Abstract
Skeletal muscle satellite cell-derived myoblasts are mainly responsible for postnatal muscle growth and injury-induced regeneration. However, the cellular signaling pathways that control proliferation and differentiation of myoblasts remain poorly defined. Recently, we found that JAK1/STAT1/STAT3 not only participate in myoblast proliferation but also actively prevent them from premature differentiation. Unexpectedly, we found that a related pathway consisting of JAK2, STAT2, and STAT3 is required for early myogenic differentiation. Interference of this pathway by either a small molecule inhibitor or small interfering RNA inhibits myogenic differentiation. Consistently, all three molecules are activated upon differentiation. The pro-differentiation effect of JAK2/STAT2/STAT3 is partially mediated by MyoD and MEF2. Interestingly, the expression of the IGF2 gene and the HGF gene is also regulated by JAK2/STAT2/STAT3, suggesting that this pathway could also promote differentiation by regulating signaling molecules known to be involved in myogenic differentiation. In summary, our current study reveals a novel role for the JAK2/STAT2/STAT3 pathway in myogenic differentiation.
Myogenic differentiation is critically dependent on two families of transcription factors: one is the myogenic regulatory factor (MRF)2 family, which includes Myf5, MyoD, myogenin, and MRF4 (1–3), and the other is the myocyte enhancer factor 2 (MEF2) family, which consists of MEF2A, MEF2B, MEF2C, and MEF2D (4, 5). Members of the MRF and MEF2 families can physically interact with each other to synergistically activate many muscle-specific genes (6). Among four MRFs, Myf5, MyoD, and MRF4 are thought to function to establish the myogenic fate in muscle precursor cells, whereas myogenin is mainly involved in execution of the differentiation program (7–10). When grown in culture, proliferating myoblasts do not express myogenin. Expression of myogenin signals that myoblasts have irreversibly withdrawn from the cell cycle and have initiated the differentiation program. Both MyoD and MEF2 are known to be involved in myogenin induction at the start of differentiation (11–13).
Skeletal muscle satellite cells, which are a group of quiescent cells located between basal lamina and plasma membrane of myofibers in mature muscles, are mainly responsible for postnatal muscle growth and injury-induced muscle regeneration. Upon muscle damage by trauma or genetic disorders, the quiescent satellite cells are activated to re-enter the cell cycle, actively proliferate, and eventually differentiate into multi-nucleated myotubes to repair the damaged muscles (14–17). Different molecular markers have been identified that can be used to characterize muscle satellite cells at different stages. For example, the quiescent muscle satellite cells are known to express Pax7, the activated satellite cells, and proliferating myoblasts tend to co-express Pax7 and MyoD, whereas the differentiating myocytes start to express myogenin with concomitant down-regulation of Pax7 (14, 15).
Multiple signaling pathways and growth factors have been found to play roles in myogenic differentiation. We and others previously showed that the p38 mitogen-activated protein kinase (MAPK) pathway is required for myogenic differentiation (18–20), whereas the extracellular signal-regulated kinase (ERK) pathway plays dual roles: it inhibits differentiation at the onset of differentiation and promotes myocyte fusion at the late stage of differentiation (18, 21, 22). Insulin-like growth factors (IGFs) potently promote myogenic differentiation via phosphatidylinositol 3-kinase (PI3K) and Akt (22–29), whereas hepatocyte growth factor (HGF) can stimulate myoblast proliferation and inhibit differentiation, presumably via the ERK pathway (30–33).
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway has been studied extensively in cytokine signaling during blood development and immune responses (34). The JAK family of tyrosine kinases has four members: JAK1, 2, and 3 and Tyk2, whereas the STAT family of transcription factors consists of seven members: STAT1–4, 5a, 5b, and 6. Among members of the JAK and STAT families, STAT3 was the first to be implicated in myoblast proliferation in muscle satellite cell-derived C2C12 cells (35, 36). Subsequently, active STAT3 was also found to be present in activated muscle satellite cells and proliferating myoblasts in regenerating rat muscles (37). In addition, STAT3 was also shown to physically interact with MyoD (38). Based on the effect of AG490, an inhibitor for JAK2 (39), JAK2 was proposed to be required for leukemia inhibitory factor (LIF)-induced C2C12 proliferation (36). However, this result has to be viewed with caution because of potential nonspecific effects of the drug (i.e. inhibition of cellular molecules other than JAK2). Recently, our group showed that a pathway consisting of JAK1/STAT1/STAT3 is involved in myoblast proliferation (40). Perturbation of this pathway by siRNAs up-regulates the expression levels of p21cip1 and p27kip1. Furthermore, we found that this pathway also inhibits the differentiation program by repressing the expression of MyoD and MEF2 genes, whose protein products are required for myogenin induction at the onset of differentiation (11–13, 40). Down-regulation of either JAK1 or STAT1 by siRNA accelerates myogenic differentiation in both C2C12 cells and primary myoblasts (40).
Although JAK2 is quite homologous to JAK1, unexpectedly, we found that JAK2 plays an opposite role during myogenic differentiation in comparison with JAK1. STAT2 and STAT3 appear to function downstream of JAK2 during myogenic differentiation. In addition to MyoD and MEF2, we showed that JAK2/STAT2/STAT3 also control the expression of HGF and IGF2, both of which are known to regulate the proliferation and differentiation of myoblasts. This finding provides additional mechanisms to explain why JAK2 is required for myogenic differentiation.
EXPERIMENTAL PROCEDURES
Isolation and Culturing of Primary Myoblasts—Isolation of primary myoblasts was carried out as described previously (57). Briefly, C57/B6 mice were sacrificed by cervical dislocation. Skeletal muscles from hind limbs and back were dissected out and minced with scissors. The muscles were then digested by 1 mg/ml Pronase (Calbiochem, San Diego, CA) in phosphate-buffered saline at 37 °C for 1 h to dissociate muscle satellite cells from myofibers. After digestion, myofibers were allowed to sediment, and satellite cells in the supernatant were passed through 40-μm cell sieves (BD Biosciences, Franklin Lakes, NJ). The cells were then subjected to centrifugation in a Percoll (Sigma-Aldrich) gradient. The satellite cells were collected in the interphase between 60 and 20% of Percoll and cultured on Matrigel (Clontech, Mountain View, CA)-coated plates. The cells were first grown for 24 h in the plating medium (DMEM with 10% horse serum, 0.5% chicken embryo extract, 1% penicillin, and streptomycin) to facilitate their attachment to plates, followed by growth in the satellite growth medium (DMEM with 20% fetal bovine serum, 2% chicken embryo extract, 1% penicillin, and streptomycin) to promote myoblast proliferation. The myoblasts were induced to differentiate in the satellite differentiation medium (DMEM with 5% horse serum, 1% penicillin, and streptomycin).
