Abstract
Many different signaling pathways share common components but nevertheless invoke distinct physiological responses. In yeast, the adaptor protein Ste50 functions in multiple mitogen-activated protein (MAP) kinase pathways, each with unique dynamical and developmental properties. Although Kss1 activity is sustained and promotes invasive growth, Hog1 activity is transient and promotes cell adaptation to osmotic stress. Here we show that osmotic stress activates Kss1 as well as Hog1. We show further that Hog1 phosphorylates Ste50 and that phosphorylation of Ste50 limits the duration of Kss1 activation and prevents invasive growth under high osmolarity growth conditions. Thus feedback regulation of a shared component can restrict the activity of a competing MAP kinase to ensure signal fidelity.
All living organisms respond to specific external cues and initiate the appropriate developmental or metabolic responses. In many cases, extracellular stimuli lead to activation of MAP2 kinases, which in turn initiate distinct, and often mutually exclusive, cellular behaviors including cell growth, movement, differentiation, and death. Therefore the regulation and coordination of multiple kinases are essential for the cell to respond appropriately to a changing environment (1). However, the underlying mechanisms ensuring pathway fidelity are not well understood.
The MAP kinases in yeast Saccharomyces cerevisiae provide a powerful model to study the mechanisms of signal specificity. Two different MAP kinases, Fus3 and Kss1, function in the mating-response pathway leading to fusion of haploid a- and α-type cells. In this case, pheromone stimulation leads to activation of a protein kinase cascade that includes Ste20, Ste11, Ste7, and ultimately Fus3 and Kss1 (2). Nutrient-poor conditions lead to activation of the same kinase components, with the exception of Fus3 (3, 4). In this alternate developmental pathway, the cells exhibit altered budding, formation of long branching filaments, as well as increased adherence and invasion of the substratum. A third pathway leads to activation of the high osmolarity glycerol (HOG) response kinase Hog1 (5, 6). The HOG response is initiated by stimulation of two putative osmosensing proteins, Sln1 and Sho1 (7, 8). Sln1 activates two partially redundant kinases, Ssk2 and Ssk22, which then activate the MAP kinase kinase Pbs2 and ultimately Hog1. Sho1 recruits a distinct kinase Ste11 to activate Pbs2 and Hog1. In either case, Hog1 induces production of glycerol that serves to balance intracellular osmotic pressure with the external environment and thereby enables cell survival (9–11).
Thus Ste11 is required for signaling by Sho1 as well as by mating pheromones. Another shared component is Ste50, which forms a stable complex with Ste11 (12–17). Although the Sho1 branch of the Hog1 pathway shares components with the Fus3 and Kss1 pathways, osmotic stimulation does not normally induce mating or invasive growth. If Hog1 expression or catalytic activity is abrogated, however, osmotic stimulation leads to inappropriate activation of the mating response (18). Thus Hog1 mediates the HOG response and simultaneously acts to prevent induction of the mating or invasive growth pathways. The manner by which Hog1 inhibits cross-talk to these parallel signaling systems has not been determined previously.
In this study, we examine the role of Hog1 in ensuring pathway fidelity. Because our interest is in mechanisms of signaling specificity, we have restricted our investigations to the Sho1 branch of the pathway (ssk1Δ). First we show that Kss1, together with Hog1, is transiently activated by osmotic stimulation. We demonstrate further that Hog1 phosphorylates a shared upstream component Ste50, and this feedback-phosphorylation event limits the duration of pathway activation. When Ste50 phosphorylation is abrogated, Kss1 activity is sustained, and the cells undergo invasive growth in response to osmotic stress. This work reveals a novel negative feedback loop that controls the dynamics of MAPK activation and thereby ensures signal fidelity.
EXPERIMENTAL PROCEDURES
Standard methods for maintenance of yeast and bacteria, manipulation of DNA, purification and analysis of protein kinases, protein immunodetection, and analysis of cellular responses to pheromone and salt stimulation were used throughout. Full experimental procedures and any associated references are available in the supplemental materials.
