Abstract
Mutations in the DJ-1 gene have been implicated in the autosomal recessive early onset parkinsonism. DJ-1 is a soluble dimeric protein with critical roles in response to oxidative stress and in neuronal maintenance. However, several lines of evidence suggest the existence of a nonfunctional aggregated form of DJ-1 in the brain of patients with some neurodegenerative diseases. Here, we show that inorganic phosphate, an important anion that exhibits elevated levels in patients with Parkinson disease, transforms DJ-1 into filamentous aggregates. According to the 2.4-Å crystal structure, DJ-1 dimers are linearly stacked through Pi-mediated interactions to form protofilaments, which are then bundled into a filamentous assembly.
Human DJ-1 is a 189-amino acid, homo-dimeric protein. It was first identified as a novel candidate for the oncogene product that transformed mouse NIH3T3 cells in concert with activated ras (1). After this initial identification, DJ-1 has been shown to have various physiological implications. DJ-1 was characterized as a protein that regulates an RNA-protein interaction (2) and positively modulates the androgen receptor (3). In addition to functions in somatic cells, DJ-1 seems to play roles in male fertility. CAP-1, or SP22, a rat homologue of DJ-1, has been identified as a key protein related to infertility observed in male rats that were exposed to sperm toxicants (4–6). DJ-1 began to attract attention after a homozygous deletion in the DJ-1 gene was revealed to be responsible for the autosomal recessive early onset Parkinson disease (PD)3 (7). Like the deletion mutation, several missense mutations of the DJ-1 gene found in inherited PD cases also appear to cause a complete lack of DJ-1 function. For example, the L166P mutation appears to destabilize the dimeric structure of DJ-1 by promoting the unfolding of its C-terminal region, subsequently enhancing its degradation via the proteasome system and leading to the loss of DJ-1 in cells (8–10).
Accumulating evidence reveals that DJ-1 plays protective functions against oxidative stress. DJ-1 is a molecular chaperone (11) that is activated in an oxidative cytoplasmic environment (12). Activated DJ-1 inhibits α-synuclein aggregation, the major component of Lewy bodies, which are the characteristic intracytoplasmic neuronal inclusions in PD and are closely associated with the progression of PD. DJ-1 also acts as a redox-sensitive negative regulator of apoptosis. DJ-1 contributes to the activation of the PI3K/Akt survival signaling pathway (13, 14) and blocks the Daxx-ASK1 proapoptotic pathway by sequestering Daxx from ASK1 (15). Studies using mice and Drosophila also suggest that DJ-1 plays critical roles in cell survival and response to oxidative stress (13, 16–20).
Structural alterations of DJ-1 are closely associated with its functions. Thus far, three structural modifications of the DJ-1 dimeric structure have been reported. First, DJ-1 is sumoylated at lysine residue 130 by PIASxα. Proper sumoylation is thought to be essential in ras-dependent cellular transformation, cell growth promotion, and anti-UV-induced apoptosis (21). Second, a cysteine residue (Cys106) of DJ-1 is oxidized to sulfinic acid under oxidative stress conditions (11, 22), which causes a relocalization of DJ-1 to mitochondria. In addition, a C106A mutant fails to prevent cell death against oxidative stress, indicating the importance of oxidation at Cys106 for DJ-1 function (22, 23). Third, the solubility of DJ-1 is altered to form insoluble aggregates in brains of patients with neurodegenerative diseases (24–29). DJ-1 colocalizes with tau-positive inclusions over a range of neurodegenerative tauopathies, including Alzheimer disease, and with α-synuclein-positive glial inclusions in multiple system atrophy (24, 25, 29). In addition, insoluble DJ-1 is dramatically increased in brains of sporadic PD patients (28, 29). These findings suggest that abnormal aggregation of DJ-1 may be involved in the pathogenesis of multiple neurodegenerative diseases. Here, we report biochemical and structural investigations of DJ-1 aggregates formed in a Pi-dependent manner.
EXPERIMENTAL PROCEDURES
Purification of DJ-1—The DJ-1 gene (encoding residues 1–189) was amplified, inserted into pET-21a (Novagen) and introduced into Escherichia coli strain B834 (DE3), and tDJ-1 was purified as described (11). For purification of pDJ-1, the cells were grown to an A600 of ∼0.7 in Luria-Bertani medium containing 0.1 mg/ml ampicillin at 37 °C, and the expression of DJ-1 was induced by 1 mm isopropyl-β-d-thiogalactoside. After 4-h induction at 37 °C, the cells were harvested and resuspended in sodium phosphate buffers (pH 7.5) containing 1 mm dithiothreitol. The cells were disrupted by sonication, and the cell debris was discarded by centrifugation. The supernatants were loaded on a nickel-nitrilotriacetic acid column (Novagen) and eluted with sodium phosphate butters containing 20 mm imidazole. The eluted fraction was loaded on a Q-Sepharose Fast Flow column (Amersham Biosciences) and eluted with a 0–1 m NaCl gradient in sodium phosphate buffers (pH 7.5) containing 1 mm dithiothreitol.
