Abstract
Laminin-332 (Lm332) is a large heterotrimeric glycoprotein that has been identified as a scattering factor, a regulator of cancer invasion as well as a prominent basement membrane component of the skin. Past studies have identified the functional domains of Lm332 and revealed the relationships between its activities and the processing of its subunits. However, there is little information available concerning the effects of N-glycosylation on Lm332 activities. In some cancer cells, an increase of β1,6-GlcNAc catalyzed by N-acetylglucosaminyltransferase V (GnT-V) is related to the promotion of cancer cell motility. By contrast, bisecting GlcNAc catalyzed by N-acetylglucosaminyltransferase III (GnT-III) suppresses the further processing with branching enzymes, such as GnT-V, and the elongation of N-glycans. To examine the effects of those N-glycosylations to Lm332 on its activities, we purified Lm332s from the conditioned media of GnT-III- and GnT-V-overexpressing MKN45 cells. Lectin blotting and mass spectrometry analyses revealed that N-glycans containing the bisecting GlcNAc and β1,6-GlcNAc structures were strongly expressed on Lm332 purified from GnT-III-overexpressing (GnT-III-Lm332) and GnT-V-overexpressing (GnT-V-Lm332) cells, respectively. Interestingly, the cell adhesion activity of GnT-III-Lm332 was apparently decreased compared with those of control Lm332 and GnT-V-Lm332. In addition, the introduction of bisecting GlcNAc to Lm332 resulted in a decrease in its cell scattering and migration activities. The weakened activities were most likely derived from the impaired α3β1 integrin clustering and resultant focal adhesion formation. Taken together, our results clearly demonstrate for the first time that N-glycosylation may regulate the biological function of Lm332. This finding could introduce a new therapeutic strategy for cancer.
Laminins (Lms)2 are large heterotrimeric glycoproteins that are prominent components of basement membranes and are involved in important biological roles, including tissue development and cell differentiation, survival, adhesion, and migration (1). Lms are heavily glycosylated molecules. It has been reported that between 13 and 30% of the total molecular weight of Lms is N-linked glycosylated (2). Laminin-111 (Lm111; previously known as laminin-1) is easily purified from mouse Engelbreth-Horm-Swarm tumor and has been intensively investigated for its carbohydrate structures. In comparison with unglycosylated Lm111, which is purified from cell lysates of tunicamycin-treated cells, glycosylation of Lm111 was shown to affect cell spreading and neurite outgrowth activities but not cell adhesion activity or heterotrimer assembly (3, 4). However, tunicamycin extensively inhibited the secretion of laminin into cell culture medium (3, 5).
Laminin-332 (Lm332; previously known as laminin-5) is composed of α3, β3, and γ2 chains. Lm332 is expressed in the skin and other stratified squamous epithelial tissues, where is associate with hemidesmosomes via integrin α6β4. Genetic absence of Lm332 causes a severe and lethal skin blistering disease, Herlitz's junctional epidermolysis bullosa (6, 7). In vitro Lm332 promotes cell motility and scattering through the association of C-terminal globular (G) domains with integrin α3β1 (8), which is thought to be a key factor during wound healing (9, 10) and cancer metastasis (11, 12). Proteolytic cleavage of the Lm332 α3 and γ2 subunits (13, 14) affects both cell adhesion and migration (15, 16). Few studies have assessed the functional significance of N-glycosylation of Lm332 subunits.
N-Acetylglucosaminyltransferase V (GnT-V) catalyzes the addition of the β1,6-linked GlcNAc branch and defines this subset of N-glycans (17, 18) (Fig. 1A). In some cancers, an increase of β1,6-GlcNAc is related to cancer metastasis. This is supported by several reports, including GnT-V overexpression in cancer (19, 20) and GnT-V-deficient mouse studies (21). By contrast, bisecting GlcNAc catalyzed by N-acetylglucosaminyltransferase III (GnT-III) (Fig. 1A) suppresses further processing with branching enzymes, such as GnT-V, and elongation of N-glycans (22, 23), resulting in down-regulating cancer metastasis (24). In addition, GnT-III modification of α3β1 integrin inhibits cell migration promoted by GnT-V on the Lm332 substrate (25).
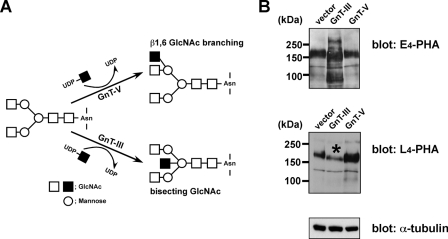
FIGURE 1.
Characterization of MKN45 transfectants overexpressing GnT-III or GnT-V. A, biosynthesis pathways of the bisecting GlcNAc and β1,6-GlcNAc branching structures by GnT-III and GnT-V, respectively. Square, N-acetylglucosamine; circle, mannose. B, 20 μg of cell lysates from vector, GnT-III, and GnT-V MKN45 transfectants were run on 7.5% SDS-polyacrylamide gels and then blotted to nitrocellulose membranes. Blotted proteins were probed with biotinylated E4-PHA lectin (top) and L4-PHA lectin (middle). E4-PHA and L4-PHA lectin blotting revealed that GnT-III and GnT-V transfectants were significantly modified with bisecting GlcNAc and β1,6-linked GlcNAc, respectively. An asterisk indicates reduced L4-PHA staining in GnT-III transfectants. α-tubulin was used as a loaded control.
In the present study, to investigate the effect of N-glycosylation on Lm332 function, we focused on GnT-III- and GnT-V-mediated N-glycosylation of Lm332. Therefore, we purified Lm332s from the conditioned media of GnT-III- and GnT-V-overexpressing MKN45 cells. The analysis of lectin blotting and mass spectrometry indicated that Lm332 could be modified by either GnT-III or GnT-V. As a result, GnT-III modification of Lm332 caused a decrease in its keratinocyte cell adhesion and migration activities. Our findings demonstrate a novel regulatory mechanism of Lm332 activities brought on by N-glycosylation.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents—Mouse monoclonal antibodies against the N-terminal regions of the human laminin α3 chain (Lsαc3) and the γ2 chain (D4B5) were a generous gift from Dr. Kaoru Miyazaki (Yokohama City University, Yokohama, Japan). Mouse monoclonal antibody against the laminin α3 chain (2B10) was prepared as previously described (26). Monoclonal antibodies against the human laminin β3 chain (kalinin B1) and paxillin were purchased from Transduction Laboratories (Lexington, KY). Control mouse and rat IgG and function-blocking anti-integrin α3 (P1B5) and α6 (GoH3) antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Alexa Fluor 488 goat anti-mouse IgG was obtained from Invitrogen. Biotinylated leukoagglutinating phytohemagglutinin (L4-PHA) and biotinylated erythroagglutinating phytohemagglutinin (E4-PHA) were from Seikagaku Biobusiness Corp. (Tokyo, Japan). A monoclonal antibody against α-tubulin was purchased from Sigma.
