Abstract
Retinoic acid (RA) is one of the major components of vitamin A. In the present study, we found that retinoic acid activated AMP-activated protein kinase (AMPK). RA induced Rac1-GTP formation and phosphorylation of its downstream target, p21-activated kinase (PAK), whereas the inhibition of AMPK blocked RA-induced Rac1 activation. Moreover, cofilin, an actin polymerization regulator, was activated when incubated with RA. We then showed that inhibition of AMPK by compound C, a selective inhibitor of AMPK, or small interfering RNA of AMPK α1 blocked RA-induced cofilin phosphorylation. Additionally, we found that retinoic acid-stimulated glucose uptake in differentiated C2C12 myoblast cells and activated p38 mitogen-activated protein kinase (MAPK). Finally, the inhibition of AMPK and p38 MAPK blocked retinoic acid-induced glucose uptake. In summary, our results suggest that retinoic acid may have cytoskeletal roles in skeletal muscle cells via stimulation of the AMPK-Rac1-PAK-cofillin pathway and may also have beneficial roles in glucose metabolism via stimulation of the AMPK-p38 MAPK pathway.
Retinoids are important regulators of differentiation and cell proliferation. Induction of differentiation by retinoic acid has been observed in various cell systems, such as endothelial, neuronal, and lung cancers (1). Retinoic acid has been shown to inhibit the growth of breast cancer cells and to reduce the number of tumors in animal models (2, 3). The anti-tumor potential of retinoids has been demonstrated by their ability to inhibit the growth of several human cancers, including colon cancer, prostate cancer, and melanoma (4, 5). Retinoic acid mediates its effects by binding to its receptors, retinoid acid receptor, or retinoid X receptor, followed by heterodimerization of the receptors and their recognition of binding to retinoid acid receptor element-containing promoters.
AMP-activated protein kinase (AMPK)2 is a phylogenetically conserved intracellular energy sensor that plays a central role in the regulation of glucose and lipid metabolism. AMPK, a heterotrimeric complex comprised of a catalytic subunit and two regulatory subunits, is activated when cellular energy is depleted (6). Upon activation by allosteric binding of AMP or phosphorylation at Thr172 of the catalytic subunit by AMPK kinase, AMPK accelerates ATP-generating catabolic pathways, including glucose and fatty acid oxidation (7–9) while simultaneously reducing ATP-consuming anabolic pathways including cholesterol, fatty acid, and triacylglycerol synthesis (10). In addition to its roles in energy homeostasis, AMPK also has been shown to regulate the endothelial nitric-oxide synthase pathway through Rac1 (11). The involvement of interaction of Rac1 with AMPK has been implicated in many of the biological effects of AMPK in cytoskeletal remodeling.
Small GTPases of the Rho family have diverse effects on cellular structure and function. Depending on the cell type, specific Rho GTPases induce particular surface protrusions generated by actin-remodeling reactions that change cell shapes and influence cell adhesion and locomotion (12, 13). Filopodia and lamellipodia are formed by the polymerization and extension of actin filaments toward the cell membrane. This polymerization at the barbed end of the filament is balanced by depolymerization at the pointed end, recycling the actin in a “treadmilling” process (14). The dynamic reorganization of actin in the cytoskeleton drives processes that include changes in cell morphology, cell migration, and phagocytosis (15). Cellular actin dynamics are regulated by complex mechanisms involving several actin-binding proteins in a spatially and temporally regulated and tissue-specific manner. The central machinery of rapid actin turnover requires actin nucleation, filament disassembly, and capping barbed ends to limit the number of elongation sites (16–19). It is therefore possible that AMPK regulates cytoskeletal rearrangement by regulating Rac1.
In this study, we investigated the effects of retinoic acid on AMPK and glucose uptake in skeletal muscle cells to gain an understanding of its cytoskeletal and metabolic roles. We found that retinoic acid activates the AMPK-Rac1-cofilin pathway in muscle cells, and further demonstrated that the activities of AMPK and p38 MAPK are involved in retinoic acid-induced glucose uptake. These findings provide novel insights into the manner in which retinoic acid contributes to the cytoskeletal and metabolic functions in skeletal muscle cells.
