Abstract
FGF19 subfamily proteins (FGF19, FGF21, and FGF23) are unique members of fibroblast growth factors (FGFs) that regulate energy, bile acid, glucose, lipid, phosphate, and vitamin D homeostasis in an endocrine fashion. Their activities require the presence of α or βKlotho, two related single-pass transmembrane proteins, as co-receptors in relevant target tissues. We previously showed that FGF19 can bind to both α and βKlotho, whereas FGF21 and FGF23 can bind only to either βKlotho or αKlotho, respectively in vitro. To determine the mechanism regulating the binding and specificity among FGF19 subfamily members to Klotho family proteins, chimeric proteins between FGF19 subfamily members or chimeric proteins between Klotho family members were constructed to probe the interaction between those two families. Our results showed that a chimera of FGF19 with the FGF21 C-terminal tail interacts only with βKlotho and a chimera with the FGF23 C-terminal tail interacts only with αKlotho. FGF signaling assays also reflected the change of specificity we observed for the chimeras. These results identified the C-terminal tail of FGF19 as a region necessary for its recognition of Klotho family proteins. In addition, chimeras between α and βKlotho were also generated to probe the regions in Klotho proteins that are important for signaling by this FGF subfamily. Both FGF23 and FGF21 require intact α or βKlotho for signaling, respectively, whereas FGF19 can signal through a Klotho chimera consisting of the N terminus of αKlotho and the C terminus of βKlotho. Our results provide the first glimpse of the regions that regulate the binding specificity between this unique family of FGFs and their co-receptors.
The FGF19 subfamily of fibroblast growth factors (FGFs),2 consisting of FGF19, FGF21, and FGF23, has been implicated in the regulation of a variety of metabolic processes (1–4). FGF19 can regulate hepatic bile acid metabolism through repression of the gene encoding cholesterol 7α-hydroxylase (CYP7A1), the first and rate-limiting step in the biosynthesis of bile acids (5). Elevation of plasma FGF19 levels either by transgenic expression or injection of recombinant protein has been shown to improve insulin sensitivity, reduce adiposity, and increase metabolic rate in rodent diabetes and obesity models (1, 2). FGF21 was found to increase the glucose uptake in mouse 3T3-L1 and primary human adipocytes (3). FGF21 transgenic mice were resistant to diet-induced obesity (3). In addition, injection of recombinant FGF21 reduced plasma glucose and triglycerides to near normal levels in both ob/ob and db/db mice (3, 6). FGF23 reduced serum phosphate levels by suppressing kidney proximal tubular phosphate re-absorption (4). FGF23 also reduced the serum levels of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] resulting in suppressed intestinal phosphate absorption (4).
Unlike other FGFs, which act locally in an autocrine or paracrine manner, FGF19 subfamily members can regulate physiological functions in an endocrine fashion. The fact that this subfamily of FGFs has very low binding affinity to heparin and heparan sulfate may help to prevent them from being trapped in the extracellular matrices (7). In addition, the presence of intramolecular disulfide bonds could increase their plasma stability and allow them to function as hormones and act on distant tissues. However, the reduced affinity of FGF19 subfamily members to heparin and heparan sulfate, thought to be required for high affinity binding between classical FGFs to FGF receptors (FGFRs) (8), correlates with their reduced affinity for FGFRs. Indeed, direct interactions between FGFRs and the FGF19 subfamily proteins in vitro have not been observed, which implies that additional cofactors are required to promote the binding of FGF19 subfamily members to their cognate FGFRs in the target tissues. Two such cofactors, αKlotho and βKlotho, were recently identified to facilitate the interaction between FGF19 subfamily proteins with FGFRs and the activation of FGF signaling (9–11). The αKlotho and βKlotho proteins are members of the Klotho family of proteins and share about 41% amino acid identity (12). They are single-pass transmembrane glycoproteins of about 130 kDa with a short cytoplasmic domain (12). The extracellular domains of both Klotho proteins have two internal repeats, each repeat sharing 20–40% sequence identity to the β-glucosidases of bacteria and plants as well as to mammalian lactase glycosylceramidase (12). αKlotho is expressed most notably in the distal convoluted tubules in the kidney (13), and βKlotho is expressed in adipose tissue, liver, and pancreas (12, 14). The first suggestion that Klotho proteins might be cofactors for FGF19 subfamily functions came from the observation that αKlotho-deficient mice and FGF23-deficient mice exhibited overlapping phenotypes, such as hyperphosphatemia (15, 16). Subsequent biochemical experiments demonstrated that αKlotho can directly interact with FGF receptors as well as with FGF19 and FGF23 (10, 11). In HEK293 cells, which normally do not respond to FGF19 subfamily members, co-expression of αKlotho conferred responsiveness of the cells to FGF19 and FGF23 (10, 11). Similar to αKlotho, βKlotho may also directly interact with multiple FGF receptors. However, in contrast to αKlotho, βKlotho interacts directly with FGF19 and FGF21 but not with FGF23 (9, 11). Both FGF19 and FGF21 activated HEK293 cells transfected with βKlotho (11). βKlotho knockout mice display increased bile acid synthesis (17), which is also observed in mice deficient in FGF15 (the mouse ortholog of human FGF19). Taken together, this evidence strongly suggests that αKlotho and βKlotho are necessary cofactors in FGF19 subfamily signaling and is consistent with the notion that restricted expression of Klotho proteins may specify the metabolic actions of FGF19 subfamily members. The requirement for cofactors could eliminate unwanted side effects, perhaps caused by indiscriminatory activation of FGFRs expressed in other tissues. Thus FGF19 subfamily proteins may be promising therapeutic targets.