Cell Culture—The human gastric cancer cell line MKN45 was cultured in RPMI 1640 medium (Nacalai Tesque, Japan). MKN45 transfectants were described previously (27). The Buffalo rat liver-derived epithelial cell line BRL was a gift from Dr. Kaoru Miyazaki (Yokohama City University, Yokohama, Japan) and was maintained in Dulbecco's modified Eagle's medium. Those media were supplemented with 10% fetal calf serum, penicillin, and streptomycin sulfate. Keratinocytes isolated from patients with junctional epidermolysis bullosa lacking Lm332 were a generous gift from Dr. M. Peter Marinkovich (Stanford University). Keratinocytes were grown in 50% defined keratinocyte medium (Invitrogen) and 50% medium 154 (Cascade Biologics, Portland, OR) containing penicillin and streptomycin sulfate.
Preparation of Conditioned Medium and Purification of Laminin-332—For purification of Lm332, the serum-free CM from MKN45 transfectants were collected every 2 or 3 days. The collected media were centrifuged at 1,000 rpm for 10 min. Finally its supernatant was collected and used as a source for purification of Lm332. The protein containing the supernatant was precipitated by 80% saturated ammonium sulfate. The precipitate was dissolved in and dialyzed against a gelatin column buffer (20 mm Tris-HCl (pH 7.5), 0.1 m NaCl, 0.1% CHAPS, 0.005% Brij 35) overnight at 4 °C. Then samples were centrifuged at 19,000 rpm for 30 min at 4 °C to remove the undissolved proteins. The precleared solution was passed through a gelatin column, and then its flow-through was directly applied to an α3 antibody (2B10) column. After washing with the antibody column buffer (20 mm Tris-HCl (pH 7.5), 0.5 m NaCl, 0.1% CHAPS, 0.005% Brij 35), followed by MilliQ water, binding proteins were eluted by 0.05% trifluoroacetic acid (v/v) and immediately neutralized with Tris-HCl (pH 8.0) containing 0.005% Brij 35 and 0.1% CHAPS.
Preparation of Cell Lysate—For preparing cell lysate, cells were washed with cold PBS twice and then lysed with lysis buffer (1% Triton X-100, 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 5 mm EDTA) containing protease inhibitor mixture (Nacalai Tesque). After incubation for 10 min on ice, cell lysates were cleared by centrifugation at 15,000 rpm for 10 min at 4 °C, and its supernatants were used in the following samples. The protein concentration was determined using a protein assay kit (Nacalai Tesque).
SDS-PAGE, Immunoblotting, and Lectin Blotting—SDS-PAGE was performed either on 6% gels or on 4.0–7.5% gradient gels under reducing or nonreducing conditions. Separated proteins were stained with Coomassie Brilliant Blue (CBB). ImageJ software was used for densitometric analysis of protein bands. For immunoblotting analyses, proteins resolved by SDS-PAGE were transferred to nitrocellulose membranes. The blots were probed with each specific antibody or biotinylated E4-PHA and L4-PHA. Immunoreactive bands were detected using an ECL detection kit (GE Healthcare) and a Vectastain ABC kit (Vector Laboratories).
Analysis of N-Glycan Structures by Liquid Chromatography/Multiple-stage Mass Spectrometry (LC/MSn)—Purified Lm332 was applied to SDS-PAGE using a 4–7.5% gradient gel under reducing conditions and visualized by CBB staining. The gel bands corresponding to α3, β3, and γ2 subunits were excised from the gel and then cut into pieces, respectively. The gel pieces were destained with 25 mm NH4HCO3 containing 30% acetonitrile and then dehydrated with 100% acetonitrile. The proteins in the gel were reduced and carboxymethylated by incubation with dithiothreitol and sodium monoiodoacetate (25). N-Glycans were released by the treatment with peptide:N-glycanase F, and extracted from the gel pieces, as reported (28). The oligosaccharides were reduced with sodium borohydride and desalted. LC/MSn was performed using a quadropole linear ion trap-Fourier transform ion cyclotron resonance mass spectrometer (Finnigan LTQ-FT™; Thermo Fisher Scientific Corp., San Jose, CA) connected to a nanoflow LC system (NanoFrontier nLC; Hitachi High-Technologies Corp., Japan). The eluents were 5 mm ammonium acetate containing 2% acetonitrile, pH 9.6 (pump A), and 5 mm ammonium acetate containing 80% acetonitrile, pH 9.6 (pump B). The borohydride-reduced N-glycans were separated on a Hypercarb column (0.075 × 150 mm; Thermo Fisher Scientific Corp.) with a linear gradient of 5–35% of pump B in 110 min. A single mass scan with Fourier transform (m/z 450–2,000) followed by data-dependent MS/MS for the most intense ions was performed in both positive and negative ion modes as previously reported (29).
Cell Adhesion Assay—Cell adhesion assay was performed as described previously (8). Briefly, each well of a 96-well enzyme-linked immunosorbent assay (ELISA) plate (Costar, Cambridge, MA) was coated with a substrate protein and then blocked with 1% bovine serum albumin (BSA). 2 × 104 cells in supplement-free keratinocyte growth medium were inoculated per well of 96-well plates. After nonadherent cells were removed, adherent cells were fixed with 25% (w/v) glutaraldehyde and stained with 0.5% crystal violet (w/v) in 20% (v/v) methanol for 10 min. The well was measured for absorbance at 590 nm using a microplate reader. For inhibition assay, the cell suspension was incubated with function-blocking anti-integrin antibodies or with the control IgG for 20 min at room temperature before inoculation.
Cell Spreading Assay—For measurement of the cell-spreading area, keratinocytes on purified Lm332 substrates were incubated in keratinocyte growth medium. After 1 h, at least 100 cells were photographed, and their cell spreading areas were measured using Axio Vision software (Carl Zeiss, Germany).
Cell Scattering Assay—A scattering assay was done as reported previously (10). Briefly, 500 μl of cell suspension (2 × 104 cells in Dulbecco's modified Eagle's medium plus 1% (v/v) fetal calf serum) were inoculated per well of 24-well plates. Test samples were directly added into the culture medium and incubated at 37 °C. After 40 h, cells were fixed with 25% (w/v) glutaraldehyde and stained with 0.5% crystal violet (w/v) in 20% (v/v) methanol for 10 min. The scattered single cells were counted, and the degree of cell scattering was expressed as the percentage of single cells in each field. At least 300 cells were counted in each field.
Cell Migration Assay—A glass bottom dish (Asahi Techno Glass, Japan) was precoated with purified Lm332 and then blocked with 1% BSA for 1 h at 37°C. 200 μl of Lm332-null keratinocyte cell suspension (4 × 104 cells/ml) in growth medium were inoculated into each Lm332-precoated glass bottom dish. After incubation for 1 h at 37°C, cell movement was monitored using time lapse video equipment (Carl Zeiss) for 8 h.