MATERIALS AND METHODS
Reagents—Anti-Phospho-ACC (Ser79), anti-phospho-AMPK (Thr172), anti-phospho-p38 MAPK, anti-phospho-PAK, anti-phospho-cofilin, anti-AMPK, anti-p38 MAPK, and anti-cofilin and anti-PAK antibodies were purchased from Cell Signaling Technology (New England Biolabs, Beverly, MA). Horseradish peroxidase-conjugated secondary antibodies were obtained from Kirkegaard and Perry Lab (Gaithersburg, MD). Glyceraldehyde-3-phosphate dehydrogenase antibodies were purchased from Sigma. Retinoic acid, NSC23766 (Rac inhibitor), SB 203580 (p38 MAPK inhibitor), insulin, compound C, and AICAR (5-aminoimidazole-4-carboxy-amide-1-d-ribofuranoside) were obtained from Calbiochem.
Cell Culture—Mouse myoblast C2C12 cell were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics at 37 °C in an incubator with 5% CO2. Cells were grown in culture medium consisting of 500 μl of Dulbecco's modified Eagle's medium (Invitrogen GIBCO), containing 0.584 g/liter of l-glutamate and 4.5 g/liter of glucose, mixed with 500 ml of F-12 medium containing 0.146 g/liter of l-glutamate, 1.8 g/liter of glucose, 100 μg/ml of gentamicin, 2.5 g/liter of sodium carbonate, and 10% heat-inactivated fetal bovine serum.
Immunoblot Analysis—Cells were grown on 24-well plates and serum-starved for 36 h prior to treatment with the indicated agents. Following treatment of cells, the media was aspirated and the cells were washed twice in ice-cold phosphate-buffered saline and lysed in 100 μl of lysis buffer. The samples were then briefly sonicated, heated for 5 min at 95 °C, and centrifuged for 5 min. The supernatants were electrophoresed on SDS-PAGE (8%) gels, and transferred to polyvinylidene difluoride membranes. The blots were incubated overnight at room temperature with primary antibodies and then washed six times in Tris-buffered saline with 0.1% Tween 20 prior to 1 h of incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature. The blots were then visualized via ECL. In some cases, the blots were stripped and reprobed using other antibodies.
Silencing AMPKα1—Mouse myoblast C2C12 cells were seeded in 6-well plates and allowed to grow to 70% confluence for 24 h. Transient transfections were performed with transfection reagent (Lipofectamine 2000; Invitrogen) according to the manufacturer's protocol. Briefly, both AMPKα1 (Dharmacon; NM_001013367) and non-targeted control siRNAs were designed. 5 μl of siRNA and 5 μl of transfection reagent (Lipofectamine 2000) were each diluted first with 95 μl of reduced serum media (Opti-MEM; Invitrogen) and then mixed. The mixtures were allowed to incubate for 30 min at room temperature and then were added by drop to each culture well containing 800 μl of reduced serum media (Opti-MEM; final siRNA concentration, 100 nm). Four hours after transfection, the medium was changed with fresh complete medium. Cells were cultivated for 24 h and were lysed, and the expression of AMPKα1 protein was assayed with Western blotting.
GTP-Rac Assay—The p21-binding domain (PBD) of PAK fused to glutathione S-transferase (GST) was expressed from pGEX-PBD. Recombinant glutathione S-transferase-PBD was purified with glutathione-Sepharose 4B beads. Immediately after treatment, cells were lysed in lysis buffer, and 800 μl of total lysate was incubated with glutathione S-transferase-PBD beads at 4 °C for 4 h. Beads were collected by centrifugation and were washed three times with washing buffer (25 mm Tris·HCl (pH 7.6), 1 mm dithiothreitol, 30 mm MgCl2, 40 mm NaCl, 1% (v/v) Nonidet P-40). Proteins were eluted by boiling beads in 2× sample buffer for 5 min, separated on a 10% SDS-polyacrylamide gel, and blotted with antibody to Rac1.
2-Deoxyglucose Uptake—The uptake of 2-deoxyglucose by mouse myoblast C2C12 cells was evaluated. In brief, cells were washed twice in phosphate-buffered saline containing 2.5 mm MgCl2, 1 mm CaCl2, and 20 mm HEPES with a pH 7.4, and then incubated with the test compounds in the same buffer at 37 °C. The transport assay was initiated via the addition of 2-[U-14C]deoxy-d-glucose (25 mm; 10 mCi/ml) to each of the wells, for 10 min at 37 °C. The assay was terminated by the addition and subsequent washing of the cells with ice-cold phosphate-buffered saline. The cells were lysed in 10% SDS or 50 mm NaOH. Radioactivity was evaluated via scintillation counting of the lysates extracted in SDS, whereas total protein contents were determined in lysates extracted in NaOH via the Bradford procedure (Bio-Rad). The glucose uptake values were corrected for non-carrier-mediated transport by measuring glucose uptake in the presence of 10 mm cytochalasin B.