So far there is little structural information available for the tertiary complex formed by the FGF receptor, FGF19/21/23, and α/βKlotho. The crystal structure of FGF19 was solved, and a model for FGF19 and FGFR4 interaction was proposed (7, 18); however, exactly how Klotho proteins interact with FGF19 subfamily members and receptors remains unknown. In this study we probed the interaction between FGF19 subfamily proteins and Klotho family proteins utilizing domain-swapped chimeras to identify the important regions on those proteins that determine the specificity of their interactions.
EXPERIMENTAL PROCEDURES
Materials—FGF receptors Fc fusion proteins, FGF21, FGF23, anti-FGF19, anti-FGF21, antiFGF23, anti-αKlotho and anti-βKlotho antibodies were purchased from R&D. Anti-V5 antibody was purchased from Invitrogen.
Klotho Constructs—Full-length human βKlotho, αKlotho, and the extracellular domain of human βKlotho and αKlotho were cloned into the pTT14 expression vector. The Klotho chimera constructs were generated by PCR with overlapping oligonucleotides overlapping PCR to fuse the 5′-end of αKlotho (residues 1–514) to the 3′-end of βKlotho (517–1044) for the α/β Klotho chimera. The β/α Klotho chimera consists of residues 1–516 of βKlotho fused to residues 515–1012 of αKlotho. They were also cloned into the pTT14 vector backbone.
Expression and Purification of Recombinant FGF19 and Chimeric FGF19 Production—The oligos for the human chimeric FGF19 molecules were synthesized by Blue Heron Bio (Bothhell, WA). Both FGF19–21C and FGF19–23C chimeras contains the core of FGF19 (residues 1–177) fused to the tail of human FGF21 (residues 177–209) and the tail of human FGF23 (residues 171–251), respectively. Wild-type FGF19 and chimeras were cloned into the pET30 vector (Novagen). DNA constructs were transformed into BL21(DE3) Escherichia coli (Novagen). Protein expression was induced with isopropyl-1-thio-β-d-galactopyranoside at 37 °C. Cells were lysed by high pressure disruption and inclusion bodies were isolated. The insolubly expressed material was extracted at ambient temperature with a reducing solution of guanidine-HCl buffered at pH 8.5 followed by dilution into chilled refolding buffer containing urea, arginine-HCl, and a redox couple buffered at pH 9.5. The solution was gently stirred until negative to Ellman's reagent to allow for complete air oxidation of the protein. Soluble proteins were exchanged into buffered solutions at pH 8 (FGF19) or pH 9.7 (FGF19–21C, FGF19–23C) and purified by anion exchange on a Q-Sepharose high performance column (GE Healthcare) resolved with an NaCl gradient. Proteins were further purified on ceramic hydroxyapatite type I (Bio-Rad) or Superdex 75 (GE Healthcare) columns resolved in PBS.
Cell Culture and Transfections—HEK293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. Cells were transfected with expression vectors using the Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's protocol.
Conditioned Medium Preparation—HEK293 cells were transfected with the extracellular domain of βKlotho and αKlotho expression vectors, and the medium was collected 2 days after transfection.
