Abstract
In chloroplasts and bacteria, the Tat (twin-arginine translocation) system is engaged in transporting folded passenger proteins across the thylakoid and cytoplasmic membranes, respectively. To date, three membrane proteins (TatA, TatB, and TatC) have been identified to be essential for Tat-dependent protein translocation in the plant system, whereas soluble factors seem not to be required. In contrast, in the bacterial system, several cytosolic chaperones were described to be involved in Tat transport processes. Therefore, we have examined whether stromal or peripherally associated membrane proteins also play a role in Tat transport across the thylakoid membrane. Analyzing both authentic precursors as well as the chimeric 16/23 protein, which allows us to study each step of the translocation process individually, we demonstrate that a soluble form of TatA is present in the chloroplast stroma, which significantly improves the efficiency of Tat-dependent protein transport. Furthermore, this soluble TatA is able to reconstitute the Tat transport properties of thylakoid membranes that are transport-incompetent due to extraction with solutions of chaotropic salts.
The twin-arginine translocation (Tat)3 pathway translocates proteins across the thylakoid membrane of chloroplasts and the plasma membranes of bacteria and archaea (1). It is specifically engaged by proteins carrying signal peptides with a characteristic twin pair of arginine residues within their N-region (2). Perhaps the most remarkable feature of the Tat pathway is its ability to translocate proteins in a fully folded conformation across an energized membrane (3–8). However, it also accepts unfolded proteins as substrates (4) unless they expose extended hydrophobic stretches at their surface (9). The energy source of the Tat transport pathway is still a matter of debate. Recent data showed that at the thylakoid membrane, transport can be driven by either ΔpH or ΔΨ, whereas in the bacterial system, it seems to be strictly dependent on the ΔΨ across the membrane (10, 11).
Three membrane proteins, namely TatA, TatB, and TatC (in the thylakoid system also called Tha4, Hcf106, and cpTatC, respectively), are essential for Tat-dependent protein translocation (12–16). Mechanistically, protein transport by the Tat pathway is initiated by the direct insertion of the transport substrate into the target membrane (17, 18) followed by association to the TatBC-receptor complex (19, 20), which in plants has an apparent size of 560–700 kDa (19, 21). The actual translocation step depends on TatA, which joins the TatBC-substrate complex (19, 22). Finally, the Tat substrate is presumably released into the membrane for proteolytic removal of the signal peptide (23).
The thylakoidal Tat pathway is generally assumed to act independently from soluble factors or nucleoside triphosphates (24). Only in the case of the Rieske protein has a requirement for stromal chaperones been described. The chaperones are presumably involved in the assembly of the iron-sulfur cluster (25). In contrast, in bacterial Tat systems, several chaperones were found to bind the precursor proteins prior to translocation (26–29). Cytosolic forms of either TatA or TatA and TatB have been reported for Bacillus subtilis, Streptomyces lividans, and Escherichia coli (30–33), which suggests that soluble components might play a more significant role in the Tat translocation process than originally assumed. Therefore, it was the goal of this study to examine in detail the potential involvement of stromal and peripherally associated thylakoid membrane proteins on the individual steps of Tat-dependent protein transport.
EXPERIMENTAL PROCEDURES
Protein Transport Experiments—Isolation of chloroplasts and thylakoids was carried out according to Ref. 17. Protein transport experiments with radiolabeled precursor proteins followed published protocols (5). Protein extraction of thylakoids with solutions of chaotropic salts prior to transport experiments was performed as described (34).
Preparation of Stromal Extract—For the preparation of stromal extract, chloroplasts were lysed by osmotic shock for 10 min on ice at 0.75 mg of chlorophyll/ml in HM buffer (10 mm Hepes/KOH, pH 8.0; 5 mm MgCl2). Thylakoids were sedimented by centrifugation for 5 min at 20,000 × g at 4 °C. To completely remove chloroplast envelope membranes and residual thylakoid membranes from the soluble fraction, the supernatant was subjected to ultracentrifugation for 1 h at 100,000 × g at 4 °C. Stromal extract was concentrated using Vivaspin 5.000 MWCO PES ultrafiltration columns (Sartorius AG, Göttingen, Germany), frozen in liquid nitrogen and stored at –80 °C until use.
TatA Depletion of Stromal Extract—50 μl of 10-fold concentrated stromal extract were supplemented with NaCl (180 mm final concentration) and 4 μg of affinity-purified TatA antibodies and incubated for 1 h at 4°C on a slowly rotating wheel. 100 μl of protein A-Sepharose CL-4B (10% (w/v) (GE Healthcare, München, Germany) in HM-NaCl buffer (10 mm Hepes/KOH, pH 8.0; 5 mm MgCl2; 180 mm NaCl) were added and incubated for 1 h at 4°C on the rotating wheel. Protein A-Sepharose was sedimented by centrifugation for 10 min at 3000 rpm in a microcentrifuge. The supernatant was removed, and the pellet was washed twice with HM-NaCl buffer. All supernatants were pooled and concentrated to a volume of 50 μl using Vivaspin 5.000 MWCO PES ultrafiltration columns (Sartorius AG, Göttingen, Germany) to yield TatA-depleted 10-fold concentrated stromal extract. The protein A-Sepharose pellet was finally resuspended in SDS-PAGE sample buffer for further analysis.
In Vitro Synthesis of TatA—TatA synthesis was carried out with the Rapid Translation System (RTS) wheat germ linear template generation set and the RTS 100 wheat germ CECF kit according to the manufacturer's instructions (Roche Diagnostics, Mannheim, Germany) using the following primers: 5′-ctttaagaaggagatataccatggccttcttcggtctaggc-3′ and 5′-tgatgatgagaaccccccccttatcatacattatcctttgtg-3′. The translation start codon was set to the predicted stromal processing peptidase cleavage site at amino acid position 56 of the precursor protein according to Ref. 35. It should be noted that the TatA cDNA used here deviates at one nucleotide position from the published sequence (accession number AAD33943), leading to a conservative amino acid exchange from glutamate to aspartate at position 127 of the precursor protein.
