Abstract
Among the many mammalian secreted phospholipase A2 (sPLA2) enzymes, PLA2G3 (group III secreted phospholipase A2) is unique in that it possesses unusual N- and C-terminal domains and in that its central sPLA2 domain is homologous to bee venom PLA2 rather than to other mammalian sPLA2s. To elucidate the in vivo actions of this atypical sPLA2, we generated transgenic (Tg) mice overexpressing human PLA2G3. Despite marked increases in PLA2 activity and mature 18-kDa PLA2G3 protein in the circulation and tissues, PLA2G3 Tg mice displayed no apparent abnormality up to 9 months of age. However, alterations in plasma lipoproteins were observed in PLA2G3 Tg mice compared with control mice. In vitro incubation of low density (LDL) and high density (HDL) lipoproteins with several sPLA2s showed that phosphatidylcholine was efficiently converted to lysophosphatidylcholine by PLA2G3 as well as by PLA2G5 and PLA2G10, to a lesser extent by PLA2G2F, and only minimally by PLA2G2A and PLA2G2E. PLA2G3-modified LDL, like PLA2G5- or PLA2G10-treated LDL, facilitated the formation of foam cells from macrophages ex vivo. Accumulation of PLA2G3 was detected in the atherosclerotic lesions of humans and apoE-deficient mice. Furthermore, following an atherogenic diet, aortic atherosclerotic lesions were more severe in PLA2G3 Tg mice than in control mice on the apoE-null background, in combination with elevated plasma lysophosphatidylcholine and thromboxane A2 levels. These results collectively suggest a potential functional link between PLA2G3 and atherosclerosis, as has recently been proposed for PLA2G5 and PLA2G10.
Secreted phospholipase A2 (sPLA2)3 enzymes represent a group of structurally related, disulfide-rich, low molecular mass (typically 14–18 kDa) enzymes with strict Ca2+ dependence and a His-Asp catalytic dyad (1, 2). To date, 10 catalytically active sPLA2 enzymes have been identified in mammals (IB, IIA, IIC, IID, IIE, IIF, III, V, X, and XIIA). These enzymes are further subdivided into three branches, namely groups I/II/V/X, group III, and group XII (1, 2). Recent studies employing transgenic (Tg) (gain-of-function) and knock-out (loss-of-function) mice for several sPLA2s have revealed that individual enzymes exert distinct functions in vivo. Thus, PLA2G1B (group IB sPLA2) plays a role in digestion of dietary phospholipids (3, 4), PLA2G2A (group IIA sPLA2) plays a role in antibacterial defense and possibly tumorigenesis (5–8), PLA2G5 (group V sPLA2) plays a role in zymosan-induced eicosanoid synthesis and phagocytosis by macrophages (9, 10) and airway hypersensitivity (11), and PLA2G10 (group X sPLA2) plays a role in allergen-induced asthma (12) and myocardial ischemia/reperfusion injury (13). Thus far, beyond the well accepted regulatory role of group IVA cytosolic PLA2 in arachidonate metabolism (14), PLA2G5 and PLA2G10 are the only two sPLA2s that have been proven to have the capacity to modulate arachidonic acid metabolism in vivo (15, 16).
PLA2G3 (group III sPLA2) is distinctive among mammalian sPLA2s. It consists of a central sPLA2 (S) domain flanked by unique N-terminal and C-terminal domains, the molecular mass of its full-length protein (55 kDa) is larger than that of other sPLA2s (14–18 kDa), and its S domain is homologous to bee venom group III sPLA2 rather than to other mammalian sPLA2s (17). The central S domain alone is sufficient for its enzymatic function (17–19). PLA2G3 undergoes proteolytic processing to produce the S domain-only form in cultured cells (19), yet it remains uncertain whether the same processing occurs in vivo. Forced expression of PLA2G3 in several cell types results in increased arachidonic acid metabolism, for which its potency is superior to that of PLA2G2A and next to that of PLA2G10 and PLA2G5 (18, 19). PLA2G3 is immunohistochemically detected in the vascular endothelium of various tissues, alveolar epithelium and macrophages, peripheral and central nervous systems, and several types of cancer (19–21). However, the pathophysiological roles and relevant substrates for PLA2G3 in vivo remain to be elucidated.
In an effort to gain new insight into the in vivo actions of this unique sPLA2, we generated Tg mice overexpressing human PLA2G3. Despite marked increases in PLA2 activity in sera and tissues, PLA2G3 Tg mice exhibit no signs of lung surfactant hydrolysis, which is a prominent phenotype of PLA2G5 Tg mice (22) and macrophage-specific PLA2G10 Tg mice (23), or hair loss, which occurs in PLA2G2A Tg mice (24). However, we found marked alterations in plasma lipoproteins in PLA2G3 Tg mice. PLA2G3-modified low density lipoprotein (LDL) promoted the formation of lipid droplet-rich foam cells from macrophages. Furthermore, atherosclerosis was exacerbated in PLA2G3 Tg × apoE-deficient mice fed a high cholesterol diet. These observations, together with the finding that PLA2G3 is located in atherosclerotic lesions in both humans and apoE-deficient mice, delineate an unexplored link of PLA2G3 with atherosclerosis, as has been proposed for several other sPLA2s, such as PLA2G5 and PLA2G10 (25–36).