Cell Lines, DNA Constructs, and Reagents—C2C12 cells were maintained in the growth medium (GM, DMEM with 20% fetal bovine serum, 1% penicillin, and streptomycin). To induce differentiation, the cells were grown in DMEM containing 2% horse serum (also called differentiation medium (DM)). The cells were kept in a 37 °C incubator with 5% CO2. JAK and STAT constructs were gifts from Dr. Zilong Wen (Hong Kong University of Science & Technology). JAK1 and 2, Tyk2, and STAT1, 2, and 3 were subcloned into pcDNA3 vector with an N-terminal FLAG tag. G133-luc, MCK-luc, 4RE-luc, 3MEF2-luc, Gal4-MyoD, Gal4-MEF2A, Gal4-MEF2C, and Gal4-MEF2D were described previously (18, 24). AG490 was purchased from LC Laboratories (Woburn, MA). Recombinant mouse IGF1 was from R & D (Minneapolis, MN).
siRNA and Plasmid Transfection—For siRNA transfection, 50% confluent C2C12 cells or primary myoblasts were transfected with 100 nm siRNA using Lipofectamine 2000 (Invitrogen). The following siRNAs (top strand sequence is shown) were used: JAK2(1) (5′-gca aac cag gaa ugc uca a), JAK2(2) (5′-gga aug gcc ugc cuu aca a), JAK2-scrambled (5′-guc aag aac cgc caa gau a), STAT2(1) (5′-gga cug guu ggc cga uua a), STAT2(2) (5′-gaa gug aau gca gag cuc uug uua g), STAT2-scrambled (5′-gug acu cag ugc gaa gug u), STAT3(1) (5′-uau cau cga ccu ugu gaa a), STAT3(2) (5′-cca acg acc ugc agc aau a), STAT3-scrambled (5′-gcc uac cgc acc aag aua a), and enhanced green fluorescent protein (EGFP) (5′-gcu gac ccu gaa guu cau c). For plasmids and siRNA co-transfection, 1 μg of a plasmid was delivered into cells in each 35-mm well with 100 mM siRNA using Lipofectamine 2000.
Luciferase Assay—For luciferase assays, the cells were first transfected and incubated in GM for 24 h, followed by growth in DM for another 24 h. The cells were then harvested in the lysis buffer (50 mm Hepes, pH 7.6, 1% (v/v) Triton X-100, 150 mm NaCl, 1 mm EGTA, 1.5 mm MgCl2, 100 mm NaF, 20 mm p-nitrophenylphosphate, 20 mm β-glycerophosphate, 50 μm sodium vanadate, 2 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 0.5 μg/ml leupeptin, and 0.7 μg/ml pepstatin) at 4 °C for 10 min. Whole cell extracts (WCEs) were obtained after centrifugation followed by removal of the debris. The luciferase activity was determined in a luminometor (Berthold Technologies, Bad Wilbad, Germany) and normalized to the amount of proteins present in each sample. The protein concentration of each sample was determined by protein assay reagent from Bio-Rad.
RT-PCR—For semi-quantitative RT-PCR, total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. 1 μg of total RNA from each sample was reverse transcribed into cDNA using the ImProm-II reverse transcription system (Promega, Madison, WI). The expression levels of various genes were then detected by PCR. The following primers were used for detection of gene expression: myogenin (forward, 5′-gac tcc cca ctc ccc att cac ata; reverse, 5′-ggc ggc agc ttt aca aac aac aca), GAPDH (forward, 5′-tga tgc tgg tgc tga gta tgt cgt; reverse, 5′-tcc ttg gag gcc atg tag gcc at), MyoD (forward, 5′-agg ctc tgc tgc gcg acc; reverse, 5′-tgc agt cga tct ctc aaa gca cc); MEF2A (forward, 5′-tga cct gtc tgc cct gca; reverse, 5′-tta ggt cac cca tgt gtc), MEF2C (forward, 5′-tga cat ccg gtg cag gca c; reverse, 5′-gag tgc tag tgc aag ctc), IGF2 (forward, 5′-tgc tgc atc gct gct tac; reverse, 5′-tga ttc act gat ggt tgc tg), and HGF (forward, 5′-gac tat gaa gct tgg ctt g; reverse, 5′-ctc aca tgg tcc tga tcc).
Antibodies, Immunoblotting, Immunoprecipitation, and Immunostaining—The JAK2 and STAT2 antisera were generated by injecting rabbits with the following protein fragments: JAK2, amino acids 820–1129; STAT2, amino acids 315–568. Antibodies from serum were first affinity-purified by incubating with polyvinylidene difluoride membranes containing transferred antigens of JAK2 or STAT2 followed by washing and elusion. The antibodies were subsequently incubated with membranes transferred with the corresponding regions of JAK1 or STAT1 to eliminate those that cross-react with other family members. The sources of commercial antibodies used in this work were listed as follows: anti-phospho-tyrosine (05-321), anti-STAT1 (06-501) and anti-STAT2 (07-140) were from Millipore (Billerica, MA); anti-myogenin (SC-12732), anti-MyoD (SC-760), anti-MEF2 (SC-313), anti-β-actin (SC-8432), and anti-Gal4-DBD (SC-510) were from Santa Cruz (Santa Cruz, CA); anti-β-tubulin (T4026) and anti-Flag (F3165) were from Sigma-Aldrich; anti-myosin heavy chain (MHC) (MF20) was from Developmental Studies Hybridoma Bank (Iowa City, IA); anti-Ser(P)473-Akt (9271), anti-Thr(P)308-Akt (9275), anti-Akt (9272), anti-Thr(P)202, Tyr(P)204-ERK (9101), anti-ERK (9102), anti-STAT3 (9132), anti-Tyr(P)705-STAT3 (9131), and anti-JAK1 (3332) were from Cell Signaling Technology (Danvers, MA). Immunoblotting was carried out according to standard procedures. To perform immunostaining, primary myoblasts or cultured C2C12 cells were first fixed with 4% paraformaldehyde in phosphate-buffered saline for 10 min and then permeabilized with 0.2% Triton in phosphate-buffered saline for 10 min at room temperature, followed by incubation with primary antibodies against either myogenin (1:10) or MHC (1:10) overnight. The cells were then incubated with appropriate secondary antibodies for 1 h, followed by incubation with 4′,6′-diamino-2-phenylindole for 10 min to counterstain the nuclei. The images were taken by a CCD camera (Spot RT; Diagnostic Instruments Inc., Sterling Heights, MI) linked to an Olympus IX70 fluorescent microscope. Immunoprecipitation was generally performed by adding 1 μg of antibody to 500 μg of whole cell lysates together with 20 μl (bed volume) of protein A-Sepharose beads. The mixture was rotated for 2 h in 4 °C followed by extensive washing with the lysis buffer.
