Abstract
The majority of plants are unable to evade unfavorable conditions such as flooding, salinity, or drought. Therefore, a fine-tuned water homeostasis appears to be of crucial importance for plant survival, and it was assumed that aquaporins play a significant role in these processes. Regulation of plant aquaporin conductivity was suggested to be achieved by a gating mechanism that involves protein phosphorylation under drought stress conditions and protonation after cytosolic acidification during flooding. The effect of protein phosphorylation or protonation of aquaporins was studied on two plasma membrane intrinsic proteins, NtPIP2;1 and NtAQP1 from tobacco, which were heterologously expressed in yeast. Our results on mutated aquaporins with serine-to-alanine exchange indicate that phosphorylation of the two key serine residues did not affect the pH-dependent modification of water permeability. Protonation on a conserved histidine residue decreased water conductivity of NtPIP2;1. Although cells expressing NtPIP2;1 with a replacement of the histidine by an alanine were found to be pH-insensitive with regard to water permeability, these maintain high water transport rates, similar to those obtained under acidic conditions. The data clearly support the role of histidine at 196 as a component of pH-dependent modification of aquaporin-facilitated water transport. The predictions of combined effects from phosphorylation at conserved serines and histidine protonation were not supported by the results of functional analysis. The obtained results challenge the gating model as a general regulation mechanism for plant plasma membrane aquaporins.
Plants answer to conditions that lead to a decrease in cytosolic pH with reduction of hydraulic permeability (1, 2). Since this plant reaction was found to be sensitive to heavy metal ions, it was suggested that aquaporin regulation caused this effect. Furthermore, it was observed that water transport in plasma membrane vesicles obtained from Arabidopsis thaliana suspension cells was blocked by H+ and Ca2+. Also, in this case, it was concluded that aquaporin regulation caused the observed changes (3). Direct evidence for the regulation of pH was noted on the Arabidopsis aquaporins PIP1;2, PIP2;2, and PIP2;3 heterologously expressed in Xenopus laevis oocytes (4). Replacement of a histidine residue by an alanine or aspartic acid in loop D at position 197 prevented pH sensitivity. In the frame of these studies, it was also noticed that anoxia induced by flooding lead to reduced water uptake due to inhibition of water uptake by roots. Because these conditions also lead to cytosolic acidification, regulation of aquaporins was supposed to be the molecular basis of the observed physiological plant response. As the histidine residue in loop D can be identified in many plant aquaporin sequences, a general mechanism for all these plant aquaporins was implied. This was supported by molecular simulation using x-ray data of the spinach SoPIP2;1 (5). The suggested aquaporin gating model circumstantiates that loop D is displaced in the closed conformation and opens a hydrophobic gate, which blocks the channel entrance. The closed conformation occurs if two highly conserved serine residues are dephosphorylated or if these are phosphorylated and the above mentioned histidine is protonated, e.g. in response to flooding. Taking these findings into account, two aquaporins from tobacco were analyzed with regard to water conductivity and the effect of intracellular acidification.
EXPERIMENTAL PROCEDURES
Cloning and Yeast Expression—cDNA of tobacco NtPIP2;1 and NtAQP1 were inserted into pYesDEST52 (Invitrogen) yeast expression vector by the Gateway™ (Invitrogen) technology. Point mutations were introduced using the QuikChange® site-directed mutagenesis kit (Stratagene, La Jolla, CA). All constructs were verified by sequencing. Saccharomyces cerevisiae strain SY1 (6) were transfected by biolistic bombardment as described elsewhere (7), and selection was based on ura3 complementation. Yeast transformants were cultured in synthetic complete medium for 24 h. Cultures were diluted to OD1, and heterologous protein expression was induced by changing the medium carbon source from glucose to galactose. For Western blot analysis, yeast membrane proteins were separated on SDS-PAGE and electrotransferred onto nitrocellulose membranes. Specific aquaporin content was verified by hybridization of nitrocellulose blotted proteins with a NtPIP2;1 or NtAQP1 specific antibody and detection by the Western-star™ system (Applied Biosystems, Foster City, CA). Determination of plasma membrane expressed proteins was done with the help of the cell surface protein biotinylation and purification kit (Pierce/Thermo Fisher Scientific) following the supplier's protocol. Intactness of protoplasts during the biotinylation procedure was verified by an antibody against phosphoglycerate kinase I. Aquaporins in purified plasma membrane protein fractions were detected with a dot blot procedure using a specific antibody toward NtPIP2;1 and NtAQP1, respectively. The concentration of detected proteins was normalized to the total content of biotinylated proteins of the respective experiment. These data were used as a factor to relate the obtained Pf values.