Miscellaneous—Gel electrophoresis of proteins under denaturing conditions was carried out according to Ref. 36. Blue Native gel electrophoresis (37) was carried out according to Ref. 21. The gels were exposed to phosphorimaging screens and analyzed with the Fujifilm FLA-3000 (Fujifilm, Düsseldorf, Germany) using the software packages BASReader (version 3.14) and AIDA (version 3.25) (Raytest, Straubenhardt, Germany). Western analysis of Tat proteins was carried out as described (38). All other methods followed published protocols (39).
RESULTS
NaBr Treatment of Thylakoid Vesicles Abolishes Tat-dependent Protein Translocation—It is generally assumed that Tat-dependent protein transport across the thylakoid membrane operates independently from soluble factors because it is observed also in isolated thylakoid vesicles resuspended in buffer (24). However, such assays are not completely devoid of stromal factors because several stromal proteins tend to associate peripherally with thylakoid membranes from the stromal side and are thus co-isolated to a certain extent with the thylakoids upon organelle fractionation (40). To examine whether such membrane-attached stroma proteins play a role in the transport process, thylakoids were additionally treated with NaBr solutions to remove all peripherally associated proteins and subsequently subjected to protein transport experiments analyzing the authentic precursor proteins of the 16- and 23-kDa subunits of the oxygen-evolving system associated with photosystem II. In neither case were significant amounts of the respective mature proteins, which are indicative of membrane transport and terminal processing, found with such thylakoids (Fig. 1A). This is in accord with previously described results (17) and confirms that treatment with solutions of chaotropic salts interferes with Tat-dependent protein translocation, presumably due to the extraction of an essential component of the transport machinery.
FIGURE 1.
Influence of stromal extract on Tat-dependent protein transport across the thylakoid membrane. The precursor proteins 16 kDa, 23 kDa, and 16/23 were obtained by in vitro transcription/translation and incubated with isolated pea thylakoids for 15 min in the light at 25 °C. After the import reaction, the thylakoids were washed with HM buffer (10 mm Hepes/KOH, pH 8.0; 5 mm MgCl2) and either treated with thermolysin (200 μg/ml, 30 min on ice, lanes +) or mock-treated (lanes –). Stoichiometric amounts of each fraction, corresponding to 15 μg of chlorophyll, were separated on 10–17.5% SDS-polyacrylamide gradient gels and visualized by phosphorimaging. In lanes t, 1 μl of the respective in vitro translation assay was loaded. The positions of the precursor (p) and mature protein (m) are indicated by filled arrowheads, whereas open arrowheads point to the positions of the stromal intermediate of the 23-kDa precursor (i) or to the two integral translocation intermediates, Ti-1 and Ti-2 observed with 16/23. A, in thylakoido transport experiments performed with the authentic 16-(left panel) and 23-kDa (right panel) precursor proteins. Prior to the import reaction, thylakoids were washed with either HM buffer (control) or 1 m NaBr (NaBr) as described (34) and subsequently resuspended in either HM buffer (HM) or stromal extract (S). B, in thylakoido import experiment analyzing the chimeric 16/23 precursor protein as detailed in A. In the right panel, a schematic representation of the topology of the early (Ti-1) and late (Ti-2) translocation intermediates observed during thylakoid membrane transport of the chimeric 16/23 protein is depicted. The open ellipsoid indicates the hydrophobic central core within the thylakoid-targeting signal peptide, and RR points to the position of the twin-arginine motif. C, C terminus; N, N terminus. C, in thylakoido transport experiment analyzing the 16/23 precursor in the presence and absence of stromal extract. Thylakoids were resuspended in either HM buffer or stromal extract prior to the transport reaction as shown on the top of the lanes (–/+). The signal intensities of Ti-1, Ti-2, and mature protein were quantified for each assay and are given as the relative amount found in the presence of stromal extract (in terms of the percentage of that found in the absence of stromal extract) in the right panel.
Thylakoid Transport Can Be Recovered by Stromal Extracts—Inhibition of transport is, however, reversible because when fractions of the NaBr-treated thylakoids are resuspended in stromal extract prior to the addition of the precursor proteins, mature 16- and 23-kDa proteins accumulate to considerable levels (Fig. 1A). This finding suggests that translocation activity had been restored. Although translocation efficiency is not fully recovered, most likely due to destruction of some thylakoids upon extraction, it is certainly high enough to demonstrate the activity of a stromal component capable of reconstituting Tat-dependent protein transport previously impaired or inhibited by extraction with chaotropic salts. The stromal extract is enzymatically active, as indicated by cleavage of the precursor of the 23-kDa protein to the stromal intermediate by the stromal processing peptidase (Fig. 1A). Furthermore, the extract is devoid of residual envelope and thylakoid membranes because it was subjected to ultracentrifugation for 1 h at 100,000 × g prior to addition to the assays. This indicates that the component capable of reconstituting Tat-dependent protein transport is soluble in aqueous solution.