Dynamic Light Scattering—Measurements were taken at 22 °C using a DynaPro instrument (Protein Solutions Inc.) equipped with a thermostatted cell. DJ-1 in 20 mm Tris buffer, pH 7.5 (tDJ-1), and DJ-1 in sodium phosphate, pH 7.5 (pDJ-1), at concentrations of 1 mg/ml were centrifuged at 14,000 rpm for 10 min at 4 °C. 60 μl of the supernatants were added to the cuvette, and the light scattering intensity was collected 30 times at an angle of 90° using a 10-s acquisition time. Data analyses were performed using the Dynapro V.5 software.
Circular Dichroism—CD experiments were performed on a Jasco J-810 spectropolarimeter using a 0.2-cm-path length cell, with a 1-nm bandwidth and a 4-s response time. Near-UV CD spectra were collected from 340 to 240 nm with a scan speed of 50 nm/min and 1-nm step resolution. Five individual scans were added and averaged.
Electron Microscopy—Purified pDJ-1 in a phosphate buffer condition was diluted and sampled 1 and 3 days after purification. pDJ-1 (0.6 mg/ml) was applied to carbon-coated grids and negatively stained with 2% uranyl acetate. The samples were visualized on a Philips CM30 electron microscope using a TVIPS FastScan-F114T CCD camera at a 47,000× magnification.
Crystallization, Data Collection, Model Building, and Refinement—The crystals of pDJ-1 were obtained by vapor diffusion from droplets containing 2 μl of protein solution plus 2 μl of precipitant solution containing 2 m ammonium sulfate, 0.4 m sodium potassium tartrate, and 0.1 m sodium cacodylate, pH 6.5, which were equilibrated against 0.5 ml of the same precipitant solution at 277 K. For data collection, the crystals were frozen at -170 °C using a cryostream cooler (Oxford Cryosystems) after a brief immersion in a cryoprotectant solution containing 20% glycerol in the same mother liquor. A 2.4 Å data set was collected at beamline 5A of the Photon Factory (Table 1). The data were integrated and scaled using HKL2000. The crystal structure of DJ-1 was determined by molecular replacement using AMoRe. Refinement was done with a maximum likelihood algorithm implemented in CNS program (Table 1). Molecular graphic manipulations were performed with QUANTA software (Molecular Simulations Inc., San Diego, CA.). After completing the adjustment of side chains according to electron density, the model was subjected to a positional refinement, causing the R value to decrease to 27.2%. At this stage, water molecules were added using the X-solvate utility of QUANTA, and Pi was incorporated into the corresponding density. The subsequent refinement and manual refitting of model reduced R and Rfree values to 23.4 and 28.7%, respectively. The ideality of the model stereochemistry was verified by PROCHECK. The Ramachandran plot indicates 90.1% of nonglycine, and nonproline residues are in the most favored regions in the final model.
TABLE 1.
Summary of crystallographic analysis
| Data collection | |
| Space group | C2 |
| Unit cell (Å) | a = 193.62, b = 102.58, c = 85.54, β = 113.7° |
| Wavelength (Å) | 1.12714 |
| Resolution (Å) | 50-2.4 |
| Rsym (%)a,b | 8.1 (34) |
| Average I/σ | 16.5 (4.7) |
| Completeness (≥3σ) (%) | 98.62 (97.8) |
| Total reflections | 170,172 |
| Unique reflections | 56,724 |
| Refinement (F > 0 σ) | |
| Resolution (Å) | 50-2.4 |
| R/Rfree (%)c | 23.4/28.7 |
| Average B-factor (Å2) | 30.98 |
| Number of protein atoms | 9496 |
| Number of water molecules | 283 |
| Number of phosphate ions | 7 |
| Root mean square deviation bonds (Å) | 0.007 |
| Root mean square deviation angles (°) | 1.44 |
The values for the highest shells are given in parentheses
Rsym = ΣhΣI|Ih,i – Ih|/ΣhΣIIh,i, where Ih is the mean intensity of the i observations of symmetry related reflections of h
r = Σ|Fo – Fc|/ΣFo, where Fo = Fp, and Fc is the calculated protein structure factor from the atomic model. Rfree was calculated with 10% of the reflections
RESULTS AND DISCUSSION
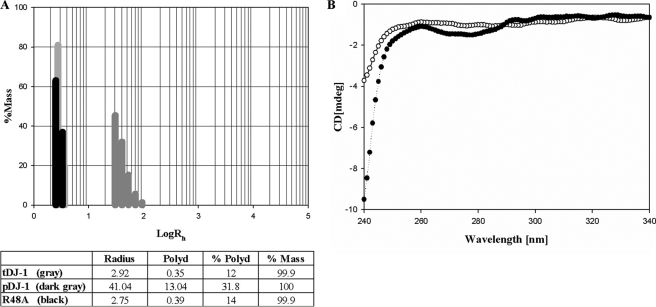