ELISA—The ELISA was as follows. The wells of a 96-well plate were coated with test proteins and then blocked with 1.2% BSA at room temperature for 1 h. The wells were washed with PBS containing 0.05% Tween 20 (washing buffer) three times and then incubated with primary antibody for 1 h at room temperature. Furthermore, the wells were washed with washing buffer three times and then incubated with secondary antibody coupled with biotin for 45 min at room temperature. Similarly, the wells were washed three times and incubated with alkaline phosphatase conjugated with avidin D for 45 min at room temperature. After five washes with washing buffer, the bound antibodies were quantified by their absorbance at 405 nm after incubation with p-nitrophenylphosphate disodium salt in 100 mm diethanolamine (pH 9.8) containing 0.24 mm MgCl2.
Immunofluorescence Microscopy—A glass bottom dish (Asahi Techno Glass) was precoated with purified Lm332 and then blocked with 1% BSA for 1 h at 37°C. 200 μl of the cell suspension (2 × 105 cells/ml) in growth medium were inoculated into each Lm332-precoated glass bottom dish. After incubation for 1 h, the cells were washed with PBS and then fixed with 4% (w/v) paraformaldehyde in PBS for 10 min. For permeabilization, the cells were treated with 0.2% (v/v) Triton X-100 in PBS. The fixed cells were blocked with 2% BSA in PBS for 1 h before staining with appropriate primary and secondary antibodies. Fluorescence images were obtained using a fluorescence microscope (Olympus, Tokyo) equipped with ×100/1.35 UPlan-Apochromat oil immersion objectives.
Statistical Analysis—Data are presented as mean ± S.D. Student's t test, with Microsoft Excel, was used to compare the two groups.
RESULTS
Characterization of GnT-III and GnT-V Transfectants—As a source for purification of Lm332 modified by GnT-III- or GnT-V-catalyzed glycosylation, we prepared GnT-III- and GnT-V-overexpressing MKN45 transfectants and also a vector-only transfectant to be used as a negative control as previously described (27). To check the changes in oligosaccharide structures for each transfectant, lectin blotting was performed against the cell lysates from those transfectants using E4-PHA, which preferentially binds to bisecting GlcNAc residues in N-glycans or L4-PHA, which preferentially binds to β1,6-branched GlcNAc residues (30). The blotting results showed that the GnT-III transfectant increased bisecting GlcNAc compared with the other two (Fig. 1B, E4-PHA) but decreased GnT-V products (Fig. 1B, L4-PHA), supporting the notion that GnT-III antagonizes the action of GnT-V for the modification of some target glycoproteins, such as α3β1 integrin (25). On the other hand, GnT-V overexpression had no effect on the GnT-III product level (Fig. 1B, E4-PHA) but led to a strong increase of β1,6-branched GlcNAc in MKN45 cells (Fig. 1B, L4-PHA). The immunoblotting using an anti-α-tubulin antibody showed that the amount of a loaded protein was almost the same among the three samples (Fig. 1B, α-tubulin).
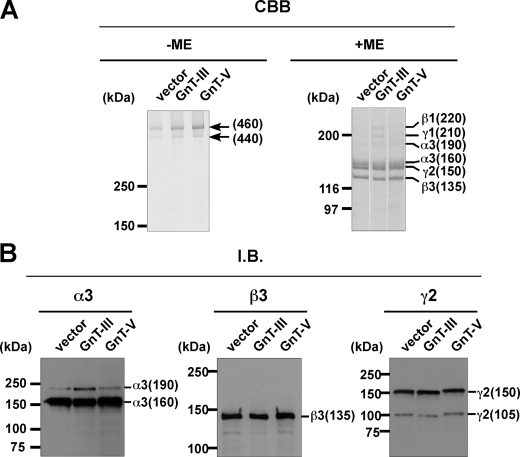
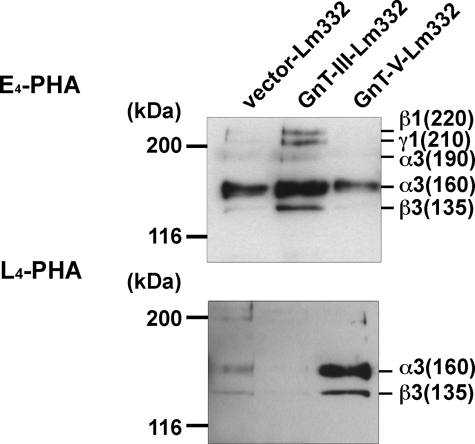
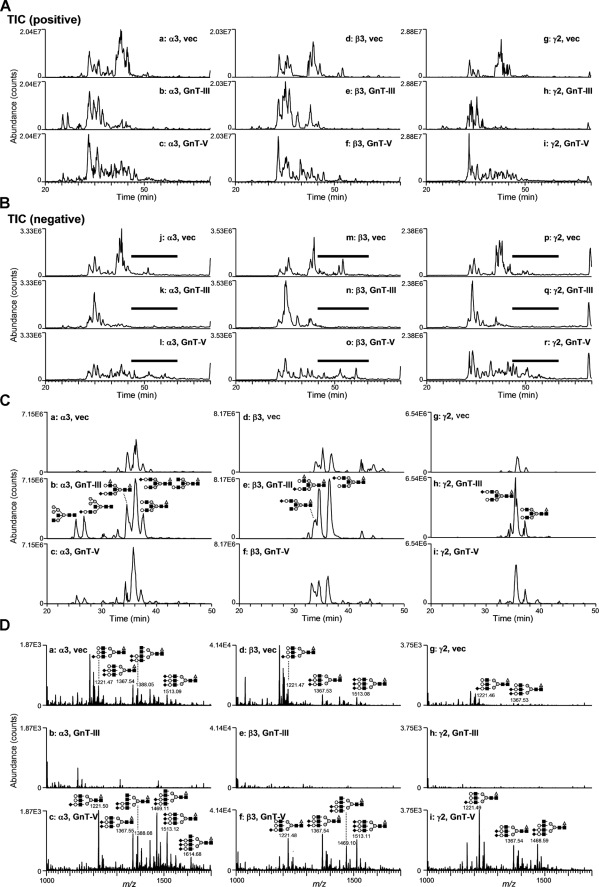
Purification and Characterization of Lm332 from MKN45 Transfectants—To examine whether Lm332 could be modified by GnT-III or GnT-V to regulate its functions, we tried to purify Lm332 from the CM of vector-, GnT-III-, and GnT-V-MKN45 transfectants using a laminin α3 chain antibody (2B10) column. Purified Lm332 was run on 4–7.5% gradient gel and 6% gel for CBB staining and immunoblotting, respectively (Fig. 2). CBB staining under nonreducing conditions revealed two major bands at ∼460 and 440 kDa, which correspond to the Lm332 forms with an unprocessed 150-kDa γ2 chain and with a processed 105-kDa γ2 chain, respectively (Fig. 2, CBB, left). SDS-PAGE under reducing conditions separated the Lm subunits. All purified Lm332s contained the three major bands of 160-, 150-, and 135-kDa proteins, which correspond to the processed α3 chain, the unprocessed γ2 chain, and the β3 chain, respectively (Fig. 2, CBB, right). In addition to those bands, there are three extra bands, which were larger than the 160-kDa processed α3 chain band. Mass spectrometry analysis showed that those bands are laminin β1, γ1, and an unprocessed α3 chain (190 kDa) (data not shown). A likely explanation is that this represented laminin-311 (Lm311; laminin-6), which is composed of α3, β1, and γ1 chains. Lm332 purified from GnT-III MKN45 transfectant (GnT-III-Lm332) contained slightly increased amounts of those extra bands rather than the Lm332 from vector MKN45 (vector-Lm332) and from GnT-V MKN45 transfectants (GnT-V-Lm332), suggesting that the processing of the α3 chain, but not the β3 and γ2 chains, could be affected by the addition of bisecting GlcNAc. The quantified band intensity upon CBB staining under reducing conditions showed that the ratio of β3 chain in Lm332 to β1 chain in Lm311 was 10:1 (data not shown). Under nonreducing conditions, it was difficult to find the bands corresponding to Lm332 with an unprocessed laminin α3 chain (490 kDa) and Lm311 (600 kDa). The compositions of the Lm332 chain were also confirmed by immunoblotting using each chain-specific antibody. Consistent with the results