RESULTS
It is well established that osmotic stress leads to activation of the MAPK Hog1 (6). It has been demonstrated previously that osmotic stress can also activate the mating pathway. This form of inappropriate cross-talk has been observed in cells lacking HOG pathway components (Pbs2- or Hog1-deficient cells). The mutant phenotype was first documented by indirect measurements of MAPK-mediated gene transcription, using a mating pheromone-responsive reporter (FUS1 promoter, lacZ reporter) (18).
More recently, it has become feasible to detect Fus3 and Kss1 activation directly using antibodies that recognize the dually phosphorylated (fully activated) form of each protein (19). Using the antibody detection method, we monitored Fus3 and Kss1 activity over time, in response to a salt stimulus. Wild-type cells and cells expressing a catalytically inactive Hog1K52R mutant were treated with 0.5 m KCl and then lysed and resolved by gel electrophoresis and immunoblotting. As expected, Kss1 was strongly activated in the hog1 mutant cells (20, 21). However, we observed very weak activation of Fus3 (Fig. 1A). Most surprisingly, we found that Kss1 is activated by salt even in wild-type cells expressing fully functional Pbs2 and Hog1 (Fig. 1, A and B). Indeed, the amplitude of Kss1 phosphorylation can be compared with that observed after treatment with pheromone at 1 μm (Fig. 1B). Moreover, there was a striking difference in the dynamics of Kss1 phosphorylation. Although pheromone stimulation normally results in sustained phosphorylation of Kss1, osmotic stress results in a transient response, with peak activity occurring 5 min after the initial stimulus. A similar time course is typically observed for Hog1 (Fig. 1, A and B). In the absence of Hog1 activity, however, a salt stimulus results in sustained phosphorylation of both Hog1 and Kss1 (Fig. 1A). From these data, we conclude that osmotic stress activates Kss1 as well as Hog1 and that activation is in each case highly transient. When Hog1 catalytic activity is abrogated, activation of both MAP kinases is sustained.
FIGURE 1.
Kss1 is activated in response to osmotic stress. A, HOG1 ssk1Δ (HOG1WT) or hog1K52R ssk1Δ mutant cells were treated with 0.5 m KCl for the times indicated. Cell lysates were then resolved by 10% SDS-PAGE and immunoblotting with anti-phospho-p42/44 antibodies, which recognize the dually phosphorylated and activated form of Fus3 (p-Fus3) and Kss1 (p-Kss1) or anti-phospho-p38 antibodies, which recognize the dually phosphorylated and activated form of Hog1 (p-Hog1) or anti-glucose-6-phosphate dehydrogenase antibodies as a loading control. Note that FUS3, KSS1, and HOG1 transcripts are unaffected by osmotic shock (45). B, wild-type (WT)(SSK1) cells were treated with 0.5 m KCl or 1 μm pheromone for the times indicated. Salt-induced Kss1 phosphorylation is ∼75% of that induced by pheromone at 5 min. Similar values were obtained in the ssk1Δ mutant cells used throughout (data not shown).
The data presented above reveal that Kss1 activation mirrors that of Hog1 and that Kss1 and Hog1 are affected equally by loss of Hog1 activity. Given the requirement for Hog1 catalytic activity, we presumed that Hog1 promotes a feedback phosphorylation event that limits the duration of pathway activation. Indeed, feedback phosphorylation of an upstream component was predicted based on genetic (18) and theoretical (22) considerations. Given that both Kss1 and Hog1 are affected equally, we presumed that the target of feedback phosphorylation is a component upstream of both MAP kinases. Upstream components shared by both pathways include Sho1, Ste11, and Ste50. We have documented previously that Sho1 is phosphorylated by Hog1. However, the result in this case is to dampen overall pathway activity, but not duration, and occurs only under extreme osmotic stress conditions (23). Thus we focused our investigations on Ste11 and Ste50.