Inorganic Phosphate Induces DJ-1 Aggregation—Despite the existence of DJ-1 aggregates in vivo, recombinant DJ-1 was known to be a dimeric protein (11, 30–33). During efforts to obtain aggregated DJ-1, we were aware that brain cells of PD, multiple system atrophy, and Alzheimer disease patients where DJ-1 aggregation has been observed also contain elevated levels of inorganic phosphate, a vital molecule involved in almost all metabolic processes (34, 35). High Pi levels in serum are directly related to some diseases (36). Thus, we tested sodium phosphate buffers containing Pi to set an environment favorable for formation of DJ-1 aggregates, instead of Tris buffers, which had been previously used to purify recombinant DJ-1 (11). For easy, effective analysis of aggregation, we performed dynamic light scattering measurements. DJ-1 purified using sodium phosphate buffers (pDJ-1) exhibited a very interesting feature compared with DJ-1 purified using Tris buffers (tDJ-1). tDJ-1 showed a hydrodynamic radius (Rh) of 2.92 nm, representing the dimeric conformation with a molecular mass of ∼40 kDa, whereas pDJ-1 had an Rh of 41.04 nm, a clear indication of aggregates (Fig. 1A). The pDJ-1 proteins purified with sodium phosphate buffers of different concentrations were subjected to dynamic light scattering to determine the effect of Pi concentration on DJ-1 aggregation. Interestingly, DJ-1 aggregates with large Rh were detected over 2 mm sodium phosphate buffers, which is a concentration comparable with the elevated Pi levels in the brains of PD patients (34, 35). We also observed time dependence of DJ-1 aggregation in buffers of low Pi concentration. Fresh pDJ-1 purified with 20–50 mm sodium phosphate buffers exhibited large Rh values. In contrast, fresh pDJ-1 in 2–10 mm sodium phosphate buffers exhibited Rh of the dimeric conformation; they showed large Rh values in a time-dependent manner. For example, pDJ-1 purified with a 2 mm sodium phosphate buffer began to display large Rh values 15 days after purification. It should be noted that tDJ-1 has the dimeric Rh value even 15 days after purification.
FIGURE 1.
The size distribution histograms from dynamic light scattering experiments and CD spectra. A, histogram of tDJ-1, pDJ-1, and R48A. The table located below the size distribution histogram lists the number of peaks and their mean value (radius equal to Rh), polydispersity (Polyd), percentage of polydispersity (%Polyd), and estimated relative amount of mass (concentration) of each peak or species (%Mass). B, near-UV CD spectra of tDJ-1 (○) in 20 mm Tris buffer and pDJ-1 (•) in 50 mm sodium phosphate buffer. The final CD spectra were obtained by subtracting the spectra of the buffers from the spectra of the samples. Protein solutions with an OD of 0.66 at 280 nm were used for CD experiments.
The structural differences between tDJ-1 and pDJ-1 are also reflected in a near-UV CD spectrum (Fig. 1B). The near-UV CD spectrum of a protein provides a valuable fingerprint of the tertiary structure of proteins, which can be used to compare protein structures (37).
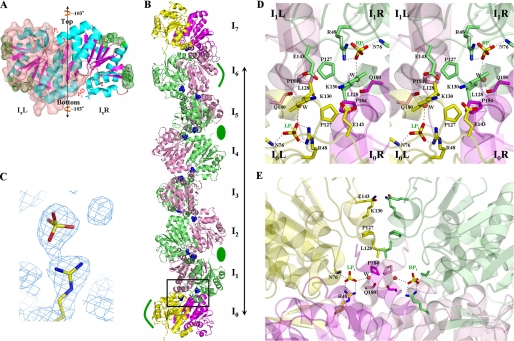
Structural Investigation of DJ-1 Aggregates—To elucidate the structural property of DJ-1 aggregates, we employed electron microscopy. Because it takes a long time for pDJ-1 in buffers of low Pi concentration to form aggregates, we used pDJ-1 purified with a 50 mm sodium phosphate buffer for structural studies. Electron micrographs (EM) of pDJ-1 solutions were obtained on the first 3 days after purification. EM revealed that DJ-1 aggregates have a filamentous shape that gets longer and thicker with time (Fig. 2). The time dependence of DJ-1 aggregates is consistent with the dynamic light scattering results. The low resolution of EM limits structural information on DJ-1 filamentous aggregates. Thus, to obtain an atomic view of DJ-1 aggregates, we grew pDJ-1 crystals. The rod-shaped crystals of pDJ-1 exhibit a filamentous arrangement of DJ-1 molecules. Three DJ-1 dimers and one monomer, which are stacked, exist in an asymmetric unit. The monomer becomes a dimer via the 2-fold crystallographic symmetry. Consequently, continuous stacking of DJ-1 dimers is achieved, resulting in the formation of straight protofilaments (Fig. 3, A and B). Protofilaments are further packed in a side-by-side manner, organized in a filamentous assembly (Fig. 4A).