To determine which step of the translocation process is impaired by NaBr treatment, the chimeric 16/23 protein, which consists of the transit peptide of the 16-kDa subunit and the mature part of the 23-kDa subunit, both from the oxygen-evolving system, was analyzed by an analogous approach. Tat-dependent thylakoid transport of this chimeric protein is known to be significantly retarded (17) so that distinct translocation intermediates indicative of individual steps in the translocation process can be identified (Fig. 1B, right panel). The early translocation intermediate (Ti-1) adopts a loop conformation within the membrane prior to the actual translocation step, whereas translocation intermediate-2 (Ti-2) is characterized by a bitopic transmembrane conformation representing the membrane-bound stage after translocation of the passenger protein across the membrane. Upon protease treatment, two characteristic degradation products of 14 and 26 kDa permit detection of these two intermediates (Fig. 1B, left panel) (17). Thylakoid transport experiments analyzing the 16/23 chimera with NaBr-treated thylakoids showed that Ti-1 still accumulated, which demonstrates that binding of the precursor protein to the membrane is not affected. Formation of Ti-1 under these conditions was actually expected because the first step in the translocation process can take place independently of proteinaceous components by direct interaction of the precursor protein with the lipid phase (17). In contrast, Ti-2 formation and subsequent maturation of the protein are prevented with NaBr-treated thylakoids, demonstrating that in such vesicles, the actual translocation step is blocked (Fig. 1B). As observed for the authentic precursors described above, translocation was recovered to a considerable degree if the NaBr-treated thylakoids were resuspended in stroma rather than in buffer. In this instance, both Ti-2 and the terminal-processing product can again be detected, proving that a component of the stromal extract is capable of compensating for the translocation arrest between Ti-1 and Ti-2 that is observed with NaBr-treated thylakoids.
Stromal Extract Improves the Efficiency of Tat-dependent Protein Translocation—To test whether stroma also has an effect on the transport activity of untreated thylakoid vesicles, thylakoids were resuspended in either buffer or stromal extract after lysis of the chloroplasts and assayed in protein transport experiments by analyzing the 16/23 chimera. Quantification of the signals obtained from the two experimental approaches showed that both Ti-2 and the mature 23-kDa protein accumulate in ∼2-fold higher amounts in those thylakoids that had been resuspended in stroma rather than in HM buffer (Fig. 1C). In line with this observation, the amount of Ti-1 is accordingly reduced to ∼50% in such assays. This apparent reduction is presumably not a result of reduced membrane binding of the protein but instead caused by an enhanced membrane translocation activity of the respective thylakoid vesicles. Thus, stromal components apparently promote the process of Tat-dependent protein transport even of untreated thylakoids by improving the efficiency of the actual membrane translocation step from Ti-1 to Ti-2.
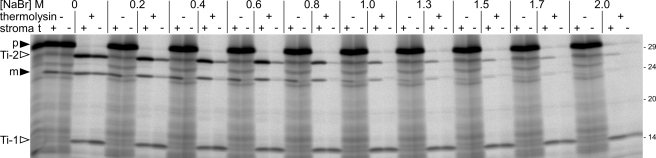
To determine the optimal NaBr concentration for membrane extraction, thylakoids were treated with NaBr solutions ranging from 0.2 to 2 m, resuspended in either buffer or stromal extract, and subjected to protein transport assays analyzing the 16/23 chimera. It turned out that even pretreatment of thylakoids with NaBr solutions of low concentration (0.2–0.4 m) had a remarkable inhibitory effect on the transport process. The accumulation of Ti-2 and the mature 23-kDa protein was significantly reduced in these assays, and protein translocation was to a large extent, although not entirely, arrested at the stage of Ti-1 (Fig. 2). In line with the results described above, this transport block was almost completely overridden in the presence of stromal extract. However, pretreatment of the thylakoids with solutions of 1.7 m NaBr or even higher concentration led to a virtually irreversible loss of transport activity, even in the presence of stromal extract (Fig. 2). For our experiments aiming to characterize the promoting effect of stroma on Tat-dependent protein transport, thylakoids treated with solutions of ∼0.8–1.0 m NaBr turned out to be most suitable because they showed only minimal residual transport activity when resuspended in buffer but still allowed significant recovery of translocation activity in the presence of stromal extracts.
FIGURE 2.
Optimization of the NaBr extraction conditions. In thykaloido import experiment analyzing the 16/23 precursor protein (p) was performed. Thylakoids were extracted with NaBr solutions of different concentrations and resuspended in either buffer or stromal extract as detailed on the top of the lanes. m, mature protein. For further details, see the legend for Fig. 1.
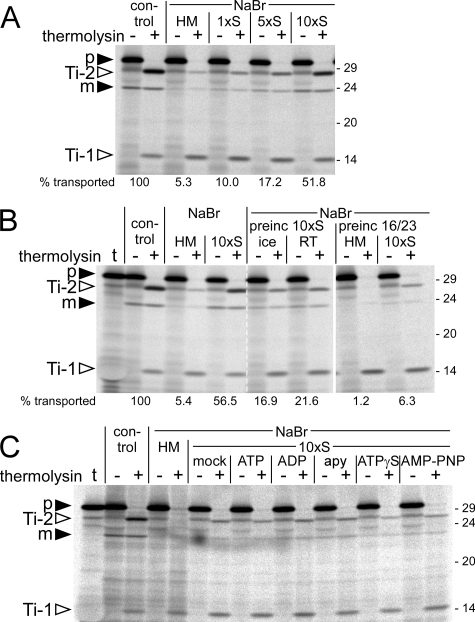
Transport Recovery Depends on Stromal Concentration and Is Also Observed with Prebound Precursor Protein—Next, we wanted to determine whether the extent to which Tat-dependent transport of NaBr-treated thylakoids can be recovered depends on the concentration of the stromal extract present in the assay. This made sense because upon preparation of the stromal extract by chloroplast lysis, the stromal components are inevitably diluted at least 10-fold. To compensate for this effect, the stromal extracts were concentrated by ultrafiltration either 5-fold or 10-fold prior to their analysis in thylakoid transport experiments. It turned out that the stimulating effect of stromal extract is indeed significantly stronger if the thylakoids were resuspended in stromal extracts of higher concentration (Fig. 3A), suggesting that recovery of transport activity is a concentration-dependent process. Although the degree of transport recovery varies to some extent between individual experiments and is presumably dependent on the residual transport activity of the thylakoids after NaBr treatment (between 3 and 10% of untreated thylakoids; data not shown), with a stromal extract concentrated 10-fold, a level of ∼50% of the control reaction analyzing untreated thylakoids was achieved (Fig. 3, A and B).
