is followed by elevated [Ca2+]m, mitochondrial NAD(P)H level and aldosterone production [152]. Store-operated Ca2+ influx both in glomerulosa [75] and ovarian luteal cells [153] also results [Ca2+]c signals never exceeding 200 nM and they are again associated with a mitochondrial NAD(P)H signal. Considering that in these cases the [Ca2+]c signal is formed very slowly whereas its rapid formation would be needed for the generation of a high Ca2+ microdomain, mitochondrial Ca2+ uptake may not be attributed to the formation of such microdomains. In support of this assumption it should be recalled that in the related adrenocortical cell line (H295R) stimulated with K+, Ca2+ uptake by subplasmalemmal mitochondria did not differ from that by perinuclear mitochondria [43]. These observations suggested that net mitochondrial Ca2+ uptake may take place without the formation of high-Ca2+ microdomains. The effect of [Ca2+]c on [Ca2+]m was examined in permeabilized cells, applying cytosol-like Ca2+ buffers. With such a protocol the formation of microdomains could be avoided. When extramitochondrial [Ca2+] was raised from 50 or 60 nM to less than 200 nM, reproducible increases in [Ca2+]m were measured in glomerulosa [154]}[155], luteal [153] and insulin-producing INS-1cells [154]. The biological significance of the measured small increases in [Ca2+]m was demonstrated by the detection of concomitant increase in NAD(P)H [154]. Further stepwise increases, still in the submicromolar range, of [Ca2+]c resulted in stepwise increases of steady-state [Ca2+]m. The [Ca2+]m elevations were ruthenium red-sensitive, developed slowly and were maintained as long as raised [Ca2+]c was present. These data provided evidence that net mitochondrial Ca2+ uptake and Ca2+ sensitive dehydrogenase activation can be enhanced by low submicromolar increases in [Ca2+]c. In harmony with these observations, in permeabilized pancreatic β cells methyl succinate, a cell permeable substrate induced mitochondrial hyperpolarization and increased [Ca2+]m, without the formation of high-Ca2+ perimitochondrial microdomain [156]. Notably, a rapid mode of Ca2+ uptake has also been documented in isolated mitochondria exposed to [Ca2+] pulses which peak at 200nM [157].
Differing from the primary glomerulosa and luteal cells (v.s.), the [Ca2+]c threshold for mitochondrial Ca2+ uptake was ~500 nM and 1 µM in H295R adrenocortical and HeLa cells, respectively [158]. This latter value is much higher than that observed by Collins et al. [159] but is lower than observed by Montero et al. [127]. Net Ca2+ uptake in T lymphocytes begins at ~400 nM [160], in ventricular myocytes at 200–500 nM [70;161] whereas supramicromolar threshold values were reported for RBL-2H3 [53] and chromaffin cells [101]. Mitochondrial Ca2+ uptake at the [Ca2+]c threshold is relatively slow, new steady-state of [Ca2+]m is attained after several tens of seconds.
The mechanisms underlying the great variance in the [Ca2+]c threshold for mitochondrial Ca2+ uptake and the unexpectedly low threshold in some cell types are unknown. Ca2+-induced potentiation of the mitochondrial Ca2+ uptake may be involved when the kinetics of the [Ca2+]m rise is slow [133;134]. It has been known for three decades that Mg2+ modifies Ca2+ uptake of isolated mitochondria [162–165]. Yet, we are not aware of any publication which would have considered cytosolic [Mg2+] as a factor influencing the [Ca2+]c threshold for net mitochondrial Ca2+ uptake. We have examined this issue in permeabilized HEK293T cells and found that in the physiological range of 0.25 – 2.5 mM Mg2+ this threshold inversely correlated with [Mg2+]. In addition, in the submicromolar range of [Ca2+]c mitochondrial Ca2+ uptake rate was also reduced by increase in [Mg2+] (G. Szanda, A. Spät & J. Garcia-Sancho, manuscript in preparation). In view of these data it may be presumed that cell-dependent Mg2+ balance and unchecked Mg2+ concentrations in cytosol-like media may be a cause of the apparent cell-to-cell variance in the mitochondrial responsiveness to submicromolar cytosolic Ca2+.