Statistics—For statistical analysis, the p value was calculated using Student's t test with p < 0.05 being considered statistically significant.
RESULTS
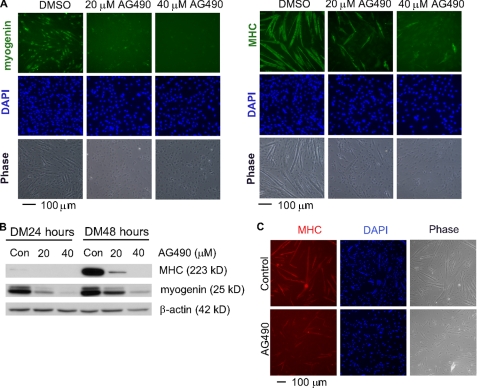
Inhibition of JAK2 with AG490 Inhibits Myogenic Differentiation— To evaluate the role of JAK2 in myogenic differentiation, we first treated C2C12 cells with AG490, a known inhibitor of JAK2, at the time when cells were induced to differentiate. Even by phase contrast microscopy, we could clearly see that the formation of multinucleated myotubes was inhibited by AG490 in a dose-dependent manner (Fig. 1A, bottom panels). Consistently, we found that the expression of myogenin and MHC, two proteins that are present at the early and late stages of differentiation, respectively, and are commonly used as stage-specific differentiation markers, was significantly inhibited by AG490 as judged by both immunostaining and immunoblotting analysis with antibodies specific for myogenin and MHC (Fig. 1A, top panels). To find out whether AG490 has a similar inhibitory effect on primary myoblasts, we applied AG490 to primary myoblasts undergoing differentiation. As shown in Fig. 1C, the differentiation of primary myoblasts was significantly inhibited as evidenced by reduced myotube formation.
FIGURE 1.
AG490 inhibits myogenic differentiation in both C2C12 and primary myoblasts. Nearly confluent C2C12 cells (A and B) or primary myoblasts (C) were induced to differentiate in DM with either dimethyl sulfoxide (DMSO, vehicle) or different doses of AG490. A, after growing in DM for 24 h (left three panels) or 48 h (right three panels), the cells were fixed and subjected to immunostaining using antibodies against either myogenin or MHC. B, equal amount of WCEs were subjected to immunoblotting to reveal the expression levels of myogenin, MHC, and β-actin (loading control). Con, dimethyl sulfoxide control. C, after growing in DM for 30 h, the primary myoblasts were fixed and subjected to immunostaining for MHC. In A and C, 4′,6′-diamino-2-phenylindole (DAPI) was added to counterstain the nuclei.
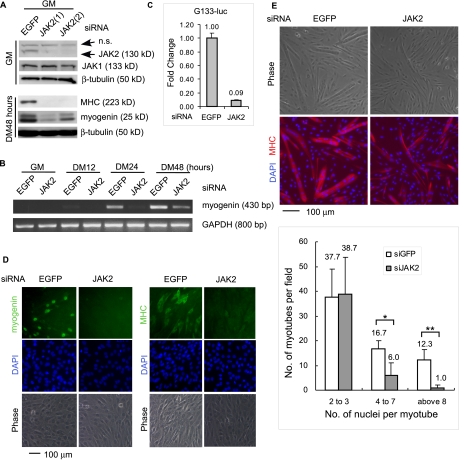
Knockdown of JAK2 by siRNA Represses Myogenic Differentiation—Although it was convenient to employ pharmacological inhibitors like AG490 in our initial studies, it was necessary to complement and verify the drug study with more specific assays because many pharmacological inhibitors may inhibit some unknown cellular targets in addition to their intended and well characterized targets. To this end, we designed two different JAK2-specific siRNAs and two control siRNAs (i.e. an EGFP-specific siRNA and a scrambled JAK2-siRNA). C2C12 cells were first transfected with individual siRNA. The cells were then either grown in the GM or induced to differentiate in the DM. By Western blotting, we showed that both JAK2-siRNAs, but not the two control siRNAs, were specific and effective in knocking down JAK2 gene expression without affecting the expression of JAK1 gene (Fig. 2A and supplemental Fig. S1B). Importantly, both JAK2-siRNAs, but not the control siRNAs, significantly inhibited myogenic differentiation, as judged by reduced expression of myogenin and MHC in immunoblotting analysis (Fig. 2A and supplemental Fig. S1D). To avoid unnecessary data duplication, the effect of one JAK2-specific siRNA was shown in subsequent assays. By semiquantitative RT-PCR, we showed that the JAK2-siRNA inhibited the expression of myogenin mRNA (Fig. 2B). Consistently, by reporter assays, we found that the JAK2-siRNA significantly inhibited the expression of a luciferase reporter gene driven by a 133-bp proximal myogenin promoter (i.e. G133-Luc) (24) (Fig. 2C). By immunostaining, we also confirmed that the expression of myogenin and MHC was inhibited by the JAK2-siRNA (Fig. 2D). Furthermore, we showed that the JAK2-siRNA also inhibited the differentiation of primary myoblasts, as evidenced by reduced number of multinucleated myotubes, especially those with four or more nuclei (Fig. 2E).
FIGURE 2.