Pull-down Assay—FGF19 or chimeric proteins (0.5 μg) were mixed with FGFR4-Fc fusion proteins (0.5 μg) in conditioned medium and applied to 50 μl of protein G-Sepharose at 4 °C for 1.5 h. The beads were washed three times with PBS and then suspended in SDS sample buffer and subjected to Western blot analysis with anti-FGF19 antibody (R&D).
Solid-phase Binding Assay—Nunc 96-well plates were coated overnight at 4 °C with 50 μl of s2 μg/ml anti-His antibody. After blocking with 200 μl per well with PBS with 3% bovine serum albumin, 45 μl of 5 μg/ml His-tagged soluble recombinant α or βKlotho proteins were added to each well at room temperature. After 1.5 h, biotinylated FGF proteins (FGF proteins were biotinylated with Pierce Sulfo-NHS-LC-Biotin) were added, and the plates were incubated for another 1.5 h at room temperature. The plates were washed 4× with PBS with 0.05% Tween 20, and streptavidin-HRP was used for detection.
Western Blot Analysis of the FGF Signaling Pathway—HEK293 cells were transfected with αKlotho, βKlotho, and chimeric Klotho constructs in 24-well plates and serum-starved overnight the day after transfection. Following treatment with various concentrations of FGF proteins for 10 min, cells were snap-frozen in liquid nitrogen, and cell lysates were prepared in SDS sample buffer and subjected to Western blot analysis using anti-phospho-p44/42 MAP kinase (ERK1/2) antibody and anti-ERK antibody (Cell Signaling). Expression levels of V5-tagged α/βKlotho and β/αKlotho chimera proteins were measured by anti-V5 antibody.
RESULTS
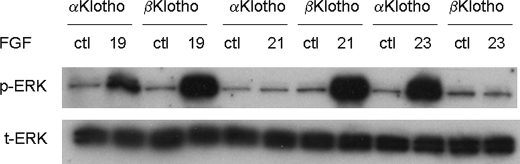
The C Terminus of FGF19 Determines the Signaling Specificity toward α or βKlotho—FGF19 subfamily members, FGF19, -21, and -23, require α or βKlotho as the co-receptor for signaling (10, 11, 14). HEK293 cells normally do not respond to the treatment by FGF19, -21, and -23 alone (as we have shown before (11) and data not shown). Therefore, using HEK293 cells transfected with either α or βKlotho, we evaluated FGF-dependent signaling by measuring ERK phosphorylation assessed by Western blot. FGF19 activated cells transfected with either α or βKlotho. FGF21 only activated βKlotho-transfected cells, while FGF23 activated only αKlotho-transfected cells (Fig. 1). Because each of the subfamily members displayed a different specificity toward Klotho family proteins, we sought to identify the FGF domains that determine this specificity. Results from a previous report suggested that the C-terminal tail of FGF23 might contribute to its interaction with αKlotho (7). To test whether the C-terminal tail domain of this subfamily of FGF proteins confers the specificity toward the Klotho family members, we generated and purified chimeric FGF19 proteins with the C-terminal tail domain of FGF21 or FGF23 (Fig. 2A). Because the ERK phosphorylation assay suggests that FGF21 and FGF23 specifically activate through βKlotho and αKlotho, respectively, if the C-terminal tail determines Klotho specificity, chimeric FGF19 proteins could be expected to show altered selective preference for either α or βKlotho.
FIGURE 1.
FGF19, -21, and -23 activate ERK1/2 phosphorylation in HEK293 cells transfected with α or βKlotho. HEK293 cells were transfected with expression vectors for α or βKlotho. Following overnight serum starvation, cells were stimulated with vehicle or 50 nm recombinant FGF19, -21, or -23 for 15 min and snap-frozen in liquid nitrogen. Cell lysates were prepared for Western blot using antibodies against phosphorylated ERK1/2 (pERK1/2) or total ERK1/2 (ERK1/2). ctl indicates reactions with no FGF added.