Future directions
The evidence on local communication between ER/SR and mitochondria presents a major challenge for microscopy. The spatial resolution required for tracking the interacting molecules both in vitro and in vivo exceeds the resolution of light microscopy therefore creative application of some current techniques (e.g. FRET) may be useful and establishing of novel means is necessary. For the in vitro studies, high-voltage electron tomography offers a promising approach. Also, labeling of the endogenous proteins needs novel technology. This direction also needs the long sought information on the molecular identity of the mitochondrial Ca2+ transport proteins. The varying affinity for Ca2+ of the mitochondrial Ca2+ uptake in different paradigms highlights the need for deciphering the mechanisms that control the Ca2+ sensitivity of the mitochondrial Ca2+ transporters in the OMM and IMM. An intriguing question remains regarding the subcellular distribution of the signalling proteins that target the IMM components. An impetus for these efforts is the growing support for the broad and fundamental biological significance of the ER-mitochondrial interactions.
Acknowledgement
This work was supported by grants from the Hungarian National Science Foundation, OTKA TS-040865 (SA) and NIH grants DK51526 & GM59419 (GH). We would like to acknowledge Dr. Suresh K. Joseph for critical readings of the manuscript. We apologize to those of our colleagues whose work could not be cited because of space restrictions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bootman MD, Petersen OH, Verkhratsky A. The endoplasmic reticulum is a focal point for co-ordination of cellular activity. Cell Calcium. 2002;32:231–234. doi: 10.1016/s0143416002002002. [DOI] [PubMed] [Google Scholar]
- 2.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 3.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium. 2005;38:291–302. doi: 10.1016/j.ceca.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Landolfi B, Curci S, Debellis L, Pozzan T, Hofer AM. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured In situ in intact cells. J Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnaudeau S, Frieden M, Nakamura K, Castelbou C, Michalak M, Demaurex N. Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J Biol Chem. 2002;277:46696–46705. doi: 10.1074/jbc.M202395200. [DOI] [PubMed] [Google Scholar]
- 6.Malli R, Frieden M, Osibow K, et al. Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem. 2003;278:44769–44779. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- 7.Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–3067. doi: 10.1242/dev.01181. [DOI] [PubMed] [Google Scholar]
- 8.Bastianutto C, Clementi E, Codazzi F, et al. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995;130:847–855. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Divet A, Paesante S, Grasso C, et al. Increased Ca(2+) storage capacity of the skeletal muscle sarcoplasmic reticulum of transgenic mice over-expressing membrane bound calcium binding protein junctate. J Cell Physiol. 2007;213:464–474. doi: 10.1002/jcp.21121. [DOI] [PubMed] [Google Scholar]
- 10.Joseph SK, Boehning D, Bokkala S, Watkins R, Widjaja J. Biosynthesis of inositol trisphosphate receptors: selective association with the molecular chaperone calnexin. Biochem J. 1999;342(Pt 1):153–161. [PMC free article] [PubMed] [Google Scholar]
- 11.Mogami H, Gardner J, Gerasimenko OV, Camello P, Petersen OH, Tepikin AV. Calcium binding capacity of the cytosol and endoplasmic reticulum of mouse pancreatic acinar cells. J Physiol. 1999;518(Pt 2):463–467. doi: 10.1111/j.1469-7793.1999.0463p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Camacho P. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J Cell Biol. 2004;164:35–46. doi: 10.1083/jcb.200307010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gyorke S, Hagen BM, Terentyev D, Lederer WJ. Chain-reaction Ca(2+) signaling in the heart. J Clin Invest. 2007;117:1758–1762. doi: 10.1172/JCI32496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura K, Zuppini A, Arnaudeau S, et al. Functional specialization of calreticulin domains. J Cell Biol. 2001;154:961–972. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Longo FJ, Wintermantel MR, Jiang X, Clark RA, DeLisle S. Calreticulin modulates capacitative Ca2+ influx by controlling the extent of inositol 1,4,5-trisphosphate-induced Ca2+ store depletion. J Biol Chem. 2000;275:36676–36682. doi: 10.1074/jbc.M002041200. [DOI] [PubMed] [Google Scholar]