FIGURE 3.
Effect of stromal concentration on the transport properties of NaBr-extracted thylakoids. A, thylakoids were extracted with 1 m NaBr, resuspended in either HM buffer (HM) or stromal extract of different concentration (1xS, 5xS, 10xS) and subjected to thylakoid transport experiments analyzing the 16/23 precursor (p). The relative amounts of translocated protein (i.e. Ti-2 plus mature protein (m), taking into account the respective number of [35S]methionine residues) were quantified (in terms of the percentage of the control reaction) and are given below the corresponding lanes. B, thylakoids extracted with 1 m NaBr were preincubated for 10 min with 10-fold concentrated stromal extract (preinc 10xS) either on ice or at room temperature (RT) and washed once with HM buffer prior to the import reaction (middle panel). Alternatively, thylakoids extracted with 1 m NaBr were preincubated for 2 min on ice with the 16/23 precursor protein and subsequently washed once with HM buffer to remove excess precursor (preinc 16/23, right panel). Thylakoids were then resuspended in either HM buffer or 10-fold concentrated stroma (10xS) upon starting the import reaction. In the left panel, import experiments analogous to that shown in A were performed in parallel for comparison. Quantification of the signal intensities was carried out as in A. In lanes t, 1 μl of the respective in vitro translation assay was loaded. C, thylakoids extracted with 1 m NaBr were resuspended in either HM buffer or 10-fold concentrated stromal extract that was further supplemented with buffer (mock), 5 mm ATP, 5 mm ADP, 2 units of apyrase (apy), 5 mm ATPγS, or 5 mm AMP-PNP and subjected to thylakoid transport assays using the 16/23 precursor protein. For further details, see the legend for Fig. 1.
Remarkably, the transport-promoting stromal component need not be present in the soluble fraction during transport but can be attached to the thylakoids prior to the actual transport experiment. If NaBr-treated thylakoids are incubated for 10 min with 10-fold concentrated stromal extract, washed extensively with HM buffer to remove loosely attached stromal components, and subsequently analyzed in thylakoid transport experiments with the 16/23 chimera, these “preloaded” thylakoids showed ∼20% of the transport activity found with untreated control thylakoids (Fig. 3B), a rate that is similar to the transport recovery obtained in the presence of 5-fold concentrated stromal extract (Fig. 3A). This “preloading” effect is apparently not temperature-dependent because it is observed irrespective of whether the incubation of the thylakoids with stromal extract is performed on ice or at room temperature (Fig. 3B).
In a reciprocal approach, we examined whether a precursor protein arrested in the Ti-1 conformation could still be recognized as substrate if the transport competence of the thylakoids was reconstituted by the addition of stromal extract. For this purpose, NaBr-treated thylakoids were incubated with the 16/23 precursor protein on ice for 2 min to allow binding and Ti-1 formation. The thylakoids were extensively washed with HM buffer to remove loosely attached precursor proteins and subsequently resuspended in either stromal extract concentrated 10-fold or HM buffer. As expected, the 16/23 chimera remained arrested in its Ti-1 conformation in the absence of stromal components. However, if the assays were supplemented with stromal extract, significant amounts of Ti-2 accumulated, which is indicative of membrane transport of the passenger protein (Fig. 3B). Even processing to the mature 23-kDa protein can be observed, although to a minor extent only.
The Transport-promoting Stromal Component Operates Independently of Nucleoside Triphosphates—The data obtained so far strongly suggest that the stromal component responsible for the transport recovery of NaBr-treated thylakoids is a soluble factor that has the capacity to interact with the thylakoid membrane. The most obvious candidates for such factors are chaperones, in particular because several chaperones were found in bacterial systems to be involved in binding of Tat substrates (41). The activity of the majority of chaperones depends on nucleoside triphosphates (42), and therefore, we examined whether the transport-stimulating effect of stromal extracts was modified by apyrase-mediated degradation of nucleoside triphosphates or by supplementing the stromal extracts with additional ATP, ADP, or the non-hydrolyzable ATP analogs ATPγS and AMP-PNP. Despite some variability in the individual assays, it is apparent that none of these substances had a significant effect on the transport recovery of NaBr-treated thylakoids by stromal extract because in all fractions, similar amounts of Ti-2 and mature protein could be detected (Fig. 3C). This demonstrates that the “stromal effect” is not directly dependent on the presence of hydrolyzable nucleoside triphosphates, which makes it unlikely that chaperones are responsible.