Hog1 Phosphorylates Ste50 in Response to Osmotic Stress—Ste11 is a MAPK kinase upstream of both Hog1 and Kss1. Ste50 is an adaptor protein required for the catalytic activity of Ste11 (14–17). Although Ste50 was originally identified as a component of the yeast mating pathway (12), further genetic studies revealed that deletion of Ste50 has a relatively modest effect on pheromone responses (13, 14). In contrast, ste50 mutants block invasive growth (24, 25) as well as the Sho1-dependent branch of the HOG pathway (13, 14, 18, 26). Given that invasive growth and HOG signaling are mediated by Kss1 and Hog1, respectively, we regarded Ste50 as a potential target for feedback regulation affecting both pathways.
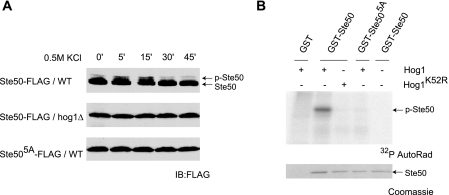
Protein phosphorylation will in many cases result in a mobility shift detectable by gel electrophoresis. Moreover, Ste50 is known to be phosphorylated at multiple sites in vivo (14, 27). To examine whether Ste50 is phosphorylated in response to osmotic stress, cells expressing FLAG-tagged Ste50 were treated with 0.5 m KCl and then lysed and resolved by gel electrophoresis and immunoblotting. As shown in Fig. 2A, Ste50 undergoes a rapid and transient reduction in electrophoretic mobility, peaking ∼15 min after osmotic stimulation. The time course of the mobility shift lags slightly behind the peak of Hog1 activation, noted in Fig. 1. Such a lag would be expected if Hog1 activation must precede phosphorylation of Ste50. Furthermore, Ste50 failed to undergo a mobility shift in the absence of Hog1 expression. The shift was also not observed after pheromone stimulation and was not affected by deletion of either Fus3 or Kss1 (data not shown). From these data, we conclude that Ste50 undergoes a salt stimulus- and Hog1-dependent post-translational modification.
FIGURE 2.
Ste50 is phosphorylated by Hog1. A, the C-terminal FLAG-tagged form of Ste50 (Ste50-FLAG) or Ste505A (Ste50-FLAG5A) was expressed under control of the ADH1 promoter in HOG1 wild-type (WT, ssk1Δ) or isogenic hog1Δ mutant cells and treated with 0.5 m KCl for the times indicated. Whole-cell extracts were resolved by 10% SDS-PAGE and immunoblotting (IB) with anti-FLAG antibodies. p-Ste50, slower migrating phosphorylated form of Ste50. B, GST-Ste50, GST-Ste505A, or GST alone were purified from Escherichia coli and mixed with Hog1 or the catalytically inactive Hog1K52R purified from yeast (previously treated with 0.5 m KCl for 15 min) and [γ-32P]ATP. Reactions were stopped by the addition of SDS-PAGE sample buffer and boiling. Expression of Ste50 was detected by Coomassie Blue staining, and phosphorylation of Ste50 was monitored by phospho-image detection (GE Healthcare, Dynamics) (32P Autorad).
To determine more directly whether Hog1 phosphorylates Ste50, we monitored phosphorylation in vitro. Hog1 was purified from yeast treated with 0.5 m KCl and then mixed with Ste50 purified from bacteria. As shown in Fig. 2B, purified Hog1 phosphorylates Ste50, whereas catalytically inactive Hog1K52R does not (23). We conclude that Hog1 phosphorylates Ste50 directly and does not require an intermediary kinase.