Data Analysis—Data are expressed as mean ± S.E. Statistical analyses were conducted using SigmaStat (SPSS Inc., Chicago, IL). p values of <0.05 were considered statistically significant.
RESULTS
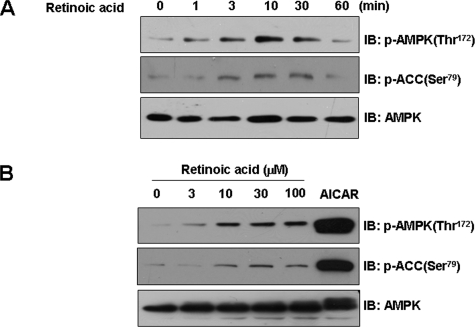
Retinoic Acid Activates AMPK-ACC Pathways in Mouse Myoblast C2C12 Cells—To characterize the molecular mechanisms of retinoic acid, we evaluated its effects on the activity of AMPK, a key metabolic sensor kinase. The administration of retinoic acid induced a time-dependent increase in AMPK phosphorylation in C2C12 cells (Fig. 1A). The phosphorylation level of Thr172, which is in the active site of AMPK-α subunit and is essential for enzyme activity, reached a maximum level at 10 min after treatment and then decreased to basal levels at 1 h. Consistent with the increase in AMPK activity, the phosphorylation of ACC-Ser79, the best characterized phosphorylation site by AMPK, increased after retinoic acid administration. In addition, retinoic acid stimulated AMPK activation in a dose-dependent manner (Fig. 1B). AICAR, a known AMPK activator, was employed as a positive control. These results demonstrate that retinoic acid has AMPK stimulatory roles in skeletal muscle cells.
FIGURE 1.
Retinoic acid activates AMPK-ACC pathways in C2C12 cells. A, time-dependent phosphorylation of AMPK by retinoic acid. C2C12 cells were stimulated for the indicated times with 10 μm retinoic acid. The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-ACC (Ser79) and anti-phospho-AMPK (Thr72) antibodies. Blotting with anti-AMPK antibody was conducted as a protein loading control. B, dose-dependent phosphorylation of AMPK by retinoic acid. C2C12 cells were stimulated with the indicated doses of either retinoic acid or 1 mm AICAR for 1 h. The cell lysates (20μg) were analyzed via Western blotting for anti-phospho-ACC (Ser79) and phospho-AMPK (Thr72) antibodies. Blotting with anti-AMPK antibody was conducted as a protein loading control. These results represent one of three independent experiments. IB, immunoblot.
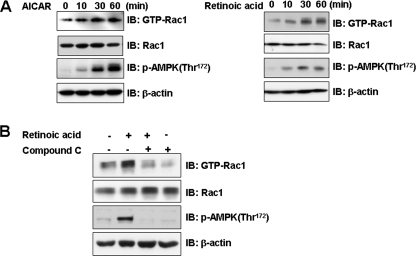
Retinoic Acid Activates Rac1—Rac1 is a serine/threonine protein kinase that leads to a translocation of the glucose transporter (GLUT4) to the plasma membrane via activation of signaling cascades (20). In an effort to understand the signal pathways involved in retinoic acid-mediated cytoskeletal rearrangement, we investigated the effects of either retinoic acid or AICAR on Rac1. Both retinoic acid and AICAR were observed to activate Rac1 in a time-dependent manner (Fig. 2A). To determine whether AMPK activity is involved in the effect of retinoic acid, we investigated Rac1 activation following treatment with compound C, an AMPK inhibitor. The retinoic acid-mediated activation of Rac1 was inhibited in cells pretreated with compound C (Fig. 2B). Together, these results indicate that Rac1 operates in the retinoic acid-mediated signaling pathway in an AMPK-dependent manner.
FIGURE 2.