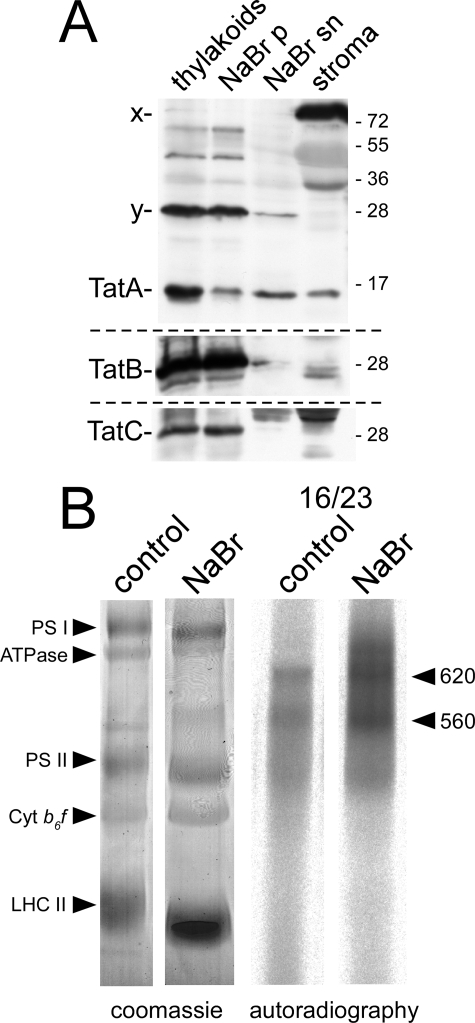
The TatBC-Receptor Complex Is Resistant to NaBr Extraction—To examine to what extent the subunits of the Tat translocase are sensitive to extraction by solutions of chaotropic salts, thylakoids were treated with NaBr solution, recovered by centrifugation, and subjected to Western analyses using polyclonal antisera raised against TatA, TatB, and TatC. The supernatant of the centrifugation step containing all extracted membrane components was analyzed in parallel, together with untreated thylakoids and stromal extracts (Fig. 4A). To allow for direct comparison of the distribution of the corresponding proteins in the different fractions, stoichiometric amounts of each fraction (equivalent to 15 μg of chlorophyll) were examined.
FIGURE 4.
BN-PAGE and Western analysis of NaBr-extracted thylakoids. A, thylakoids were extracted with 1 m NaBr and sedimented by centrifugation. Stoichiometric amounts equivalent to 15 μg of chlorophyll of both the thylakoid pellet (NaBr p) and the supernatant (NaBr sn) were separated on 15% SDS-polyacrylamide gels and subjected to Western analyses with polyclonal antisera raised against TatA, TatB, and TatC. For comparison, stoichiometric amounts of untreated thylakoids, as well as of stromal extracts (stroma), were analyzed in parallel. x and y indicate the locations of proteins cross-reacting with the TatA antibody. B, in thykaloido transport experiment analyzing the 16/23 precursor with untreated (control) or NaBr-extracted thylakoids (NaBr). Thylakoids were washed twice with HM buffer, solubilized with digitonin, separated on a 5–13.5% Blue Native polyacrylamide gradient gel, and strained with Coomassie Blue (coomassie) or visualized by phosphorimaging (autoradiography). PS I, photosystem I; PS II, photosystem II; Cyt b6f, cytochrome b6f complex; LHC II, light-harvesting complex II.
It turned out that neither TatB nor TatC was extracted to a significant extent from the thylakoids by NaBr treatment. In accord with this finding, neither of the two proteins could be detected in the stromal fraction, demonstrating that they cannot be responsible for the transport-promoting activity of stromal extracts. Nevertheless, NaBr treatment might disturb the functional properties of the thylakoidal TatBC-receptor complex, e.g. its affinity to Tat substrates like the 16/23 precursor protein (21, 23). To examine such substrate binding properties, both NaBr-treated thylakoids and untreated thylakoids were incubated with radiolabeled 16/23 protein and subjected to Blue Native-PAGE and phosphorimaging. Remarkably, it turned out that NaBr pretreatment led to increased amounts of receptor-associated 16/23 protein (Fig. 4B). Thus, prevention of the actual membrane translocation step apparently causes a kind of “traffic jam” within the Tat translocase, which results in increased precursor accumulation at the TatBC-receptor complex. This demonstrates that NaBr extraction does not affect the receptor function of TatB and TatC.
Stromal TatA Restores Transport Activity to NaBr-extracted Thylakoids—In contrast to what was found above for TatBC, the majority of TatA was removed from thylakoid membranes upon NaBr treatment (Fig. 4A), which could explain the block in protein translocation observed. Unexpectedly, significant amounts of TatA were even detected in the stromal fraction (Fig. 4A), which is the first hint that TatA might be the stromal component that is capable of restoring the transport properties to NaBr-treated thylakoids.
To examine this possibility in more detail, mature TatA was generated by in vitro translation in the wheat germ RTS. The resulting TatA protein showed a slightly greater mobility upon SDS-PAGE when compared with TatA isolated from pea chloroplasts (Fig. 5C). This might either be a consequence of the initiating methionine residue added to the N terminus of the protein or indicate a mistake in the prediction of the cleavage site for the stromal processing peptidase. However, because an identical TatA protein has previously been shown to be active in translocation (35), in neither case was the function of the protein apparently affected.
FIGURE 5.
TatA is responsible for the functional complementation of NaBr-treated thylakoids. A, thylakoids were extracted with 1 m NaBr and resuspended in HM buffer (HM), stromal extract concentrated 10-fold (10xS), HM buffer supplemented with 25 μl of wheat germ RTS, or HM buffer supplemented with TatA protein generated in 25 μl of wheat germ rapid translation system (TatA). For the control reactions, untreated thylakoids were resuspended in either HM buffer or HM buffer supplemented with 25 μl wheat germ RTS. All thylakoid fractions were subsequently assayed for protein transport of the chimeric 16/23 precursor protein as detailed in the legend for Fig. 1. Lanes t, 1 μl of the respective in vitro translation assay was loaded. B, thylakoids were extracted with 1 m NaBr and resuspended in HM buffer, stromal extract concentrated 10-fold, or stromal extract concentrated 10-fold and additionally depleted from TatA (depl). For the control reactions, untreated thylakoids were resuspended in either HM buffer or HM-NaCl buffer (10 mm Hepes/KOH, pH 8.0; 5 mm MgCl2; 180 mm NaCl) (NaCl). All thylakoid fractions were subsequently assayed as in A. C, 10-fold concentrated stromal extract was depleted from TatA as described under “Experimental Procedures.” Stoichiometric amounts of TatA-depleted stromal extract (depletion sn) and of the depleted TatA protein (depletion p) as well as a lower amount (40%) of depleted TatA (depletion 0.4p) were separated on a 15% SDS-polyacrylamide gel and subjected to Western analysis with polyclonal antibodies raised against TatA. For comparison, stoichiometric amounts of untreated thylakoids and stromal fraction (stroma) were analyzed in parallel. Likewise, 2 μl of the wheat germ rapid translation system (RTS control), as well as of TatA protein generated in this system (RTS TatA), were analyzed. x and y indicate the locations of stromal and thylakoidal proteins, respectively, cross-reacting with the TatA antibody, whereas hc indicates the position of the heavy chain of TatA antibodies from the depletion assays. For further details, see the legends for Figs. 1, 3, and 4.