To identify candidate Ste50 phosphorylation sites, we mutated each of seven Ser or Thr residues located within a MAPK consensus sequence (Ser or Thr followed by Pro). Among these individual mutants, five (Ser-155, Ser-196, Ser-202, Ser-248, Thr-341) exhibited a reduced mobility shift upon osmotic stress. In addition, all five mutants conferred elevated induction of a Kss1-responsive transcription reporter (data not shown, see below). We then replaced all five sites in combination (Ste505A). This mutant failed to undergo any mobility shift in vivo (Fig. 2A) and was no longer phosphorylated in vitro (Fig. 2B). Thus Hog1 appears to phosphorylate Ste50 directly, at multiple sites, and in response to salt stimulation. The mobility shift observed in vivo is entirely dependent on Hog1 catalytic activity and on the integrity of substrate phosphorylation sites. The same sites required in vivo are also required in vitro.
Ste50 Phosphorylation Limits the Duration of Pathway Activation—To this point, we have shown that Hog1 phosphorylates Ste50 and that Hog1 limits the duration of pathway activation. To investigate whether Ste50 phosphorylation per se contributes to regulation of signaling dynamics, we genetically replaced wild-type STE50 with the phosphorylation-deficient mutant STE505A and monitored Hog1 and Kss1 activity over time. As shown in Fig. 3A, cells expressing Ste505A exhibit sustained Hog1 and Kss1 phosphorylation upon osmotic stimulation. Indeed, the initial time course of phosphorylation resembles that seen in the absence of Hog1 catalytic activity (hog1Δ or hog1K52R) (Fig. 1). Again, no Fus3 phosphorylation was detected in either the wild-type or the mutant strains. Phosphorylation of Hog1K52R is more sustained at later time points, possibly due to the loss of later adaptation responses including new transcription and increased glycerol production, as documented previously (9–11). Thus although Ste50 phosphorylation contributes substantially to the early adaptation response, it is also evident that other sites or targets must also exist, particularly for the later responses.
FIGURE 3.
Ste50 phosphorylation limits duration of pathway activation. A, STE50 wild-type or STE505A mutant cells were treated with 0.5 m KCl for the times indicated, and whole-cell extracts were resolved by 10% SDS-PAGE and immunoblotting with anti-phospho-p42/44 antibodies (p-Kss1), or anti-phospho-p38 antibodies (p-Hog1). Anti-glucose-6-phosphate dehydrogenase antibodies were used for loading controls. p-Kss1 and pHog1, dually phosphorylated and activated form of Kss1 and Hog1. B, the same cells co-transformed with a PFUS1-lacZ reporter and treated with 0.5 m KCl for 90 min, as indicated (+). The resulting β-galactosidase activity was measured spectrofluorimetrically. Error bars, ± S.E. C, the same cells co-transformed with a PGPD1-lacZ reporter. D, STE50 wild-type, STE505A, or ste50Δ mutant cells were grown to saturation in liquid medium, serially diluted, and spotted onto solid medium containing 0.5 m KCl. Cell growth was recorded after 2–3 days (top). The plates were then rubbed under a stream of water to detect invasive growth (middle). Bottom, photograph of remaining (adherent) STE505A cells. All experiments were performed in an ssk1Δ strain background.
To corroborate the kinase activation results, we examined Hog1- and Kss1-mediated gene induction. Activation of Hog1 can be monitored using a transcription reporter containing the GPD1 promoter (28). Activation of Kss1 is commonly monitored using the Ty1 or TEC1 promoters (29). However, in our experience, these reporters exhibit high basal activity and in some cases are activated in a Kss1-independent manner. As an alternative, we used the FUS1-lacZ reporter. The FUS1 promoter is strongly and selectively activated by Fus3 or Kss1 (30); FUS1-lacZ can be used in this case given that Kss1 is activated by osmotic stress. As shown in Fig. 3B, Kss1-mediated gene transcription is significantly elevated in the Ste505A mutant following salt stimulation. In contrast, Hog1-mediated transcriptional responses are unaffected by Ste505A despite the fact that Hog1 phosphorylation is sustained (Fig. 3C). We conclude that the high osmolarity glycerol pathway is fully activated, whether activation of Hog1 is transient or sustained.