EXPERIMENTAL PROCEDURES
Animals—All mice were housed in climate-controlled (21 °C) specific pathogen-free facilities with a 12-h light-dark cycle, with free access to standard laboratory food (Picolab mouse diet 20; Laboratory Diet, Brentwood) and water. All procedures involving animals were performed according to protocols approved by the faculties.
Generation of PLA2G3 Tg Mice—The strategy for the generation of sPLA2 Tg mice has been reported previously (22). In brief, the cDNA for human PLA2G3 was inserted into the EcoRI site of the pCALNL5 vector (37). The plasmid, containing the transgene downstream of a neomycin cassette with LoxP sites at both ends, was excised at the HindIII and SalI sites to produce a 6-kb CAG-loxP-neor-loxP-PLA2G3 (LNL-PLA2G3) fragment. The fragment was then microinjected into 0.5-day fertilized eggs with a micromanipulator (Nikon), and the eggs were transferred to the fimbriae of the uterine tubes of female ICR mice (Japan SLC) that had been mated with vasoligated male mice 1 day before. The tails (5 mm in length) of the pups (4 weeks old) were cut off and homogenized in 500 μl of tissue lysis buffer comprising 50 mm Tris-HCl (pH 8.0), 100 mm EDTA, 0.5% SDS, and 0.4 mg/ml proteinase K (Sigma) at 55 °C overnight. DNA was purified from the lysates with an automated DNA isolation system (NA-2000; Kurabo Industries), and aliquots were taken for PCR for genotyping (see below). Male founders were mated with female C57BL/6 mice to confirm germ line transmission by PCR genotyping, and those with successful germ line transmission (LNL-PLA2G3 Tg mice) were then crossed with female CAG-Cre Tg mice, which carry the Cre recombinase transgene under control of the CAG (cytomegalovirus immediate early enhancer-chicken β-actin hybrid) promoter (38, 39). This step resulted in removal of the neor cassette from the LNL-PLA2G3 transgene, thereby allowing activation of the PLA2G3 transgene in the whole body of the offspring (Fig. 1). All of the PLA2G3 Tg mice were inbred with C57BL/6 mice. Phenotypes that appeared in PLA2G3 Tg mice, which carry the active PLA2G3 transgene, but not in LNL-PLA2G3 Tg mice, in which the PLA2G3 transgene remains silent, were regarded as events caused by the overexpressed PLA2G3. Generation of PLA2G10 Tg mice was described previously (36).
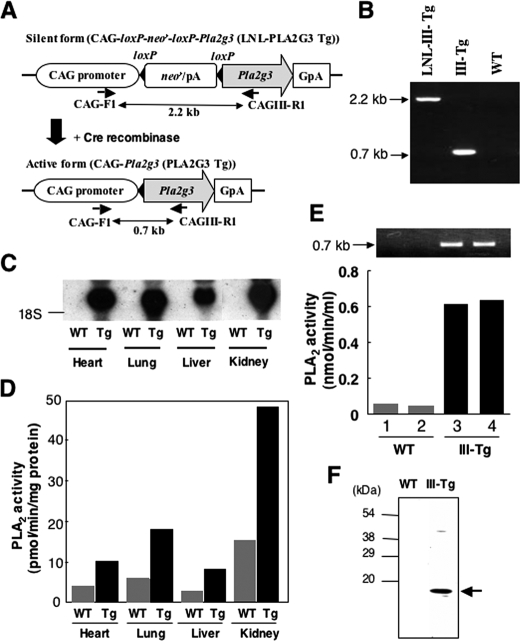
FIGURE 1.
Generation of PLA2G3 Tg mice. A, schematic diagram of the construction of PLA2G3 Tg mice. The human PLA2G3 transgene was inserted at the EcoRI site downstream of the neor/pA cassette. This fragment, in which the PLA2G3 transgene is silent, was introduced into mice, and the transgene-positive offspring were then mated with CAG-Cre Tg mice. At this stage, the LNL (for loxP-neor/pA-loxP) cassette was excised by Cre recombinase, and the PLA2G3 transgene was activated under control of the CAG promoter in the transgene-positive pups. The positions of primers for PCR genotyping and the sizes of the amplified fragments are shown. B, PCR genotyping of PLA2G3 Tg mice. After mating of LNL-III Tg mice with CAG-Cre Tg mice, PCR genotyping was performed on their F1 progeny. Fragments of 2.2 and 0.7 kb were amplified for LNL-III Tg and CAG-PLA2G3 Tg (III-Tg) mice, respectively, whereas these products were not detected in WT littermates. C, detection of PLA2G3 mRNA in tissues of WT and III-Tg neonates by Northern blotting. The membrane was hybridized with human PLA2G3 cDNA and exposed to x-ray film for 1 day. D, PLA2 enzymatic activity in the homogenates of tissues from WT and III-Tg mice shown in C. E, PLA2 enzymatic activity in sera of WT and III-Tg mice. PCR genotyping of the corresponding mice is shown in the top. F, detection of PLA2G3 protein in the serum. Sera from WT and PLA2G3 Tg mice were immunoprecipitated and immunoblotted with anti-PLA2G3 antibodies, as described under “Experimental Procedures.” The major band is indicated by an arrow on the right.
