Abstract
Aging is one major risk factor for numerous diseases. The enzyme telomerase reverse transcriptase (TERT) plays an important role for aging and apoptosis. Previously, we demonstrated that inhibition of oxidative stress-induced Src kinase family-dependent nuclear export of TERT results in delayed replicative senescence and reduced apoptosis sensitivity. Therefore, the aim of this study was to investigate mechanisms inhibiting nuclear export of TERT. First, we demonstrated that H2O2-induced nuclear export of TERT was abolished in Src, Fyn, and Yes-deficient embryonic fibroblasts. Next, we wanted to identify one potential negative regulator of this export process. One candidate is the protein tyrosine phosphatase Shp-2 (Shp-2), which can counteract activities of the Src kinase family. Indeed, Shp-2 was evenly distributed between the nucleus and cytosol. Nuclear Shp-2 associates with TERT in endothelial cells and dissociates from TERT prior to its nuclear export. Overexpression of Shp-2 wt inhibited H2O2-induced export of TERT. Overexpression of the catalytically inactive, dominant negative Shp-2 mutant (Shp-2(C459S)) reduced endogenous as well as overexpressed nuclear TERT protein and telomerase activity, whereas it had no influence on TERT(Y707F). Binding of TERT(Y707F) to Shp-2 is reduced compared with TERTwt. Ablation of Shp-2 expression led only to an increased tyrosine phosphorylation of TERTwt, but not of TERT(Y707F). Moreover, reduced Shp-2 expression decreased nuclear telomerase activity, whereas nuclear telomerase activity was increased in Shp-2-overexpressing endothelial cells. In conclusion, Shp-2 retains TERT in the nucleus by regulating tyrosine 707 phosphorylation.
Telomeres are the physical ends of the chromosomes. They maintain chromosome stability, genetic integrity and cell viability in a variety of different species (1, 2). Telomeres can also function as a mitotic clock, because telomeres are progressively shortened during each cell division. The enzyme telomerase, with its catalytic subunit telomerase reverse transcriptase (TERT),5 counteracts the shortening of telomeres. Introduction of TERT into human cells extended both their life-span and their telomeres to lengths typical of young cells (3–5). In addition to this well known function of TERT, functions independent of telomere shortening have been described. TERT has been shown to inhibit apoptosis (6, 7). Recently, it has been demonstrated that TERT is also localized in the mitochondria, but its function there is discussed controversially (8–10). TERT is regulated by transcriptional and post-translational mechanisms. Phosphorylation, binding proteins, and cellular localization have been described for post-translational modifications of TERT (11–15). TERT can be phosphorylated and its activity is regulated by kinases like c-Abl, PKC, ERK1/2, and Akt (16–20). We demonstrated that TERT is tyrosine-phosphorylated by the Src kinase family under conditions of oxidative stress (21, 22). Functionally, this results in nuclear export of TERT disrupting the anti-apoptotic and telomere-extending potential of the enzyme, which subsequently leads to enhanced apoptosis sensitivity and accelerated senescence of cells (21, 22).
One important regulator of the Src kinase family activity is the protein tyrosine phosphatase Shp-2 (Shp-2). The importance of Shp-2 for survival has been documented by the Shp-2 knockout mice which are embryonic lethal (23). All the described mechanisms for the regulatory mechanisms of Shp-2 are associated with its ability to dephosphorylate target molecules such as Src kinase family members, which are bound to growth factor receptors at the membrane to inhibit the permanent activation of the receptors by kinase phosphorylation (for review see Ref. 24). Recently, it has been demonstrated that Shp-2 is also localized in the nucleus, where it binds to the transcription factor STAT5a and thereby regulates its function (25). Because the regulation, which leads to nuclear export of TERT has to occur in the nucleus, we hypothesized that Shp-2 might counteract the nuclear export of TERT.
Our data demonstrate that nuclear export of TERT is triggered by Src and/or Yes. One negative regulator of this export is Shp-2. Shp-2 is localized in the nucleus, is associated with TERT and dissociates from TERT prior to its export. The catalytic activity of Shp-2 is crucial for retaining TERT in the nucleus.
EXPERIMENTAL PROCEDURES
Cell Culture—Endothelial cells (EC) were cultured in endothelial basal medium supplemented with hydrocortisone (1 μg/ml), bovine brain extract (12 μg/ml), gentamycin (50 μg/ml), amphotericin B (50 ng/ml), epidermal growth factor (10 ng/ml), and 10% fetal calf serum. After detachment with trypsin, cells were grown for at least 18 h (26, 27). Human embryonic kidney cells (HEK) were cultured in DMEM basal medium with 10% heat-inactivated fetal calf serum. Mouse embryonic fibroblasts deficient of Src, Fyn, and Yes and their wild-type counterparts were cultured in Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal calf serum and gentamycin.
Plasmids—Human Shp-2 was cloned out of endothelial cell-derived cDNA incorporating EcoRI and BamHI restriction sites. The amplified PCR product was subcloned into pcDNA3.1 (–) vector containing a Myc tag (Invitrogen) or into pGFP vector creating a Shp-2 GFP fusion. The catalytically inactive mutant of Shp-2 (Shp-2(C459S)) was generated by site-directed mutagenesis (Stratagene) out of Shp-2 wt. The hTERT construct was kindly donated by Dr. Weinberg (28). TERT was subcloned into pcDNA3.1(–)Myc-His vector (TERTwt) containing the Myc tag at the C terminus. TERT(Y707F) was generated by site-directed mutagenesis. ShRNA vectors were obtained from Sigma (Mission shRNA series).