Knockdown of JAK2 by siRNA inhibits myogenic differentiation. 50% confluent C2C12 cells (A–D) or primary myoblasts (E) were transfected with either an EGFP-siRNA (control) or JAK2-siRNAs as indicated followed by growth in GM for 24 h. Nearly confluent cells were induced to differentiate in DM for various times as indicated. A, WCEs were subjected to immunoblotting by various antibodies. n.s., nonspecific. B, after cell harvest, total RNA was extracted. The mRNA levels of myogenin were determined by semi-quantitative RT-PCR. GAPDH served as a loading control. C, C2C12 cells were co-transfected with siRNAs and G133-luc. After growth in GM for 24 h and DM for 24 h, the cells were harvested, and WCE were subjected to luciferase assays. Fold change was calculated as the ratio of the luciferase activity of cells transfected with the JAK2-siRNA over that with the EGFP-siRNA. D, C2C12 cells were fixed and subjected to immunostaining for either myogenin (left two columns, DM36 h) or MHC (right two columns, DM48 h). E, primary myoblasts were fixed and subjected to immunostaining for MHC. In D and E, 4′,6′-diamino-2-phenylindole (DAPI) was added to counterstain the nuclei. Among MHC-positive myotubes in three randomly chosen fields (10× magnification), those with 2–3, 4–7, and >8 nuclei were separately counted. The data are presented as the means ± S.D. *, p < 0.05. **, p < 0.01.
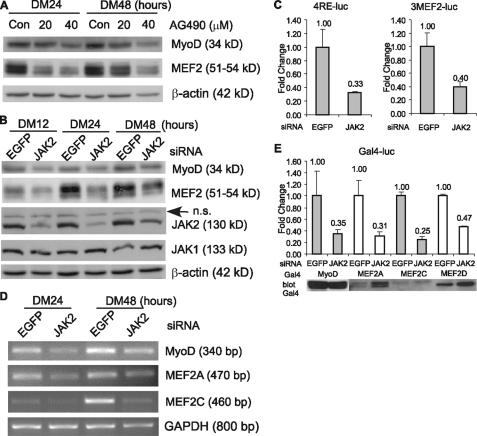
JAK2 Is Required for Both the Expression and Transactivation Function of MyoD and MEF2—To explain at the molecular level why the interference of the JAK2 function by either AG490 or siRNA represses myogenic differentiation, we examined the status of MyoD and MEF2, because both are indispensable for myogenic differentiation and myogenic gene expression. As shown in Fig. 3A, compared with nontreated control cells, the AG490-treated cells had significantly reduced levels of MyoD and MEF2. Similarly, the cells treated with the JAK2-siRNA also had reduced levels of MyoD and MEF2 when compared with the control cells transfected with the EGFP-siRNA (Fig. 3B). As a control, we found that the levels of either β-actin or JAK1 were not affected by the JAK2-siRNA. Consistently, both MRF- and MEF2-dependent reporter genes (i.e. 4RE-luc and 3MEF2-luc respectively) displayed obviously reduced activities when cells were co-transfected with the JAK2-siRNA (Fig. 3C). By RT-PCR, we further determined that the JAK2-siRNA-mediated reduction in MyoD and MEF2 protein levels was mainly due to a reduction in their mRNA levels (Fig. 3D). This suggested that JAK2 normally regulates MyoD and MEF2 gene expression at the transcription level. In addition, we also tested whether the transcriptional activity of MyoD and MEF2 was affected by the JAK2-siRNA. To do this, we first fused MyoD or several MEF2 genes to a cDNA fragment encoding the DNA-binding domain (i.e. amino acids 1–147) of yeast Gal4. When these constructs were co-transfected into C2C12 cells with a luciferase reporter gene under the control of multiple Gal4-binding sites (i.e. Gal4-luc), the reporter gene expression was mainly determined by the transactivation domain of MyoD or MEF2 (41). Interestingly, in the presence of the JAK2-siRNA, the reporter activity was considerably reduced compared with that in the presence of the EGFP-siRNA (Fig. 3E). This suggests that JAK2 also regulates the transcriptional activity of both MyoD and MEF2, in addition to its effect on the transcription of MyoD and MEF2 genes.
FIGURE 3.
Inhibition of JAK2 reduces both the expression levels of MyoD and MEF2 and their transactivating activities. A, C2C12 cells were induced to differentiate for 24 and 48 h in the presence or absence of different doses of AG490. B and C, C2C12 cells were transfected with either the EGFP- or JAK2-siRNA followed by growth in GM for 24 h. The cells were then induced to differentiate for various times as indicated. For A and B, WCEs were subjected to immunoblotting by various antibodies. n.s., nonspecific. For D, mRNA levels of MyoD, MEF2A, and MEF2C were determined by RT-PCR and normalized to that of GAPDH. C and E, C2C12 cells were transfected with various reporter constructs together with either the EGFP- or JAK2-siRNAs followed by growth in GM for 24 h. For C, the cells were induced to differentiate for 18 h before harvest. WCEs were subjected to luciferase assays, and the fold change was determined the same way as that described in the legend for Fig. 2C. For E, after luciferase assays, the remaining WCEs were subjected to immunoblotting to reveal the levels of Gal4 fusion proteins in cells transfected with either the EGFP- or JAK2-siRNA.
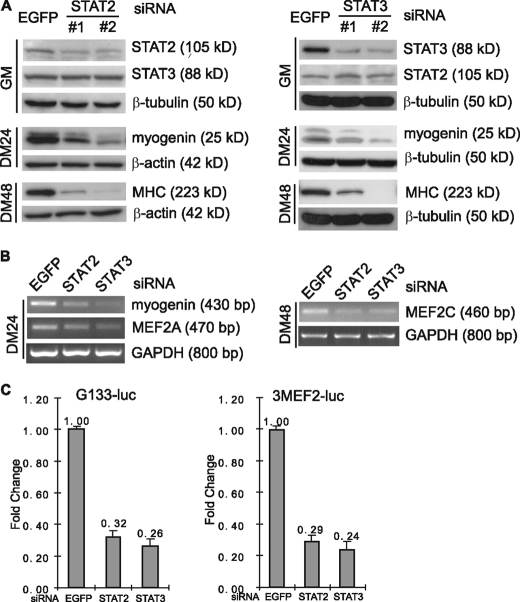
STAT2 and STAT3 Act Downstream of JAK2 to Regulate Myogenic Differentiation—STAT proteins are the best characterized downstream targets of JAKs. Of the seven STAT family members, we recently demonstrated that STAT1 functions downstream of JAK1 to promote proliferation and to inhibit premature differentiation of myoblasts (40). To identify the STATs that function downstream of JAK2 in myogenic differentiation, we first designed siRNAs specifically targeting different STATs. We found that knockdown of STAT2 and STAT3 consistently inhibited myogenic differentiation, and we decided to focus on these two STATs. As shown in Fig. 4A, two different siRNAs targeting either STAT2 or STAT3 were both effective and specific. Both STAT2-siRNAs and STAT3-siRNAs considerably inhibited myogenic differentiation in C2C12 cells as judged by reduced expression of myogenin and MHC. Apart from the EGFP-siRNA, we also used scrambled STAT2 or STAT3 siRNA as another negative control. As shown in the supplemental Fig. S1E, the scrambled siRNAs had no obvious effect on the expression of STAT2 or STAT3 genes. Consequently, they did not affect the expression of myogenin or MHC genes. This further proved that the myogenic effects caused by STAT2 or STAT3 siRNAs were specific. In addition, knockdown of STAT2 or STAT3 also resulted in reduced levels of MEF2A and MEF2C mRNA, as judged by RT-PCR (Fig. 4B). Consistently, both STAT2- and STAT3-siRNAs specifically inhibited the expression of several myogenic reporter genes including G133-luc and 3MEF2-luc (Fig. 4C). Because the effect of both STAT2-siRNA and STAT3-siRNA was very similar to that of the JAK2-siRNA, our data suggested that STAT2 and STAT3 function downstream of JAK2 to regulate myogenic differentiation.