shown in CBB staining, GnT-III-Lm332 contained slightly more unprocessed α3 chains (190-kDa) than those of vector-Lm332 and GnT-V-Lm332 (Fig. 2, I.B. α3). By contrast, there were no differences between the 135-kDa β3 chain, the unprocessed 150-kDa γ2 chain, and the processed 105-kDa γ2 chain among three Lm332s (Fig. 2, I.B. β3 and γ2). To establish whether Lm332 was modified by GnT-III or GnT-V, lectin blotting using E4-PHA and L4-PHA lectin was performed against three purified Lm332s. A comparison of bands corresponding to α3 and β3 chains among three purified Lm332s indicated that increased GnT-III and GnT-V products presented on GnT-III-Lm332 and GnT-V-Lm332, respectively (Fig. 3). Upon E4-PHA lectin blotting against GnT-III-Lm332, bisecting GlcNAc was added to β1 and γ1 chains as well as to the unprocessed α3A chain (Fig. 3, E4-PHA). To ascertain the N-glycan structures on Lm332 subunits directly, we also performed liquid chromatography/multiple-stage mass spectrometry (LC/MSn) analyses. Each subunit of Lm332 was separated by SDS-PAGE under reducing conditions, and N-glycans in each subunit were released by in-gel digestion with peptide:N-glycanase F. The oligosaccharides were extracted from the gel and subjected to LC/MSn as described under “Experimental Procedures.” Total ion chromatograms were acquired by single mass scans (m/z 450–2,000) in positive (Fig. 4A) and negative (Fig. 4B) ion modes, respectively. Structures of N-glycans in major peaks were deduced from m/z values of protonated molecules acquired by Fourier transform ion cyclotron resonance-MS and fragment ions in MSn spectra. Additional bisected N-glycans were confirmed based on the fragment ions at m/z 792 ([HexNAc-Hex-Hex-NAc-HexNAc-OH + H]+) and m/z 938 ([HexNAc-Hex-HexNAc-(dHex-)HexNAc-OH + H]+) in MS/MS and MS/MS/MS spectra. The extracted ion chromatograms of representative bisected N-glycans were acquired by single mass scans, and the structures in each peak were deduced (Fig. 4C). The peaks of the bisected N-glycans from all three subunits of GnT-III-Lm332 (Fig. 4, C, b, e, and h) were more intense than those from both the vec-Lm332 (Fig. 4C, a, d, and g) and GnT-V-Lm332 (Fig. 4C, c, f, and i). We also examined the β1,6-GlcNAc structures on all subunits of three Lm332s (Fig. 4D). The majority of N-glycans in all three subunits of vec-Lm332 (Fig. 4D, a, d, and g) and GnT-V-Lm332 (Fig. 4D, c, f, and i) were sialylated triantennary, whereas few triantennary forms were found in those of GnT-III-Lm332 (Fig. 4D, b, e, and h). These results, taken together, suggest that all subunits of Lm332 were modified by GnT-III and GnT-V, and they also support the notion that introduction of GnT-III inhibits GnT-V products.
FIGURE 2.
Characterization of laminin-332 purified from conditioned media of vector-, GnT-III-, or GnT-V-MKN45 transfectants. Purified laminin-332s, vector-Lm332 (vector), GnT-III-Lm332 (GnT-III), and GnT-V-Lm332 (GnT-V), were run on 4–7.5% gradient gels and 6% gels under nonreducing (–ME) and reducing conditions (+ME), respectively. After SDS-PAGE, separated proteins were stained with CBB (A; CBB) or transferred to the nitrocellulose membranes. Blotted proteins were probed with monoclonal antibodies against laminin α3 (Lsαc3), β3 (kalinin B1), and γ2 (D4B5) chain (B; I.B.). Ordinates indicate molecular sizes in kDa of marker proteins and laminin chains.
FIGURE 3.
Lectin blotting analysis of purified laminin-332s. 100 ng of laminin-332s (vector-, GnT-III-, and GnT-V-Lm332) was separated on 6% SDS-PAGE gel and then blotted onto the nitrocellulose membranes. Blotted proteins were probed with E4-PHA (top) and L4-PHA (bottom) lectin. Ordinates indicate molecular sizes in kDa of marker proteins and laminin chains.
FIGURE 4.
Analysis of N-glycan structures of purified laminin-332s by LC/MSn. Total ion chromatograms (TICs) obtained by single mass scans of N-glycans extracted from the gel separated Lm332 subunits (left, α3; middle, β3; right, γ2) of vec-Lm332 (top, vec), GnT-III-Lm332 (middle, GnT-III), and GnT-V-Lm332 (bottom, GnT-V) in positive (A) and negative (B) ion modes. C, extracted ion chromatograms of representative bisected N-glycans acquired by single mass scans. The extracted ion chromatogram of α3 subunit is shown at m/z 822.3, 915.9, 996.9, 1048.9, 1,142.4, and 1,215.5 (a, vector; b, GnT-III-Lm332; c, GnT-V-Lm332). The extracted ion chromatogram of β3 subunit is shown at m/z 1,061.4, 1,142.4, and 1,215.5 (d, vector; e, GnT-III-Lm332; f, GnT-V-Lm332). The extracted ion chromatogram of γ2 subunit is shown at m/z 1,142.4 and 996.9 (g, vector; h, GnT-III-Lm332; i, GnT-V-Lm332). The insets show deduced structures (gray triangle, fucose; open circle, galactose; gray circle, mannose; black square, N-acetylglucosamine; black diamond, N-acetylneuraminic acid). D, integrated mass spectra (m/z 1,000–1,700) acquired at elution positions indicated by boldface lines in total ion chromatograms in negative ion mode (Fig. 4B) (left, α3; middle, β3; right, γ2; top, vector; middle, GnT-III-Lm332; bottom, GnT-V-Lm332). The insets show deduced structure (gray triangle, fucose; open circle, galactose; gray circle, mannose; black square, N-acetylglucosamine; black diamond, N-acetylneuraminic acid).