Transfection—HEK were transiently transfected with Lipofectamine/Plus according to the manufacturer's protocol (Invitrogen) as previously described (29) with a transfection efficiency of 90 ± 4%. EC were transiently transfected with Superfect (Qiagen) as described previously. Mouse embryonic fibroblasts were transiently transfected with Lipofectamine/Plus according to the manufacturer's protocol (Invitrogen). Transfection of siRNA was performed by using INTERFERRin™ according to the manufacturer's protocol (POLYPLUS transfection). Sequence of scrambled siRNA: 5′-AACUUGAGAAUCGCCUGAA-3′, sequence of Shp-2 siRNA: 5′-GAAGCACAGUACCGAUUUA-3′.
Telomerase Enzyme Activity Measurement—Telomerase enzyme activity was measured using a commercially available PCR-based assay according to the manufacturer's protocol (Roche Applied Science) as previously described (30). In brief, after PCR amplification, PCR products were either used for detection of telomerase enzyme activity by 1) ELISA or by 2) telomerase-mediated DNA laddering. 1) For ELISA, PCR products are immobilized via the biotin-labeled TS primers (provided with the assay) to a streptavidin-coated microtiter plate. The linearity of the assay was assured by the positive controls provided by the company, and as negative controls heat (95 °C, 2 min) and RNase-treated samples were used in the presence of the biotinylated primers (inset, Fig. 5b).
FIGURE 5.
Nuclear retention of TERT requires catalytically active Shp-2. a, EC were transfected with empty vector (EV) or Shp-2(C459S). Immunoblot was performed with an anti-TERT antibody (left panel, top), an anti-topoisomerase I antibody (left panel, middle) and an anti-GAPDH antibody (left panel, bottom). TERT levels were determined by semi-quantitative analysis and normalized to topoisomerase I (right panel). Data are means ± S.E. of four independent experiments, the relative TERT levels in EV-transfected cells are set to 1 (*, p < 0.05). b, EC were transfected with empty vector (EV), Myc-tagged Shp-2 wt or Shp-2(C459S) and incubated with 200 μm H2O2 for 6 h. Nuclear telomerase activity was measured and is shown relative to the level in EV-transfected cells not treated with H2O2. Data are means ± S.E. (n = 3; *, p < 0.05 versus EV; #. p < 0.05 versus EV + H2O2; $, p < 0.05 versus Shp2(C459S)). Lower inset shows absolute values (A450) of negative and positive controls; the measured absorbances of all samples were within the range of these controls. To assess the levels of both Shp-2 wt and Shp-2(C459S) immunoblot of whole cell lysates was performed with an anti-Myc antibody (upper inset, top panel), equal loading was confirmed using GAPDH (upper inset, bottom panel).
Separation of Nuclear and Cytosolic Extracts—Nuclear and cytosolic extracts were separated using a commercially available kit according to the manufacturer's protocol (Pierce). In brief, cells were scraped off the dish and centrifuged at 800 × g for 5 min at 4 °C. The resulting pellet was resolved in cytosolic extraction reagent I (CERI buffer) and incubated for 10 min at 4 °C. After adding cytosolic extraction reagent II (CERII buffer) and further incubation for 1 min at 4 °C, samples were centrifuged at 16,000 × g for 5 min at 4 °C. The resulting supernatant contained the cytosolic fraction. The resulting pellet was washed with phosphate-buffered saline and resuspended in nuclear extraction reagent (NER buffer) and incubated for 60 min at 4 °C. After centrifugation for 15 min at 16,000 × g at 4 °C, the resulting supernatant was obtained as nuclear fraction. Purity of the nuclear and cytosolic extracts was always assured by immunoblotting with topoisomerase 1 (nuclear) and HSP70 (cytosolic).
Immunoprecipitation and Immunoblotting—Lysates (250 μg) were immunoprecipitated with 2.5 μg of Shp-2 antibody or 2.5 μg of Myc antibody overnight at 4 °C. After incubation with A- and G-Sepharose (Amersham Biosciences) for 2 h at 4°C, resulting beads were washed, subjected to SDS-PAGE sample buffer, and resolved on a 10% SDS-PAGE.
Immunoblotting was performed with antibodies directed against TERT (1:200, overnight, 4 °C, Calbiochem or 1:500, overnight, 4 °C, Rockland), actin (1:8000, overnight 4 °C, Sigma) and Shp-2, Myc, Hsp70, Ref-1, topoisomerase I (2 h, 1:250, all Santa Cruz). Antibodies were detected by the enhanced chemiluminescence system (Amersham Biosciences). Semi-quantitative analyses were performed on scanned immunoblots using Scion Image 1.6 (Scion Corp.).
Immunostaining—Cells were fixed in 4% paraformaldehyde and permeabilized using 0.3% Triton X-100 and 3% bovine serum albumin in phosphate-buffered saline. For immunostaining, cells were incubated with an antibody against TERT and stained with an anti-rabbit Texas-Red-conjugated Fab fragment. Nuclei were counterstained with Sytox-Blue or DAPI (1:1000, 5 min, Molecular Probes). Cells were visualized with confocal microscopy (Zeiss, LSM 510 META, magnification 1:40 oil).