FIGURE 4.
Knockdown of either STAT2 or STAT3 inhibits myogenic differentiation. C2C12 cells were transfected with either siRNAs alone (A and B) or siRNAs plus reporter constructs (C) followed by growth in GM for 24 h. The cells were then induced to differentiate for various times as indicated. A, WCEs were subjected to immunoblotting by various antibodies. #1 and #2 denote different sets of siRNA. B, the mRNA levels of myogenin, MEF2A and MEF2C were determined by RT-PCR and normalized to that of GAPDH. C, cells were induced to differentiate for 24 h before harvest followed by luciferase assays. The fold change was determined the same way as that described in the legend for Fig. 2C.
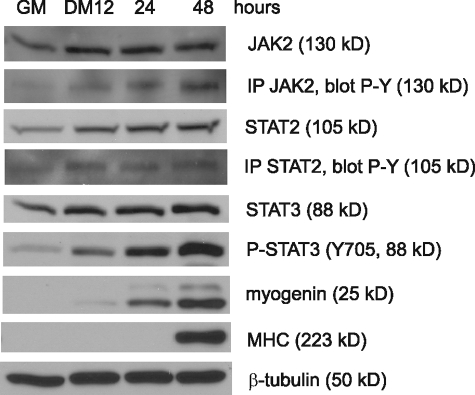
JAK2, STAT2, and STAT3 Are Activated upon Myogenic Differentiation—Because individual knockdown of the endogenous JAK2, STAT2, and STAT3 all inhibited myogenic differentiation (Figs. 2 and 4), this suggested that JAK2, STAT2, and STAT3 are normally activated during myogenic differentiation. To test whether this is the case, we examined the expression levels and the activation status of these three proteins in C2C12 cells undergoing differentiation. However, except for the antibodies against STAT3, we found that many commercial antibodies for JAK2 and STAT2 cross-reacted with other members of the JAK/STAT family and failed to specifically pull down JAK2 and STAT2 in immunoprecipitation assays.3 To overcome the problem, we generated our own antibodies against JAK2 and STAT2, purified them, and proved that they were specific and suitable for immunoblotting and immunoprecipitation assays (supplemental Fig. S1, A, C, E, and F). As indicated in Fig. 5, our immunoblotting assays revealed that the total protein levels of JAK2, STAT2, and STAT3 gradually increased during differentiation. As to the levels of the tyrosine-phosphorylated forms of JAK2, STAT2, and STAT3 that represent the active forms of these proteins (34), we found that it was easy to detect the tyrosine-phosphorylated (i.e. active) STAT3 in direct immunoblotting assays because of its abundance in C2C12 cells. Unlike STAT3, the active JAK2 and STAT2 were much more difficult to detect because of their low abundance and a lack of specific antibodies recognizing the active JAK2 and STAT2. We had to enrich them first by immunoprecipitation followed by immunoblotting with a well characterized anti-phospho-tyrosine antibody (i.e. 4G10) (42). By doing so, we found that the levels of the active JAK2 and STAT2 also increased upon differentiation, as was the case for STAT3.
FIGURE 5.
JAK2, STAT2 and STAT3 are activated during myogenic differentiation. Near confluent C2C12 cells were induced to differentiate for various times as indicated. WCEs were subjected to immunoblotting by various antibodies as indicated. To determine the levels of P-JAK2 and P-STAT2, immunoprecipitation (IP) was first performed using our homemade JAK2 and STAT2 antibodies followed by immunoblotting with an antibody against phospho-tyrosine (P-Y).
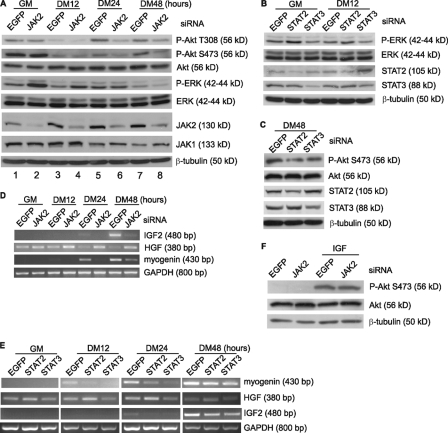
JAK2/STAT2/STAT3 Regulate the Expression of IGF2 and HGF—Multiple intracellular signaling pathways have been implicated in the process of myogenic differentiation, including the IGF/PI3K/Akt pathway and the p38 MAPK pathway that promote differentiation and the ERK pathway that inhibits the early differentiation (18, 19, 21, 22, 24, 43, 44). Thus, it would be interesting to test whether modulation of JAK2/STAT2/STAT3 affects these well characterized pathways. We found that knockdown of JAK2 in C2C12 cells increased the levels of the active ERK (i.e. p-ERK) both before and at the early stage of differentiation (Fig. 6A, lanes 2 and 4). In contrast, JAK2 knockdown significantly reduced the levels of the active Akt mainly at the late stage of differentiation (Fig. 6A, lanes 6 and 8). As a control, the JAK2-siRNA did not affect the expression of β-tubulin and JAK1. Similarly, when C2C12 cells were individually transfected with siRNAs against STAT2 or STAT3, we found that the STAT2-siRNA had more obvious effect on the levels of the active ERK during early differentiation (Fig. 6B), whereas both STAT2- and STAT3-siRNAs affected the levels of the active Akt during late differentiation (Fig. 6C). To understand what caused such changes in the levels of the active Akt and ERK, we examined the mRNA levels of IGF2 and HGF, both of which are known to be capable of activating Akt and ERK, respectively, in myogenic cells. As shown in Fig. 6D, knockdown of JAK2 induced HGF expression even in proliferating myoblasts but repressed IGF2 expression at the late stage of differentiation (e.g. DM24 and DM48 h). Consistent with their differential effects on the status of the active ERK and Akt (Fig. 6, B and C), knockdown of STAT2 preferentially induced HGF expression during early differentiation, whereas that of either STAT2 or STAT3 repressed IGF2 expression during late differentiation (Fig. 6E). We further demonstrated that Akt phosphorylation induced by the exogenous IGF was not affected by the JAK2-siRNA (Fig. 6F), suggesting that the decreased levels of the active Akt by JAK2-siRNA is not due to direct interruption of the IGF/PI3K/Akt pathway. Rather, our data suggested that the JAK2/STAT2/STAT3 pathway regulates the expression of both IGF2 and HGF, which in turn affects the status of the active Akt and ERK, respectively.