FIGURE 2.
Electron micrographs of DJ-1 filaments. A, DJ-1 was negatively stained 1 day after purification and visualized. The thickness of protofilaments is 55–65 Å, which is similar to the 60 Å width of our structural model for protofilaments shown in Fig. 3C. B, the enlarged view of the boxed region in A contains protofilaments with their lateral contact marked by an arrow. C, filaments 2–5 times thicker than the protofilaments in A were observed when the sample was stained 3 days after purification.
FIGURE 3.
Structure of DJ-1. A, ribbon diagram of a DJ-1 dimer in the reference orientation. IxL and IxR stand for the left and right monomers of an Ix dimer in this orientation, respectively. To distinguish monomers in the dimer, IxL is covered with transparent surface. The 2-fold axis is vertical to the figure. Green dots represent sites involved in lateral interactions, sticks represent residues implicated in the F contacts, and red spheres represent Pi. The vertical line with arrows at both ends represents the protofilament axis, that is, the rotational axis. B, ribbon drawing of a protofilament. I1–6L and I1–6R monomers are shown in pink and lime, respectively. For emphasis on the identity between I0 and I7 dimers, the two dimers are differently colored; the left and right monomers of I0 and I7 dimers are in yellow and magenta, respectively. The long line shows one longitudinal repeat unit of a protofilament. Green ellipses indicate the location of grooves involved in the lateral interactions between protofilaments, and curved green lines show the prominence regions. Pis are shown as blue spheres. The boxed region is the F interface between I0 and I1 dimers. The 2-fold axis of the F interface is vertical to the figure. C, the final 2Fo - Fc electron density map, contoured at 1 σ, showing Arg48 and an inorganic phosphate. D, front view of the boxed region of B. Residues implicated in the F contacts are labeled and represented by sticks. Oxygen, nitrogen, and sulfur atoms are shown in red, blue, and yellow, respectively. The color scheme for carbon atoms is identical to that in Fig. 1B. Polar interactions between atoms are shown as dashed white lines. E, side view of the boxed region of B. The color scheme for carbon atoms is identical to that in Fig. 1B.
FIGURE 4.
Lateral arrangements among protofilaments. A, top, schematic drawing of the top view. Each circle represents one protofilament, and red lines show the contacts between protofilaments. Bottom, packing model of seven protofilaments simulated in QUANTA. The green ellipsoids show that the central protofilament in red makes no contact with two neighboring protofilaments. B, left panel, surface representation showing a lateral contact between the central protofilament in red and the blue protofilament in A. The three long lines indicate the longitudinal repeat units of protofilaments, and the four short arrows represent the protofilament axes. The green box covers the only contact between the longitudinal repeat unit of the central protofilament and the adjacent protofilament in blue. There are no lateral interactions in the green circle, although slight contacts between protofilaments are seen because of the figure orientation. Right panel, the close-up view of the region covered by the green box in the left figure. The surfaces are transparently represented to show the interior. The residues implicated in the lateral interactions are represented by sticks and labeled. Oxygen and nitrogen are shown in red and blue, respectively. Polar interactions between atoms are shown as dashed black lines.