Statistics—Statistical analysis was performed with Student's t test or Wilcoxon, Mann-Whitney test using XLSTAT 2008.
RESULTS
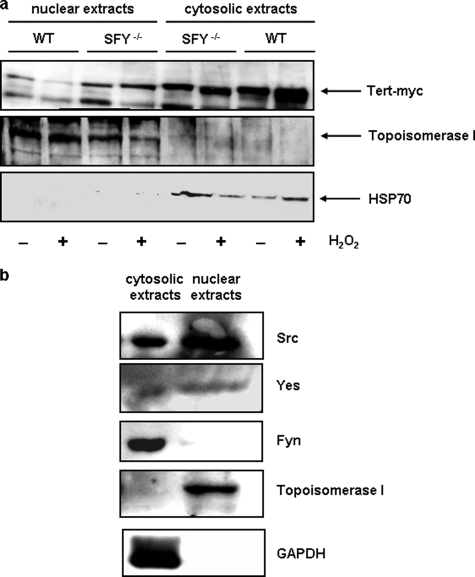
Nuclear Export of TERT Depends on Src and Yes—Recently, we demonstrated that short term exposure to oxidative stress as well as aging-induced reactive oxygen species formation led to a Src kinase family-dependent nuclear export of TERT. Loss of nuclear TERT resulted in increased apoptosis sensitivity and accelerated senescence (21, 22). To reduce the number of candidates of the Src kinase family, which are responsible for oxidative stress-induced nuclear export of TERT, we used embryonic mouse fibroblasts deficient for Src, Fyn, and Yes and overexpressed TERTwt in these cells. Treatment with 500 μm H2O2 for 6 h resulted in a reduction of nuclear TERT protein and an increase in cytosolic TERT protein in wildtype fibroblasts. In contrast, in fibroblasts deficient for Src, Fyn, and Yes, nuclear export of TERT was completely abolished (Fig. 1a) demonstrating that these three kinases play a role in this process.
FIGURE 1.
Absence of Src, Fyn, and Yes completely abrogates nuclear export of TERT. a, Src, Fyn, Yes-dependent nuclear export of TERT. Embryonic fibroblasts from mice deficient for Src, Fyn, and Yes (SFY–/–) and from their wild-type counterparts (WT) were transfected with TERT-myc and incubated with 500 μm H2O2 for 6 h. Nuclear and cytosolic extracts were prepared as described under “Experimental Procedures” and used for immunoblotting; shown is a representative blot of three independent experiments. Upper panel, detection of TERT-myc with an anti-Myc antibody. The middle and lower panel show the purity of the nuclear and cytosolic extracts with the nuclear marker topoisomerase I and the cytosolic protein Hsp70. b, nuclear localization of Src and Yes. Nuclear and cytosolic extracts were prepared from EC and used for immunoblotting. The following proteins were detected with specific antibodies (from top to bottom): Src, Yes, Fyn, topoisomerase I (nuclear marker), and GAPDH (cytosolic marker).
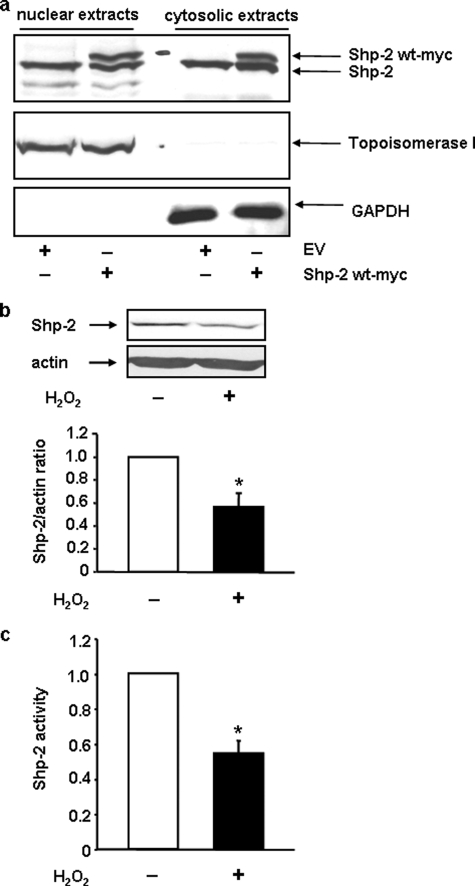
The functional relevance of these kinases in EC is substantiated by the fact that Src and Yes could be detected in nuclear extracts of them (Fig. 1b). Having demonstrated that Src and/or Yes are responsible for oxidative stress induced nuclear export of TERT, we next wanted to identify one of the counter players, which inhibits nuclear export of TERT. One known inhibitor of Src kinase family functions is Shp-2. However, to inhibit nuclear export of TERT, Shp-2, like Src and Yes must be localized in the nucleus. Therefore, we first investigated the cellular localization of Shp-2 in EC. We found that Shp-2 is evenly distributed between the nuclear and cytosolic extracts (Fig. 2a). Thus, we hypothesized that Shp-2 could be indeed the counter player for oxidative stress-induced nuclear export of TERT. Similar to endogenous Shp-2, overexpressed, myc- or GFP-tagged Shp-2 were also localized in the nucleus (Fig. 2a and supplemental Fig. S1).