FIGURE 6.
JAK2/STAT2/STAT3 regulate the expression of IGF2 and HGF at distinct phases of differentiation. C2C12 cells were transfected with different siRNAs as indicated. The cells were induced to differentiate for various times. For F, after growth in GM for 24 h, the cells were starved in serum-free DMEM for 1 h and then treated with or without 50 ng/ml IGF1 for 15 min. For A–C and F, WCEs were subjected to immunoblotting with various antibodies as indicated. For D and E, the mRNA levels of myogenin, HGF, and IGF2 were determined by RT-PCR and normalized to that of GAPDH.
DISCUSSION
Multiple JAK/STAT Pathways Regulate Myogenic Differentiation with Opposing Effects—Previous studies by several groups have shown that several JAK and STAT members are activated in both LIF-treated proliferating myoblasts and regenerating muscles (35–37). However, it was unclear how these JAKs and STATs function during myogenic differentiation. Recently, we carried out detailed analysis in both immortalized C2C12 cells and primary myoblasts. We found that JAK1, acting via STAT1 and STAT3, is involved in myoblast proliferation (40). Concomitantly, the JAK1/STAT1 pathway represses differentiation process to prevent premature differentiation of myoblasts. They do so by regulating the expression of MyoD, MEF2, Id1, p21cip1, and p27kip1, all of which are key players in myogenic differentiation (40). Unexpectedly, we find in this report that JAK2, a close relative of JAK1, plays a positive role in myogenic differentiation. Because of embryonic lethality in JAK2 knock-out mice (45, 46), we could not directly analyze the myogenic role of JAK2 in mice. Instead, we focused on myogenic cell cultures. Interference of the JAK2 function either by a small-molecule inhibitor (i.e. AG490) or by JAK2-siRNA inhibits myogenic differentiation in both C2C12 and primary myoblasts (Figs. 1 and 2). We further suggest that the pro-differentiation effect of JAK2 is mediated by STAT2 and STAT3, because the knockdown of either STAT2 or STAT3 partially recapitulates the effects generated by that of JAK2. In addition, both STAT2 and STAT3 are increasingly activated upon differentiation, which parallels the changes in the activity of JAK2 (Fig. 5). Like the JAK1/STAT1/STAT3 pathway, the JAK2/STAT2/STAT3 pathway also regulates the expression of MyoD and MEF2. Consistent with their opposing effects on differentiation, these two JAK/STAT pathways regulate the expression of MyoD and MEF2 in opposite manners: whereas the JAK1/STAT1/STAT3 pathway represses the expression of MyoD and MEF2 (40), the JAK2/STAT2/STAT3 pathway enhances their expression. It remains unclear how different STAT complexes can bring about opposite effects on the same set of target genes. Presumably, they can do so by cooperating with different co-factors and act at different stages in the course of differentiation. Although we show that LIF activates the JAK1/STAT1/STAT3 pathway (40), we do not know at present which ligand engages the JAK2/STAT2/STAT3 pathway during myogenic differentiation.
STAT3 Functions at Multiple Steps during Myogenic Differentiation— STAT3 is arguably the most important STAT during mouse embryo development, because only the loss of STAT3 causes embryonic lethality (47). Among many diverse roles revealed for STAT3, its most prominent role is in cell proliferation. It is well established that STAT3 is an essential mediator downstream of LIF in maintaining self-renewal and pluripotency of ES cells (48, 49). In addition to cytokines, many growth factors including IGF and epidermal growth factor also result in STAT3 phosphorylation and activation (50, 51). STAT3 has also been linked to uncontrolled cell growth based on the following evidence: a constitutively activated STAT3 is capable of transforming fibroblasts (52). Activated STAT3 is frequently detected in various tumors (53). In addition, STAT3 can also be activated by various oncogenes such as Src (54). In myogenic cells, activated STAT3 has been detected in LIF-treated proliferating myoblasts and regenerating muscles (35–37). In addition, knockdown of STAT3 reduced the LIF-induced myoblast proliferation (40). These data are consistent with the role of STAT3 in proliferation.
Consistent with our previous notion and a recent report (40, 55), we find that STAT3 is also required for myogenic differentiation. This is supported by the following results: First, STAT3 is significantly activated during differentiation (Fig. 5). Second, knockdown of STAT3 inhibits myogenic differentiation (Fig. 4) (55). Our data suggest that STAT3 can function at both proliferation and differentiation steps presumably by associating with different co-factors that facilitate its differential binding to different subset of target genes. Further confirmation of the dual role of STAT3 in vivo awaits the generation of satellite cell/myoblast-specific STAT3 knock-out mice.
JAK2/STAT2/STAT3 Regulate Expression of IGF2 and HGF— In addition to MyoD and MEF2, we show that the JAK2/STAT2/STAT3 pathway could also regulate the expression of HGF and IGF2; it represses HGF at the early stage of differentiation and enhances IGF2 at the late stage of differentiation. Both HGF and IGF2 are secreted growth factors known to influence proliferation and differentiation of myoblasts (26, 27, 29–32). HGF exerts its biological effect through c-Met, a member of the receptor tyrosine kinase family, with ERK being a main intracellular pathway activated by HGF (33, 56), whereas IGF exerts its effect through IGF receptor, also a member of the receptor tyrosine kinase family, with PI3K/Akt being the main pathway activated by IGF in myogenic cells (22–24, 29). Consistent with a positive role for HGF in cell proliferation and a negative role in early differentiation (30–32), down-regulation of HGF gene by JAK2 or STAT2 at the early stage of differentiation facilitates myogenic differentiation. Because IGF2 is an autocrine factor known to promote differentiation (26, 27, 29), an induction of IGF2 by JAK2, STAT2, or STAT3 during late differentiation may facilitate myocyte fusion and formation of multinucleated myotubes. Another interesting point to note is that STAT2 preferentially mediates the effect of JAK2 on the expression of HGF, whereas both STAT2 and STAT3 regulate IGF2. This indicates that the roles of STAT2 and STAT3 are not completely redundant.