Comparison of GnT-III- and GnT-V-Lm332-induced Cell Adhesion Activities—The localization of laminin isoform can be defined by the laminin α subunit, which is expressed in a tissue-specific distribution. For example, the laminin α2 chain containing laminin and the laminin α4 chain containing laminin localizes to the skeletal muscle and blood vessels, respectively. On the other hand, Lm332, which includes the laminin α3 chain, extensively localizes to the skin and stratified squamous tissues in vivo. To understand the biological activities of Lm332, we used the keratinocyte as a model cell, since Lm332 is a major component of keratinocyte extracellular matrix (ECM).
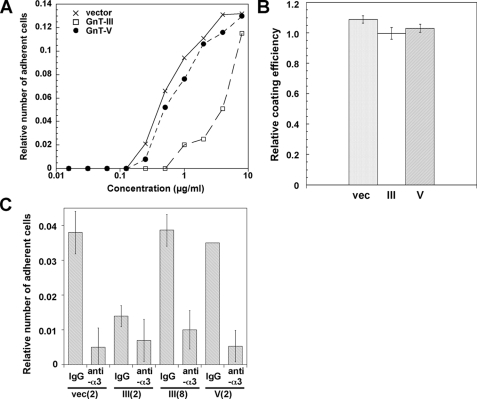
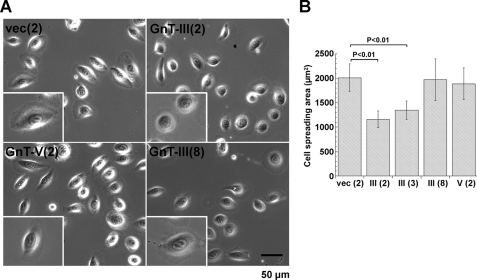
Lm332 promotes keratinocyte or other epithelial cell spreading and adhesion more effectively than other ECM proteins, such as collagen, fibronectin, and Lm111 (31). To examine the effect of GnT-III and GnT-V modification on Lm332 activities, we tried to perform a cell adhesion assay among three Lm332s. Generally, it is difficult to measure the biological functions of other ECMs in Lm332-expressing cells because the endogenous Lm332 strongly affects the experimental results. In order to prevent this, we used purified Lm332 and the Lm332-null keratinocytes established from patients with genetic mutations of the laminin β3 chain. Cell adhesion activity of GnT-V-Lm332 toward Lm332-null keratinocytes was similar to that of vector-Lm332 but was decreased in GnT-III-Lm332 (Fig. 5A). Cells displayed round morphology and formed filopodia around cells plated on culture dishes coated with 2 μg/ml GnT-III-Lm332 (Fig. 6A). In contrast, cells cultured on 2 μg/ml vector-Lm332 or GnT-V-Lm332 exhibited well spreading morphology with lamellipodia (Fig. 6A). The effect of 8 μg/ml GnT-III-Lm332 on cell morphology and spreading was similar to those of 2 μg/ml vector-Lm332 and GnT-V-Lm332 (Fig. 6, A and B). To ascertain whether GnT-III modification on Lm332 caused a decrease in its binding to the plates, coating efficiency was checked by ELISA using laminin γ2 chain monoclonal antibody (D4B5). As a result, there were no differences in coating efficiencies among the three Lm332s (Fig. 5B). Lm332 mainly binds integrin α3β1 and α6β4 as a cell surface receptor (6, 31). To ensure whether N-glycosylation affects the integrin binding function of Lm332, an inhibition assay was done using integrin α3 (P1B5) and α6 (GoH3) inhibitory antibodies. In all conditions, the anti-α3 antibody treatment completely diminished cell attachment toward the Lm332 substrate (Fig. 5C). Anti-α6 antibody (GoH3) (data not shown) as well as control IgG did not show inhibitory effects on the cell adhesion toward all Lm332 substrates. The possibility still remained that the effects of GnT-III-Lm332 could be due to the small amount of contaminating non-Lm332 subunits. To exclude this possibility, we attempted the cell adhesion assay using immunocaptured Lm332s to the plates with the laminin γ2 antibody (D4B5). A similar result to that in Fig. 5A was obtained in the experiment (data not shown). These results suggest that GnT-III products on Lm332 decreased its cell adhesion and cell spreading activities but had no influence on the integrin usage.
FIGURE 5.
Cell adhesion activities of purified laminin-332s. A, cell adhesion activities of vector-Lm332 (crosses), GnT-III-Lm332 (open squares), and GnT-V-Lm332 (closed circles). The 96-well plates were coated with the indicated concentrations of each Lm332. After 1.2% BSA blocking, Lm332-null keratinocytes suspended in supplement-free keratinocyte medium were plated to each well and incubated for 20 min. Each point represents the mean of triplicate assays. Other experimental conditions are described in “Experimental Procedures.” B, 2 μg/ml Lm332s were coated to the 96-well ELISA plates at 4 °C overnight. After blocking those wells with 1.2% BSA, coating efficiency of three kinds of Lm332s was estimated by ELISA using anti-laminin γ2 chain antibody. C, inhibitory effects of function blocking antibody against integrin α3(anti-α3) or control mouse IgG (IgG) on cell adhesion of Lm332-null keratinocytes to different kinds of Lm332s. The numbers in parentheses indicate the concentrations for Lm332 (μg/ml). Each bar represents the mean ± S.D. of triplicate assays.
FIGURE 6.
Effects of N-glycosylation on laminin-332-mediated cell spreading. Lm332-null keratinocytes were plated to the wells, which were precoated with vector-Lm332 (vec), GnT-III-Lm332 (GnT-III), and GnT-V-Lm332 (GnT-V), and then incubated for 1.5 h. A, cell morphology of Lm332-null keratinocytes on each substrate. Inset, a representative cell morphology. B, the cell spreading area was calculated using computer software (AxioVision). The numbers in parentheses are the concentrations for Lm332 (μg/ml). Each bar represents the mean ± S.D. of triplicate assays.