Within the protofilaments, DJ-1 dimers are piled in a rotational head-to-tail manner. The rotational axis of this conformation coincides with the protofilament axis (Fig. 3A), giving the protofilaments the appearance of circular cylinders when viewed from above (Fig. 4A). The top side of a DJ-1 dimer makes tight contact with the bottom side of the next dimer, displaying a ∼103° (720°/7) rotational difference (Fig. 3A). As a result, the I7 dimer exhibits the same orientation as the I0 dimer after 720° rotation; hence, the seven (from I0 to I6) dimers constitute one longitudinal repeat unit of DJ-1 protofilaments (Fig. 3B). No detectable structural differences were observed among the dimers stacked in the protofilaments. In addition, the dimers in the protofilaments are nearly structurally identical to free DJ-1 dimers (11); their backbone atoms align with an root mean square deviation of 0.175 Å. The contacts between dimers in the protofilaments, hereafter referred to as “F contacts,” bury 1809 Å2 of the solvent-accessible surface area of a DJ-1 dimer. A closer look at the F interfaces reveals their inherent 2-fold symmetry, as well as three major F contacts along the 2-fold axis: ion pairs, a hydrophobic core, and Pi-mediated contacts (Fig. 3, D and E). Because all of the F interfaces in protofilaments are identical, we describe only one F interface, which is located between the I0 and I1 dimers. Lys130 and Glu143 of the I0L monomer form ion pairs with Glu143 and Lys130 of the I1R monomer, respectively. Pro127 and Leu128 from the I0L monomer and I1R monomer, coupled with Pro184 from the I0R and I1L monomers, form the hydrophobic core. Two Pis, related by the F 2-fold axis, take positions at the F interface, mediating extensive contact: one is located between the I0L and I1L monomers (LPi), and the other is located between the I0R and I1R monomers (RPi) (Fig. 3, C–E). LPi directly forms polar interactions with the guanidium group of Arg48 and the amide nitrogen of Asn76 in the I0L monomer and makes a water-mediated hydrogen bond with the carbonyl oxygen of Gln180 in the I1L monomer. RPi is coordinated in an identical fashion. Because the guanidium group of Arg48 participates in coordinating Pi (Fig. 3, C–E), its elimination would prevent the binding of Pi at the F interfaces, affecting the formation of filamentous aggregates. To establish the role of Pi as an inducer of filamentous aggregates, we generated a R48A mutant. The mutant exhibits an Rh of 2.75 nm even in a high Pi concentration (Fig. 1A), pointing to the critical role of Pi in DJ-1 aggregation.
Protofilaments run parallel with one another in crystals. Although one protofilament is surrounded by six others, two of these do not actually contact the central protofilament (Fig. 4A, bottom panel), thus displaying a parallelogram pattern when viewed from the top (Fig. 4A, top panel). The lateral interactions between protofilaments exhibit good geometric complementarities, such that prominence regions in protofilaments fit into grooves in adjacent protofilaments (Fig. 4B). The I0L and I6L monomers function as prominences, and the grooves participating in these lateral interactions are located both between the I1R and I2L monomers and between the I4R and I5L monomers (Fig. 3B). Consequently, four lateral contact sites are located in each longitudinal repeat unit of a protofilament. Each site in a longitudinal repeat unit interacts with one of the surrounding protofilaments, and thus each longitudinal repeat unit makes contact with four protofilaments. The lateral interface between a prominence and a groove is composed of two contacting parts. Because all the lateral interfaces are identical, for convenience we describe only one lateral interface between the I0L prominence of a protofilament and the groove between the I4R and I5L monomers of an adjacent protofilament. One component of the interface is formed by the contact between the I0L prominence and the I5L monomer of the groove (Fig. 4), where Glu116 and His138 of I0L form ion pairs with Arg98 and the carbonyl carbon of Ala63 of I5L in the groove, respectively. The other interface component is formed by the contact between the I0L prominence and the I4R monomer of the groove (Fig. 4). The only interaction occurring in the second component is the ion pairing between Arg98 of I0L and Glu64 of I4R in the groove. Compared with the F contacts, lateral contacts are not extensive. Lateral interactions between a prominence and a groove bury only 357 Å2 of the solvent-accessible surface. This nonextensive lateral contact would allow DJ-1 to be assembled into a multitude of different aggregates through a different arrangement of lateral interactions, in the same manner as other filamentous proteins that form crystalline, curved, or branched aggregates (38, 39).
Comparison between EM and Crystal Structure—Although detailed comparisons between the EM and crystal structure are not possible because of the low resolution of the EM, the overall nature of the crystal structure seems to be well reflected in the EM. Similar to the protofilament structure (Fig. 3B), the EM protofilaments resemble the anterior view of a human spine, i.e. a row of small bones (Fig. 2, A and B). The dimensions (∼60 × ∼50 Å) of the objects that correspond to small bones in the spine are comparable with those of DJ-1 dimers, representing the stacking of DJ-1 dimers in the protofilaments (Fig. 3B). In addition, the contact between protofilaments, which is marked by an arrow in Fig. 2B), is reminiscent of the aforementioned lateral interactions, exhibiting geometric complementarity between prominences and grooves (Fig. 4B).