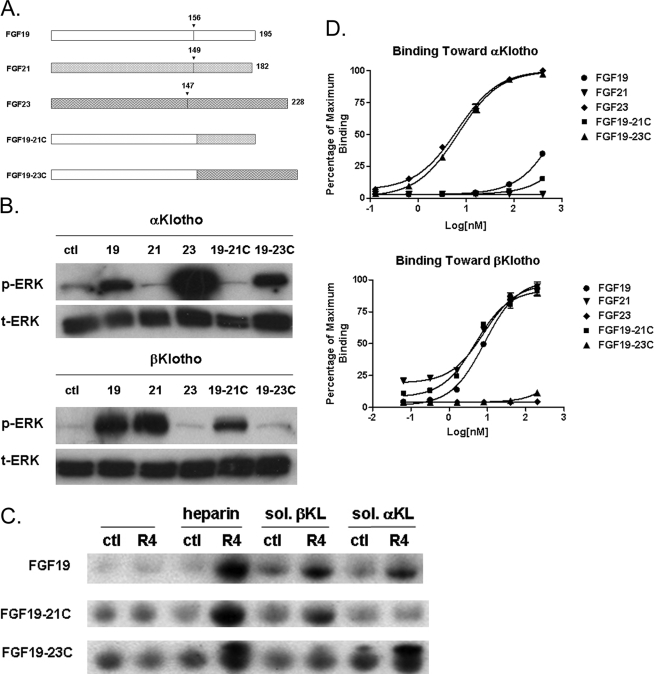
FIGURE 2.
Activity of FGF19 chimeric proteins. A, schematic diagram showing FGF19, -21, -23, and two FGF19 chimeric proteins. In FGF19–21C, the FGF19 C-terminal region is replaced with that of FGF21. In FGF19–23C, the FGF19 C-terminal region is replaced with that of FGF23. B, ERK1/2 phosphorylation in HEK293 cells transfected with α or βKlotho. FGF19–21C fails to activate ERK phosphorylation in HEK293 cells transfected with αKlotho, and FGF19–23C did not activate cells transfected with βKlotho. C, in the pull-down assay, FGF19 requires either heparin, αKlotho, or βKlotho to bind to FGFR4. FGF19–21C did not bind to FGFR4 in the presence of αKlotho, and FGF19–23C did not bind to FGFR4 in the presence of βKlotho. R4 indicates reactions containing FGFR4, ctl indicates reactions with no FGFR added. D, solid-phase binding assay measuring the direct interaction between FGF molecules and α or βKlotho.
We transfected HEK293 cells with either α or βKlotho, subsequently treated the cells with FGF19, -21, -23, 19–21C, and 19–23C, and evaluated FGF signaling by measuring ERK1/2 phosphorylation by Western blot. As shown in Fig. 2B, unlike FGF19, which responds to both αKlotho and βKlotho proteins, FGF19–21C, which contains the C-terminal region of FGF21, only activated cells transfected with βKlotho; thus more similar to FGF21. Conversely, FGF19–23C, which contains the C-terminal region of FGF23, behaved more like FGF23 and activated only αKlotho-containing cells. These results provided the first direct evidence that the C terminus of FGF19 determines its specificity for Klotho-dependent signaling.
FGF19 Chimeric Proteins Display Altered Binding Specificity in the Pull-down Assay toward α or βKlotho—We previously used a pull-down assay to evaluate the interaction between FGF19 and FGFR4 receptor assessed by anti-FGF19 Western blot of FGFR4-immunoprecipitated complexes (11). The FGF19-FGFR4 interaction could only be observed in the presence of heparin, αKlotho, or βKlotho (Fig. 2C, upper panel), consistent with the requirement for either α or βKlotho in HEK293 ERK phosphorylation assays shown in Fig. 1. In pull-down assays, an FGF19 chimeric protein with the FGF21 tail (FGF19–21C) bound to the FGFR4 receptor in the presence of βKlotho. However, distinct from wild-type FGF19, this chimera could not bind to the receptor with αKlotho (Fig. 2C, middle panel). Conversely, an FGF19 chimera with the FGF23 tail (FGF19–23C) lost its ability to bind to the receptor FGFR4 with βKlotho, but bound to the receptor in the presence of αKlotho (Fig. 2C, lower panel). These results suggest that the specificity toward different Klotho proteins in HEK293 cell signaling activation by the treatment of different FGF19 chimeric molecules (Fig. 1) is the result of altered direct binding specificity toward Klotho proteins. Therefore, this suggests that the C-terminal regions of FGF19 subfamily members are essential for interactions with α or βKlotho.