We then examined the effect of the Ste505A mutant with respect to pheromone signaling. Activation in this case is mediated by both Fus3 and Kss1 and is normally sustained. Again we found minimal differences comparing the wild-type and Ste505A mutant. We also found small differences in the magnitude or duration of Fus3 and Kss1 phosphorylation and transcriptional induction (data not shown). Thus a mutant that cannot be phosphorylated exhibits sustained Hog1 and Kss1 activation but is otherwise fully competent to activate the pheromone- and osmotic stress-response pathways.
Finally we examined how Ste50 phosphorylation affects Hog1- and Kss1-mediated cellular differentiation responses. It was demonstrated previously that Ste50 is required for growth in high salt media (13, 14, 18, 26). Although ste50 mutant cells grew poorly in the presence of 0.5 m KCl, cells expressing the Ste505A mutant grew as well as wild-type (Fig. 3D). These results confirm that Ste505A is fully competent with respect to high osmolarity responses despite sustained activation of Hog1. On the other hand, sustained Kss1 activation has been shown to induce invasive growth, characterized by an elongated cell morphology and penetration into solid medium (21, 31, 32). Thus we examined whether loss of feedback phosphorylation promotes the invasive growth response. We spotted wild-type, ste50Δ, and STE505A mutant cells on solid medium containing 0.5 m KCl, and after 1 or 2 days, washed off non-adherent cells to detect invasion. As shown in Fig. 3D, the Ste505A mutant cells exhibited invasive growth behavior, whereas the wild-type strain did not. Moreover, these adherent cells exhibited an elongated morphology characteristic of invasive growth (Fig. 3D).
Taken together, our results indicate that Hog1 phosphorylates Ste50 in response to osmotic stress. This feedback phosphorylation event leads to more transient activation of Kss1, as well as of Hog1. When feedback phosphorylation is abrogated, Ste50 can sustain normal cellular responses to osmotic stress and mating pheromone but no longer restricts salt activation of the invasive growth pathway.
DISCUSSION
It has long been held that the mating, invasive, and high osmolarity response pathways each act via distinct MAP kinases. Although pheromone-induced growth arrest requires Fus3, nutrient-driven invasive growth requires Kss1, and growth under osmotic stress conditions requires Hog1. The underlying assumption has been that cross-activation would occur only in the absence of the primary MAPK. For example, Kss1 can act in place of Fus3 to sustain mating (33). Likewise, osmotic stress will induce mating, but only if Hog1 activity is absent (18).
More recently, it was demonstrated that Kss1 is activated by pheromone even in wild-type cells that express Fus3 (19). Here we show that Kss1 is activated by osmotic stress even in cells that express functional Hog1; activation is transient, however, and under normal circumstances, fails to promote Kss1-mediated invasive growth.
Given that both Kss1 and Hog1 require the same upstream kinase Ste11, we considered whether Ste11 or its adaptor protein Ste50 contributes to pathway fidelity. We showed that Hog1 phosphorylates Ste50 in vivo, as well as in vitro, and mapped the sites of phosphorylation. When these sites are mutated, Kss1 activity is more sustained, and the cells exhibit invasive growth behavior under osmotic stress conditions.
Although precise regulation is required to ensure signaling specificity, limited activation of multiple MAP kinases may be beneficial under some circumstances. For example, new evidence has revealed that Kss1, once believed to be redundant with Fus3, promotes unique mating-related responses such as elongated growth at very low doses of pheromone. In contrast to Kss1, Fus3 activation occurs at higher doses of pheromone, elicits a more switch-like dose-response profile, and is required for elongated growth toward a pheromone gradient (34, 35). Such elongated or chemotropic growth could allow yeasts, which are otherwise non-motile, to orient new bud formation in the direction of a weak pheromone stimulus and thus toward a distant mating partner (36). Similarly, Kss1 activation in the HOG response might be advantageous in certain circumstances. For instance, high osmotic stress together with external cAMP induces pseudohyphal growth in diploid cells (37). Even if osmostress-stimulated Kss1 does not normally lead to chemotropic or invasive growth, it may “prime” the cellular signaling system in preparation for a possible shift to poor growth conditions and the need for invasion (38). Recent studies have shown that transcription induction is not required for yeast to respond to an initial hyperosmotic stress stimulus, although it does allow cells to better adapt to subsequent stress stimuli (22, 39). Likewise, simultaneous activation of Kss1 might prepare cells for the need to undergo invasive growth, and thereby, escape further stress stimuli. In this way, Kss1 activation could help to provide an appropriate alternative response in the face of a rapidly changing environment.