In summary, we show here that JAK2, STAT2, and STAT3 positively regulate myogenic differentiation by regulating the expression of MyoD, MEF2, HGF, and IGF2. Our results further reinforce our previous notion that multiple members of the JAK/STAT family participate in myogenic differentiation via distinct pathways and with distinct effects.
Supplementary Material
Acknowledgments
We thank Dr. Zilong Wen for various JAK and STAT constructs and Carol Wong for technical assistance.
This work was supported by Hong Kong Research Grant Council Grants 663007 and CA06/07.SC02 (to Z. W.) and Area of Excellence Scheme AoE/B-15/01 from the Hong Kong University Grant Committee. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: MRF, myogenic regulatory factor; STAT, signal transducers and activators of transcription; siRNA, small interfering RNA; MEF, myocyte enhancer factor; ERK, extracellular signal-regulated kinase; IGF, insulin-like growth factor; PI3K, phosphatidylinositol 3-kinase; HGF, hepatocyte growth factor; JAK, Janus kinase; LIF, leukemia inhibitory factor; DMEM, Dulbecco's modified Eagle's medium; GM, growth medium; DM, differentiation medium; EGFP, enhanced green fluorescent protein; WCE, whole cell extract; RT, reverse transcription; MHC, myosin heavy chain; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
K. Wang and Z. Wu, unpublished data.
References
- 1.Molkentin, J. D., and Olson, E. N. (1996) Curr. Opin. Genet. Dev. 6 445-453 [DOI] [PubMed] [Google Scholar]
- 2.Sabourin, L. A., and Rudnicki, M. A. (2000) Clin. Genet. 57 16-25 [DOI] [PubMed] [Google Scholar]
- 3.Tapscott, S. J. (2005) Development 132 2685-2695 [DOI] [PubMed] [Google Scholar]
- 4.Black, B. L., and Olson, E. N. (1998) Annu. Rev. Cell Dev. Biol. 14 167-196 [DOI] [PubMed] [Google Scholar]
- 5.McKinsey, T. A., Zhang, C. L., and Olson, E. N. (2002) Trends Biochem. Sci. 27 40-47 [DOI] [PubMed] [Google Scholar]
- 6.Molkentin, J. D., Black, B. L., Martin, J. F., and Olson, E. N. (1995) Cell 83 1125-1136 [DOI] [PubMed] [Google Scholar]
- 7.Kassar-Duchossoy, L., Gayraud-Morel, B., Gomes, D., Rocancourt, D., Buckingham, M., Shinin, V., and Tajbakhsh, S. (2004) Nature 431 466-471 [DOI] [PubMed] [Google Scholar]
- 8.Rudnicki, M. A., Schnegelsberg, P. N., Stead, R. H., Braun, T., Arnold, H. H., and Jaenisch, R. (1993) Cell 75 1351-1359 [DOI] [PubMed] [Google Scholar]
- 9.Hasty, P., Bradley, A., Morris, J. H., Edmondson, D. G., Venuti, J. M., Olson, E. N., and Klein, W. H. (1993) Nature 364 501-506 [DOI] [PubMed] [Google Scholar]
- 10.Nabeshima, Y., Hanaoka, K., Hayasaka, M., Esumi, E., Li, S., Nonaka, I., and Nabeshima, Y. (1993) Nature 364 532-535 [DOI] [PubMed] [Google Scholar]
- 11.Yee, S. P., and Rigby, P. W. (1993) Genes Dev. 7 1277-1289 [DOI] [PubMed] [Google Scholar]
- 12.Cheng, T. C., Hanley, T. A., Mudd, J., Merlie, J. P., and Olson, E. N. (1992) J. Cell Biol. 119 1649-1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmondson, D. G., Cheng, T. C., Cserjesi, P., Chakraborty, T., and Olson, E. N. (1992) Mol. Cell. Biol. 12 3665-3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Grand, F., and Rudnicki, M. A. (2007) Curr. Opin. Cell Biol. 19 628-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckingham, M. (2006) Curr. Opin. Genet. Dev. 16 525-532 [DOI] [PubMed] [Google Scholar]
- 16.Wagers, A. J., and Conboy, I. M. (2005) Cell 122 659-667 [DOI] [PubMed] [Google Scholar]
- 17.Dhawan, J., and Rando, T. A. (2005) Trends Cell Biol. 15 666-673 [DOI] [PubMed] [Google Scholar]
- 18.Wu, Z., Woodring, P. J., Bhakta, K. S., Tamura, K., Wen, F., Feramisco, J. R., Karin, M., Wang, J. Y., and Puri, P. L. (2000) Mol. Cell. Biol. 20 3951-3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zetser, A., Gredinger, E., and Bengal, E. (1999) J. Biol. Chem. 274 5193-5200 [DOI] [PubMed] [Google Scholar]
- 20.Cuenda, A., and Cohen, P. (1999) J. Biol. Chem. 274 4341-4346 [DOI] [PubMed] [Google Scholar]
- 21.Bennett, A. M., and Tonks, N. K. (1997) Science 278 1288-1291 [DOI] [PubMed] [Google Scholar]
- 22.Coolican, S. A., Samuel, D. S., Ewton, D. Z., McWade, F. J., and Florini, J. R. (1997) J. Biol. Chem. 272 6653-6662 [DOI] [PubMed] [Google Scholar]
- 23.Tureckova, J., Wilson, E. M., Cappalonga, J. L., and Rotwein, P. (2001) J. Biol. Chem. 276 39264-39270 [DOI] [PubMed] [Google Scholar]
- 24.Xu, Q., and Wu, Z. (2000) J. Biol. Chem. 275 36750-36757 [DOI] [PubMed] [Google Scholar]
- 25.Jiang, B. H., Aoki, M., Zheng, J. Z., Li, J., and Vogt, P. K. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 2077-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart, C. E., and Rotwein, P. (1996) J. Biol. Chem. 271 11330-11338 [DOI] [PubMed] [Google Scholar]