Retinoic acid activates Rac1. A, time-dependent Rac1 activation by retinoic acid and AICAR. C2C12 cells were stimulated for the indicated times with either 10 μm retinoic acid or 1 mm AICAR. The cell lysates were affinity precipitated with GTP-PBD bound to glutathione-agarose beads. Precipitated GTP-Rac1 was detected by immunoblotting with anti-Rac1 antibody. Blotting with anti-phospho-AMPK and Rac1 antibodies was conducted as an experimental control. Blotting with anti-β-actin antibody was conducted as a protein loading control. B, AMPK-dependent Rac1 activation by retinoic acid. C2C12 cells were stimulated with retinoic acid in the presence of compound C (10 μm), a selective inhibitor of AMPK. The cell lysates (20 μg) were affinity precipitated with GTP-PBD bound to glutathione-agarose beads. Precipitated GTP-Rac1 was detected by immunoblotting (IB) with anti-Rac1 antibody. Blotting with anti-phospho-AMPK and Rac1 antibodies was conducted as an experimental control. Blotting with anti-β-actin antibody was conducted as a protein loading control. These results represent one of three independent experiments.
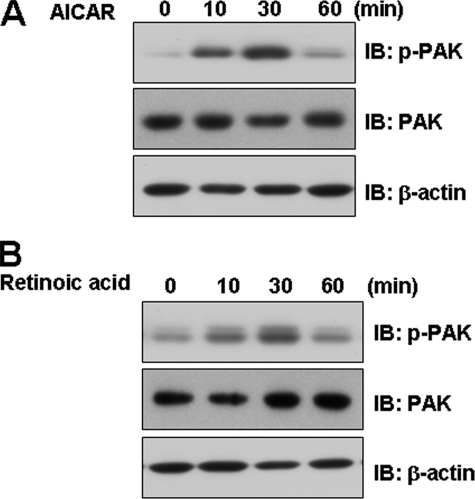
PAK Is Involved in Retinoic Acid-mediated Signaling—To corroborate the roles of retinoic acid in the Rac1-mediated signaling pathway, we assessed the effects of AMPK on molecules downstream of Rac1. To this end, C2C12 cells were treated with AICAR before measuring phosphorylation of PAK. AICAR increased PAK phosphorylation (Fig. 3A), indicating that AMPK may be involved in Rac1-mediated signaling. Furthermore, PAK phosphorylation was clearly observed in cells treated with retinoic acid (Fig. 3B). Together, our findings indicate that retinoic acid increases PAK-mediated signaling by acting on AMPK.
FIGURE 3.
Retinoic acid activates the PAK pathways in C2C12 cells. C2C12 cells were stimulated for the indicated times with either 1 mm AICAR (A) or 10 μm retinoic acid (B). The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-PAK antibody. Blotting with anti-PAK and β-actin antibodies were conducted as protein loading control. These results represent one of three independent experiments. IB, immunoblot.
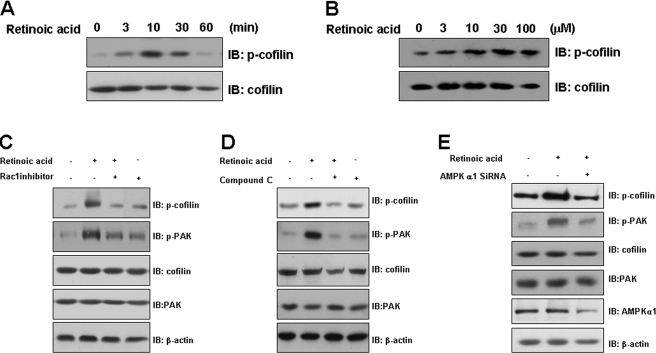
Cofilin Is Activated by a Retinoic Acid-mediated Pathway—To verify the roles of retinoic acid in the PAK-mediated signaling pathway, we assessed the effects of retinoic acid on cofilin activation. The phosphorylation of cofilin was increased in C2C12 cells that had been incubated with retinoic acid in a time- and dose-dependent manner (Figs. 4, A and B). Furthermore, the retinoic acid-mediated phosphorylation of cofilin and PAK was clearly suppressed in cells pretreated with either Rac1 inhibitor or AMPK inhibitor (Fig. 4, C and D). Next, to better understand the signaling mechanisms of AMPK regulation on the retinoic acid-induced pathway, we tested whether AMPK regulates retinoic acid-induced phosphorylation of Rac1 downstream molecules. Knockdown of AMPKα1 by siRNA transfection significantly reduced retinoic acid-induced phosphorylation of cofilin and PAK (Fig. 4E). This result suggests that AMPK mediates retinoic acid-induced Rac1 activation.