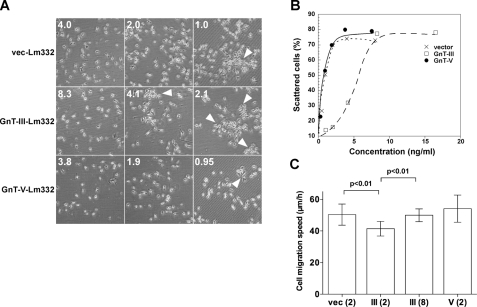
Effects of GnT-III and GnT-V Modification of Lm332 on Cell Scattering and Migration Activities—Cell scattering, which represents epithelial cell dissociation, is a well known Lm332 function (10, 11). To test whether GnT-III or GnT-V modification of Lm332 can influence its cell scattering activity, we investigated the scattering morphology using a Buffalo rat liver epithelial cell line (BLR cells), which is sensitive to the scattering activity of Lm332 (10). When the final concentration of vector-Lm332 at 2.0 ng/ml was added to the culture medium of BLR cells, more than 70% of the BLR cells markedly exhibited scattering morphology, as shown in Fig. 7A. GnT-V-Lm332 showed similar scattering activity to vector-Lm332. In contrast, GnT-III-Lm332 at 2.0 ng/ml did not show scattering activity. The concentration for the half-maximal scattering activity (ED50) was ∼0.5 ng/ml for vector-Lm332 and GnT-V-Lm332 but 5.0 ng/ml for GnT-III-Lm332 (Fig. 7B). We also compared cell migration activity among three Lm332s. When coated at 2 μg/ml, GnT-III-Lm332 showed decreased cell migration activity compared with vec-Lm332 and GnT-V-Lm332. However, when GnT-III-Lm332 was coated at 8 μg/ml for each plate, the activity was recovered to a level similar to those of 2 μg/ml vec-Lm332 and GnT-V-Lm332 (Fig. 7C). These results strongly support the notion that remodeling of N-glycans plays an important biological role.
FIGURE 7.
Cell scattering and migration activities of N-glycosylated laminin-332s. BLR cells were incubated with the indicated concentrations of Lm332s. After 40 h of incubation, cells were fixed and stained with crystal violet for observing cell scattering. A, cell morphology of BLR cells in each indicated condition. The numbers on the left are the concentrations for Lm332 (ng/ml) in cell culture medium. The arrowheads indicate the cluster of cells. B, scattered cells were counted and indicated as a percentage against total number of the cells. Crosses, vector-Lm332; open squares, GnT-III-Lm332; closed circles, GnT-V-Lm332. C, effects of vector-Lm332 (vec), GnT-III-Lm332 (III), and GnT-V-Lm332 (V) on migration of Lm332-null keratinocytes. The migration on each substrate was monitored by time lapse microscopy for 8 h. Each bar represents the mean ± S.D. of the migration speeds of nine cells. The numbers in parentheses are the concentrations for Lm332 (μg/ml). Other experimental conditions are under “Experimental Procedures.”
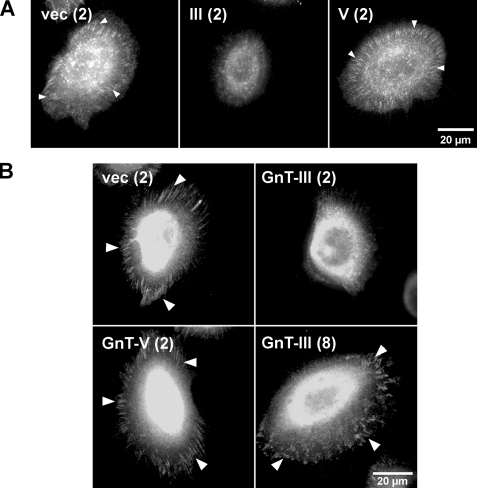
Effects of N-Glycosylation on Lm332-mediated α3β1 Integrin Association and Focal Complex Formation—Upon cell binding to ECM proteins, integrins undergo conformational changes, and their cytoplasmic domains interact through linker molecules, such as paxillin with the actin cytoskeleton and with signaling proteins (32). Furthermore, ECM induces integrin clustering, which can amplify various cell signaling from ECM (33). To clarify the reason why GnT-III-Lm332 showed lower activities than either vec-Lm332 or GnT-V-Lm332, we examined whether the modification of Lm332 by GnT-III or GnT-V affects the α3β1 integrin clustering or focal contact formation using fluorescence microscopy. Integrin α3 clustering (Fig. 8A) and focal contacts (Fig. 8B, paxillin) were distinctly observed in those cells cultured on 2 μg/ml vec-Lm332 as well as on GnT-V-Lm332 but not those cultured on GnT-III-Lm332. When cells were plated on 8 μg/ml GnT-III-Lm332, the focal contacts were clearly visualized by paxillin staining (Fig. 8B). These results suggest that modification of Lm332 by bisecting GlcNAc reduces the efficiency of integrin α3β1 clustering, which may in turn suppress focal contact formation.
FIGURE 8.
Effects of N-glycosylation on laminin-332-mediated α3β1 integrin association and focal contact formation. For visualizing α3 integrin (A) and paxillin (B), Lm332-null keratinocytes were plated on the indicated Lm332s and then incubated for 1.5 h. After fixation, cells were stained with appropriate primary and secondary antibodies. The numbers in parentheses are the concentrations for Lm332 (μg/ml). The arrowheads indicate α3β1 integrin clustering (A) and focal contacts (B).
DISCUSSION
In this study, we intensively investigated the effects of N-glycosylation on the biological functions of Lm332. GnT-III-Lm332 and GnT-V-Lm332 were modified with bisecting GlcNAc and β1,6-branched GlcNAc, respectively. Our further analyses demonstrated that introduction of bisecting GlcNAc to Lm332 decreased its cell spreading, adhesion, and scattering activities as well as its migration activity. Although three kinds of Lm332s showed no significant differences concerning integrin α3β1 binding function, GnT-III-Lm332 decreased α3β1 integrin clustering and the resultant focal contact formation.
In some cancer tissues, overexpression of GnT-V was observed by immunohistochemistry (20, 34, 35). Deficient GnT-V expression suppressed tumor growth and metastasis in animal models (21). Furthermore, Patridge et al. (36) reported that modification of growth factor receptors, such as epidermal growth factor and transforming growth factor-β receptors, with N-glycans using poly-N-acetyllactosamine produced by GnT-V caused preferential receptor binding to galectins, resulting in formation of a lattice that opposes constitutive endocytosis. These data suggest that GnT-V affects the cancer by promoting cell motility and changing the stability for growth factor receptors at the cell surface. Ly6 family member C4.4A can bind to Lm332, and its association of galectin-3 influences Lm332 adhesion (37). In addition, galectin-3-dependent oligomerization potentiates NG2-mediated activation of α3β1 integrin (38). Recently, we found that galectin-3 could bind to Lm332.3 It is possible that galectin-3 links GnT-V-modified-Lm332 and α3β1 integrin as well as growth factor receptors via poly-N-acetyllactosamine, resulting in the clustering and activation of the Lm332-α3β1 integrin-growth factor receptor complexes as well as causing resistance to their endocytosis. In contrast, modification of GnT-III suppresses further processing with branching enzymes, such as GnT-V, and elongation of N-glycans. Our previous study showed that the increase of GnT-V products on integrin α3 promoted cell migration on Lm332, whereas introduction of bisecting GlcNAc into the integrin α3 subunit resulted in a decrease of α3β1 integrin-mediated cancer cell motility by inhibition of β1,6-branched GlcNAc modification on the α3 integrin subunit (25). Lm332, which is a specific substrate for α3β1 integrin, promotes cell motility during wound healing and tumor progression (11, 12). In contrast to integrin α3β1, although introduction of bisecting GlcNAc significantly reduced the activities of Lm332, the modification of GnT-V does not affect the activities of Lm332 compared with the activities of vector-Lm332. It could be argued that intrinsic GnT-V-mediated glycosylation is enough for occupation of functional N-glycosylation sites of vector-Lm332 as well as of GnT-V-Lm332. As shown in both Fig. 3 and Fig. 4, β1,6-branched GlcNAc was expressed on vector-Lm332. Actually, we found that the integrin α5 subunit has fewer important N-glycosylation sites for its biological functions and expression on its cell surface, although it has 14 potential N-glycosylation sites (39).