Indeed, supplementation of NaBr-treated thylakoids with TatA generated in vitro led to a clear increase in protein transport activity (Fig. 5A). This increase was all the more remarkable considering the fact that wheat germ RTS not expressing TatA caused inhibition of Tat-dependent protein transport to some extent (36% of the control reaction, Fig. 5A). This inhibition is probably due to reduced binding of the precursor protein to the thylakoids in the presence of RTS because formation of Ti-1 is likewise reduced to ∼40% (Fig. 5A).
To examine the presumed function of TatA as the stromal transport recovery factor of NaBr-treated thylakoids using an independent approach, TatA was depleted from stromal extracts by immunoprecipitation with affinity-purified TatA antibodies. Analysis of NaBr-treated thylakoids that were resuspended in TatA-depleted stromal extracts showed that they were incapable of translocating the 16/23 chimera, i.e. transport of this protein remained arrested at the stage of Ti-1 (Fig. 5B). Thus, stromal TatA is indeed the factor that is responsible for the recovery of the protein transport activity of NaBr-treated thylakoids by stromal extracts.
A series of control experiments was conducted to verify these findings. First, NaCl, which had to be added to the stromal extracts upon TatA depletion to prevent unspecific binding of proteins to the affinity matrix, did not show any inhibitory effect if added to thylakoid transport assays in which the 16/23 chimera was analyzed (Fig. 5B). Second, it was important to examine the efficiency of both the depletion reaction and the subsequent removal of the TatA antibodies. Because neither residual TatA nor antibody heavy chains could be detected in the supernatant of the stroma depletion assays (Fig. 5C), both reactions were apparently quantitative. Furthermore, the fact that a stromal protein with a molecular mass of ∼80 kDa, which cross-reacts with TatA antibodies under denaturing conditions, is detected in comparable amounts in untreated and TatA-depleted stromal extracts (Fig. 5C, marked as x) demonstrates that the depletion reaction was specific for TatA and confirms that these stromal extracts contain equal amounts of proteins, except for TatA.
DISCUSSION
In the present study, we examined the involvement of stromal components on Tat-dependent protein transport across the thylakoid membrane. We could show that a stromal extract improves thylakoidal transport efficiency (Fig. 1C) and can even reconstitute Tat-dependent protein transport of thylakoids previously rendered transport-inactive by extraction with NaBr solutions (Figs. 1, 2, 3). Interestingly, this effect is apparently not caused by stromal chaperones because it is independent of the presence of hydrolyzable nucleoside triphosphates (Fig. 3C), which distinguishes it from the observed influence of cytosolic chaperones on Tat-dependent protein transport in bacteria (41). Instead, our data demonstrate that the component responsible for this effect is soluble TatA, which is capable of triggering the actual membrane translocation step during Tat-dependent protein transport (Fig. 5). Although TatA is described as an integral thylakoid membrane component (13), it is easily extracted from thylakoid membranes by weak chaotropic salts such as NaBr (Fig. 4). Furthermore, it is also present in the chloroplast stroma, in line with previous reports describing soluble cytoplasmic forms of TatA in B. subtilis, S. lividans, and E. coli (30–33). The fact that the topology of TatA within the membrane is also a matter of debate (43, 44) suggests that the localization and/or topology of TatA might change during the transport process, possibly depending on its activity status. Loosely anchored TatA might thus represent an intermediate stage between stromal and tightly anchored membrane forms of the protein. Taken together, the presumed role of TatA as an obligatory membrane component of the thylakoidal Tat machinery needs to be scrutinized, and the role of stromal factors during Tat-dependent transport has to be reconsidered.
Stromal TatA Is Not Required for Precursor Targeting to the Thylakoid Membrane—In bacterial systems, soluble forms of TatA were proposed to function in targeting of precursor proteins to the membrane-bound Tat transport machinery (31, 33). In chloroplasts, however, a similar targeting function of stromal TatA appears unlikely. The early steps of thylakoidal Tat transport, i.e. targeting and binding of the precursor protein to the TatBC-receptor complex, can efficiently take place in the absence of stromal extract even with NaBr-treated thylakoids significantly depleted of membrane-bound TatA (Fig. 4B). Furthermore, an interaction of Tat substrate and TatA in stromal extracts could not be detected upon co-immunoprecipitation experiments (data not shown). Thus, it seems that membrane targeting of precursor proteins by soluble TatA is restricted to bacterial Tat systems, possibly even to those from Gram-positive bacteria.