PCR Genotyping—Approximately 0.1 μg of genomic DNA obtained from the mouse tails was subjected to PCR amplification with ExTaq polymerase (Takara Biomedicals) and a set of primers (CAG-F1 (5′-ctgctaaccatgttcatgcc-3′) and CAG-III-R1 (5′-gttgtactgcaagggtgaga-3′) for PLA2G3; CAG-F1 and CAGX-R1 (5′-gggctaagcagttagcaatc-3′) for PLA2G10) obtained from Fasmac. The PCR conditions were 95 °C for 5 min and then 35 cycles of 95 °C for 30 s and 68 °C for 3 min on a thermal cycler (Applied Biosystems). The PCR products were analyzed by 1% agarose gel electrophoresis with ethidium bromide.
Northern Blotting—Equal amounts (∼10 μg) of total RNA obtained from tissues by use of TRIzol reagent (Invitrogen) were applied to separate lanes of 1.2% (w/v) formaldehyde-agarose gels, electrophoresed, and transferred to Immobilon-N membranes (Millipore). The resulting blots were then probed with appropriate cDNA probes that had been labeled with [32P]dCTP (PerkinElmer Life Sciences) by random priming (Takara Biomedicals). Hybridization and subsequent membrane washing were carried out as described previously (15).
Purification of Recombinant Human PLA2G3—The cDNA encoding the S domain of human PLA2G3 (III-S; amino acid residues 136–289) was inserted after a coding sequence for the His6 tag of the pMSNHT expression vector (Katakura Industries). Baculovirus carrying the cDNA for the N-terminally His6-tagged III-S was injected into silkworms (Katakura Industries). After 6 days, 2 ml of the silkworm body fluid was diluted in 40 ml of 100 mm phosphate buffer (pH 7.4) containing 1.5 m NaCl, 0.01% Tween 20, and protease inhibitors (catalogue number 1 873 580; Roche Applied Science). The soluble fraction of the homogenate was applied to HisTrap HP (1-ml column volume; GE Healthcare) on an AKTA system (Amersham Biosciences). The column was sequentially washed with phosphate buffer containing 1.5 m NaCl and 0.01% Tween 20 and then with the same buffer containing 0.5 m NaCl, followed by elution of the bound proteins with phosphate buffer (pH 7.4) with a 0–100% gradient of NaCl and imidazole (both up to 0.5 m) for 90 min at a flow rate of 1 ml/min. Fractions containing III-S protein (∼20% purity as assessed by SDS-PAGE followed by staining with Coomassie Brilliant Blue; not shown) were collected and dialyzed against 20 mm Tris-HCl (pH 8.0).
Measurement of PLA2 Activity—Tissues (100 μg) were soaked in 500 μl of SET buffer comprising 20 mm Tris-HCl (pH 7.4), 0.25 m sucrose, 1 mm EDTA, and 1 mm phenylmethylsulfonyl fluoride (Sigma) and then homogenized with a Polytron homogenizer and sonicator. PLA2 activities in the tissue homogenates and sera were assayed by measuring the amounts of radiolabeled linoleic acid released from the substrate 1-palmitoyl-2-[14C]linoleoyl-phosphatidylethanolamine (PerkinElmer Life Science). The substrate in ethanol was dried under a stream of N2 and dispersed in water by sonication. Each reaction mixture (total volume 250 μl) consisted of appropriate amounts of the required samples, 100 mm Tris-HCl (pH 7.4), 4 mm CaCl2 and 2 μmol of substrate. After incubation for 30 min at 37 °C, [14C]linoleic acid was extracted, and the radioactivity was quantified with a liquid scintillation counter, as described previously (15).
Preparation of Anti-PLA2G3 Antibodies—The cDNA encoding the S domain of human PLA2G3 (amino acid residues 150–290) was inserted after a coding sequence for His6 tag of the pPROExHTb expression vector (Invitrogen). The expressed protein was purified by nickel-Sepharose and further purified by the Prep Cell system (Bio-Rad), followed by removal of the His6 tag from the S domain. Two rabbit antisera against the purified S domain were prepared by Kitayama Laboratories (designated Nov1 and Nov2). Alternatively, rabbit antiserum against the synthetic peptide CPQNISPLQYNYGIRN (corresponding to amino acid residues 187–202, a portion that shows high homology between human and mouse PLA2G3 proteins) was prepared by BioLogica (designated S3). The specificity of the antibodies thus obtained was evaluated by immunoblotting (Fig. S1A) using Sf9 cells (cell homogenates and culture supernatants) infected with baculovirus expressing III-S of human PLA2G3. The procedure for immunoblotting has been described previously (15).