It is noteworthy that pheromone stimulation activates Fus3 and Kss1 but not Hog1, whereas osmotic stress activates Hog1 and Kss1 but not Fus3. Thus only a subset of MAP kinases is activated at any given time, although all three share upstream signaling proteins including Ste11 and Ste50. This suggests that signal identity is determined by additional components upstream of Fus3 or Hog1. For example, the scaffold protein Ste5 is required for Fus3 activation and likely limits cross-activation of Fus3 by other external stimuli (21). The scaffold protein Pbs2 might serve a similar function for Hog1 (6).
It is also noteworthy that Kss1 is activated as part of the invasive growth, mating, and HOG pathways; however, no cross-talk among these pathways has been observed. Treatment of cells with pheromone or salt does not normally induce invasive growth (6, 33). Given that Kss1 is activated by multiple stimuli, how does Kss1 interpret these different input signals? We have shown that the dynamics of Kss1 activation vary depending on the nature of the stimulus. Although Kss1 activation is transient upon osmotic stress, it is somewhat prolonged in response to pheromone stimulation (Fig. 1A). When Kss1 activity is fully sustained, the cells undergo invasive growth (21, 31, 32). These observations imply that signaling dynamics are particularly important for dictating signaling specificity (supplemental Fig. S1). Indeed, the importance of signaling dynamics in yeast has striking parallels with signaling specificity in mammalian cells. In one oft-cited example, epidermal growth factor induces transient activation of the ERK MAPK and leads to cell proliferation, whereas nerve growth factor promotes sustained ERK activation and results in cell differentiation (40). The mammalian ortholog of Hog1 (p38) is regulated by an adaptor protein called OSM (osmosensing scaffold for MEKK3). Given the strong similarities between OSM and Ste50 (41), it will be interesting to determine whether OSM is subject to feedback regulation by p38.
Currently, we are investigating how Ste50 function is altered by phosphorylation. Ste50 has been reported to bind to nearly two dozen proteins;3 many of these binding partners are required for Hog1 signaling, including Ste11 and Ste50 itself (in the form of a dimer) (14, 24, 42–44). Thus it will be interesting to determine whether Ste50 phosphorylation alters the expression, catalytic activity, subcellular localization, or trafficking of any of the reported binding partners. Another question is how Ste50 cooperates with other adaptation mechanisms that are likely to regulate Kss1 or Hog1 activation. For example, Hog1 phosphorylates proteins that contribute to pathway adaptation, including transcription factors that (over a longer time scale) up-regulate components, leading to glycerol production (11).
Taken together, these findings suggest that signal identity is encoded to a large extent by the dynamics of kinase activation. We have shown how one MAPK can limit the activity of a competing MAPK through feedback phosphorylation of a shared upstream component, and more generally, how feedback inhibition contributes to signal fidelity. Given that the MAPK signaling apparatus is highly conserved among all eukaryotes, the mechanisms outlined here are likely applicable to other signaling pathways in yeast as well as in more complex organisms.
Supplementary Material
Acknowledgments
We thank Peter Pryciak (University of Massachusetts) and Cunle Wu (McGill University) for generously providing plasmids.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures and supplemental references and a supplemental figure.
Footnotes
The abbreviations used are: MAP, mitogen-activated protein; MAPK, MAP kinase; ERK, extracellular signal-regulated kinase; HOG, high osmolarity glycerol; OSM, osmosensing scaffold for MEKK3; GST, glutathione S-transferase.