FIGURE 4.
Retinoic acid activates cofilin. A, time-dependent phosphorylation of cofilin by retinoic acid. C2C12 cells were stimulated for the indicated times with 10 μm retinoic acid. The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-cofilin antibody. Blotting with anti-cofilin antibody was conducted as a protein loading control. B, dose-dependent phosphorylation of cofilin by retinoic acid. C2C12 cells were stimulated with the indicated doses of retinoic acid for 30 min. The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-cofilin antibody. Blotting with anti-cofilin antibody was conducted as a protein loading control. C, Rac1-dependent cofilin activation by retinoic acid. C2C12 cells were stimulated with retinoic acid in the presence of NSC23766, a Rac1 inhibitor. The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-cofilin and anti-phospho-PAK antibodies. Blotting with anti-cofilin, anti-PAK, and β-actin antibodies were conducted as protein loading control. D, AMPK-dependent Rac1 activation by retinoic acid. C2C12 cells were stimulated with retinoic acid in the presence of compound C. The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-cofilin and anti-phospho-PAK antibodies. Blotting with anti-cofilin, anti-PAK, and β-actin antibodies were conducted as protein loading control. E, AMPK-dependent Rac1 activation by retinoic acid. C2C12 cells were transfected with AMPKα1 siRNA for 24 h and stimulated with retinoic acid for 10 min. Cell lysates (20 μg) were analyzed by immunoblotting (IB) using antibodies specific to phospho-cofilin, phospho-PAK, PAK, cofilin, AMPKα1, and β-actin. These results represent one of three independent experiments.
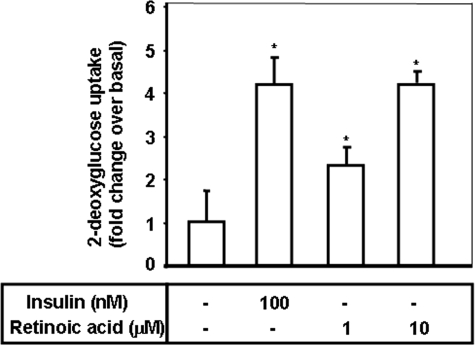
Retinoic Acid Stimulates Glucose Uptake in Differentiated Mouse Myoblast C2C12 Cells—To determine the role of retinoic acid in glucose metabolism, we examined the dose-dependent effects of retinoic acid on glucose uptake in differentiated myoblast C2C12 cells. We observed that retinoic acid initiated an increase in 2-deoxyglucose uptake in differentiated C2C12 myoblast cells when used at a concentration of 1 μm, with a maximal increase occurring at 10 μm (Fig. 5). Retinoic acid did not influence the viability of C2C12 cells at 10 μm as assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide staining (data not shown). Insulin, used at 100 nm, caused an increase in glucose uptake, and was utilized as a positive control. The effects of 10 μm retinoic acid on 2-deoxyglucose uptake were comparable with those of insulin, suggesting that retinoic acid may have metabolic roles in skeletal muscle cells. Taken together, our results indicate that retinoic acid can act in the metabolic pathways in skeletal muscle cells.
FIGURE 5.
Retinoic acid stimulates glucose uptake in differentiated mouse myoblast C2C12 cells. Differentiated C2C12 cells were incubated in 60-mm dishes for 1 h with either retinoic acid at 1 or 10μm, or insulin at 100 nm and then assayed for 2-deoxyglucose uptake, as described under “Materials and Methods.” *, p < 0.05, as compared with the control values (one-way analysis of variance and Holm-Sidak comparisons). Each value is expressed as the mean ± S.D. of four determinations.