Interestingly, both FGF19–21C and FGF19–23C chimeric proteins are still able to interact with FGFR4 in the presence of heparin and in the absence of Klotho proteins (Fig. 2C), similar to wild-type FGF19 itself. Such heparin-dependent Klotho-independent interaction with FGFR4 is unique to FGF19 and has not been observed for FGF21 and FGF23 (10, 11, 14). The observation that the two chimeric FGF19 molecules with alternative C-terminal regions preserved this unique heparin-dependent interaction with FGFR4 suggests that the specificity toward FGF receptors is likely determined by domains outside of the C terminus of FGF19. Swapping the C-terminal tail of FGF19 with that of either FGF21 or FGF23 changed its specificity toward either α or βKlotho without affecting FGF19/receptor interactions; therefore, the C-terminal regions of FGF19 subfamily members are responsible for determining the specificity of FGF19/Klotho family protein interactions.
Direct Interactions between FGF19 Subfamily Members, FGF19 Chimeric Proteins with α and βKlotho—To provide more direct evidence for the interactions between the C-terminal region of FGF19 subfamily members and Klotho proteins and to gain further insight into the relationship between the binding affinity toward α and βKlotho and signaling activities, we developed a solid-phase assay to provide a more quantitative measurement of the relative binding affinities between FGF19, -21, -23, FGF19 chimeras and recombinant soluble α and βKlotho proteins (Fig. 2D). Results show that consistent with activation of receptor signaling in the cell-based ERK phosphorylation assay and biochemical binding with soluble receptor complexes in the pull-down assay, FGF19 subfamily members and chimeric proteins could indeed interact directly with the Klotho family of proteins. Consistent with the predicted specificity shown in Figs. 1 and 2, FGF19, FGF23, and FGF19–23C formed direct interactions with αKlotho, and FGF19, FGF21, and FGF19–21C formed direct interactions with βKlotho in a dose-dependent manner (Fig. 2D). The interaction between FGF19 and αKlotho appeared weaker than FGF23 and FGF19 with the FGF23 C-terminal tail (FGF19–23C), suggesting that the FGF23 C-terminal region may form tighter interactions with the αKlotho protein. On the other hand, FGF19, FGF21, and FGF19 with the FGF21 C-terminal region (FGF19–21C) all seem to interact equally well with βKlotho (Fig. 2D). Given that FGF21 and FGF19–21C did not form a significant interaction with αKlotho and FGF23 and FGF19–23C did not bind βKlotho (Fig. 2D), these results are also consistent with our hypothesis that the C-terminal regions of FGF19 subfamily members are the predominant interaction and specificity determination site for Klotho interactions.
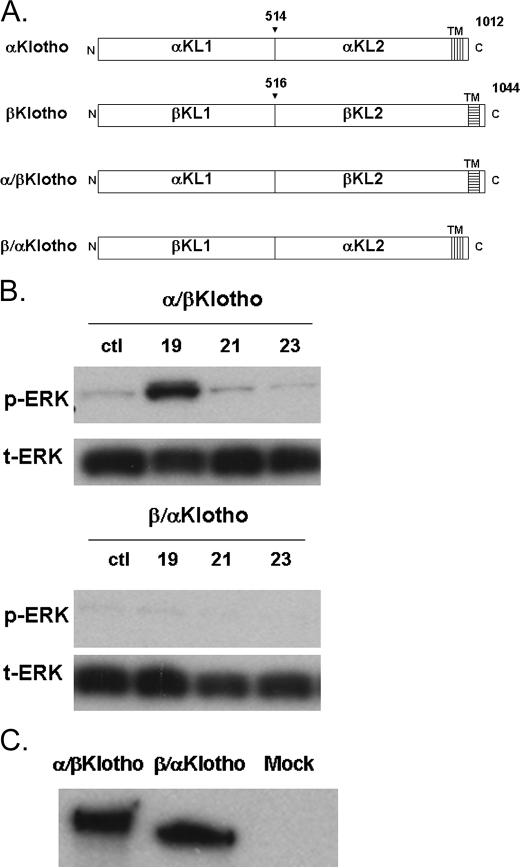
Klotho Domain and the Specificity of the FGF19 Family—The extracellular domains of Klotho proteins have two internal repeats (αKL1 and αKL2 for αKlotho, βKL1 and βKL2 for βKlotho) each sharing sequence homology to β-glucosidases. To investigate the contribution of each of these domains to interaction and activation by FGF19 subfamily proteins, we generated chimeras between α and βKlotho (Fig. 3A). α/βKlotho contains the KL1 domain from αKlotho, and KL2 domain from βKlotho, whereas β/αKlotho contains the KL1 domain from βKlotho, and KL2 domain from αKlotho. Following transfection with α/βKlotho or β/αKlotho, HEK293 cells were treated with FGF19, -21, and -23 proteins, and signaling was measured by an anti-ERK Western blot. As shown in Fig. 3B, only FGF19-activated cells transfected with α/βKlotho, and no FGF proteins were able to activate β/αKlotho-containing cells even though both Klotho chimeric proteins were expressed equally well in these cells (Fig. 3C). FGF- and Klotho-dependent signaling activation data are summarized in Table 1. FGF21 and FGF23 required intact βKlotho and αKlotho, respectively, for signaling, whereas αKlotho, βKlotho, or the α/βKlotho chimera can promote signaling by FGF19.