Water Permeability Measurements—Water permeability of intact yeast protoplasts was measured by stopped flow spectrophotometry (SFM300, BioLogic, Claix, France) as described elsewhere (8). The protoplasts were exposed to a 300 mosmol outwardly directed osmotic gradient to induce protoplast swelling. Volume change was followed by the decrease of scattered light intensity in the stopped flow spectrophotometer. Quantification of water conductivity was achieved by fitting a single exponential function on the initial 100 ms on an average curve of 5–10 protoplast swelling kinetics using the Biokine (BioLogic) software. The osmotic water permeability coefficients (Pf) were calculated using the rate constant of the exponential decay, the partial volume of water, the external osmolarity after the mixing event, and the initial mean protoplast volume and surface described by van Heeswijk and van Os (9). The initial size of protoplasts was determined by light microscopy.
Change of Yeast Cytosolic pH—Prior to permeability measurements, yeast protoplasts were incubated in incubation buffer (IB)2 A (1.8 m sorbitol, 50 mm NaCl, 5 mm CaCl2, 10 mm Tris/HCl, pH 7.5). To lower cytosolic pH, protoplasts were resuspended in IB-B (1.8 m sorbitol, 50 mm NaCl, 5 mm CaCl2, 10 mm Mes/HCl, pH 5). Further reduction of intracellular pH of yeast protoplasts in IB-B was achieved by a 10-min incubation with 50 mm NaAc preliminary to stopped flow measurements. Cytosolic pH of yeast protoplasts was measured using fluorescein as a pH-sensitive dye as described elsewhere (10). Briefly, yeast protoplasts were loaded with fluorescein and resuspended in IB-A, IB-B, or IB-B + NaAc. Intracellular fluorescence intensity was recorded in a spectrofluorometer (LS50B, PerkinElmer Life Sciences) at 520 nm after excitation at 490 and 435 nm. The data for cytosolic pH were calculated from a calibration curve. Fluorescein was dissolved in a series of McIlvain buffers from pH 5 to 9. At each pH, the fluorescein intensity was measured at 520 nm after excitation at 435 as well as 490 nm. The ratio of these figures to the intensity values was plotted against pH. The authors are aware that the intracellular pH determination can be considered as semiquantitative since no information of the pH indicator in an intracellular environment on changes in pH was available.
RESULTS
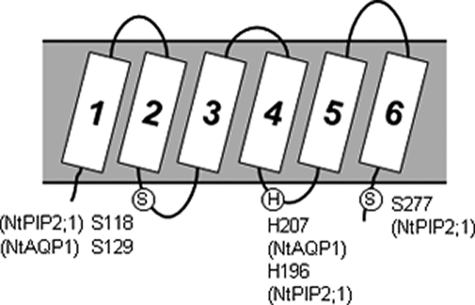
The Aquaporins under Investigation, NtAQP1 and NtPIP2;1, Belong to the Plasma Membrane Intrinsic Protein Family 1 (PIP1) and 2 (PIP2), Respectively—Both show characteristic serine residues in the cytosolic loop B at position Ser-129 or Ser-118 (Fig. 1). NtPIP2;1 has a second serine at position 277, whereas the homologous site is lacking in PIP1 aquaporins. Both serine residues are a component of the plant aquaporin gating mechanism, which was supposed to be relevant for plant aquaporin regulation if the cytoplasm acidifies. Another amino acid with a key function in this mechanism is a histidine located at 207 in NtAQP1 or at position 196 in NtPIP2;1. These become protonated under acidic conditions, and together with phosphorylation of the serine residues, lead to channel closure (5).
FIGURE 1.
Aquaporin topology. Conserved key residues for a postulated gating mechanism of spinach SoPIP2;1, tobacco NtPIP2;1 and NtAQP1 in loop B, loop D, and at the C terminus are depicted. The circled S indicates conserved serine; the circled H indicates conserved histidine.