- 16.Mendes CC, Gomes DA, Thompson M, et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005 doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 17.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laver DR. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys J. 2007;92:3541–3555. doi: 10.1529/biophysj.106.099028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph SK, Hajnoczky G. IP(3) receptors in cell survival and apoptosis: Ca(2+) release and beyond. Apoptosis. 2007;12:951–968. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- 20.Missiaen L, De Smedt H, Droogmans G, Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992;357:599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- 21.Oldershaw KA, Taylor CW. Luminal Ca2+ increases the affinity of inositol 1,4,5-trisphosphate for its receptor. Biochem J. 1993;292(Pt 3):631–633. doi: 10.1042/bj2920631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horne JH, Meyer T. Luminal calcium regulates the inositol trisphosphate receptor of rat basophilic leukemia cells at a cytosolic site. Biochemistry. 1995;34:12738–12746. doi: 10.1021/bi00039a033. [DOI] [PubMed] [Google Scholar]
- 23.Bezprozvanny I, Ehrlich BE. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sienaert I, De Smedt H, Parys JB, et al. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271:27005–27012. doi: 10.1074/jbc.271.43.27005. [DOI] [PubMed] [Google Scholar]
- 25.Thrower EC, Mobasheri H, Dargan S, Marius P, Lea EJ, Dawson AP. Interaction of luminal calcium and cytosolic ATP in the control of type 1 inositol (1,4,5)-trisphosphate receptor channels. J Biol Chem. 2000;275:36049–36055. doi: 10.1074/jbc.M000970200. [DOI] [PubMed] [Google Scholar]
- 26.Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 27.Sitsapesan R, Williams AJ. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by luminal Ca2+ J Membr Biol. 1994;137:215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- 28.Tripathy A, Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J. 1996;70:2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ching LL, Williams AJ, Sitsapesan R. Evidence for Ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res. 2000;87:201–206. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- 31.Pinton P, Ferrari D, Magalhaes P, et al. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 33.Chen R, Valencia I, Zhong F, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White C, Li C, Yang J, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- 36.Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- 37.Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca(2+) signals. J Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- 38.Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 39.Smaili SS, Stellato KA, Burnett P, Thomas AP, Gaspers LD. Cyclosporin A inhibits inositol 1,4,5-trisphosphate-dependent Ca2+ signals by enhancing Ca2+ uptake into the endoplasmic reticulum and mitochondria. J Biol Chem. 2001;276:23329–23340. doi: 10.1074/jbc.M100989200. [DOI] [PubMed] [Google Scholar]
- 40.Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 41.Vay L, Hernandez-Sanmiguel E, Santo-Domingo J, et al. Modulation of Ca(2+) release and Ca(2+) oscillations in HeLa cells and fibroblasts by mitochondrial Ca(2+) uniporter stimulation. J Physiol. 2007;580:39–49. doi: 10.1113/jphysiol.2006.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishii K, Hirose K, Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006;7:390–396. doi: 10.1038/sj.embor.7400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szanda G, Koncz P, Várnai P, Spät A. Mitochondrial Ca(2+) uptake with and without the formation of high-Ca(2+) microdomains. Cell Calcium. 2006;40:527–538. doi: 10.1016/j.ceca.