STE50/YCL032W Summary in the Saccharomyces Genomic Database.
References
- 1.Turjanski, A. G., Vaque, J. P., and Gutkind, J. S. (2007) Oncogene 26 3240–3253 [DOI] [PubMed] [Google Scholar]
- 2.Dohlman, H. G., and Thorner, J. W. (2001) Annu. Rev. Biochem. 70 703–754 [DOI] [PubMed] [Google Scholar]
- 3.Roberts, R. L., and Fink, G. R. (1994) Genes Dev. 8 2974–2985 [DOI] [PubMed] [Google Scholar]
- 4.Liu, H., Styles, C. A., and Fink, G. R. (1993) Science 262 1741–1744 [DOI] [PubMed] [Google Scholar]
- 5.Brewster, J. L., de Valoir, T., Dwyer, N. D., Winter, E., and Gustin, M. C. (1993) Science 259 1760–1763 [DOI] [PubMed] [Google Scholar]
- 6.Posas, F., and Saito, H. (1997) Science 276 1702–1705 [DOI] [PubMed] [Google Scholar]
- 7.Maeda, T., Wurgler-Murphy, S. M., and Saito, H. (1994) Nature 369 242–245 [DOI] [PubMed] [Google Scholar]
- 8.Maeda, T., Takekawa, M., and Saito, H. (1995) Science 269 554–558 [DOI] [PubMed] [Google Scholar]
- 9.O'Rourke, S. M., Herskowitz, I., and O'Shea, E. K. (2002) Trends Genet 18 405–412 [DOI] [PubMed] [Google Scholar]
- 10.Saito, H., and Tatebayashi, K. (2004) J. Biochem. 136 267–272 [DOI] [PubMed] [Google Scholar]
- 11.Hohmann, S. (2002) Microbiol. Mol. Biol. Rev. 66 300–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rad, M. R., Xu, G., and Hollenberg, C. P. (1992) Mol. Gen. Genet. 236 145–154 [DOI] [PubMed] [Google Scholar]
- 13.Xu, G., Jansen, G., Thomas, D. Y., Hollenberg, C. P., and Ramezani Rad, M. (1996) Mol. Microbiol. 20 773–783 [DOI] [PubMed] [Google Scholar]
- 14.Wu, C., Leberer, E., Thomas, D. Y., and Whiteway, M. (1999) Mol. Biol. Cell 10 2425–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truckses, D. M., Bloomekatz, J. E., and Thorner, J. (2006) Mol. Cell. Biol. 26 912–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatebayashi, K., Yamamoto, K., Tanaka, K., Tomida, T., Maruoka, T., Kasukawa, E., and Saito, H. (2006) EMBO J. 25 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu, C., Jansen, G., Zhang, J., Thomas, D. Y., and Whiteway, M. (2006) Genes Dev. 20 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Rourke, S. M., and Herskowitz, I. (1998) Genes Dev. 12 2874–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbagh, W., Jr., Flatauer, L. J., Bardwell, A. J., and Bardwell, L. (2001) Mol. Cell 8 683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davenport, K. D., Williams, K. E., Ullmann, B. D., and Gustin, M. C. (1999) Genetics 153 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flatauer, L. J., Zadeh, S. F., and Bardwell, L. (2005) Mol. Cell. Biol. 25 1793–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mettetal, J. T., Muzzey, D., Gomez-Uribe, C., and van Oudenaarden, A. (2008) Science 319 482–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao, N., Behar, M., Parnell, S. C., Torres, M. P., Borchers, C. H., Elston, T. C., and Dohlman, H. G. (2007) Curr. Biol. 17 659–667 [DOI] [PubMed] [Google Scholar]
- 24.Jansen, G., Buhring, F., Hollenberg, C. P., and Ramezani Rad, M. (2001) Mol. Gen. Genomics 265 102–117 [DOI] [PubMed] [Google Scholar]