Mouse monoclonal antibodies for human PLA2G3 were prepared by a standard protocol using recombinant human III-S protein as an antigen (see above). Anti-PLA2G3 mouse monoclonal antibody was produced by culturing the hybridoma cell line 7B5B7, 4A10C5, 1F4E2, or 5D2F1 in BD Cell mAb Serum-free Medium with CELLine CL-1000 flasks (BD Biosciences) at 37 °C under a 5% CO2 atmosphere for 7 days. Culture supernatant was centrifuged at 1000 rpm for 10 min to remove cells and then filtered through a 0.45-μm filter. Phosphate buffer (pH 7.4) was added to the filtered culture supernatant at a final concentration of 20 mm before loading the sample onto a Protein G-Sepharose column (GE Healthcare). After washing the column with 10 times the column volume of 20 mm phosphate buffer, the anti-PLA2G3 antibody was eluted with 100 mm citric acid buffer (pH 2.7) and dialyzed against 10 mm Tris-HCl (pH 7.4) containing 150 mm NaCl (TBS). The specificity of each monoclonal antibody was evaluated by Western blotting (Fig. S1B).
Histochemistry—Immunohistochemistry of mouse and human tissue sections was performed as described previously (19). Use of human tissues was approved by the ethics committee of our faculty. In brief, formalin-fixed tissues were embedded in paraffin, sectioned, mounted on glass slides, deparaffinized in xylene, and rehydrated in ethanol with increasing concentrations of water. The tissue sections (4 μm thick) were incubated with Target Retrieval Solution (Dako Cytomation) as required, incubated for 10 min with 3% (v/v) H2O2, washed three times with TBS for 5 min each, incubated with 5% (v/v) skim milk in TBS for 30 min, washed three times with TBS for 5 min each, and incubated with anti-sPLA2 antibodies or control serum at a 1:200–500 dilution in TBS overnight at 4 °C. The sections were then treated with a CSA (catalyzed signal-amplified) system staining kit (Dako Cytomation) with diaminobenzidine substrate, followed by counterstaining with hematoxylin and eosin. The cell type was identified by conventional hematoxylin and eosin staining of serial sections adjacent to the specimen used for immunohistochemistry. Rabbit antisera for human and mouse PLA2G2A, PLA2G5, and PLA2G10 have been described previously (15, 19). Immunostaining for human α-smooth muscle actin (SMA) (clone 1A4; Dako Cytomation) and human macrophages (clone HAM56; Dako Cytomation) was also performed using the EnVision+ kit (Dako Cytomation) for identification of smooth muscle cells and monocytes/macrophages, respectively (40). Double immunostaining with antibodies against α-SMA or HAM56 and PLA2G3 was performed on the sections to determine the localization of PLA2G3 in intimal cells. In brief, the sections were first immunostained for α-SMA or HAM56 and visualized by diaminobenzidine. They were then reimmunostained with antibody against PLA2G3 using EnVision System-alkaline phosphatase (Dako Cytomation) and visualized with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate system (Dako Cytomation). When colocalized, the color turned into dark blue.
Immunoprecipitation and Western Blotting—A monoclonal antibody for PLA2G3 (5D2F1) or control mouse IgG1 (2 mg) was conjugated with 0.5 ml of formyl Cellulofine (Seikagaku Kogyo). The beads (20 μl) were incubated with 250 μl of the plasma obtained from PLA2G3 Tg mice as well as control mice at 4 °C overnight. After centrifugation, the beads were washed five times with 1 ml of phosphate-buffered saline (PBS) and boiled for 5 min in 20 μl of SDS-PAGE sample buffer. Then the resulting supernatants were applied to 12.5% SDS-polyacrylamide gels, and separated proteins were transferred to nitrocellulose membranes. The membranes were subjected to immunoblotting with rabbit anti-PLA2G3 polyclonal antibody, as described previously (15, 19).
Measurement of Serum Biochemical Markers—With or without an overnight fast, 10-week-old mice were anesthetized, and blood samples were immediately collected by cardiac puncture. Sera were applied to a clinical chemistry analyzer VetScan with V-DPP rotors (Abaxis).
Foam Cell Formation from Macrophages—The mouse macrophage cell line J774 (American Type Culture Collection) was maintained in DMEM (Wako) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. LDL (d = 1.019–1.063 g/ml) was prepared from human plasma by the ultracentrifugation method modified by Hatch (38). LDL (2 mg/ml) was treated with partially purified human PLA2G3 (200 μg/ml) or human PLA2G5 (5 μg/ml) (Cayman Chemicals) in a buffer comprising 6 mm HEPES (pH 7.4), 6 mm CaCl2, 84 mm NaCl, 2.4 mm MgCl2, and 20 mg/ml bovine serum albumin). After incubation for 24 h at 37 °C, the reaction was terminated by the addition of 10 mm EDTA. Subsequently, the concentrations of nonesterified fatty acids (NEFA) released were determined by colorimetric assay with an NEFA C Kit (Wako).