FIGURE 2.
Subcellular localization of Shp-2 and regulation by H2O2. a, endogenous and overexpressed Shp-2 are evenly distributed between nucleus and cytoplasm in EC. Shp-2 wt-myc was overexpressed in EC. Nuclear and cytosolic extracts were prepared as described under “Experimental Procedures” and used for immunoblots. Upper panel shows an immunoblot with an anti-Shp-2 antibody, middle and lower panels demonstrate the purity of the extracts using antibodies against the nuclear marker topoisomerase I and the cytosolic marker GAPDH. One representative immunoblot is shown (n = 6). EV, empty vector; b, H2O2 reduces Shp-2 protein levels. Upper panel, EC were incubated with 200 μm H2O2 for 6 h and immunoblots were performed with an anti-Shp-2 antibody and anti-actin for normalization. Lower panel, semi-quantitative analysis of four independent experiments. Shown are the Shp-2 levels relative to actin, data are means ± S.E. (relative levels in untreated cells set to 1; *, p < 0.05). c, H2O2 reduces Shp-2 activity. EC were incubated with 200 μm H2O2 for 6 h, lysed, and Shp-2 activity was measured in the lysates. Data are means ± S.E. (n = 6) and are shown relative to Shp-2 activity in untreated cells (*, p < 0.05).
If Shp-2 is involved in TERT nuclear export by oxidative stress, we hypothesized that Shp-2 itself must be regulated by H2O2 in EC. Indeed, incubation with 200 μm H2O2, which led to a nuclear export of TERT, resulted in a minor, but significant decrease of total Shp-2 protein and activity (Fig. 2, b and c).
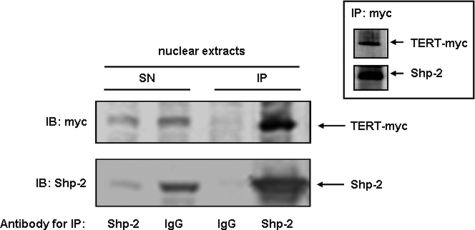
Shp-2 Associates with TERT and Retains It in the Nucleus—To get first insights whether Shp-2 could be a direct player in the regulation of TERT, we next determined whether TERT and Shp-2 associate with each other in the nucleus. Therefore, we co-immunoprecipitated endogenous Shp-2 and TERT-myc from nuclear extracts of TERT-myc-transfected cells. Indeed, the majority of TERT is associated with Shp-2 in the nucleus (Fig. 3). This association seems to be specific as we did not find complexes between TERT-myc, Shp-2, and the transcription factor Ref-1 (supplemental Fig. S2). Having demonstrated that nuclear Shp-2 interacts with TERT, we next examined whether Shp-2 has an inhibitory effect on oxidative stress-induced nuclear export of TERT.
FIGURE 3.
Shp-2 associates with TERT. HEK cells were transfected with TERT-myc and endogenous Shp-2 was immunoprecipitated from 250 μg of nuclear protein. Immunoblots with the precipitate (IP) and 25 μg of total protein from the supernatant (SN) were performed with an anti-Myc antibody (TERT-myc, upper panel) and anti-Shp-2 (lower panel). IgG served as negative control. The inset shows the reciprocal immunoprecipitation with an anti-Myc antibody.
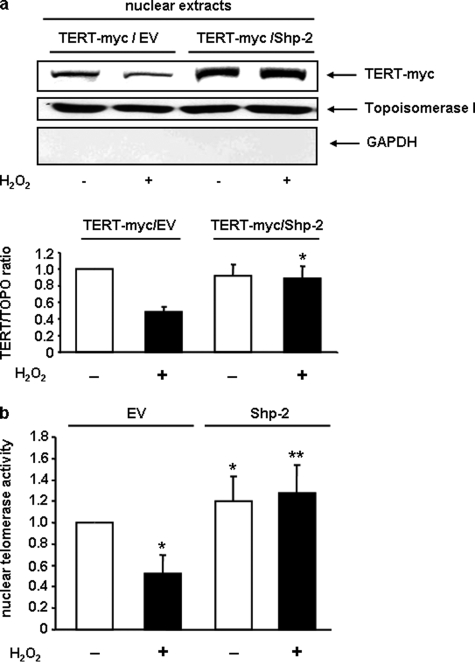
We overexpressed Shp-2 and TERT-myc in EC and incubated them for 6 h with 200 μm H2O2 to induce nuclear export of TERT. As shown in Fig. 4a, overexpression of Shp-2 completely abolished nuclear export of TERT. In line with this finding, overexpression of Shp-2 inhibited H2O2-induced reduction of endogenous, nuclear telomerase activity (Fig. 4b).
FIGURE 4.