Matsui et al. (40) showed that loss of N-glycosylation was not necessary for Lm332 assembly and secretion. The present study consistently showed that the modification of either GnT-III or GnT-V had no effect on Lm332 assembly and secretion. However, GnT-III-Lm332 was slightly resistant to the proteolytic processing of the laminin α3 subunit compared with the vector-Lm332 and GnT-V-Lm332. A slight increase in unprocessed laminin α3 subunits (190-kDa form) might result in the up-regulation of Lm311 formation, since laminin β1 and γ1 preferentially assembled with the unprocessed α3 chain rather than with the processed α3 chain (41). These results may also suggest that N-glycosylation affects trimeric formation among laminin subunits in vivo. In this study, since purified GnT-III-Lm332 contained a very small amount of the unprocessed α3 chain, the effect of those Lms in purified GnT-III-Lm332 was thought to be negligible. Moreover, the activities of Lm311 and Lm332 with the unprocessed form of the α3 chain are not so different from those of Lm332 with the processed α3 subunit (160-kDa form) (16, 41), compared with the differences between GnT-III-Lm332 and either vector-Lm332 or GnT-V-Lm332. Therefore, the decreased activities of GnT-III-Lm332 were mainly caused by the addition of bisecting GlcNAc to Lm332. In previous studies, Lm311 slightly stimulated cell spreading in the presence of a low concentration of Lm332, whereas Lm311 showed no cell spreading activities by itself (41). Although it is not the case for this study, the clarification of the molecular mechanism underlying the association of GnT-III-Lm332 with Lm311 could be a very interesting theme. Moreover, since syndecan-1, -2, and -4 can bind to the G4-G5 domain in the unprocessed laminin α3 chain (42, 43), we cannot definitely exclude the effect of syndecan on Lm332 activities, although the vast majority of Lm332 was processed (without the G4-G5 domain), compared with unprocessed Lm332 (with the G4-G5 domain) in this study. A previous study (44) showed that deglycosylation of recombinant Lm332 containing the heterotrimeric, C-terminal part of the coiled-coil domain and G domains did not affect its binding to integrin α3β1. However, we found that the addition of bisecting GlcNAc to Lm332 diminished Lm332-mediated α3β1 integrin clustering and the subsequent cell adhesion, spreading and scattering as well as the migration induced by Lm332. It is reasonable to assume that galectin-3 can link molecules via poly-N-acetyllactosamine, because galectin-3 binding to GnT-III-Lm332 is probably much less than its binding to either vector- or GnT-V-Lm332. Details of the molecular mechanism in play here will require further study.
In the basement membranes of the skin and of other tissues, there are many components, such as type IV collagen, nidogens, proteoglycans, agrin, and laminin-511 (Lm511; laminin-10), that contribute to structure and receptor interactions. Since most ECM proteins are glycoproteins, alteration of carbohydrates could change carbohydrate/carbohydrate or carbohydrate/protein interactions in the basement membrane, which presumably affects functional activities of diverse ECM. To understand how carbohydrate modifications affect the skin and other tissues, therefore, the analysis of carbohydrate functions of individual or combined ECM proteins is quite important. This will be further addressed in future studies.
In conclusion, this study reports for the first time that Lm332 can be modified by both GnT-III and GnT-V, resulting in the regulation of cell spreading, adhesion, scattering, and migration activities induced by Lm332. In addition, N-glycosylation of Lm332 can influence the proteolytic processing of laminin subunits and α3β1 integrin clustering. Given the importance of Lm332 and the dynamic changes in N-glycosylation during tumor formation and cancer metastasis, N-glycan could represent a new therapeutic target.
Acknowledgments
We thank Dr. M. Peter Marinkovich (Stanford University) for critical reading of the manuscript.
This work was supported in part by Core Research for Evolutional Science and Technology (CREST), the Japan Science and Technology Agency (JST), and the Academic Frontier Project for Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Lm, laminin; BRL, buffalo rat liver; BSA, bovine serum albumin; CBB, Coomassie Brilliant Blue; ECM, extracellular matrix; E4-PHA, erythroagglutinating phytohemagglutinin; ELISA, enzyme-linked immunosorbent assay; GnT-III, N-acetylglucosaminyltransferase III; GnT-V, N-acetylglucosaminyltransferase V; LC, liquid chromatography; L4-PHA, leukoagglutinating phytohemagglutinin; Lmxyz, laminin-xyz; MS, mass spectrometry; PBS, phosphate-buffered saline; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