J774 cells were seeded on 24-well plates at a cell density of 105 cells/well. After incubation for 2 h, the medium was replaced with Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 200 μg/ml LDL with or without pretreatment with human PLA2G3 or PLA2G5. Alternatively, 50 μg/ml acetylated LDL (Ac-LDL) (Biomedical Technology Inc.) was used as a positive control for foam cell formation. After 24 h, the cells were fixed in 10% formalin in PBS for 10 min and washed with PBS. Saturated oil red O solution was prepared by dilution of 3 mg/ml oil red O in 60% isopropyl alcohol, followed by filtration through a 0.45-μm filter. Following equilibration in 60% isopropyl alcohol, the cells were stained in saturated oil red O. After a brief wash with 60% isopropyl alcohol, the cells were equilibrated in PBS.
For quantification of cholesterol, the cells were washed with 0.3% bovine serum albumin in PBS and then twice with PBS. Cellular cholesterol was extracted with hexane/isopropyl alcohol (3:2) for 30 min at room temperature. Subsequently, cellular protein was extracted with 0.5 m NaOH for 30 min at 37 °C. The solvent samples containing the extracted cholesterol were evaporated under N2 gas, and the remaining cellular lipids were dissolved in propanol. Cholesterol contents were determined by the cholesterol quantification method described by Heider and Boyett (41). Cellular protein contents were determined by BCA protein assay (Pierce).
Peritoneal cells isolated from mice were seeded in 2 × 106 cells/well in 12-well plates (Iwaki) for 2 h in RPMI1640 containing 10% lipoprotein-deficient fetal bovine serum (Sigma), and adherent cells were used as primary macrophages. The cells were then incubated with or without 200 μg/ml LDL or 50 μg/ml Ac-LDL. After 24 h, the cells were stained with oil red O. Replicate cells were washed twice with PBS and lysed in 0.5% SDS to determine protein concentrations. The supernatants were subjected to PGE2 enzyme immune assay (Cayman Chemicals) or interleukin-6 enzyme immune assay (eBioscience).
Separation of Lipoproteins—Lipoproteins in mouse sera (100 μl/run after a 1:10 dilution) were separated by high performance liquid chromatography (HPLC) on TSK gel Lipopropak XL (TOSOH) with TSK Eluent PL1 (TOSOH) as a running buffer at a flow rate of 0.35 ml/min. Total cholesterol, phospholipids, and lysophosphatidylcholine (LPC) levels were determined with Determinar TC2 (Kyowa Medex), a phospholipid C test (Wako), and the LPC assay kit (AZWELL), respectively. Triglyceride levels were measured using the biochemical analyzer Fuji Drychem. Alternatively, LDL and high density lipoprotein (HDL) were separated by discontinuous ultracentrifugation (42), and lipids extracted from them were applied to electrospray ionization-mass spectrometry (ESI-MS).
Lipoprotein Electrophoresis—LDL and HDL obtained from mice were electrophoresed (1-μl aliquot/lane) on TITAN GEL Lipoprotein gels (Helena Laboratories) at 90 V for 25 min. Then the gels were stained with detection reagent for 15 min at 30 °C, followed by incubation with 5% acetic acid for 15 min.
ESI-MS—Recombinant human PLA2G2A, PLA2G2E, PLA2G2F, PLA2G5, PLA2G10 (kindly provided by Dr. M. Gelb, University of Washington, Seattle), or PLA2G3 (see above) (0.2 and 1 μg/ml) was incubated with 1 mg/ml human LDL or HDL (both from Sigma) for 4 h in 100 mm Tris-HCl, pH 7.4, containing 10 mm CaCl2. After incubation, lipids were extracted from the reaction mixtures by the method of Bligh and Dyer (43). The ESI-MS analyses were performed using a 4000Q TRAP, quadrupole-linear ion trap hybrid mass spectrometer (MDS Sciex; Applied Biosystems) with a UltiMate 3000 nano/cap/micro-liquid chromatography system (Dionex Corp., Sunnyvale, CA) combined with an HTS PAL autosampler (CTC Analytics AG, Zwingen, Switzerland). Phospholipids were subjected directly to ESI-MS analysis by flow injection; typically, 3 μl (3 nmol of phosphorus equivalent) of sample was applied. The mobile phase composition was acetonitrile/methanol/water (6:7:2) (plus 0.1% ammonium formate, pH 6.8) at a flow rate of 10 μl/min. The scan range of the instrument was set at m/z 200–1000 at a scan speed of 1000 Da/s. The trap fill time was set at 1 ms in the positive ion mode and at 5 ms in the negative ion mode. The ion spray voltage was set at 5500 V in the positive ion mode and at -4500 V in the negative ion mode. Nitrogen was used as curtain gas (setting of 10, arbitrary units) and as collision gas (set to “high”). The detailed procedure for ESI-MS was described previously (44, 45). A peak of sphingomyelin (m/z 703 in the positive ion mode) was regarded as an internal standard. The measurement of fatty acids and their oxygenated products in mouse plasma was carried out by TrueMass Profiling (Lipomics Technologies; available on the World Wide Web).