Shp-2 wt rescues H2O2-induced loss of nuclear TERT protein and telomerase activity. a, EC were transfected with either empty vector (EV) and TERT-myc or with Shp-2 and TERT-myc. After incubation with 200 μm H2O2, immunoblot against TERT-myc and topoisomerase I was performed in nuclear extracts. a, upper panel shows a representative immunoblot with an Myc antibody, the upper middle panel shows purity of nuclear fraction with an anti-topoisomerase I antibody and upper lower panel shows purity of nuclear fraction with an anti-GAPDH antibody (n = 4). Lower panel shows the semi-quantitative analysis of four independent experiments. TERT levels were normalized to topoisomerase I and are shown relative to the levels of cells transfected with EV/TERT-myc not treated with H2O2. Data are means ± S.E. (*, p < 0.05 versus TERT-myc/EV + H2O2). b, overexpression of Shp-2 rescued H2O2-induced reduction of nuclear telomerase activity. EC were transfected and treated as in a. Nuclear telomerase activity was measured as described under “Experimental Procedures” and is shown relative to the level in EV-transfected cells not treated with H2O2. Data are means ± S.E. (n = 6; *, p < 0.05 versus EV-H2O2. **, p < 0.05 versus EV + H2O2).
Nuclear Retention of TERT Requires Catalytically Active Shp-2—To determine whether the catalytic activity of Shp-2 is required for retaining TERT in the nucleus, we investigated the effects of the catalytically inactive, dominant negative Shp-2 mutant, Shp-2(C459S). First we verified that this mutant like the wild-type protein is evenly distributed between nucleus and cytoplasm (supplemental Fig. S3).
Overexpression of Shp-2(C459S) induced already under basal conditions reduction of nuclear TERT protein (Fig. 5a). In addition, we transfected EC with Shp-2 wt or Shp-2(C459S), incubated them with H2O2 and measured telomerase activity in nuclear extracts. Shp-2 wt increased, whereas Shp-2(C459S) reduced endogenous, nuclear telomerase activity under basal conditions. This effect seems to be specific for Shp-2, because overexpression of another phosphatase, namely the protein phosphatase 2a (PP2A) did not alter nuclear TERT protein and telomerase activity (supplemental Fig. S4). Shp-2 wt completely abrogated the effect of H2O2 on endogenous, nuclear telomerase activity, which is in accordance with our data presented in Fig. 4a. Interestingly Shp-2(C459S)-overexpressing cells showed a stronger effect on endogenous, nuclear telomerase activity compared with empty vector transfected cells under H2O2 treatment (Fig. 5b). These data clearly demonstrate that nuclear retention of TERT depends on the presence of Shp-2 protein and its activity. This is in accordance with the findings shown in Fig. 2 that H2O2 treatment reduces total Shp-2 protein and activity, providing one possible explanation for the H2O2-induced nuclear export of TERT.
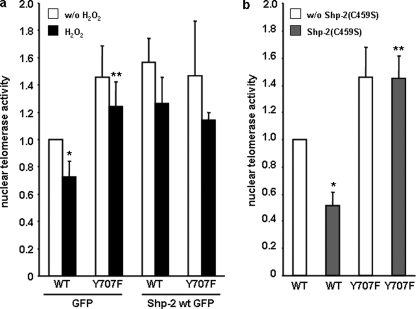
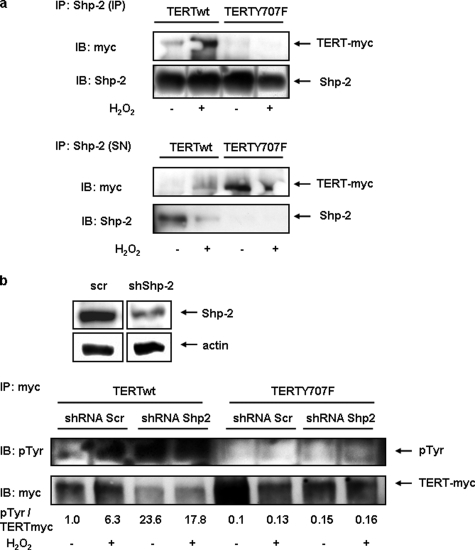
We previously reported that TERT(Y707F) is retained in the nucleus under H2O2 treatment, suggesting that this tyrosine is crucial for nuclear export (21). The involvement of Shp-2 in this process suggests that phosphorylation/dephosphorylation regulates subcellular TERT localization. As expected, Shp-2 wt did not alter nuclear telomerase activity in TERT(Y707F)-overexpressing cells even after H2O2 treatment because this mutant cannot be phosphorylated at position 707 and thus does not require dephosphorylation for nuclear retention (Fig. 6a and Ref. 21). Moreover, under basal conditions, overexpression of dominant negative Shp-2(C459S) reduced nuclear telomerase activity in TERT-wt transfected cells, whereas it had no influence on TERT(Y707F) (Fig. 6b), suggesting that tyrosine 707 in TERT is the target for Shp-2 phosphatase activity. Next, we investigated the influence of tyrosine 707 on the TERT Shp-2 association in the nucleus in the presence and absence of H2O2. Association of TERT(Y707F) to Shp-2 is markedly reduced compared with TERT-wt. This is also reflected in the supernatants of the immunoprecipitations where more unbound TERT(Y707F) is found (Fig. 7a). Finally, ablation of Shp-2 increased tyrosine phosphorylation of nuclear TERT-wt, whereas TERT(Y707F) was unaffected (Fig. 7b). Similarly, reduced Shp-2 expression decreased nuclear telomerase activity and TERT protein already under basal conditions (supplemental Fig. S5 and Fig. 7b).
FIGURE 6.