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Hirose K, Iino M. Heterogeneity of channel density in inositol-1,4,5-trisphosphate-sensitive Ca2+ stores. Nature. 1994;372:791–794. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- 45.Shuai J, Rose HJ, Parker I. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys J. 2006;91:4033–4044. doi: 10.1529/biophysj.106.088880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson BS, Pfeiffer JR, Smith AJ, Oliver JM, Oberdorf JA, Wojcikiewicz RJ. Calcium-dependent clustering of inositol 1,4,5-trisphosphate receptors. Mol Biol Cell. 1998;9:1465–1478. doi: 10.1091/mbc.9.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tateishi Y, Hattori M, Nakayama T, et al. Cluster formation of inositol 1,4,5-trisphosphate receptor requires its transition to open state. J Biol Chem. 2005;280:6816–6822. doi: 10.1074/jbc.M405469200. [DOI] [PubMed] [Google Scholar]
- 49.Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isaeva EV, Shkryl VM, Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J Physiol. 2005;565:855–872. doi: 10.1113/jphysiol.2005.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchant JS, Ramos V, Parker I. Structural and functional relationships between Ca2+ puffs and mitochondria in Xenopus oocytes. Am J Physiol Cell Physiol. 2002;282:1374–1386. doi: 10.1152/ajpcell.00446.2001. [DOI] [PubMed] [Google Scholar]
- 52.Means S, Smith AJ, Shepherd J, et al. Reaction diffusion modeling of calcium dynamics with realistic ER geometry. Biophys J. 2006;91:537–557. doi: 10.1529/biophysj.105.075036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. Embo J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bezprozvanny I, Ehrlich BE. ATP modulates the function of inositol 1,4,5-trisphosphategated channels at two sites. Neuron. 1993;10:1175–1184. doi: 10.1016/0896-6273(93)90065-y. [DOI] [PubMed] [Google Scholar]
- 55.Maes K, Missiaen L, De Smet P, et al. Differential modulation of inositol 1,4,5-trisphosphate receptor type 1 and type 3 by ATP. Cell Calcium. 2000;27:257–267. doi: 10.1054/ceca.2000.0121. [DOI] [PubMed] [Google Scholar]
- 56.Mak DO, McBride S, Foskett JK. ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca(2+) activation. J Biol Chem. 1999;274:22231–22237. doi: 10.1074/jbc.274.32.22231. [DOI] [PubMed] [Google Scholar]
- 57.Wagner LE2, Betzenhauser MJ, Yule DI. ATP binding to a unique site in the type-1 S2-inositol 1,4,5-trisphosphate receptor defines susceptibility to phosphorylation by protein kinase A. J Biol Chem. 2006;281:17410–17419. doi: 10.1074/jbc.M601340200. [DOI] [PubMed] [Google Scholar]
- 58.Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 59.Boehning D, van Rossum DB, Patterson RL, Snyder SH. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc Natl Acad Sci U S A. 2005;102:1466–1471. doi: 10.1073/pnas.0409650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 61.Madesh M, Hawkins BJ, Milovanova T, et al. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005;170:1079–1090. doi: 10.1083/jcb.200505022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Y, Shen X. H2O2 directly activates inositol 1,4,5-trisphosphate receptors in endothelial cells. Redox Rep. 2005;10:29–36. doi: 10.1179/135100005X21660. [DOI] [PubMed] [Google Scholar]
- 63.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 64.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 65.Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1, 4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 66.Franzini-Armstrong C. ER-Mitochondria Communication. How Privileged? Physiology (Bethesda) 2007;22:261–268. doi: 10.1152/physiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- 67.Tinel H, Cancela JM, Mogami H, et al. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca(2+) signals. Embo J. 1999;18:4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mannella CA, Buttle K, Rath BK, Marko M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors. 1998;8:225–228. doi: 10.1002/biof.5520080309. [DOI] [PubMed] [Google Scholar]