Y. Kariya and J. Gu, unpublished data.
References
- 1.Colognato, H., and Yurchenco, P. D. (2000) Dev. Dyn. 218 213–234 [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara, S., Shinkai, H., Deutzmann, R., Paulsson, M., and Timpl, R. (1988) Biochem. J. 252 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean, J. W., III, Chandrasekaran, S., and Tanzer, M. L. (1990) J. Biol. Chem. 265 12553–12562 [PubMed] [Google Scholar]
- 4.Green, T. L., Hunter, D. D., Chan, W., Merlie, J. P., and Sanes, J. R. (1992) J. Biol. Chem. 267 2014–2022 [PubMed] [Google Scholar]
- 5.Howe, C. C. (1984) Mol. Cell Biol. 4 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter, W. G., Ryan, M. C., and Gahr, P. J. (1991) Cell 65 599–610 [DOI] [PubMed] [Google Scholar]
- 7.Ryan, M. C., Christiano, A. M., Engvall, E., Wewer, U. M., Miner, J. H., Sanes, J. R., and Burgeson, R. E. (1996) Matrix Biol. 15 369–381 [DOI] [PubMed] [Google Scholar]
- 8.Kariya, Y., Tsubota, Y., Hirosaki, T., Mizushima, H., Puzon-McLaughlin, W., Takada, Y., and Miyazaki, K. (2003) J. Cell Biochem. 88 506–520 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen, B. P., Gil, S. G., and Carter, W. G. (2000) J. Biol. Chem. 275 31896–31907 [DOI] [PubMed] [Google Scholar]
- 10.Kariya, Y., and Miyazaki, K. (2004) Exp. Cell Res. 297 508–520 [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki, K. (2006) Cancer Sci. 97 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinkovich, M. P. (2007) Nat. Rev. Cancer 7 370–380 [DOI] [PubMed] [Google Scholar]
- 13.Giannelli, G., Falk-Marzillier, J., Schiraldi, O., Stetler-Stevenson, W. G., and Quaranta, V. (1997) Science 277 225–228 [DOI] [PubMed] [Google Scholar]
- 14.Veitch, D. P., Nokelainen, P., McGowan, K. A., Nguyen, T. T., Nguyen, N. E., Stephenson, R., Pappano, W. N., Keene, D. R., Spong, S. M., Greenspan, D. S., Findell, P. R., and Marinkovich, M. P. (2003) J. Biol. Chem. 278 15661–15668 [DOI] [PubMed] [Google Scholar]
- 15.Koshikawa, N., Giannelli, G., Cirulli, V., Miyazaki, K., and Quaranta, V. (2000) J. Cell Biol. 148 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsubota, Y., Yasuda, C., Kariya, Y., Ogawa, T., Hirosaki, T., Mizushima, H., and Miyazaki, K. (2005) J. Biol. Chem. 280 14370–14377 [DOI] [PubMed] [Google Scholar]
- 17.Cummings, R. D., Trowbridge, I. S., and Kornfeld, S. (1982) J. Biol. Chem. 257 13421–13427 [PubMed] [Google Scholar]
- 18.Shoreibah, M., Perng, G. S., Adler, B., Weinstein, J., Basu, R., Cupples, R., Wen, D., Browne, J. K., Buckhaults, P., and Fregien, N. (1993) J. Biol. Chem. 268 15381–15385 [PubMed] [Google Scholar]
- 19.Dennis, J. W., and Laferte, S. (1989) Cancer Res. 49 945–950 [PubMed] [Google Scholar]
- 20.Handerson, T., and Pawelek, J. M. (2003) Cancer Res. 63 5363–5369 [PubMed] [Google Scholar]
- 21.Granovsky, M., Fata, J., Pawling, J., Muller, W. J., Khokha, R., and Dennis, J. W. (2000) Nat. Med. 6 306–312 [DOI] [PubMed] [Google Scholar]
- 22.Gu, J., Nishikawa, A., Tsuruoka, N., Ohno, M., Yamaguchi, N., Kangawa, K., and Taniguchi, N. (1993) J. Biochem. (Tokyo) 113 614–619 [DOI] [PubMed] [Google Scholar]
- 23.Schachter, H. (1986) Biochem. Cell Biol. 64 163–181 [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura, M., Nishikawa, A., Ihara, Y., Taniguchi, S., and Taniguchi, N. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 8754–8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao, Y., Nakagawa, T., Itoh, S., Inamori, K., Isaji, T., Kariya, Y., Kondo, A., Miyoshi, E., Miyazaki, K., Kawasaki, N., Taniguchi, N., and Gu, J. (2006) J. Biol. Chem. 281 32122–32130 [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara, H., Kikkawa, Y., Sanzen, N., and Sekiguchi, K. (2001) J. Biol. Chem. 276 17550–17558 [DOI] [PubMed] [Google Scholar]
- 27.Ihara, S., Miyoshi, E., Ko, J. H., Murata, K., Nakahara, S., Honke, K., Dickson, R. B., Lin, C. Y., and Taniguchi, N. (2002) J. Biol. Chem. 277 16960–16967 [DOI] [PubMed] [Google Scholar]
- 28.Kuster, B., Wheeler, S. F., Hunter, A. P., Dwek, R. A., and Harvey, D. J. (1997) Anal. Biochem. 250 82–101 [DOI] [PubMed] [Google Scholar]
- 29.Itoh, S., Kawasaki, N., Hashii, N., Harazono, A., Matsuishi, Y., Hayakawa, T., and Kawanishi, T. (2006) J. Chromatogr. A. 1103 296–306 [DOI] [PubMed] [Google Scholar]
- 30.Cummings, R. D., and Kornfeld, S. (1982) J. Biol. Chem. 257 11230–11234 [PubMed] [Google Scholar]
- 31.Rousselle, P., and Aumailley, M. (1994) J. Cell Biol. 125 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes, P. E., and Pfaff, M. (1998) Trends Cell Biol. 8 359–364 [DOI] [PubMed] [Google Scholar]
- 33.Carman, C. V., and Springer, T. A. (2003) Curr. Opin. Cell Biol. 15 547–556 [DOI] [PubMed] [Google Scholar]
- 34.Fernandes, B., Sagman, U., Auger, M., Demetrio, M., and Dennis, J. W. (1991) Cancer Res. 51 718–723 [PubMed] [Google Scholar]
- 35.Murata, K., Miyoshi, E., Kameyama, M., Ishikawa, O., Kabuto, T., Sasaki, Y., Hiratsuka, M., Ohigashi, H., Ishiguro, S., Ito, S., Honda, H., Takemura, F., Taniguchi, N., and Imaoka, S. (2000) Clin. Cancer Res. 6 1772–1777 [PubMed] [Google Scholar]
- 36.Partridge, E. A., Le Roy, C., Di Guglielmo, G. M., Pawling, J., Cheung, P., Granovsky, M., Nabi, I. R., Wrana, J. L., and Dennis, J. W. (2004) Science 306 120–124 [DOI] [PubMed] [Google Scholar]
- 37.Paret, C., Bourouba, M., Beer, A., Miyazaki, K., Schnolzer, M., Fiedler, S., and Zoller, M. (2005) Int. J. Cancer 115 724–733 [DOI] [PubMed] [Google Scholar]
- 38.Fukushi, J., Makagiansar, I. T., and Stallcup, W. B. (2004) Mol. Biol. Cell 15 3580–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isaji, T., Sato, Y., Zhao, Y., Miyoshi, E., Wada, Y., Taniguchi, N., and Gu, J. (2006) J. Biol. Chem. 281 33258–33267 [DOI] [PubMed] [Google Scholar]
- 40.Matsui, C., Wang, C. K., Nelson, C. F., Bauer, E. A., and Hoeffler, W. K. (1995) J. Biol. Chem. 270 23496–23503 [DOI] [PubMed] [Google Scholar]
- 41.Hirosaki, T., Tsubota, Y., Kariya, Y., Moriyama, K., Mizushima, H., and Miyazaki, K. (2002) J. Biol. Chem. 277 49287–49295 [DOI] [PubMed] [Google Scholar]
- 42.Utani, A., Nomizu, M., Matsuura, H., Kato, K., Kobayashi, T., Takeda, U., Aota, S., Nielsen, P. K., and Shinkai, H. (2001) J. Biol. Chem. 276 28779–28788 [DOI] [PubMed] [Google Scholar]
- 43.Okamoto, O., Bachy, S., Odenthal, U., Bernaud, J., Rigal, D., Lortat-Jacob, H., Smyth, N., and Rousselle, P. (2003) J. Biol. Chem. 278 44168–44177 [DOI] [PubMed] [Google Scholar]
- 44.Kunneken, K., Pohlentz, G., Schmidt-Hederich, A., Odenthal, U., Smyth, N., Peter-Katalinic, J., Bruckner, P., and Eble, J. A. (2004) J. Biol. Chem. 279 5184–5193 [DOI] [PubMed] [Google Scholar]