- 25.Ramezani-Rad, M. (2003) Curr. Genet. 43 161–170 [DOI] [PubMed] [Google Scholar]
- 26.Posas, F., Witten, E. A., and Saito, H. (1998) Mol. Cell. Biol. 18 5788–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, C., Arcand, M., Jansen, G., Zhong, M., Iouk, T., Thomas, D. Y., Meloche, S., and Whiteway, M. (2003) Eukaryot. Cell 2 949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris, K., Lamson, R. E., Nelson, B., Hughes, T. R., Marton, M. J., Roberts, C. J., Boone, C., and Pryciak, P. M. (2001) Curr. Biol. 11 1815–1824 [PubMed] [Google Scholar]
- 29.Madhani, H. D., and Fink, G. R. (1997) Science 275 1314–1317 [DOI] [PubMed] [Google Scholar]
- 30.Gartner, A., Nasmyth, K., and Ammerer, G. (1992) Genes Dev. 6 1280–1292 [DOI] [PubMed] [Google Scholar]
- 31.Maleri, S., Ge, Q., Hackett, E. A., Wang, Y., Dohlman, H. G., and Errede, B. (2004) Mol. Cell. Biol. 24 9221–9238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson, J., Simpson, D. M., Qi, M., Wang, Y., and Elion, E. A. (2004) EMBO J. 23 2564–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madhani, H. D., Styles, C. A., and Fink, G. R. (1997) Cell 91 673–684 [DOI] [PubMed] [Google Scholar]
- 34.Paliwal, S., Iglesias, P. A., Campbell, K., Hilioti, Z., Groisman, A., and Levchenko, A. (2007) Nature 446 46–51 [DOI] [PubMed] [Google Scholar]
- 35.Hao, N., Nayak, S., Behar, M., Shanks, R. H., Nagiec, M. J., Errede, B., Hasty, J., Elston, T. C., and Dohlman, H. G. (2008) Mol. Cell 30 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erdman, S., and Snyder, M. (2001) Genetics 159 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaragoza, O., and Gancedo, J. M. (2000) Antonie Leeuwenhoek 78 187–194 [DOI] [PubMed] [Google Scholar]
- 38.McClean, M. N., Mody, A., Broach, J. R., and Ramanathan, S. (2007) Nat. Genet. 39 409–414 [DOI] [PubMed] [Google Scholar]
- 39.Westfall, P. J., Patterson, J. C., Chen, R. E., and Thorner, J. (2008) Proc. Natl. Acad. Sci. U. S. A 105 12212–12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall, C. J. (1995) Cell 80 179–185 [DOI] [PubMed] [Google Scholar]
- 41.Uhlik, M. T., Abell, A. N., Johnson, N. L., Sun, W., Cuevas, B. D., Lobel-Rice, K. E., Horne, E. A., Dell'Acqua, M. L., and Johnson, G. L. (2003) Nat. Cell Biol. 5 1104–1110 [DOI] [PubMed] [Google Scholar]
- 42.Ramezani Rad, M., Jansen, G., Buhring, F., and Hollenberg, C. P. (1998) Mol. Gen. Genet. 259 29–38 [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharjya, S., Xu, P., Gingras, R., Shaykhutdinov, R., Wu, C., Whiteway, M., and Ni, F. (2004) J. Mol. Biol. 344 1071–1087 [DOI] [PubMed] [Google Scholar]
- 44.Grimshaw, S. J., Mott, H. R., Stott, K. M., Nielsen, P. R., Evetts, K. A., Hopkins, L. J., Nietlispach, D., and Owen, D. (2004) J. Biol. Chem. 279 2192–2201 [DOI] [PubMed] [Google Scholar]
- 45.Gasch, A. P., Spellman, P. T., Kao, C. M., Carmel-Harel, O., Eisen, M. B., Storz, G., Botstein, D., and Brown, P. O. (2000) Mol. Biol. Cell 11 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.