Concluding Remarks—DJ-1 is implicated in PD pathogenesis (7), and insoluble DJ-1 aggregates have been observed in brains of patients with neurodegenerative diseases (24–29). Because protein aggregates not only are diagnostic hallmarks but have also been implicated in the pathogenesis of neurodegenerative diseases (40), physicochemical information on in vivo DJ-1 aggregates bears potential for further understanding of the etiology of PD. Here, we describe a biochemical and structural investigation of in vitro DJ-1 aggregates whose formation is dependent only on Pi. Unfortunately, there have been no reports on the characterization of in vivo DJ-1 aggregates, which precludes direct comparison between in vivo aggregates and the Pi-induced DJ-1 aggregates presented in this study. Therefore, we cannot assert that in vivo DJ-1 aggregates share characteristics with the Pi-induced aggregates. However, considering the observation that recombinant proteins forming pathological aggregates in brains can assemble into aggregates in vitro that closely resemble corresponding in vivo aggregates (41), the physicochemical properties of the Pi-induced aggregates might provide some insights into those of in vivo DJ-1 aggregates.
The loss of DJ-1 function induced by mutations in the DJ-1 gene has been reported to lead to neurodegeneration (7). There is an emerging consensus that DJ-1 may provide protective roles against various stresses such as the oxidative damage involved in PD pathogenesis (22, 42–44). DJ-1 aggregation, which removes soluble DJ-1, would lead cells to lose functional DJ-1 that normally performs neuroprotective roles. As such, DJ-1 aggregation may be compatible with the loss-of-function pathogenic mechanism of DJ-1 (7). To determine the pathological role of the Pi-dependent DJ-1 aggregation, however, it is prerequisite to reveal the in vivo relationship between fragmentary observations of the Pi level and the DJ-1 aggregation. The increased Pi level in the brains of patients with neurodegenerative diseases (34, 35) shows that their physiological conditions can be thought to have been shifted to more suitable environments for DJ-1 aggregation. Nevertheless, because of the lack of any information on the cellular factors affecting protein aggregation, it is not certain that the observed DJ-1 aggregates in vivo (24–29) are correlated with the Pi level. It should also be noted that the Pi-dependent DJ-1 aggregation is described here for the first time. Consequently, at present we cannot determine whether Pi elevation induces DJ-1 aggregation in the brain, culminating in neurodegeneration. A future challenge is to address this issue.
Although the implication of the Pi-dependent DJ-1 aggregation in the pathogenesis of PD remains to be elucidated, the novel finding regarding the critical role of Pi in DJ-1 aggregation and the detailed view of a DJ-1 filamentous assembly at atomic resolution extends our knowledge of molecular mechanisms behind protein aggregation and provides an important frame-work for future works in unveiling the physiological meaning of the Pi-dependent DJ-1 aggregation.
The atomic coordinates and structure factors (code 3BWE) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported by the Functional Proteomics Center Program, Korea Ministry of Science and Technology and by the Marine & Extreme Genome Research Center Program, Ministry of Land, Transport, and Maritime Affairs, Republic of Korea. This work was also supported by Korea Institute of Science and Technology institutional grants and a Research Fellowship of the BK21 Project, Republic of Korea (to I. K. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PD, Parkinson disease; EM, electron micrograph(s).
References
- 1.Nagakubo, D., Taira, T., Kitaura, H., Ikeda, M., Tamai, K., Iguchi-Ariga, S. M., and Ariga, H. (1997) Biochem. Biophys. Res. Commun. 231 509-513 [DOI] [PubMed] [Google Scholar]