The Potential Role of Stromal TatA in the Translocation Process—It appears that stromal TatA is involved in the actual membrane transport step, probably the transition of Tat substrates from Ti-1 to Ti-2. If soluble TatA is added to thylakoids having precursor protein in the Ti-1 conformation bound to the TatBC-receptor complex, membrane translocation of the mature part is initiated, and both Ti-2 and mature protein can be detected (Fig. 3B). It is widely assumed that a hydrophilic pore (or channel) composed predominantly or exclusively of TatA is required for this transport step (41), and it was even suggested that pore complexes of different sizes exist as preformed units within the membrane (45). Because TatA is found in several high molecular weight complexes in the stroma (data not shown), similar to TatA from B. subtilis (32), one could speculate that those might represent such pore complexes, in analogy to the detergent-solubilized TatA complexes described for bacteria (45). However, the following considerations argue against such a scenario. In contrast to detergent-solubilized TatA complexes, which can expose their membrane anchors to the hydrophobic parts of the detergent molecules, the N-terminal hydrophobic regions of stromal TatA must somehow be shielded against the hydrophilic stroma to prevent precipitation of the protein. It appears likely that such shielding is facilitated by orienting the hydrophobic regions of several TatA molecules to each other, resulting in TatA complexes of high molecular weight that expose only their hydrophilic domains to the aqueous solution. This would be in line with the soluble micelle-like TatA complexes that were described for TatA in B. subtilis (32). Alternatively, the stromal TatA complexes might also be related to the polymeric tubular TatA structures that were recently found in the cytoplasm of E. coli after overproduction of TatA (30). In both scenarios, stromal TatA would have to be significantly restructured upon integration into the thylakoid membrane to provide the hydrophilic environment of a gated pore for the protein to be translocated, which strongly argues against their function as preformed pore complexes. Instead, a kind of reservoir or storage function of yet unused TatA molecules appears more likely. Nevertheless, it remains to be shown whether the stromal form of TatA is directly involved in protein translocation or whether it first has to be inserted into the thylakoid membrane to exert its function.
However, there is an even more plausible explanation for the function of stromal TatA. Assuming that TatA indeed accumulates in the stroma in analogy to detergent molecules in micelle-like structures, these TatA micelles presumably also share the membrane-interacting properties of detergents, i.e. they should be able to reversibly insert into lipid bilayers. Such spontaneous insertion of thylakoidal TatA into lipid bilayers has previously been described (35). Together with the remarkable stoichiometric variability of the Tat subunits in different systems, which can vary from excess to substoichiometric amounts of TatA versus TatB and TatC (46), these findings rather favor a model in which TatA mediates Tat-dependent protein translocation by a yet undefined “membrane weakening” mechanism (47).
Acknowledgments
We thank Gary Sawers for review of the manuscript.
This work was supported by grants from the state Sachsen-Anhalt (Exzellenzcluster Biowissenschaften, Research Cluster B) and the Deutsche Forschungsgemeinschaft (KL 862/2-1). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Tat, twin-arginine translocation; Ti-1/Ti-2, translocation intermediate 1/2; RTS, Rapid Translation System; ATPγS, adenosine 5′-O-(thiotriphosphate); AMP-PNP, 5′-adenylyl-β,γ-imidodiphosphate.
References
- 1.Müller, M., and Klösgen, R. B. (2005) Mol. Membr. Biol. 22 113–121 [DOI] [PubMed] [Google Scholar]
- 2.Chaddock, A. M., Mant, A., Karnauchov, I., Brink, S., Herrmann, R. G., Klösgen, R. B., and Robinson, C. (1995) EMBO J. 14 2715–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark, S. A., and Theg, S. M. (1997) Mol. Biol. Cell 8 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynds, P. J., Robinson, D., and Robinson, C. (1998) J. Biol. Chem. 273 34868–34874 [DOI] [PubMed] [Google Scholar]
- 5.Marques, J. P., Dudeck, I., and Klösgen, R. B. (2003) Mol. Genet. Genomics 269 381–387 [DOI] [PubMed] [Google Scholar]
- 6.Marques, J. P., Schattat, M. H., Hause, G., Dudeck, I., and Klösgen, R. B. (2004) J. Exp. Bot. 55 1697–1706 [DOI] [PubMed] [Google Scholar]
- 7.Santini, C. L., Ize, B., Chanal, A., Müller, M., Giordano, G., and Wu, L. F. (1998) EMBO J. 17 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargent, F., Bogsch, E. G., Stanley, N. R., Wexler, M., Robinson, C., Berks, B. C., and Palmer, T. (1998) EMBO J. 17 3640–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter, S., Lindenstrauss, U., Lücke, C., Bayliss, R., and Brüser, T. (2007) J. Biol. Chem. 282 33257–33264 [DOI] [PubMed] [Google Scholar]