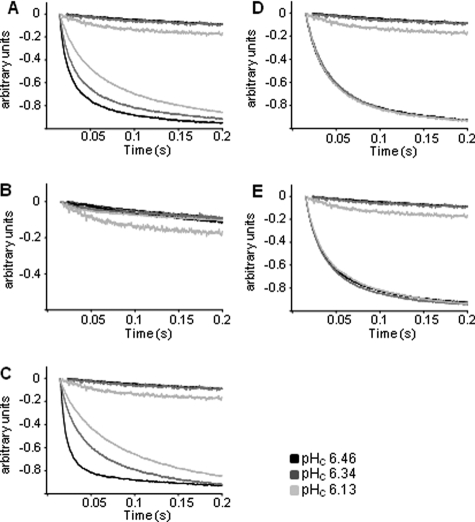
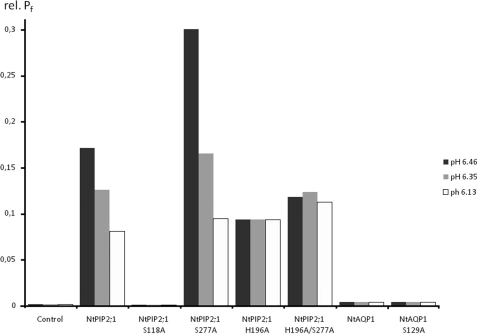
For Functional Analysis and Inspection of Functional Changes after Intracellular Acidification, the Respective Aquaporins Were Expressed in Yeast and Assayed in a Stopped Flow Spectrophotometer—The intracellular pH of yeast clones was determined according to Slavik et al. (10). A stepwise change in external pH induced an intracellular pH decrease from 6.46 to 6.34 and to 6.13. NtAQP1 and NtPIP2;1 water transport activity as well as that of the individual mutants was determined under these conditions. NtPIP2;1 shows 88% similarity to SoPIP2;1, the aquaporin on which the gating model resides. Accordingly, it has all the required structural features of the gating mechanism. In fact, the data obtained in the yeast system clearly indicate for a pH sensitivity of NtPIP2;1-facilitated water transport (Figs. 2A and 3). The overall water permeability of the yeast cellular membrane was reduced roughly by 2/3 from 0.17 to 0.08 cm/s (Fig. 3, Table 1). However, a complete closure of all expressed NtPIP2;1 aquaporins would reveal values similar to the non-induced controls, which were about 250 times lower. Thus, the pH-dependent regulation mechanism causes just a slight reduction of the conductivity and could not be relevant for a complete and effective closure of all channel molecules as would be anticipated from the proposed gating mechanism. Replacement of the histidine at position 196 by an alanine eliminated the pH sensitivity of the water transport and left the transport rates around 0.09 cm/s under all experimental conditions (Fig. 2D, Table 1). Values below those obtained for the unaltered NtPIP2;1 under acidic conditions were not obtained. Since it was suggested that phosphorylation at serines 118 and 277 is in favor of the open state, the water permeability of yeasts expressing aquaporins with serine-to-alanine replacement were analyzed (Figs. 2 and 3). A respective mutation at serine 118 reduces the water permeability to that of mock controls, irrespective of pH (Fig. 2B). This and the fact that the mutated protein was inserted into the plasma membrane indicated that the S118A turns the protein into a non-functional aquaporin. S277A, representing a mutation of one of the key phosphorylated residues, should remove the pH regulation phenomena. However, it increased the water conductivity to about 0.3 cm/s at pH 6.46 or to 0.16 at pH 6.35. The Pf value under more alkaline pH conditions was not affected by the mutation and appeared in a range similar to the unaltered protein (Fig. 2C, Table 1). Replacement of the histidine residue by alanine in this mutant caused the loss of pH sensitivity, although on a slightly higher level as the replacement mutation H196A with a serine at position 277 (Fig. 2E). The model would predict the aquaporin to be complete open, and Pf values like those under more alkaline conditions would be expected. The analysis of NtAQP1 revealed that water conductivity was insensitive to pH, although one of the relevant serines as well as the histidine residue was present (Fig. 3, Table 1). According to the model, the protonated histidine at position 207 should corroborate aquaporin closure together with the phosphorylated serine at 129. A deletion of this single important serine renders this aquaporin almost impermeable for water in the yeast expression system. Since NtAQP1 induces only low water permeability throughout the experiments, it remains questionable whether conclusions about a regulative mechanism could be drawn.
FIGURE 2.