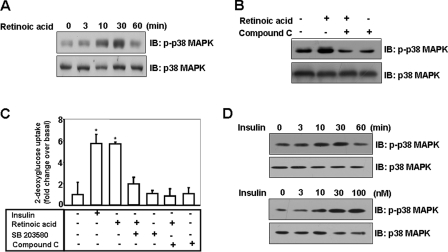
Retinoic Acid Activates the p38 MAPK Pathway—In an effort to understand the signaling pathways involved in retinoic acid-mediated glucose uptake, we investigated the effects of retinoic acid on p38 MAPK. Retinoic acid was observed to activate p38 MAPK in a time-dependent manner (Fig. 6A). The phosphorylation of p38 MAPK reached a maximum level at 30 min and then returned to basal level at 60 min. To determine whether AMPK activity was involved in this effect of retinoic acid, we investigated p38 MAPK activation following AMPK inhibition by compound C. The retinoic acid-mediated activation of p38 MAPK was inhibited in cells pretreated with 10 μm compound C (Fig. 6B). Together, these results indicate that p38 MAPK operates in the retinoic acid-mediated signaling pathway in an AMPK-dependent manner. To corroborate the roles of p38 MAPK and AMPK in the retinoic acid-mediated signaling pathway, we assessed the effects of inhibitors of these kinases on glucose uptake. C2C12 cells were pretreated with either SB203580, a p38 MAPK inhibitor, or compound C in the presence of retinoic acid. The effect of retinoic acid on glucose uptake was attenuated in C2C12 cells that had been incubated with these inhibitors (Fig. 6C), indicating that both of these pathways are involved in retinoic acid-induced glucose uptake. Finally, to address to role of p38 MAPK, we examined the effect of insulin on p38 MAPK. The phosphorylation of p38 MAPK was increased in a time- and dose-dependent manner upon insulin treatment (Fig. 6D). Together, our findings show that retinoic acid stimulates glucose uptake via activation of p38 MAPK.
FIGURE 6.
Retinoic acid activates p38 MAPK pathway. A, time-dependent phosphorylation of p38 MAPK by retinoic acid. C2C12 cells were stimulated for the indicated times with 10 μm retinoic acid. The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-p38 MAPK antibody. Blotting with anti-p38 MAPK antibody was conducted as a protein loading control. B, AMPK-dependent phosphorylation of p38 MAPK by retinoic acid. C2C12 cells were stimulated for 30 min with 10 μm retinoic acid in the presence of compound C. The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-p38 MAPK antibody. Blotting with anti-p38 MAPK antibody was conducted as a protein loading control. C, p38 MAPK/AMPK dependence of retinoic acid-induced glucose uptake. Differentiated C2C12 cells were incubated in 60-mm dishes for 1 h with the indicated experimental conditions, and then assayed for 2-deoxyglucose uptake, as described under “Materials and Methods.” *, p < 0.05, as compared with the insulin alone sample values (one-way analysis of variance and Holm-Sidak comparisons). Each value is expressed as the mean ± S.D. of four determinations. D, time and dose-dependent phosphorylation of p38 MAPK by insulin. C2C12 cells were stimulated for the indicated times with 100 nm insulin or the indicated doses for 30 min. The cell lysates (20 μg) were analyzed via Western blotting for anti-phospho-p38 MAPK antibody. Blotting with anti-p38 MAPK antibody was conducted as a protein loading control. These results represent one of three independent experiments. IB, immunoblot.
DISCUSSION
The principal finding of this study was that retinoic acid is involved in cytoskeletal rearrangement and metabolic function in skeletal muscle cells. Specifically, we demonstrated that AMPK is instrumental in retinoic acid-mediated signaling in these cells.
The primary assertion of this study is that AMPK mediates some of the metabolic and cytoskeletal effects of retinoic acid. The role of retinoic acid has previously been evaluated in conjunction with differentiation (1) and anti-tumor functions (2–5). The cytoskeleton-altering properties of retinoic acid appear to be responsible for its role in differentiation, and may also contribute to its observed anti-tumor effects. The contribution of retinoic acid to differentiation has raised questions as to which of the activities of retinoic acid may be relevant to its cytoskeletal role. The mechanisms of retinoic acid-induced cytoskeletal changes have previously been suggested (21–24). In the present study, we have established that retinoic acid induces cytoskeletal rearrangement through the AMPK pathway. Additionally, we demonstrated that retinoic acid activates the uptake of glucose through the AMPK pathway. The relationship of retinoic acid with glucose regulation has been suggested (25, 26). Collectively, our results indicate that AMPK may play crucial roles in retinoic acid-mediated cytoskeletal changes and metabolic functions.