FIGURE 3.
Activation of FGF signaling in HEK293 cells transfected with Klotho chimeras. A, schematic diagram showing α and βKlotho chimeric constructs (TM, transmembrane region). B, FGF19 activates cells transfected with α/βKlotho constructs. None of the ligands activates β/αKlotho cells. ctl indicates reactions with no FGF molecule added. C, because α/βKlotho and β/αKlotho chimeric constructs contained the V5 tag, their expression levels in HEK293 cells after transfection were detected by Western analysis using anti-V5 antibody. MOCK indicates untransfected cells.
TABLE 1.
Summary of the FGF19, -21, and -23 activities on HEK293 cells transfected with different Klotho constructs
| αKlotho | βKlotho | α/βKlotho | β/αKlotho | |
|---|---|---|---|---|
| FGF19 | + | + | + | – |
| FGF21 | – | + | – | – |
| FGF23 | + | – | – | – |
DISCUSSION
FGF19 subfamily members require Klotho family cofactors to activate signaling by FGF receptors. We and others have previously shown that members of the FGF19 subfamily display different specificities toward α and βKlotho. In this current study, we have utilized these specificity differences to understand the interactions between the FGF19 subfamily of proteins and Klotho proteins.
Based on the study of a naturally occurring C-terminally truncated FGF23 protein, it was previously suggested that the C-terminal tail of FGF23 might contribute to its interaction with αKlotho (7). To test the importance of C-terminal regions in FGF19 subfamily member interaction with Klotho proteins, we constructed C-terminal domain chimeric proteins between FGF19, -21, and -23 (Fig. 2A). We hypothesized that if the C-terminal domain is important for Klotho interaction, then a FGF19/FGF21 chimeric protein in which the FGF19 C-terminal region is replaced with that of FGF21 will result in specific binding to βKlotho. Alternatively, a FGF19/FGF23 chimeric protein in which the FGF19 C-terminal region is replaced with that of FGF23 will result in specific binding to αKlotho. Indeed, both in cell-based signaling assay and in biochemical pull-down assay with soluble receptor complexes, the novel FGF19 chimeric molecules resulted in altered Klotho specificity compared with wild-type FGF19 molecule (Fig. 2, B and C). These results strongly suggested that the C-terminal region is critical for Klotho specificity determination and interaction.
To provide more evidence for a direct interaction between the C-terminal region of FGF19 and Klotho molecules, a solidphase assay was developed to provide a more quantitative measurement of the relative binding affinity of various native and chimeric FGF proteins to Klotho proteins (Fig. 2D). These results show that FGF19, FGF21, and FGF23 can interact directly with Klotho proteins. In addition, the altered binding specificity of FGF19–21C to bind βKlotho only and FGF19–23C to bind αKlotho only provided direct evidence supporting the hypothesis that the C-terminal region is the predominant site of interaction on FGF19 molecules to Klotho proteins and may determine the specificity toward either α or βKlotho. The involvement of the N-terminal region in Klotho interaction cannot be completely ruled out. Interestingly, the results also seem to show that the N-terminal portion of FGF19, but not FGF21 and FGF23, may contribute additional weaker interactions to Klotho proteins as well under the conditions tested, since very weak signals were observed between FGF19–21C to αKlotho and FGF19–23C to βKlotho (Fig. 2D). However, these interactions must be very weak, and on their own, could not trigger activation of the signaling pathway (Fig. 2B).