PLA2G3 Tg × apoE-/- Mice—PLA2G3 Tg (PLA2G3tg/0) mice were crossed with ApoE knock-out (apoE-/-) mice (Charles River). PLA2G3tg/0 × apoE+/- mice at the F1 generation were mated again with apoE-/- mice, and PLA2G3tg/0 × apoE-/- mice among six genotypes at the F2 generation thus obtained were crossed with apoE-/- mice. The resulting PLA2G3tg/0 × apoE-/- and littermate PLA2G3non-Tg × apoE-/- mice at the F3 were used in subsequent studies. In the same manner, LNL-PLA2G3 Tg (LNL-PLA2G3tg/0) mice and apoE-/- mice were crossed to obtain LNL-PLA2G3tg/0 × apoE-/- mice. These mice were fed with a high fat/high cholesterol diet (16% fat, 1.14% cholesterol, and 0.4% cholic acid; Oriental Yeast Co.) for 10–14 weeks starting at 10–11 weeks of age. After blood was collected from each mouse, the circulation system was perfused with PBS and fixed with PBS containing 4% paraformaldehyde. The aorta was then excised from the root to the abdominal area, and the connective tissue was carefully removed. The aorta was stained with oil red O, and the positive areas in the specimen were calculated using Photoshop CS2 and Canvas software. The root area of the aorta tissue was fixed using a 4% paraformaldehyde-lysine-sodium periodate solution. For histochemistry, the aortas were embedded in paraffin (Wako), and cross-sections (5 μm thick) were then prepared.
LDL fraction was separated by ultracentrifugation as described previously (46). An aliquot of the fraction (10 mg of protein equivalent) was subjected to native PAGE (1.8–13% acrylamide). The particle size was estimated using ferritin (12.2 nm), thyroglobulin (17 nm), and latex beads (30 nm) as standards (47).
RESULTS
Generation of PLA2G3 Tg Mice—The Tg construct for PLA2G3 (Fig. 1A) was microinjected into the pronuclei of fertilized eggs of C57BL/6 females and transferred into the oviducts of ICR pseudopregnant females. The offspring were examined for expression of the transgene (LNL-PLA2G3) by PCR genotyping. Tail biopsies were taken on day 28, and the genomic DNAs isolated were subjected to PCR genotyping with a CAG forward primer and a reverse primer specific for PLA2G3. A founder male mouse showed the presence of a 2.2 kb band for LNL-PLA2G3 (Fig. 1B). The founder mouse, in which the PLA2G3 transgene was still silent, was mated with female CAG-Cre Tg mice to allow the removal of the neor cassette from the LNL-PLA2G3 transgene by the Cre/LoxP reaction. Following this, the transcription of the PLA2G3 transgene was directly regulated by the CAG promoter and was thereby activated in all tissues of the offspring. A representative result of PCR genotyping of the F1 progeny is shown in Fig. 1B. As a result of the Cre/LoxP reaction, the band for the PLA2G3 transgene shifted from 2.2 to 0.7 kb whereas no band was detected in the littermate siblings (Fig. 1B). Mice carrying the active PLA2G3 transgene were bred with C57BL/6 mice. The ratio of transgene-positive to -negative pups was ∼1:1, and these pairs (hereafter designated as PLA2G3 Tg mice (PLA2G3tg/0) and non-Tg, wild-type (WT) littermate controls) were used in each experiment. Note that mice carrying the inactive transgene (LNN-PLA2G3) showed no apparent abnormality, implying that the phenotypes observed in PLA2G3 Tg mice described below were indeed caused by overexpression of PLA2G3.
PLA2G3 Tg mice showed no neonatal or postnatal mortality and remained healthy until adulthood. RNA blotting showed expression of human PLA2G3 mRNA in all tissues from PLA2G3 Tg mice (Fig. 1C). When PLA2 enzymatic activities in tissue homogenates were measured with 1-palmitoyl-2-linoleoyl-phosphatidylethanolamine as substrate, the activities in Tg tissues were increased 2–3-fold compared with those in control tissues (which reflect the combined activities of the various PLA2 enzymes intrinsically present in each tissue) (Fig. 1D) and roughly correlated with the result of RNA blotting (Fig. 1C). PLA2 activity in the sera of PLA2G3 Tg mice was >10-fold higher than that of WT mice (Fig. 1E). On the basis of enzyme activity, the concentration of PLA2G3 in PLA2G3 Tg mice appeared to be similar to that of PLA2G5 in the PLA2G5 Tg mice we had generated previously (estimated to be within the range 10–100 ng/ml, depending on the tissue) (22).
Expression of PLA2G3 in the Tg mice was further assessed by subjecting sera from PLA2G3 Tg and WT mice to immunoprecipitation with mouse anti-PLA2G3 monoclonal antibody, followed by immunoblotting with rabbit anti-PLA2G3 polyclonal antibody. As shown in Fig. 1F, a main 18 kDa band was detected in the serum of PLA2G3 Tg but not control mice. As evaluated from molecular sizes, this band appeared to correspond to the S domain-only form (III-S) (19, 20), suggesting that PLA2G3 is processed to a fully processed III-S form in the blood circulation. This is, to our knowledge, the first demonstration that PLA2G3 undergoes proteolytic processing in vivo. Although it has been shown that PLA2G3 can be N-glycosylated in several PLA2G3-transfected cell lines in culture (19), bands corresponding to the N-glycosylated forms were barely detected in the serum of PLA2G3 Tg mice (Fig. 1F), arguing that the majority of PLA2G3 does not undergo N-glycosylation in vivo.