- 69.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3- sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 70.Szalai G, Csordás G, Hantash BM, Thomas AP, Hajnóczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- 71.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 72.Shkryl VM, Shirokova N. Transfer and tunneling of Ca2+ from sarcoplasmic reticulum to mitochondria in skeletal muscle. J Biol Chem. 2006;281:1547–1554. doi: 10.1074/jbc.M505024200. [DOI] [PubMed] [Google Scholar]
- 73.Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 74.Szanda G, Koncz P, Várnai P, Spät A. Mitochondrial Ca(2+) uptake with and without the formation of high-Ca(2+) microdomains. Cell Calcium. 2006;40:527–538. doi: 10.1016/j.ceca.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 75.Rohács T, Tory K, Dobos A, Spät A. Intracellular calcium release is more efficient than calcium influx in stimulating mitochondrial NAD(P)H formation in adrenal glomerulosa cells. Biochemical Journal. 1997;328:525–528. doi: 10.1042/bj3280525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rizzuto R, Pinton P, Carrington W, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 77.Filippin L, Magalhaes PJ, Di Benedetto G, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J Biol Chem. 2003;278:39224–39234. doi: 10.1074/jbc.M302301200. [DOI] [PubMed] [Google Scholar]
- 78.Shore GC, Tata JR. Two fractions of rough endoplasmic reticulum from rat liver. I. Recovery of rapidly sedimenting endoplasmic reticulum in association with mitochondria. J Cell Biol. 1977;72:714–725. doi: 10.1083/jcb.72.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meier PJ, Spycher MA, Meyer UA. Isolation and characterization of rough endoplasmic reticulum associated with mitochondria from normal rat liver. Biochim Biophys Acta. 1981;646:283–297. doi: 10.1016/0005-2736(81)90335-7. [DOI] [PubMed] [Google Scholar]
- 80.Csordas G, Renken C, Varnai P, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu X, Forbes JG, Colombini M. Actin modulates the gating of Neurospora crassa VDAC. J Membr Biol. 2001;180:73–81. doi: 10.1007/s002320010060. [DOI] [PubMed] [Google Scholar]
- 84.Carre M, Andre N, Carles G, et al. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- 85.Linden M, Karlsson G. Identification of porin as a binding site for MAP2. Biochem Biophys Res Commun. 1996;218:833–836. doi: 10.1006/bbrc.1996.0148. [DOI] [PubMed] [Google Scholar]
- 86.Kusano H, Shimizu S, Koya RC, et al. Human gelsolin prevents apoptosis by inhibiting apoptotic mitochondrial changes via closing VDAC. Oncogene. 2000;19:4807–4814. doi: 10.1038/sj.onc.1203868. [DOI] [PubMed] [Google Scholar]
- 87.Boncompagni S, Protasi F. Tethers: Structural Connections between SR and the Outer Mitochondrial Membrane. Biophys J. 2007:313a–314a. [Google Scholar]
- 88.Suhara W, Kobayashi M, Sagara H, et al. Visualization of inositol 1,4,5-trisphosphate receptor by atomic force microscopy. Neurosci Lett. 2006;391:102–107. doi: 10.1016/j.neulet.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 89.Pitts KR, Yoon Y, Krueger EW, McNiven MA. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol Biol Cell. 1999;10:4403–4417. doi: 10.1091/mbc.10.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Varadi A, Cirulli V, Rutter GA. Mitochondrial localization as a determinant of capacitative Ca2+ entry in HeLa cells. Cell Calcium. 2004;36:499–508. doi: 10.1016/j.ceca.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Wang HJ, Guay G, Pogan L, Sauve R, Nabi IR. Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum. J Cell Biol. 2000;150:1489–1498. doi: 10.1083/jcb.150.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simmen T, Aslan JE, Blagoveshchenskaya AD, et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. Embo J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rutter GA. Moving Ca2+ from the endoplasmic reticulum to mitochondria: is spatial intimacy enough? Biochem Soc Trans. 2006;34:351–355. doi: 10.1042/BST0340351. [DOI] [PubMed] [Google Scholar]
- 94.Goetz JG, Genty H, St-Pierre P, et al. Reversible interactions between smooth domains of the endoplasmic reticulum and mitochondria are regulated by physiological cytosolic Ca2+ levels. J Cell Sci. 2007 doi: 10.1242/jcs.03486. [DOI] [PubMed] [Google Scholar]