Tyrosine 707 is a target for Shp-2. a, EC were transfected with Myc-tagged TERT-wt (WT) or TERT(Y707F) (Y707F) together with GFP or Shp-2 wt GFP. After incubation with H2O2 nuclear extracts were prepared and nuclear telomerase activity was measured. Data are means ± S.E. (n = 4) relative to the values obtained with TERT-wt/GFP in untreated cells (*, p < 0.05 versus TERT wt/GFP w/o H2O2; **, p < 0.05 versus TERT wt/GFP with H2O2. b, EC were transfected with Myc-tagged TERT-wt (WT) or TERT Y707F (Y707F) together with GFP or Shp-2(C459S)-GFP. 24 h after transfection, nuclear extracts were prepared, and nuclear telomerase activity was measured. Data are means ± S.E. (n = 4) relative to the values obtained with TERT-wt/GFP (*, p < 0.05 versus TERT-wt/GFP; **, p < 0.05 versus TERT-wt/Shp2(C459S)-GFP).
FIGURE 7.
Tyrosine phosphorylation of TERT depends on Shp-2. a, HEK cells were transfected with Myc-tagged TERT-wt (WT) or TERT(Y707F) (Y707F), incubated with H2O2 for 1 h as indicated and immunoprecipitated with an anti-Shp-2-antibody from nuclear extracts. Immunoblots with the precipitates (IP) and the supernatants of the IP (SN) were performed with an anti-Myc antibody (upper panel) or an anti-Shp-2 (lower panel). b, HEK cells were first transfected with a shRNA vector targeting Shp-2 (shShp-2) or a scrambled control (scr) and 24 h later with TERT-wt (WT) or TERT(Y707F) (Y707F). Another 24 h later, cells were treated with H2O2 for 1 h, and nuclear lysates used for immunoprecipitation (IP) with an anti-Myc antibody. Immunoblots with the precipitates were performed with an anti-phosphotyrosine antibody (upper panel) or an anti-Myc antibody (lower panel).
DISCUSSION
The present study demonstrates that nuclear Shp-2 is associated with nuclear TERT and dissociates from TERT prior to its export, that Shp-2 inhibits nuclear export of TERT and that tyrosine 707 in TERT is a target for Shp-2.
Shp-2 was identified as a cytosolic SH2 domain containing tyrosine phosphatase, which is ubiquitously expressed. The wide distribution of the enzyme indicates that it might regulate various physiological functions. Homozygous Shp-2 knockout mice are embryonic lethal underscoring the importance of the enzyme. It is clear that Shp-2 plays a critical role in regulating signal transduction; however, a profound biochemical basis for the different functions of this phosphatase remains to be elucidated. Our data now add another important issue to the list of unaddressed questions: What is the function for nuclear Shp-2? Chughtai et al. (25) reported that Shp-2 exists in a complex with the transcription factor Stat5a. The authors speculate that active Shp-2 and a tyrosine-phosphorylated Stat5a translocate to the nucleus and potentially function in the nucleus to regulate transcription as both Shp-2 and Stat5a as a complex bind DNA. Our data now provide evidence that Shp-2 is localized in the nucleus already under basal conditions, where it is associated with TERT. Thus, it is tempting to speculate that some of the physiological functions of Shp-2 have to be addressed to its nuclear localization. The question remains how Shp-2 is imported into the nucleus, because it lacks a nuclear localization sequence. Therefore, a binding protein for Shp-2 could be required to induce its nuclear import. A possible candidate is Gab1, which has a nuclear import sequence (31) and has been shown to bind to Shp-2 (32). Preliminary data from our laboratory indicate that overexpression of a nuclear localization deficient Gab1 mutant prevents nuclear import of Shp-2.6
Under oxidative stress, TERT is tyrosine-phosphorylated and thereby exported from the nucleus in a Src kinase family/CRM1-dependent manner (21). In this study we demonstrate that Src and Yes are also localized in the nucleus of EC and thereby could be responsible for tyrosine phosphorylation of TERT. Moreover, the Src kinase family has been implicated in aging processes. This is mainly due to the fact that reactive oxygen species, which accumulate with age and induce lipid peroxidation, protein modification, DNA strand breaks, and cause oxidative damage, enhance the activity of the Src kinase family, which results in a progressive loss of cell function, a hallmark for aging processes (22, 33–35). We provide functional evidence that Src, Fyn, and Yes could play a role in aging processes, because cells deficient in these kinases can retain TERT in the nucleus under oxidative stress.
It has to be assumed that the export of nuclear TERT is regulated. Here, we demonstrate that Shp-2 is associated with TERT in the nucleus under basal conditions. More importantly, endogenous Shp-2 dissociates from nuclear TERT prior to its export and down-regulation of endogenous Shp-2 expression reduced nuclear telomerase activity. Thus, one may speculate that Shp-2 protects TERT from nuclear export by complex formation with TERT. Our data also suggest that tyrosine 707 in TERT is a target for the phosphatase activity of Shp-2. Therefore, Shp-2 might indeed be the unknown counterplayer for oxidative stress-induced nuclear export of TERT. However, we still do not know whether it acts solely via dephosphorylation. However, our data presented here strongly suggested that tyrosine 707 in TERT is a target of Shp-2 because the phosphorylation status of TERT(Y707F) is unaffected by Shp-2 and more importantly tyrosine phosphorylation of TERT-wt is negatively regulated by Shp-2. As a functional consequence nuclear Shp-2 could protect the cell either against accelerated senescence or increased apoptosis sensitivity or even both.