The activation of the signaling pathway by FGF19, -21, and -23 requires both Klotho protein and FGFRs. It is interesting to note that although FGF19–23C and FGF23 appeared to have a similar binding strength to αKlotho (Fig. 2D), the FGF23-induced activation of signaling pathway was consistently stronger than FGF19–23C (Fig. 2B). Conversely, although FGF19–23C appeared to have a stronger interaction with αKlotho than FGF19, they seem to result in similar levels of signaling in cells (Fig. 2, D and B). In addition, Klotho-independent heparin-induced FGF19/FGFR4 interaction was not affected by changes in its C-terminal region (Fig. 2C) suggesting that the N-terminal region of FGF19 molecules is responsible for heparin and FGFR interactions. It was also observed previously that, in HEK293 cells, FGF19 was not able to activate signaling in the presence of heparin alone; however, heparin could potentially synergize with Klotho for receptor activation (11), which should also be the case for the chimeric FGF molecules since they share the same N-terminal region with FGF19. These results suggest that though the C-terminal region might be important for Klotho binding and perhaps act as an anchor for FGF binding to the receptor complexes, the N-terminal regions of FGF19 molecules might be important for FGFR interactions and activation of downstream signaling events. The precise mechanism of how the N-terminal region interacts and triggers the FGFR conformation changes for activation will need to be studied further in the future.
Biochemical and cell-based studies so far demonstrated that an FGF19 chimera with an FGF21 C-terminal tail qualitatively resembled FGF21 with respect to βKlotho interaction and receptor signaling, and a chimera with an FGF23 C-terminal tail behaved similarly to FGF23 with respect to αKlotho interactions and signaling. Given that FGF19, -21, and -23 have similar FGF receptor specificity, with the preference of binding to the c-isoform of the FGFRs (10, 11, 14), it is intriguing to speculate that Klotho co-receptor specificity to a large extent might dictate physiological functions. This hypothesis predicts that chimeric molecules among these subfamily members might behave similarly to the subfamily members with which they share the C-terminal tails. For example, an FGF21 molecule with an FGF23 tail might be able to decrease serum phosphate levels, and an FGF23 molecule with an FGF21 tail could reduce blood glucose.
Both α and βKlotho contain two β-glucosidase-like domains. To further probe FGF and Klotho structure-function relationships, we made chimeric expression constructs with one β-glucosidase-like domain from each Klotho and evaluated FGF-dependent signaling. FGF19 activated cells transfected with a chimeric Klotho consisting of the N terminus of αKlotho (αKL1) and C terminus of βKlotho (βKL2). FGF19 also activated cells transfected with full-length αKlotho and βKlotho. In contrast, FGF21 and FGF23 required intact β or αKlotho to facilitate signaling. Klotho protein domains responsible for selective interactions with FGF19 subfamily members may be complex. In addition, the sequence homology between the C-terminal regions of FGF19, FGF21, and FGF23 are weak, suggesting that they may form distinct interactions with Klotho proteins or perhaps even bind to different surfaces/regions on Klotho co-receptor molecule. Further studies will be required to define necessary and sufficient regions of Klotho proteins that contribute to FGF C-terminal tail selectivity and ultimately their role in metabolic control.
Acknowledgments
We thank Hongfei Ge, Jing Xu, Murielle Veniant-Ellison, Ming Wang, Claudia Baikalov, Raj Haldankar, John Lamerdin, Margret Karow, Randy Hecht, Grant Shimamoto, Luke Li, Helen Kim, Jackie Sheng, and Tom Boone for helpful discussions and critical reading of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: FGF, fibroblast growth factor; PBS, phosphate-buffered saline; ERK, extracellular signal-regulated kinase; HEK, human embryonic kidney.