- 95.Szabadkai G, Bianchi K, Varnai P, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mignery GA, Sudhof TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- 97.Satoh T, Ross CA, Villa A, et al. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990;111:615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rizzuto R, Bastianutto C, Brini M, Murgia M, Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. J Cell Biol. 1994;126:1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simpson PB, Mehotra S, Langley D, Sheppard CA, Russell JT. Specialized distributions of mitochondria and endoplasmic reticulum proteins define Ca2+ wave amplification sites in cultured astrocytes. J Neurosci Res. 1998;52:672–683. doi: 10.1002/(SICI)1097-4547(19980615)52:6<672::AID-JNR6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 100.Drummond RM, Mix TC, Tuft RA, Walsh JV, Jr, Fay FS. Mitochondrial Ca2+ homeostasis during Ca2+ influx and Ca2+ release in gastric myocytes from Bufo marinus. J Physiol. 2000;522(Pt 3):375–390. doi: 10.1111/j.1469-7793.2000.t01-2-00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montero M, Alonso MT, Carnicero E, et al. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol. 2000;2:57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- 102.Dai J, Kuo KH, Leo JM, Van Breemen C, Lee CH. Rearrangement of the close contact between the mitochondria and the sarcoplasmic reticulum in airway smooth muscle. Cell Calcium. 2005;37:333–340. doi: 10.1016/j.ceca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53 Suppl 1:S96–S102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- 104.Koncz P, Szanda G, Rajki A, Spat A. Reactive oxygen species, Ca2+ signaling and mitochondrial NAD(P)H level in adrenal glomerulosa cells. Cell Calcium. 2006;40:347–357. doi: 10.1016/j.ceca.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 105.Fontaine EM, Keriel C, Lantuejoul S, Rigoulet M, Leverve XM, Saks VA. Cytoplasmic cellular structures control permeability of outer mitochondrial membrane for ADP and oxidative phosphorylation in rat liver cells. Biochem Biophys Res Commun. 1995;213:138–146. doi: 10.1006/bbrc.1995.2108. [DOI] [PubMed] [Google Scholar]
- 106.Saks VA, Kaambre T, Sikk P, et al. Intracellular energetic units in red muscle cells. Biochem J. 2001;356:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:209–216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 108.Csordas G, Madesh M, Antonsson B, Hajnoczky G. tcBid promotes Ca(2+) signal propagation to the mitochondria: control of Ca(2+) permeation through the outer mitochondrial membrane. Embo J. 2002;21:2198–2206. doi: 10.1093/emboj/21.9.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rapizzi E, Pinton P, Szabadkai G, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salnikov V, Lukyanenko YO, Frederick CA, Lederer WJ, Lukyanenko V. Probing the outer mitochondrial membrane in cardiac mitochondria with nanoparticles. Biophys J. 2007;92:1058–1071. doi: 10.1529/biophysj.106.094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J Biol Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 112.Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Israelson A, Abu-Hamad S, Zaid H, Nahon E, Shoshan-Barmatz V. Localization of the voltage-dependent anion channel-1 Ca(2+)-binding sites. Cell Calcium. 2006;41:235–244. doi: 10.1016/j.ceca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 115.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Negre-Salvayre A, Hirtz C, Carrera G, et al. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. Faseb J. 1997;11:809–815. [PubMed] [Google Scholar]
- 117.McCarty MF. High mitochondrial redox potential may promote induction and activation of UCP2 in hepatocytes during hepatothermic therapy. Med Hypotheses. 2005;64:1216–1219. doi: 10.1016/j.mehy.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 118.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 119.Produit-Zengaffinen N, Davis-Lameloise N, Perreten H, et al. Increasing uncoupling protein-2 in pancreatic beta cells does not alter glucose-induced insulin secretion but decreases production of reactive oxygen species. Diabetologia. 2007;50:84–93. doi: 10.1007/s00125-006-0499-6. [DOI] [PubMed] [Google Scholar]