The objective of the present study was to ascertain whether or not the cytoskeleton of skeletal muscle cells is directly regulated by retinoic acid and, if so, to determine which molecules are involved in this process. Our data have revealed a novel role for Rac1 downstream of AMPK activation by retinoic acid. Our identification of an AMPK-Rac1-PAK-cofilin axis in retinoic acid-treated skeletal muscle cells led us to hypothesize that Rac1 might play a role in the AMPK-mediated modulation of actin cytoskeleton remodeling. To our knowledge, this is the first report of a link between AMPK and Rac1 in skeletal muscle cells. Indeed, it is tempting to speculate that AMPK-mediated regulation of Rac1 plays a role in cellular differentiation, because both retinoic acid and Rac1 have been implicated in differentiation and cytoskeletal rearrangement. In addition to its cytoskeletal roles, we further demonstrated that retinoic acid plays a metabolic role by activating glucose uptake. Overall, although the mechanism by which AMPK influences skeletal muscle cell differentiation remains unknown, the findings in this report suggest that retinoic acid may induce rearrangement of the cytoskeleton through the AMPK pathway as part of the process of retinoic acid-mediated differentiation.
Various reports have suggested the importance of calcium signaling in myogenesis (27, 28). Calcium signaling has the potential to enable activity of MyoD heterodimers with E-protein in myogenesis through calcium/calmodulin inhibition of DNA binding (29). Retinoids mediate a wide spectrum of calcium regulating activities through calcium-binding protein (30), intracellular calcium fluctuation, and store-operated calcium influx (31, 32). Because retinoic acid has no capability to induce calcium in the present study, calcium might not play a role in retinoic acid-mediated glucose uptake and Rac1-mediated signaling.
In conclusion, we have determined that retinoic acid activates AMPK in skeletal muscle cells and stimulates glucose uptake as well. We further demonstrated that the AMPK pathway exerts a profound influence on retinoic acid-mediated cytoskeletal rearrangement. Future studies should focus on elucidating the relationship of AMPK to glucose uptake within the context of a retinoic acid-mediated cytoskeletal signaling pathway. We are currently investigating this relationship.
This work was supported Korea University College of Medicine and Korea Science and Engineering Foundation Grant KOSEF, R01-2008-000-11180-0. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: AMPK, AMP-activated protein kinase; ACC, acetyl-CoA carboxylase; AICAR, 5-aminoimidazole-4-carboxy-amide-1-d-ribofuranoside; MAPK, mitogen-activated protein kinase; PAK, p21-activated kinase; siRNA, small interfering RNA; PBD, p21-binding domain.
References
- 1.Hong, W. K., Benner, S. E., and Lippman, S. M. (1994) Leukemia 8 Suppl. 3, S33–S37 [PubMed] [Google Scholar]
- 2.Fitzgerald, P., Teng, M., Chandraratna, R. A., Heyman, R. A., and Allegretto, E. A. (1997) Cancer Res. 57 2642–2650 [PubMed] [Google Scholar]
- 3.Sheikh, M. S., Shao, Z. M., Li, X. S., Dawson, M., Jetten, A. M., Wu, S., Conley, B. A., Garcia, M., Rochefort, H., and Fontana, J. A. (1994) J. Biol. Chem. 269 21440–21447 [PubMed] [Google Scholar]
- 4.O'Dwyer, P. J., Ravikumar, T. S., McCabe, D. P., and Steele, G., Jr. (1987) J. Surg. Res. 43 550–557 [DOI] [PubMed] [Google Scholar]
- 5.Blutt, S. E., Allegretto, E. A., Pike, J. W., and Weigel, N. L. (1997) Endocrinology 138 1491–1497 [DOI] [PubMed] [Google Scholar]
- 6.Hardie, D. G., and Carling, D. (1997) Eur. J. Biochem. 246 259–273 [DOI] [PubMed] [Google Scholar]