Unlike PLA2G5 Tg mice, which die shortly after birth from a lung disorder resulting from aberrant hydrolysis of the lung surfactant phospholipids phosphatidylcholine (PC) and phosphatidylglycerol (22), the absence of respiratory disorders in PLA2G3 Tg mice suggests that PLA2G3 hardly hydrolyzes lung surfactant under physiological conditions. Indeed, ESI-MS analysis of lung surfactant phospholipids did not show any appreciable difference between control and PLA2G3 Tg mice (data not shown). Furthermore, although PLA2G2A Tg mice show alopecia (24) as well as male infertility because of impaired spermatogenesis (48), PLA2G3 mice had normal pelage hairs and showed no sign of reproductive defects up to 9 months of age.
Altered Plasma Lipoproteins in PLA2G3 Tg Mice—To identify in vivo effects of Tg expression of PLA2G3, various biochemical markers were analyzed in sera from 8–15-week-old PLA2G3 Tg and control mice. Although most parameters, including serum ions and biomarkers for liver and kidney function, did not differ appreciably between PLA2G3 Tg and WT mice, the level of total cholesterol was significantly lower in PLA2G3 Tg mice than in control mice (Fig. 2A and Table S1). The decrease in cholesterol in PLA2G3 Tg mice relative to WT mice was more prominent after overnight fasting than under normally fed conditions and also more prominent in males than in females; the difference between normally fed female Tg and WT mice was subtle.
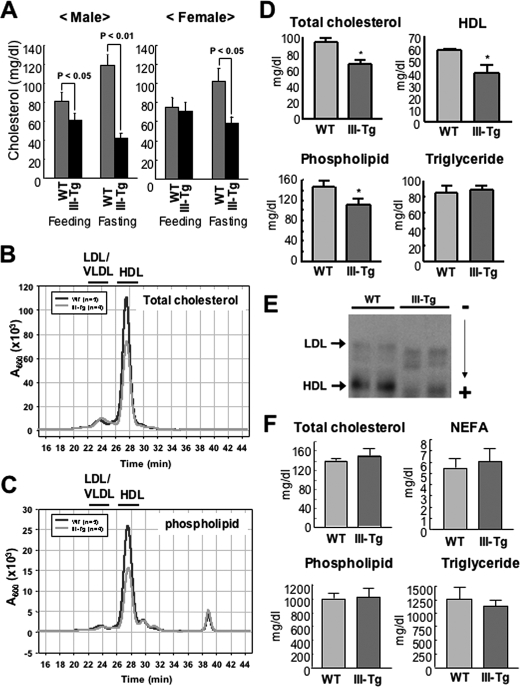
FIGURE 2.
Altered plasma lipoproteins in PLA2G3 Tg mice. A, 8–15-week-old male (n = 10; left) and female (n = 9; right) WT and PLA2G3 Tg (III-Tg) mice were maintained on a normal diet (Feeding) or without food (Fasting) for 12 h. Then the levels of total cholesterol (cholesterol and cholesteryl ester) in sera of these mice were quantified by use of a VetScan. B and C, lipoproteins obtained from WT and III-Tg mice after overnight fasting were separated on HPLC with monitoring of total cholesterol (B) and phospholipids (C) (see “Experimental Procedures”). Fractions containing LDL/VLDL and HDL are shown at the top. D, HDL fractions were prepared from the sera of male WT and III-Tg mice after overnight fasting, and the concentrations of HDL as well as HDL-associated total cholesterol, phospholipids, and triglyceride were determined. Values are mean ± S.D. (n = 5; *, p < 0.05 versus WT). E, agarose gel electrophoresis of HDL and LDL from WT and PLA2G3 Tg mice (two animals for each). Positions of LDL and HDL are shown by arrows. F, phospholipids, NEFA, cholesterol, and triglyceride levels in livers of III-Tg mice were similar to those of WT mice. Values are mean ± S.D. (n = 5).
Since serum cholesterol is predominantly present in lipoprotein particles, it was anticipated that the decrease in total cholesterol in PLA2G3 Tg mice might result from the action of PLA2G3 on phospholipids in lipoprotein particles. To address this issue, we used HPLC to separate lipoprotein fractions from the sera of male mice after overnight fasting. As shown in Fig. 2B, the major peak corresponding to HDL cholesterol was smaller in PLA2G3 Tg mice than in control mice. Likewise, the HDL of PLA2G3 Tg mice contained less phospholipid than did that of control mice (Fig. 2C). Quantitatively, serum HDL as well as HDL-associated cholesterol and phospholipids were decreased by as much as 25–30% in PLA2G3 Tg mice compared with WT mice (Fig. 2D), whereas phospholipid contents in the VLDL/LDL fraction of PLA2G3 Tg and WT mice were similar (data not shown). There was no appreciable difference in triglyceride level between the genotypes (Fig. 2D). Conversely, the amounts of the PLA2-hydrolytic products LPC and NEFA in sera of PLA2G3 Tg mice tended to be higher than those in control mice (470 ± 4.7 and 515 ± 46 μm LPC and 890 ± 10 and 1030 ± 40 μeq/liter NEFA in WT and PLA2G3 Tg mice, respectively (n = 5)), although these differences were not statistically significant because of the high background (probably albumin-bound) pools.