- 2.Hod, Y., Pentyala, S. N., Whyard, T. C., and El-Maghrabi, M. R. (1999) J. Cell. Biochem. 72 435-444 [PubMed] [Google Scholar]
- 3.Takahashi, K., Taira, T., Niki, T., Seino, C., Iguchi-Ariga, S. M., and Ariga, H. (2002) J. Biol. Chem. 276 37556-37563 [DOI] [PubMed] [Google Scholar]
- 4.Klinefelter, G. R., Laskey, J. W., Ferrell, J., Suarez, J. D., and Roberts, N. L. (1997) J. Androl. 18 139-150 [PubMed] [Google Scholar]
- 5.Wagenfeld, A., Yeung, C. H., Strupat, K., and Cooper, T. G. (1998) Biol. Reprod. 58 1257-1265 [DOI] [PubMed] [Google Scholar]
- 6.Welch, J. E., Barbee, R. R., Roberts, N. L., Suarez, J. D., and Klinefelter, G. R. (1998) J. Androl. 19 385-393 [PubMed] [Google Scholar]
- 7.Bonifati, V., Rizzu, P., van Baren, M. J., Schaap, O., Breedveld, G. J., Krieger, E., Dekker, M. C. J., Squitieri, F., Ibanez, P., Joosse, M., van Dongen, J. W., Vanacore, N., van Swieten, J. C., Brice, A., Meco, G., van Duijn, C. M., Oostra, B. A., and Heutink, P. (2003) Science 299 256-259 [DOI] [PubMed] [Google Scholar]
- 8.Miller, D. W., Ahmad, R., Hague, S., Baptista, M. J., Canet-Aviles, R., McLendon, C., Carter, D. M., Zhu, P. P., Stadler, J., Chandran, J., Klinefelter, G. R., Blackstone, C., and Cookson, M. R. (2003) J. Biol. Chem. 278 36588-36595 [DOI] [PubMed] [Google Scholar]
- 9.Moore, D. J., Zhang, L., Dawson, T. M., and Dawson, V. L. (2003) J. Neurochem. 87 1558-1567 [DOI] [PubMed] [Google Scholar]
- 10.Olzmann, J. A., Brown, K., Wilkinson, K. D., Rees, H. D., Huai, Q., Ke, H., Levey, A. I., Li, L., and Chin, L. S. (2004) J. Biol. Chem. 279 8506-8515 [DOI] [PubMed] [Google Scholar]
- 11.Lee, S. J., Kim, S. J., Kim, I. K., Ko, J., Jeong, C. S., Kim, G. H., Park, C., Kang, S. O., Suh, P. G., Lee, H. S., and Cha, S. S. (2003) J. Biol. Chem. 278 44552-44559 [DOI] [PubMed] [Google Scholar]
- 12.Shendelman, S., Jonason, A., Martinat, C., Leete, T., and Abeliovich, A. (2004) PLoS Biol. 2 e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang, Y., Gehrke, S., Haque, M. E., Imai, Y., Kosek, J., Yang, L., Beal, M. F., Nishimura, I., Wakamatsu, K., Ito, S., Takahashi, R., and Lu, B. (2005) Proc. Natl. Acad. Sci. U. S. A 102 13670-13675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, R. H., Peters, M., Jang, Y., Shi, W., Pintilie, M., Fletcher, G. C., De-Luca, C., Liepa, J., Zhou, L., Snow, B., Binari, R. C., Manoukian, A. S., Bray, M. R., Liu, F. F., Tsao, M. S., and Mak, T. W. (2005) Cancer Cell 7 263-273 [DOI] [PubMed] [Google Scholar]
- 15.Junn, E., Taniguchi, H., Jeong, B. S., Zhao, X., Ichijo, H., and Mouradian, M. M. (2005) Proc. Natl. Acad. Sci. U. S. A 102 9691-9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meulener, M., Whitworth, A. J., Armstrong-Gold, C. E., Rizzu, P., Heutink, P., Wes, P. D., Pallanck, L. J., and Bonini, N. M. (2005) Curr. Biol. 15 1572-1577 [DOI] [PubMed] [Google Scholar]
- 17.Menzies, F. M., Yenisetti, S. C., and Min, K. T. (2005) Curr. Biol. 15 1578-1582 [DOI] [PubMed] [Google Scholar]
- 18.Goldberg, M. S., Pisani, A., Haburcak, M., Vortherms, T. A., Kitada, T., Costa, C., Tong, Y., Martella, G., Tscherter, A., Martins, A., Bernardi, G., Roth, B. L., Pothos, E. N., Calabresi, P., and Shen, J. (2005) Neuron 45 489-496 [DOI] [PubMed] [Google Scholar]
- 19.Kim, R. H., Smith, P. D., Aleyasin, H., Hayley, S., Mount, M. P., Pownall, S., Wakeham, A., You-Ten, A. J., Kalia, S. K., Horne, P., Westaway, D., Lozano, A. M., Anisman, H., Park, D. S., and Mak, T. W. (2005) Proc. Natl. Acad. Sci. U. S. A 102 5215-5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, L., Cagniard, B., Mathews, T., Jones, S., Koh, H. C., Ding, Y., Carvey, P. M., Ling, Z., Kang, U. J., and Zhuang, X. (2005) J. Biol. Chem. 280 21418-21426 [DOI] [PubMed] [Google Scholar]