- 7.Makinde, A. O., Gamble, J., and Lopaschuk, G. D. (1997) Circ. Res. 80 482–489 [DOI] [PubMed] [Google Scholar]
- 8.Ai, H., Ihlemann, J., Hellsten, Y., Lauritzen, H. P., Hardie, D. G., Galbo, H., and Ploug, T. (2002) Am. J. Physiol. 282 E1291–E1300 [DOI] [PubMed] [Google Scholar]
- 9.Zong, H., Ren, J. M., Young, L. H., Pypaert, M., Mu, J., Birnbaum, M. J., and Shulman, G. I. (2002) Proc. Natl. Acad. Sci. U. S. A 99 15983–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henin, N., Vincent, M. F., Gruber, H. E., and Van den Berghe, G. (1995) FASEB J. 9 541–546 [DOI] [PubMed] [Google Scholar]
- 11.Levine, Y. C., Li, G. K., and Michel, T. (2007) J. Biol. Chem. 282 20351–20364 [DOI] [PubMed] [Google Scholar]
- 12.Nobes, C. D., and Hall, A. (1995) Biochem. Soc. Trans. 23 456–459 [DOI] [PubMed] [Google Scholar]
- 13.Gauthier-Rouviere, C., Vignal, E., Meriane, M., Roux, P., Montcourier, P., and Fort, P. (1998) Mol. Biol. Cell 9 1379–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, J., Godt, D., Gunsalus, K., Kiss, I., Goldberg, M., and Laski, F. A. (2001) Nat. Cell Biol. 3 204–209 [DOI] [PubMed] [Google Scholar]
- 15.Cowieson, N. P., King, G., Cookson, D., Ross, I., Huber, T., Hume, D. A., Kobe, B., and Martin, J. L. (2008) J. Biol. Chem. 283 16187–16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper, J. A., and Schafer, D. A. (2000) Curr. Opin. Cell Biol. 12 97–103 [DOI] [PubMed] [Google Scholar]
- 17.Carlier, M. F., Le Clainche, C., Wiesner, S., and Pantaloni, D. (2003) Bioessays 25 336–345 [DOI] [PubMed] [Google Scholar]
- 18.Pollard, T. D., and Borisy, G. G. (2003) Cell 112 453–465 [DOI] [PubMed] [Google Scholar]
- 19.Nicholson-Dykstra, S., Higgs, H. N., and Harris, E. S. (2005) Curr. Biol. 15 R346–R357 [DOI] [PubMed] [Google Scholar]
- 20.Ishikura, S., Koshkina, A., and Klip, A. (2008) Acta Physiol. (Oxf.) 192 61–74 [DOI] [PubMed] [Google Scholar]
- 21.Oreffo, R. O., Teti, A., Triffitt, J. T., Francis, M. J., Carano, A., and Zallone, A. Z. (1988) J. Bone Miner. Res. 3 203–210 [DOI] [PubMed] [Google Scholar]
- 22.Menard, C., Pupier, S., Mornet, D., Kitzmann, M., Nargeot, J., and Lory, P. (1999) J. Biol. Chem. 274 29063–29070 [DOI] [PubMed] [Google Scholar]
- 23.Zitterbart, K., and Veselska, R. (2001) Neoplasma 48 456–461 [PubMed] [Google Scholar]
- 24.Pang, J., Kao, Y-L., Joshi, S., Jeetendran, S., DiPette, D., and Singh, U. S. (2005) J. Neuorochem. 93 571–583 [DOI] [PubMed] [Google Scholar]
- 25.Hong, S.-E., Ahn, I.-S., Jung, H.-S., Rayner, D. V., and Do, M.-S. (2004) J. Med. Food 7 320–326 [DOI] [PubMed] [Google Scholar]
- 26.Montessuit, C., Papageorgiou, I., and Lerch, R. (2008) Endocrinology 149 1064–1074 [DOI] [PubMed] [Google Scholar]
- 27.Delling, U., Tureckova, J., Lim, H. W., De Windt, L. J., Rotwein, P., and Molkentin, J. D. (2000) Mol. Cell. Biol. 20 6600–6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinsey, T. A., Zhang, C. L., and Olson, E. N. (2002) Trends Biochem. Sci. 27 40–47 [DOI] [PubMed] [Google Scholar]
- 29.Corneliussen, B., Holm, M., Watersson, Y., Onions, J., Hallberg, B., Thornell, A., and Grundstrom, T. (1994) Nature 368 760–764 [DOI] [PubMed] [Google Scholar]
- 30.Shyu, R. Y., Huang, S. L., and Jiang, S. Y. (2003) J. Biomed. Sci. 10 313–319 [DOI] [PubMed] [Google Scholar]
- 31.Varani, J., Burmeister, B., Perone, P., Bleavins, M., and Johnson, K. J. (1995) Am. J. Pathol. 147 718–727 [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, W., Meng, H., Li, Z. H., Shu, Z., Ma, X., and Zhang, B. X. (2007) Am. J. Physiol. 292 F1054–F1064 [DOI] [PubMed] [Google Scholar]