- 120.Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 121.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 122.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: Transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005 doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 123.Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH. The mitochondrial ryanodine receptor in rat heart: a pharmaco-kinetic profile. Biochim Biophys Acta. 2007;1768:1784–1795. doi: 10.1016/j.bbamem.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 124.Jaburek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99:878–883. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 125.Szanda G, Koncz P, Rajki A, Spat A. Participation of p38 MAPK and a novel-type protein kinase C in the control of mitochondrial Ca(2+) uptake. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 126.De Chiara G, Marcocci ME, Torcia M, et al. Bcl-2 Phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J Biol Chem. 2006;281:21353–21361. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- 127.Montero M, Lobaton CD, Moreno A, Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. Faseb J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- 128.Montero M, Lobaton CD, Hernandez-Sanmiguel E, et al. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem J. 2004;384:19–24. doi: 10.1042/BJ20040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kroner H. Ca2+ ions, an allosteric activator of calcium uptake in rat liver mitochondria. Arch Biochem Biophys. 1986;251:525–535. doi: 10.1016/0003-9861(86)90360-7. [DOI] [PubMed] [Google Scholar]
- 130.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca(2+) uptake depends on the spatial and temporal profile of cytosolic Ca(2+) signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 131.Moreau B, Nelson C, Parekh AB. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr Biol. 2006;16:1672–1677. doi: 10.1016/j.cub.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 132.Maechler P, Kennedy ED, Wang H, Wollheim CB. Desensitization of mitochondrial Ca2+ and insulin secretion responses in the beta cell. J Biol Chem. 1998;273:20770–20778. doi: 10.1074/jbc.273.33.20770. [DOI] [PubMed] [Google Scholar]
- 133.Rohacs T, Tory K, Dobos A, Spat A. Intracellular calcium release is more efficient than calcium influx in stimulating mitochondrial NAD(P)H formation in adrenal glomerulosa cells. Biochem J. 1997;328(Pt 2):525–528. doi: 10.1042/bj3280525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Csordas G, Hajnoczky G. Plasticity of mitochondrial calcium signaling. J Biol Chem. 2003;278:42273–42282. doi: 10.1074/jbc.M305248200. [DOI] [PubMed] [Google Scholar]
- 135.Putney JW, Jr, Thomas AP. Calcium signaling: double duty for calcium at the mitochondrial uniporter. Curr Biol. 2006;16:812–815. doi: 10.1016/j.cub.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 137.Bianchi K, Vandecasteele G, Carli C, Romagnoli A, Szabadkai G, Rizzuto R. Regulation of Ca2+ signalling and Ca2+-mediated cell death by the transcriptional coactivator PGC-1alpha. Cell Death Differ. 2006;13:586–596. doi: 10.1038/sj.cdd.4401784. [DOI] [PubMed] [Google Scholar]
- 138.Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brough D, Schell MJ, Irvine RF. Agonist-induced regulation of mitochondrial and endoplasmic reticulum motility. Biochem J. 2005;392:291–297. doi: 10.1042/BJ20050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of CRAC channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006 doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 141.Gerencser AA, Adam-Vizi V. Mitochondrial Ca2+ dynamics reveals limited intramitochondrial Ca2+ diffusion. Biophys J. 2005;88:698–714. doi: 10.1529/biophysj.104.050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1- dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 143.Subramanian K, Meyer T. Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell. 1997;89:963–971. doi: 10.1016/s0092-8674(00)80281-0. [DOI] [PubMed] [Google Scholar]
- 144.Hom JR, Gewandter JS, Michael L, Sheu SS, Yoon Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol. 2007;212:498–508. doi: 10.1002/jcp.21051. [DOI] [PubMed] [Google Scholar]