- 21.Shinbo, Y., Niki, T., Taira, T., Ooe, H., Takahashi-Niki, K., Maita, C., Seino, C., Iguchi-Ariga, S. M., and Ariga, H. (2006) Cell Death Differ 13 96-108 [DOI] [PubMed] [Google Scholar]
- 22.Canet-Aviles, R. M., Wilson, M. A., Miller, D. W., Ahmad, R., McLendon, C., Bandyopadhyay, S., Baptista, M. J., Ringe, D., Petsko, G. A., and Cookson, M. R. (2004) Proc. Natl. Acad. Sci. U. S. A 101 9103-9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taira, T., Saito, Y., Niki, T., Iguchi-Ariga, S. M., Takahashi, K., and Ariga, H. (2004) EMBO Rep. 5 213-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann, M., Muller, V., Gorner, K., Kretzschmar, H. A., Haass, C., and Kahle, P. J. (2004) Acta Neuropathol. (Berl.) 107 489-496 [DOI] [PubMed] [Google Scholar]
- 25.Rizzu, P., Hinkle, D. A., Zhukareva, V., Bonifati, V., Severijnen, L. A., Martinez, D., Ravid, R., Kamphorst, W., Eberwine, J. H., Lee, V. M., Trojanowski, J. Q., and Heutink, P. (2004) Ann. Neurol. 55 113-118 [DOI] [PubMed] [Google Scholar]
- 26.Baulac, S., LaVoie, M. J., Strahle, J., Schlossmacher, M. G., and Xia, W. (2004) Mol. Cell Neurosci. 27 236-246 [DOI] [PubMed] [Google Scholar]
- 27.Jin, J., Meredith, G. E., Chen, L., Zhou, Y., Xu, J., Shie, F. S., Lockhart, P., and Zhang, J. (2005) Brain Res. Mol. Brain Res. 134 119-138 [DOI] [PubMed] [Google Scholar]
- 28.Moore, D. J., Zhang, L., Troncoso, J., Lee, M. K., Hattori, N., Mizuno, Y., Dawson, T. M., and Dawson, V. L. (2005) Hum. Mol. Genet. 14 71-84 [DOI] [PubMed] [Google Scholar]
- 29.Moore, D. J., West, A. B., Dawson, V. L., and Dawson, T. M. (2005) Annu. Rev. Neurosci. 28 57-87 [DOI] [PubMed] [Google Scholar]
- 30.Tao, X., and Tong, L. (2003) J. Biol. Chem. 278 31372-31379 [DOI] [PubMed] [Google Scholar]
- 31.Wilson, M. A., Collins, J. L., Hod, Y., Ringe, D., and Petsko, G. A. (2003) Proc. Natl. Acad. Sci. U. S. A 100 9256-9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honbou, K., Suzuki, N. N., Horiuchi, M., Niki, T., Taira, T., Ariga, H., and Inagaki, F. (2003) J. Biol. Chem. 278 31380-31384 [DOI] [PubMed] [Google Scholar]
- 33.Huai, Q., Sun, Y., Wang, H., Chin, L. S., Li, L., Robinson, H., and Ke, H. (2003) FEBS Lett. 549 171-175 [DOI] [PubMed] [Google Scholar]
- 34.Barbiroli, B., Martinelli, P., Patuelli, A., Lodi, R., Iotti, S., Cortelli, P., and Montagna, P. (1999) Mov. Disord. 14 430-435 [DOI] [PubMed] [Google Scholar]
- 35.Brown, G. G., Levine, S. R., Gorell, J. M., Pettegrew, J. W., Gdowski, J. W., Bueri, J. A., Helpern, J. A., and Welch, K. M. (1989) Neurology 39 1423-1427 [DOI] [PubMed] [Google Scholar]
- 36.Dhingra, R., Sullivan, L. M., Fox, C. S., Wang, T. J., D'Agostino, R. B., Sr., Gaziano, J. M., and Vasan, R. S. (2007) Arch. Intern Med. 167 879-885 [DOI] [PubMed] [Google Scholar]
- 37.Kelly, S. M., Jess, T. J., and Price, N. C. (2005) Biochim. Biophys. Acta 1751 119-139 [DOI] [PubMed] [Google Scholar]
- 38.Lowe, J., and Amos, L. A. (1999) EMBO J. 18 2364-2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aebi, U., Fowler, W. E., Isenberg, G., Pollard, T. D., and Smith, P. R. (1981) J. Cell Biol. 91 340-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J., Taddei, N., Ramponi, G., Dobson, C. M., and Stefani, M. (2002) Nature 416 507-511 [DOI] [PubMed] [Google Scholar]
- 41.Crowther, R. A., Jakes, R., Spillantini, M. G., and Goedert, M. (1998) FEBS Lett. 436 309-312 [DOI] [PubMed] [Google Scholar]
- 42.Batelli, S., Albani, D., Rametta, R., Polito, L., Prato, F., Pesaresi, M., Negro, A., and Forloni, G. (2008) PLoS ONE 3 e1884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Yanagisawa, D., Kitamura, Y., Inden, M., Takata, K., Taniguchi, T., Morikawa, S., Morita, M., Inubushi, T., Tooyama, I., Taira, T., Iguchi-Ariga, S. M., Akaike, A., and Ariga, H. (2008) J. Cereb Blood Flow Metab. 28 563-578 [DOI] [PubMed] [Google Scholar]
- 44.Liu, F., Nguyen, J. L., Hulleman, J. D., Li, L., and Rochet, J. C. (2008) J. Neurochem.