References
- 1.Fu, L., John, L. M., Adams, S. H., Yu, X. X., Tomlinson, E., Renz, M., Williams, P. M., Soriano, R., Corpuz, R., Moffat, B., Vandlen, R., Simmons, L., Foster, J., Stephan, J. P., Tsai, S. P., and Stewart, T. A. (2004) Endocrinology 145 2594-2603 [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson, E., Fu, L., John, L., Hultgren, B., Huang, X., Renz, M., Stephan, J. P., Tsai, S. P., Powell-Braxton, L., French, D., and Stewart, T. A. (2002) Endocrinology 143 1741-1747 [DOI] [PubMed] [Google Scholar]
- 3.Kharitonenkov, A., Shiyanova, T. L., Koester, A., Ford, A. M., Micanovic, R., Galbreath, E. J., Sandusky, G. E., Hammond, L. J., Moyers, J. S., Owens, R. A., Gromada, J., Brozinick, J. T., Hawkins, E. D., Wroblewski, V. J., Li, D. S., Mehrbod, F., Jaskunas, S. R., and Shanafelt, A. B. (2005) J. Clin. Investig. 115 1627-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito, H., Kusano, K., Kinosaki, M., Ito, H., Hirata, M., Segawa, H., Miyamoto, K., and Fukushima, N. (2003) J. Biol. Chem. 278 2206-2211 [DOI] [PubMed] [Google Scholar]
- 5.Inagaki, T., Choi, M., Moschetta, A., Peng, L., Cummins, C. L., McDonald, J. G., Luo, G., Jones, S. A., Goodwin, B., Richardson, J. A., Gerard, R. D., Repa, J. J., Mangelsdorf, D. J., and Kliewer, S. A. (2005) Cell Metab. 2 217-225 [DOI] [PubMed] [Google Scholar]
- 6.Inagaki, T., Dutchak, P., Zhao, G., Ding, X., Gautron, L., Parameswara, V., Li, Y., Goetz, R., Mohammadi, M., Esser, V., Elmquist, J. K., Gerard, R. D., Burgess, S. C., Hammer, R. E., Mangelsdorf, D. J., and Kliewer, S. A. (2007) Cell Metab. 5 415-425 [DOI] [PubMed] [Google Scholar]
- 7.Goetz, R., Beenken, A., Ibrahimi, O. A., Kalinina, J., Olsen, S. K., Eliseenkova, A. V., Xu, C., Neubert, T. A., Zhang, F., Linhardt, R. J., Yu, X., White, K. E., Inagaki, T., Kliewer, S. A., Yamamoto, M., Kurosu, H., Ogawa, Y., Kuro-o, M., Lanske, B., Razzaque, M. S., and Mohammadi, M. (2007) Mol. Cell. Biol. 27 3417-3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadi, M., Olsen, S. K., and Ibrahimi, O. A. (2005) Cytokine Growth Factor Rev. 16 107-137 [DOI] [PubMed] [Google Scholar]
- 9.Kurosu, H., Choi, M., Ogawa, Y., Dickson, A. S., Goetz, R., Eliseenkova, A. V., Mohammadi, M., Rosenblatt, K. P., Kliewer, S. A., and Kuro-o, M. (2007) J. Biol. Chem. 282 26687-26695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurosu, H., Ogawa, Y., Miyoshi, M., Yamamoto, M., Nandi, A., Rosenblatt, K. P., Baum, M. G., Schiavi, S., Hu, M. C., Moe, O. W., and Kuro-o, M. (2006) J. Biol. Chem. 281 6120-6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu, X., Ge, H., Gupte, J., Weiszmann, J., Shimamoto, G., Stevens, J., Hawkins, N., Lemon, B., Shen, W., Xu, J., Veniant, M. M., Li, Y. S., Lindberg, R., Chen, J. L., Tian, H., and Li, Y. (2007) J. Biol. Chem. 282 29069-29072 [DOI] [PubMed] [Google Scholar]
- 12.Ito, S., Kinoshita, S., Shiraishi, N., Nakagawa, S., Sekine, S., Fujimori, T., and Nabeshima, Y. I. (2000) Mech. Dev. 98 115-119 [DOI] [PubMed] [Google Scholar]
- 13.Tsujikawa, H., Kurotaki, Y., Fujimori, T., Fukuda, K., and Nabeshima, Y. (2003) Mol. Endocrinol. 17 2393-2403 [DOI] [PubMed] [Google Scholar]
- 14.Ogawa, Y., Kurosu, H., Yamamoto, M., Nandi, A., Rosenblatt, K. P., Goetz, R., Eliseenkova, A. V., Mohammadi, M., and Kuro-o, M. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 7432-7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada, T., Kakitani, M., Yamazaki, Y., Hasegawa, H., Takeuchi, Y., Fujita, T., Fukumoto, S., Tomizuka, K., and Yamashita, T. (2004) J. Clin. Investig. 113 561-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida, T., Fujimori, T., and Nabeshima, Y. (2002) Endocrinology 143 683-689 [DOI] [PubMed] [Google Scholar]
- 17.Ito, S., Fujimori, T., Furuya, A., Satoh, J., Nabeshima, Y., and Nabeshima, Y. (2005) J. Clin. Investig. 115 2202-2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmer, N. J., Pellegrini, L., Chirgadze, D., Fernandez-Recio, J., and Blundell, T. L. (2004) Biochemistry 43 629-640 [DOI] [PubMed] [Google Scholar]