To our knowledge Shp-2, or more precise nuclear Shp-2, has not been implicated in aging processes until now. It has only been suggested that Shp-1 is responsible for aging-related attenuation of EGF receptor signaling in dermal fibroblasts (36), which suggests that Shp-1 negatively contributes to aging processes. This is in line with findings that Shp-1 plays a negative role in transducing signals for cellular responses (24). Previous biochemical evidence has shown that the enzymatic activity of Shp-2 is required for its function in signal transduction (37, 38). Replacing cysteine 459 with serine completely abolished its enzymatic activity. Binding of this mutant to other proteins via its SH2 domains remained unaltered. However, introduction of this mutant markedly inhibited the activation of MAP kinases in response to EGF and insulin (37, 39). This is in line with our data presented here that Shp-2(C459S) has also a nuclear localization (data not shown), but reduced nuclear telomerase activity and TERT protein already under basal conditions.
In summary, our study demonstrates a new important function for nuclear Shp-2 in retaining TERT in the nucleus. In addition, tyrosine 707 is a target for Shp-2. It counteracts the Src and Yes effects on TERT protein and telomerase activity in the nucleus under conditions of oxidative stress, most likely by regulating the net phosphorylation status of tyrosine 707 in TERT. This suggests that keeping Shp-2 in the nucleus delays aging processes and inhibits apoptosis.
Supplementary Material
Acknowledgments
We thank Christine Goy, Barbara Wiegand, Kerstin Kunze, Jennifer Birke, and Diane Schmiegelt for expert technical assistance.
This study was supported by the Deutsche Forschungsgemeinschaft (SFB728/B5 and HA2868/2-3, to J. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
Footnotes
The abbreviations used are: TERT, telomerase reverse transcriptase; Shp-2, protein tyrosine phosphatase Shp-2; EC, human umbilical venous endothelial cells; HEK, human embryonic kidney cells; MEF, mouse embryonic fibroblasts; wt, wild type; GFP, green fluorescent protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
S. Jakob, unpublished observation.
References
- 1.Blackburn, E. H. (2000) Nature 408 53–56 [DOI] [PubMed] [Google Scholar]
- 2.de Lange, T. (1998) Nature 392 753–754 [DOI] [PubMed] [Google Scholar]
- 3.Bodnar, A. G., Ouellette, M., Frolkis, M., Holt, S. E., Chiu, C. P., Morin, G. B., Harley, C. B., Shay, J. W., Lichtsteiner, S., and Wright, W. E. (1998) Science 279 349–352 [DOI] [PubMed] [Google Scholar]
- 4.Counter, C. M., Hahn, W. C., Wei, W., Caddle, S. D., Beijersbergen, R. L., Lansdorp, P. M., Sedivy, J. M., and Weinberg, R. A. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 14723–14728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang, J., Chang, E., Cherry, A. M., Bangs, C. D., Oei, Y., Bodnar, A., Bronstein, A., Chiu, C. P., and Herron, G. S. (1999) J. Biol. Chem. 274 26141–26148 [DOI] [PubMed] [Google Scholar]
- 6.Oh, H., Taffet, G. E., Youker, K. A., Entman, M. L., Overbeek, P. A., Michael, L. H., and Schneider, M. D. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudolph, K. L., Chang, S., Millard, M., Schreiber-Agus, N., and DePinho, R. A. (2000) Science 287 1253–1258 [DOI] [PubMed] [Google Scholar]
- 8.Ahmed, S., Passos, J. F., Birket, M. J., Beckmann, T., Brings, S., Peters, H., Birch-Machin, M. A., von Zglinicki, T., and Saretzki, G. (2008) J. Cell Sci. 121 1046–1053 [DOI] [PubMed] [Google Scholar]
- 9.Santos, J. H., Meyer, J. N., Skorvaga, M., Annab, L. A., and Van Houten, B. (2004) Aging Cell 3 399–411 [DOI] [PubMed] [Google Scholar]
- 10.Santos, J. H., Meyer, J. N., and Van Houten, B. (2006) Hum. Mol. Genet. 15 1757–1768 [DOI] [PubMed] [Google Scholar]
- 11.Kang, S. S., Kwon, T., Kwon, D. Y., and Do, S. I. (1999) J. Biol. Chem. 274 13085–13090 [DOI] [PubMed] [Google Scholar]
- 12.Kharbanda, S., Kumar, V., Dhar, S., Pandey, P., Chen, C., Majumder, P., Yuan, Z. M., Whang, Y., Strauss, W., Pandita, T. K., Weaver, D., and Kufe, D. (2000) Curr. Biol. 10 568–575 [DOI] [PubMed] [Google Scholar]