- 145.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007 doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pivovarova NB, Hongpaisan J, Andrews SB, Friel DD. Depolarization-induced mitochondrial Ca accumulation in sympathetic neurons: Spatial and temporal characteristics. J Neurosci. 1999;19:6372–6384. doi: 10.1523/JNEUROSCI.19-15-06372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Colegrove SL, Albrecht MA, Friel DD. Dissection of mitochondrial Ca2+ uptake and release fluxes in situ after depolarization-evoked [Ca2+]i elevations in sympathetic neurons. J Gen Physiol. 2000;115:351–369. doi: 10.1085/jgp.115.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kamishima T, Quayle JM. Mitochondrial Ca2+ uptake is important over low [Ca2+]i range in arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002;238:H2431–H2439. doi: 10.1152/ajpheart.00865.2001. [DOI] [PubMed] [Google Scholar]
- 150.Szanda G, Koncz P, Várnai P, Spät A. Mitochondrial Ca(2+) uptake with and without the formation of high-Ca(2+) microdomains. Cell Calcium. 2006;40:527–538. doi: 10.1016/j.ceca.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 151.Várnai P, Petheõ GL, Makara JK, Spät A. Electrophysiological study on the high K+ sensitivity of rat glomerulosa cells. Pflügers Arch. 1998;435:429–431. doi: 10.1007/s004240050534. [DOI] [PubMed] [Google Scholar]
- 152.Pralong W-F, Hunyady L, Várnai P, Wollheim CB, Spät A. Pyridine nucleotide redox state parallels production of aldosterone in potassium-stimulated adrenal glomerulosa cells. Proc Natl Acad Sci USA. 1992;89:132–136. doi: 10.1073/pnas.89.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Szabadkai G, Pitter JG, Spät A. Cytoplasmic Ca2+ at low submicromolar concentration stimulates mitochondrial metabolism in rat luteal cells. Pflügers Arch. 2001;441:678–685. doi: 10.1007/s004240000466. [DOI] [PubMed] [Google Scholar]
- 154.Pitter JG, Maechler P, Wollheim CB, Spät A. Mitochondria respond to Ca2+ already in the submicromolar range: correlation with redox state. Cell Calcium. 2002;31:97–104. doi: 10.1054/ceca.2001.0264. [DOI] [PubMed] [Google Scholar]
- 155.Szanda G, Koncz P, Várnai P, Spät A. Mitochondrial Ca(2+) uptake with and without the formation of high-Ca(2+) microdomains. Cell Calcium. 2006;40:527–538. doi: 10.1016/j.ceca.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 156.Maechler P, Kennedy ED, Pozzan T, Wollheim CB. Mitochondrial activation directly triggers the exocytosis of insulin in permeabilized pancreatic β-cells. EMBO J. 1997;16:3833–3841. doi: 10.1093/emboj/16.13.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 158.Szanda G, Koncz P, Várnai P, Spät A. Mitochondrial Ca(2+) uptake with and without the formation of high-Ca(2+) microdomains. Cell Calcium. 2006;40:527–538. doi: 10.1016/j.ceca.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 159.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca2+ uptake depends on the spatial and temporal profile of cytosolic Ca2+ signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 160.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou Z, Matlib MA, Bers DM. Cytosolic and mitochondrial Ca2+ signals in patch clamped mammalian ventricular myocytes. J Physiol. 1998;507:379–403. doi: 10.1111/j.1469-7793.1998.379bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hutson SM, Pfeiffer DR, Lardy HA. Effect of cations and anions on the steady state kinetics of energy-dependent Ca2+ transport in rat liver mitochondria. J Biol Chem. 1976;251:5251–5258. [PubMed] [Google Scholar]
- 163.Akerman KE. Effect of Mg2+ and spermine on the kinetics of Ca2+ transport in rat-liver mitochondria. J Bioenerg Biomembr. 1977;9:65–72. doi: 10.1007/BF00745043. [DOI] [PubMed] [Google Scholar]
- 164.Nicholls DG. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978;176:463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Prentki M, Janjic D, Wollheim CB. The regulation of extramitochondrial steady state free Ca2+ concentration by rat insulinoma mitochondria. J Biol Chem. 1983;258:7597–7602. [PubMed] [Google Scholar]