- 10.Bageshwar, U. K., and Musser, S. M. (2007) J. Cell Biol. 179 87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun, N. A., Davis, A. W., and Theg, S. M. (2007) Biophys. J. 93 1993–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori, H., Summer, E. J., and Cline, K. (2001) FEBS Lett. 501 65–68 [DOI] [PubMed] [Google Scholar]
- 13.Mori, H., Summer, E. J., Ma, X., and Cline, K. (1999) J. Cell Biol. 146 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motohashi, R., Nagata, N., Ito, T., Takahashi, S., Hobo, T., Yoshida, S., and Shinozaki, K. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 10499–10504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Settles, A. M., Yonetani, A., Baron, A., Bush, D. R., Cline, K., and Martienssen, R. (1997) Science 278 1467–1470 [DOI] [PubMed] [Google Scholar]
- 16.Walker, M. B., Roy, L. M., Coleman, E., Voelker, R., and Barkan, A. (1999) J. Cell Biol. 147 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou, B., Frielingsdorf, S., and Klösgen, R. B. (2006) J. Mol. Biol. 355 957–967 [DOI] [PubMed] [Google Scholar]
- 18.Shanmugham, A., Wong Fong Sang, H. W., Bollen, Y. J., and Lill, H. (2006) Biochemistry 45 2243–2249 [DOI] [PubMed] [Google Scholar]
- 19.Mori, H., and Cline, K. (2002) J. Cell Biol. 157 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter, S., and Brüser, T. (2005) J. Biol. Chem. 280 42723–42730 [DOI] [PubMed] [Google Scholar]
- 21.Berghöfer, J., and Klösgen, R. B. (1999) FEBS Lett. 460 328–332 [DOI] [PubMed] [Google Scholar]
- 22.Alami, M., Lüke, I., Deitermann, S., Eisner, G., Koch, H. G., Brunner, J., and Müller, M. (2003) Mol. Cell 12 937–946 [DOI] [PubMed] [Google Scholar]
- 23.Frielingsdorf, S., and Klösgen, R. B. (2007) J. Biol. Chem. 282 24455–24462 [DOI] [PubMed] [Google Scholar]
- 24.Mould, R. M., Shackleton, J. B., and Robinson, C. (1991) J. Biol. Chem. 266 17286–17289 [PubMed] [Google Scholar]
- 25.Molik, S., Karnauchov, I., Weidlich, C., Herrmann, R. G., and Klösgen, R. B. (2001) J. Biol. Chem. 276 42761–42766 [DOI] [PubMed] [Google Scholar]
- 26.Graubner, W., Schierhorn, A., and Brüser, T. (2007) J. Biol. Chem. 282 7116–7124 [DOI] [PubMed] [Google Scholar]
- 27.Maillard, J., Spronk, C. A., Buchanan, G., Lyall, V., Richardson, D. J., Palmer, T., Vuister, G. W., and Sargent, F. (2007) Proc. Natl. Acad. Sci. U. S. A 104 15641–15646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Rodríguez, R., Fisher, A. C., Perlmutter, J. D., Hicks, M. G., Chanal, A., Santini, C. L., Wu, L. F., Palmer, T., and DeLisa, M. P. (2007) J. Mol. Biol. 367 715–730 [DOI] [PubMed] [Google Scholar]
- 29.Schubert, T., Lenz, O., Krause, E., Volkmer, R., and Friedrich, B. (2007) Mol. Microbiol. 66 453–467 [DOI] [PubMed] [Google Scholar]
- 30.Berthelmann, F., Mehner, D., Richter, S., Lindenstrauss, U., Lünsdorf, H., Hause, G., and Brüser, T. (2008) J. Biol. Chem. 283 25281–25289 [DOI] [PubMed] [Google Scholar]
- 31.Pop, O. I., Westermann, M., Volkmer-Engert, R., Schulz, D., Lemke, C., Schreiber, S., Gerlach, R., Wetzker, R., and Müller, J. P. (2003) J. Biol. Chem. 278 38428–38436 [DOI] [PubMed] [Google Scholar]
- 32.Westermann, M., Pop, O. I., Gerlach, R., Appel, T. R., Schlörmann, W., Schreiber, S., and Müller, J. P. (2006) Biochim. Biophys. Acta 1758 443–451 [DOI] [PubMed] [Google Scholar]
- 33.De Keersmaeker, S., Van Mellaert, L., Schaerlaekens, K., Van Dessel, W., Vrancken, K., Lammertyn, E., Anné, J., and Geukens, N. (2005) FEBS Lett. 579 797–802 [DOI] [PubMed] [Google Scholar]
- 34.Karnauchov, I., Herrmann, R. G., and Klösgen, R. B. (1997) FEBS Lett. 408 206–210 [DOI] [PubMed] [Google Scholar]
- 35.Fincher, V., Dabney-Smith, C., and Cline, K. (2003) Eur. J. Biochem. 270 4930–4941 [DOI] [PubMed] [Google Scholar]
- 36.Laemmli, U. K. (1970) Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- 37.Schägger, H., and von Jagow, G. (1991) Anal. Biochem. 199 223–231 [DOI] [PubMed] [Google Scholar]
- 38.Vachereau, A. (1989) Anal. Biochem. 179 206–208 [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, Second Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 40.Reisinger, V., and Eichacker, L. A. (2007) Proteomics 7 Suppl. 1, 6–16 [DOI] [PubMed] [Google Scholar]
- 41.Sargent, F. (2007) Biochem. Soc. Trans. 35 835–847 [DOI] [PubMed] [Google Scholar]
- 42.Netzer, W. J., and Hartl, F. U. (1998) Trends Biochem. Sci. 23 68–73 [DOI] [PubMed] [Google Scholar]
- 43.Chan, C. S., Zlomislic, M. R., Tieleman, D. P., and Turner, R. J. (2007) Biochemistry 46 7396–7404 [DOI] [PubMed] [Google Scholar]
- 44.Gouffi, K., Gérard, F., Santini, C. L., and Wu, L. F. (2004) J. Biol. Chem. 279 11608–11615 [DOI] [PubMed] [Google Scholar]
- 45.Gohlke, U., Pullan, L., McDevitt, C. A., Porcelli, I., de Leeuw, E., Palmer, T., Saibil, H. R., and Berks, B. C. (2005) Proc. Natl. Acad. Sci. U. S. A 102 10482–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakob, M., Kaiser, S., Gutensohn, M., Hanner, P., and Klösgen, R. B. (September 30, 2008) Biochim. Biophys. Acta–Mol. Cell Res. 10.1016/j.bbamcr.2008.09.006 [DOI] [PubMed]
- 47.Natale, P., Brüser, T., and Driessen, A. J. (2008) Biochim. Biophys. Acta 1778 1735–1756 [DOI] [PubMed] [Google Scholar]