- 13.Li, H., Zhao, L., Yang, Z., Funder, J. W., and Liu, J. P. (1998) J. Biol. Chem. 273 33436–33442 [DOI] [PubMed] [Google Scholar]
- 14.Liu, J. P. (1999) Faseb J. 13 2091–2104 [DOI] [PubMed] [Google Scholar]
- 15.Seimiya, H., Tanji, M., Oh-hara, T., Tomida, A., Naasani, I., and Tsuruo, T. (1999) Biochem. Biophys. Res. Commun. 260 365–370 [DOI] [PubMed] [Google Scholar]
- 16.Hemann, M. T., Rudolph, K. L., Strong, M. A., DePinho, R. A., Chin, L., and Greider, C. W. (2001) Mol. Biol. Cell 12 2023–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattson, M. P., and Klapper, W. (2001) J. Neurosci. Res. 63 1–9 [DOI] [PubMed] [Google Scholar]
- 18.Wong, K. K., Chang, S., Weiler, S. R., Ganesan, S., Chaudhuri, J., Zhu, C., Artandi, S. E., Rudolph, K. L., Gottlieb, G. J., Chin, L., Alt, F. W., and DePinho, R. A. (2000) Nat. Genet. 26 85–88 [DOI] [PubMed] [Google Scholar]
- 19.Zhu, H., Fu, W., and Mattson, M. P. (2000) J Neurochem. 75 117–124 [DOI] [PubMed] [Google Scholar]
- 20.Haendeler, J., Hoffmann, J., Rahman, S., Zeiher, A. M., and Dimmeler, S. (2003) FEBS Lett. 536 180–186 [DOI] [PubMed] [Google Scholar]
- 21.Haendeler, J., Hoffmann, J., Brandes, R. P., Zeiher, A. M., and Dimmeler, S. (2003) Mol. Cell. Biol. 23 4598–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haendeler, J., Hoffmann, J., Diehl, J. F., Vasa, M., Spyridopoulos, I., Zeiher, A. M., and Dimmeler, S. (2004) Circ. Res. 94 768–775 [DOI] [PubMed] [Google Scholar]
- 23.Saxton, T. M., Henkemeyer, M., Gasca, S., Shen, R., Rossi, D. J., Shalaby, F., Feng, G. S., and Pawson, T. (1997) EMBO J. 16 2352–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu, C. K. (2002) Biochim. Biophys. Acta 1592 297–301 [DOI] [PubMed] [Google Scholar]
- 25.Chughtai, N., Schimchowitsch, S., Lebrun, J. J., and Ali, S. (2002) J. Biol. Chem. 277 31107–31114 [DOI] [PubMed] [Google Scholar]
- 26.Haendeler, J., Weiland, U., Zeiher, A. M., and Dimmeler, S. (1997) Nitric Oxide 1 282–293 [DOI] [PubMed] [Google Scholar]
- 27.Dimmeler, S., Haendeler, J., Rippmann, V., Nehls, M., and Zeiher, A. M. (1996) FEBS Lett. 399 71–74 [DOI] [PubMed] [Google Scholar]
- 28.Meyerson, M., Counter, C. M., Eaton, E. N., Ellisen, L. W., Steiner, P., Caddle, S. D., Ziaugra, L., Beijersbergen, R. L., Davidoff, M. J., Liu, Q., Bacchetti, S., Haber, D. A., and Weinberg, R. A. (1997) Cell 90 785–795 [DOI] [PubMed] [Google Scholar]
- 29.Haendeler, J., Ishida, M., Hunyady, L., and Berk, B. C. (2000) Circ. Res. 86 729–736 [DOI] [PubMed] [Google Scholar]
- 30.Vasa, M., Breitschopf, K., Zeiher, A. M., and Dimmeler, S. (2000) Circ. Res. 87 540–542 [DOI] [PubMed] [Google Scholar]
- 31.Osawa, M., Itoh, S., Ohta, S., Huang, Q., Berk, B. C., Marmarosh, N. L., Che, W., Ding, B., Yan, C., and Abe, J. (2004) J. Biol. Chem. 279 29691–29699 [DOI] [PubMed] [Google Scholar]
- 32.Huang, Q., Lerner-Marmarosh, N., Che, W., Ohta, S., Osawa, M., Yoshizumi, M., Glassman, M., Yan, C., Berk, B. C., and Abe, J. (2002) J. Biol. Chem. 277 29330–29341 [DOI] [PubMed] [Google Scholar]
- 33.Griendling, K. K., Sorescu, D., Lassegue, B., and Ushio-Fukai, M. (2000) Arterioscler. Thromb. Vasc. Biol. 20 2175–2183 [DOI] [PubMed] [Google Scholar]
- 34.Geserick, C., and Blasco, M. A. (2006) Mech. Ageing Dev. 127 579–583 [DOI] [PubMed] [Google Scholar]
- 35.Wang, J. F., Zhang, X., and Groopman, J. E. (2004) J. Biol. Chem. 279 27088–27097 [DOI] [PubMed] [Google Scholar]
- 36.Tran, K. T., Rusu, S. D., Satish, L., and Wells, A. (2003) Exp. Cell Res. 289 359–367 [DOI] [PubMed] [Google Scholar]
- 37.Milarski, K. L., and Saltiel, A. R. (1994) J. Biol. Chem. 269 21239–21243 [PubMed] [Google Scholar]
- 38.Yamauchi, K., Milarski, K. L., Saltiel, A. R., and Pessin, J. E. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett, A. M., Hausdorff, S. F., O'Reilly, A. M., Freeman, R. M., and Neel, B. G. (1996) Mol. Cell. Biol. 16 1189–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.