Effects of decreased cytosolic pH on water permeability of intact yeast protoplasts expressing native or mutant NtPIP2;1. Kinetics of protoplast swelling was recorded as time courses of decreased scattered light intensity (arbitrary units) in a stopped flow spectrophotometer. Yeast protoplasts expressing no extra aquaporin exhibit very low water permeability rates (upper three curves in a chart), whereas those expressing NtPIP2;1 completed swelling in 0.2 s following osmotic shift. A change of cytosolic pH was achieved by incubation of yeast spheroblasts expressing unaltered NtPIP2;1 (A) or mutant NtPIP2;1-S118A (B), NtPIP2;1-S277A (C), NtPIP2;1-H196A (D), or NtPIP2;1-H196A/S277A NtPIP2;1 (E) in buffers at pH 7.5 and pH 5, respectively, and an additional acid load treatment with 50 mm NaAc at pH 5. (n = 30 ± S.E.).
FIGURE 3.
Relative Pf (rel. Pf) values of yeast protoplasts expressing NtPIP2;1 or a mutated form of the protein. Permeability measurements at cytosolic pH of 6.46 are indicated by black bars; those performed at cytosolic pH 6.35 and 6.13 are represented by gray and light gray bars, respectively. (n = 30).
TABLE 1.
Relative Pf values and relative aquaporin plasma membrane expression rates. Pf values were calculated as described under “Experimental Procedures” and obtained in cm × s–1. Aquaporins in purified plasma membrane protein fractions were detected and quantified by dot blot and a specific antibody. The concentration of detected proteins was normalized to the total content of biotinylated proteins and the values given as relative aquaporin content. These were used as a factor to calculate the provided relative Pf values
| pH 6.46 | pH 6.35 | pH 6.13 | Relative aquaporin content | |
|---|---|---|---|---|
| Control | 0.0007 ± 0.0001 | 0.0007 ± 0 | 0.0007 ± 0 | 0.5 |
| NtPIP2;1 | 0.1708 ± 0.0028 | 0.1256 ± 0.0043 | 0.0804 ± 0.0026 | 1.99 |
| NtPIP2;1 S118A | 0.0004 ± 0 | 0.0004 ± 0 | 0.0004 ± 0 | 0.9 |
| NtPIP2;1 S277A | 0.3 ± 0.0094 | 0.1647 ± 0.0159 | 0.0941 ± 0.0082 | 1.7 |
| NtPIP2;1 H196A | 0.0930 ± 0.0019 | 0.0930 ± 0.0031 | 0.0930 ± 0.0015 | 1.78 |
| NtPIP2;1 H196A/S277A | 0.1176 ± 0.0012 | 0.1229 ± 0.0025 | 0.1122 ± 0.0045 | 1.82 |
| NtAQP1 | 0.0033 ± 0.0003 | 0.0034 ± 0.0003 | 0.0032 ± 0.0004 | 0.12 |
| NtAQP1 S129A | 0.0032 ± 0.0004 | 0.0033 ± 0.0004 | 0.0031 ± 0.0002 | 0.13 |
DISCUSSION
NtPIP2;1 shares 72 and 83% amino acid sequence identity with SoPIP2;1 or AtPIP2;2, respectively. It is the PIP2 family member equivalent from tobacco. The key amino acids of the postulated gating mechanism (serines in loop B and at the C terminus, histidine in loop D) are located at positions 118 (serine in loop B), 277 (serine at C terminus), and 196 (histidine in loop D). Due to the high sequence identity including the key elements of the gating mechanism, a similar regulation mechanism for NtPIP2;1 can be expected. In fact, it was claimed by Törnroth-Horsefield that gating is relevant for all plant aquaporins (5).
The effect of aquaporin protonation was investigated by lowering the yeast cytosolic pH prior to water permeability measurements. Yeast protoplasts expressing the wild type NtPIP2;1 showed a concomitant decrease of water permeability with changing cytosolic pH. We could also show that mutation of the highly conserved histidine at position 196 abolished pH-dependent water conductivity. Thus, our results are consistent with those obtained for AtPIP2;2 and confirm the role of the histidine in loop D as a central component of a pH-sensing mechanism. Surprisingly, acidification renders the membrane water permeability at a high level. It was reduced by just one-half of that under more alkaline conditions. In the case of an effective pH-dependent aquaporin closure by gating, Pf values similar to that of controls would be expected. These would be about 250 times lower than those of NtPIP2;1, rather than only one-half. Since the protonated histidine is in the core of the mechanism, its deletion should keep the aquaporin completely open, inducing Pf values similar to those obtained under more alkaline conditions. However, the obtained values were almost identical to those under acidic conditions, which are reflected in merely half-maximal water permeability.