- 27.Stewart, C. E., James, P. L., Fant, M. E., and Rotwein, P. (1996) J. Cell. Physiol. 169 23-32 [DOI] [PubMed] [Google Scholar]
- 28.Kaliman, P., Vinals, F., Testar, X., Palacin, M., and Zorzano, A. (1996) J. Biol. Chem. 271 19146-19151 [DOI] [PubMed] [Google Scholar]
- 29.Florini, J. R., Ewton, D. Z., and Coolican, S. A. (1996) Endocr. Rev. 17 481-517 [DOI] [PubMed] [Google Scholar]
- 30.Miller, K. J., Thaloor, D., Matteson, S., and Pavlath, G. K. (2000) Am. J. Physiol. 278 C174-C181 [DOI] [PubMed] [Google Scholar]
- 31.Gal-Levi, R., Leshem, Y., Aoki, S., Nakamura, T., and Halevy, O. (1998) Biochim. Biophys. Acta 1402 39-51 [DOI] [PubMed] [Google Scholar]
- 32.Anastasi, S., Giordano, S., Sthandier, O., Gambarotta, G., Maione, R., Comoglio, P., and Amati, P. (1997) J. Cell Biol. 137 1057-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponzetto, C., Bardelli, A., Zhen, Z., Maina, F., dalla Zonca, P., Giordano, S., Graziani, A., Panayotou, G., and Comoglio, P. M. (1994) Cell 77 261-271 [DOI] [PubMed] [Google Scholar]
- 34.O'Shea, J. J., Gadina, M., and Schreiber, R. D. (2002) Cell 109 (suppl.) S121-S131 [DOI] [PubMed] [Google Scholar]
- 35.Megeney, L. A., Perry, R. L., LeCouter, J. E., and Rudnicki, M. A. (1996) Dev. Genet. 19 139-145 [DOI] [PubMed] [Google Scholar]
- 36.Spangenburg, E. E., and Booth, F. W. (2002) Am. J. Physiol. 283 C204-C211 [DOI] [PubMed] [Google Scholar]
- 37.Kami, K., and Senba, E. (2002) J. Histochem. Cytochem. 50 1579-1589 [DOI] [PubMed] [Google Scholar]
- 38.Kataoka, Y., Matsumura, I., Ezoe, S., Nakata, S., Takigawa, E., Sato, Y., Kawasaki, A., Yokota, T., Nakajima, K., Felsani, A., and Kanakura, Y. (2003) J. Biol. Chem. 278 44178-44187 [DOI] [PubMed] [Google Scholar]
- 39.Meydan, N., Grunberger, T., Dadi, H., Shahar, M., Arpaia, E., Lapidot, Z., Leeder, J. S., Freedman, M., Cohen, A., Gazit, A., Levitzki, A., and Roifman, C. M. (1996) Nature 379 645-648 [DOI] [PubMed] [Google Scholar]
- 40.Sun, L., Ma, K., Wang, H., Xiao, F., Gao, Y., Zhang, W., Wang, K., Gao, X., Ip, N., and Wu, Z. (2007) J. Cell Biol. 179 129-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, L., Liu, L., Yang, X. J., and Wu, Z. (2004) J. Cell Sci. 117 3021-3029 [DOI] [PubMed] [Google Scholar]
- 42.Druker, B. J., Mamon, H. J., and Roberts, T. M. (1989) N. Engl. J. Med. 321 1383-1391 [DOI] [PubMed] [Google Scholar]
- 43.Tamir, Y., and Bengal, E. (2000) J. Biol. Chem. 275 34424-34432 [DOI] [PubMed] [Google Scholar]
- 44.Xu, Q., Yu, L., Liu, L., Cheung, C. F., Li, X., Yee, S. P., Yang, X. J., and Wu, Z. (2002) Mol. Biol. Cell 13 1940-1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parganas, E., Wang, D., Stravopodis, D., Topham, D. J., Marine, J. C., Teglund, S., Vanin, E. F., Bodner, S., Colamonici, O. R., van Deursen, J. M., Grosveld, G., and Ihle, J. N. (1998) Cell 93 385-395 [DOI] [PubMed] [Google Scholar]
- 46.Neubauer, H., Cumano, A., Muller, M., Wu, H., Huffstadt, U., and Pfeffer, K. (1998) Cell 93 397-409 [DOI] [PubMed] [Google Scholar]
- 47.Takeda, K., Noguchi, K., Shi, W., Tanaka, T., Matsumoto, M., Yoshida, N., Kishimoto, T., and Akira, S. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 3801-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuda, T., Nakamura, T., Nakao, K., Arai, T., Katsuki, M., Heike, T., and Yokota, T. (1999) EMBO J. 18 4261-4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niwa, H., Burdon, T., Chambers, I., and Smith, A. (1998) Genes Dev. 12 2048-2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zong, C. S., Zeng, L., Jiang, Y., Sadowski, H. B., and Wang, L. H. (1998) J. Biol. Chem. 273 28065-28072 [DOI] [PubMed] [Google Scholar]
- 51.Zhong, Z., Wen, Z., and Darnell, J. E., Jr. (1994) Science 264 95-98 [DOI] [PubMed] [Google Scholar]
- 52.Bromberg, J. F., Wrzeszczynska, M. H., Devgan, G., Zhao, Y., Pestell, R. G., Albanese, C., and Darnell, J. E., Jr. (1999) Cell 98 295-303 [DOI] [PubMed] [Google Scholar]
- 53.Garcia, R., and Jove, R. (1998) J. Biomed. Sci. 5 79-85 [DOI] [PubMed] [Google Scholar]
- 54.Yu, C. L., Meyer, D. J., Campbell, G. S., Larner, A. C., Carter-Su, C., Schwartz, J., and Jove, R. (1995) Science 269 81-83 [DOI] [PubMed] [Google Scholar]
- 55.Snyder, M., Huang, X. Y., and Zhang, J. J. (2008) J. Biol. Chem. 283 3791-3798 [DOI] [PubMed] [Google Scholar]
- 56.Rosario, M., and Birchmeier, W. (2003) Trends Cell Biol. 13 328-335 [DOI] [PubMed] [Google Scholar]
- 57.Beauchamp, J. R., Morgan, J. E., Pagel, C. N., and Partridge, T. A. (1999) J. Cell Biol. 144 1113-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.