These studies support the notion that the histidine in loop D modifies aquaporin conductivity depending on the internal pH. It did not, however, lead to a complete closure as originally proposed and rather appears to modify aquaporin activity to a minor extent. It is indicated that the role of this residue is different from that suggested by the gating mechanism because its deletion did not lead to a completely open channel and keeps the water permeability at a 50% level.
In the postulated pH-dependent gating, an aquaporin channel closure due to protonation also involves two highly conserved phosphorylated serine residues in loop B and at the C terminus. Correspondingly, preventing NtPIP2;1 from phosphorylation at both sites should avoid relocation of loop D and a concomitant inhibition of water conductivity. Water permeability can be expected to show maximum values irrespectively of pH. However, neither a mutation of the serine to alanine in loop B of NtPIP2;1 nor the exchange of S277A, the serine residue close to the C terminus, led to the expected changes in Pf values. Because the pH sensitivity of the water transport was also observed in cells expressing the aquaporins that lack the key serines, the results imply that modification of aquaporin conductivity due to protonation is independent of the phosphorylation state of the protein, which is inconsistent with the suggested gating mechanism as well.
NtAQP1, as a member of the PIP1 subfamily, induced only low water permeability when expressed in heterologous systems (11). Lowering the cytosolic pH or alteration of the highly conserved phosphorylation site in loop B (Ser-129) did not affect water conductivity of NtAQP1 at all. Since NtAQP1, like the other aquaporins and the corresponding mutated proteins, was clearly integrated into yeast plasma membranes (Table 1), regulation by cellular trafficking is improbable, and it remains open wheher a non-functional aquaporin was inserted into the membrane or whether another gating mechanism rendered the aquaporin in a closed state.
In summary, the data clearly support the role of histidine at 196 as a component of pH-dependent modification of aquaporin-facilitated water transport. The gating mechanism prediction of the combined effects from phosphorylation at conserved serines and histidine protonation were not supported by the functional analysis. Removal of serine 277 did not, as predicted, cause closure of the aquaporin and is consequently not crucial in this context. Thus, it is evident that NtPIP2;1 and possibly also NtAQP1, both representing major plant aquaporins in tobacco, do not fulfill the requirements for the pH-dependent gating as predicted.
Acknowledgments
We are indebted to W. Kukulski and A. Engel (University of Basel, Swiss) for help in establishing the biotinylation procedure.
The work was supported by a grant from the Deutsche Forschungsgemeinschaft. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IB, incubation buffer; Mes, 4-morpholineethanesulfonic acid.
References
- 1.Kamaluddin, M., and Zwiazek, J. J. (2004) Tree Physiol. 24 1173–1180 [DOI] [PubMed] [Google Scholar]
- 2.Zhang, W. H., and Tyerman, S. D. (1999) Plant Physiol. 120 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerbeau, P., Amodeo, G., Henzler, T., Santoni, V., Ripoche, P., and Maurel, C. (2002) Plant J. 30 71–81 [DOI] [PubMed] [Google Scholar]
- 4.Tournaire-Roux, C., Sutka, M., Javot, H., Gout, E., Gerbeau, P., Luu, D. T., Bligny, R., and Maurel, C. (2003) Nature 425 393–397 [DOI] [PubMed] [Google Scholar]
- 5.Tornroth-Horsefield, S., Wang, Y., Hedfalk, K., Johanson, U., Karlsson, M., Tajkhorshid, E., Neutze, R., and Kjellbom, P. (2006) Nature 439 688–694 [DOI] [PubMed] [Google Scholar]
- 6.Nakamoto, R. K., Rao, R., and Slayman, C. W. (1991) J. Biol. Chem. 266 7940–7949 [PubMed] [Google Scholar]
- 7.Sanford, J. C., Smith, F. D., and Russell, J. A. (1993) Methods Enzymol. 217 483–509 [DOI] [PubMed] [Google Scholar]
- 8.Bertl, A., and Kaldenhoff, R. (2007) FEBS Lett. 581 5413–5417 [DOI] [PubMed] [Google Scholar]
- 9.van Heeswijk, M. P., and van Os, C. H. (1986) J Membr. Biol. 92 183–193 [DOI] [PubMed] [Google Scholar]
- 10.Slavik, J. (1982) FEBS Lett. 140 22–26 [DOI] [PubMed] [Google Scholar]
- 11.Biela, A., Grote, K., Otto, B., Hoth, S., Hedrich, R., and Kaldenhoff, R. (1999) Plant J. 18 565–570 [DOI] [PubMed] [Google Scholar]