Next, we separated the lipoprotein fractions from PLA2G3 Tg and control mice by agarose gel electrophoresis. Consistent with the results from the HPLC analyses (Fig. 2, B and C), significant reduction in the intensity of the band for HDL was apparent in PLA2G3 Tg mice as compared with WT mice (Fig. 2E). Moreover, the HDL of PLA2G3 Tg mice showed faster migration than that of control mice. In addition, the band for LDL in PLA2G3 Tg mice appeared broader and also moved faster than that in control mice (Fig. 2E). These findings suggest increased net negative charge on both HDL and LDL particles in PLA2G3 Tg mice. Since the lipid content of the liver, a tissue that plays a central role in lipoprotein metabolism, of PLA2G3 Tg mice was similar to that of WT mice (Fig. 2F), it is likely that the observed changes in PLA2G3 Tg mice shown above are a result of the direct action of PLA2G3 on phospholipids in HDL and LDL.
Lipoprotein Hydrolysis by Various sPLA2s in Vitro—To compare the hydrolytic action of PLA2G3 on lipoprotein phospholipids with that of other sPLA2s, recombinant PLA2G3 (III-S), PLA2G5, PLA2G10, PLA2G2A, PLA2G2E, and PLA2G2F were each incubated for 4 h with 1 mg/ml LDL or HDL, and phospholipids extracted from the reaction mixtures were subjected to ESI-MS analyses. Both LDL (Fig. 3) and HDL (Fig. 4) particles contained five major PC molecular species (C16:0–18:2, C16:0–18:1, C16:0–20:4, C18:0–18:2, and C18:0–20:4) and trace levels of LPC molecular species (C16:0 and C18:0). When LDL was treated with 0.2 μg/ml (Fig. 3A) and 1 μg/ml (Fig. 3B) PLA2G3, PLA2G5, or PLA2G10, there were robust and dose-dependent increases in both LPC species. The release of LPC from LDL was accompanied by concomitant decreases in all PC molecular species, an event that was particularly obvious when each of these three enzymes was added at 1 μg/ml (Fig. 3B). Judging from the peak areas of remaining PC species, the most active enzyme was PLA2G10, with which partial and preferential reduction in PC species with C20:4 was seen at a low concentration (0.2 μg/ml). The ability of PLA2G3 to hydrolyze LDL-associated PC was slightly weaker than that of PLA2G5. LPC release from LDL after treatment with PLA2G2A or PLA2G2E was minimal, whereas PLA2G2F increased LPC species modestly at a high concentration (1 μg/ml) (Fig. 3B).
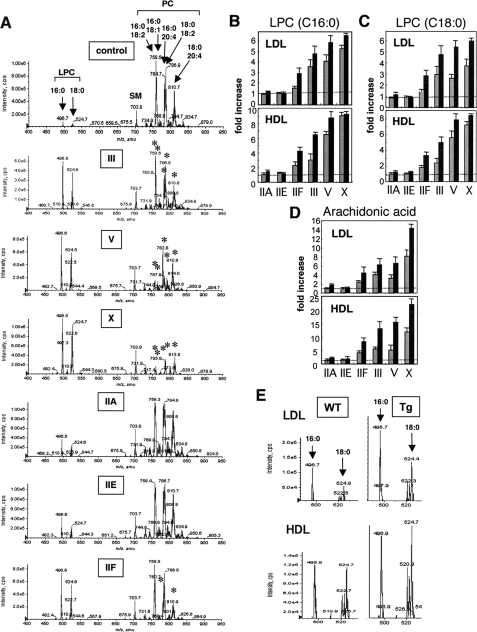
FIGURE 3.
Hydrolysis of LDL by various sPLA2s in vitro. Human LDL (1 mg/ml) was incubated for 4 h with 0.2 μg/ml (A) or 1 μg/ml (B) PLA2G3 (III), PLA2G5 (V), PLA2G10 (X), PLA2G2A (IIA), PLA2G2E (IIE), or PLA2G2F (IIF). Lipids were extracted and subjected to ESI-MS (see “Experimental Procedures”). Representative results of two reproducible experiments are shown. The asterisks indicate the PC peaks that were significantly decreased in the Tg mice compared with control mice. SM, sphingomyelin.
FIGURE 4.
Hydrolysis of lipoproteins by sPLA2s in vitro (A–D) and in vivo (E). Human HDL (1 mg/ml) was incubated for 4 h with 1 μg/ml PLA2G3 (III), PLA2G5 (V), PLA2G10 (X), PLA2G2A (IIA), PLA2G2E (IIE), or PLA2G2F (IIF). Lipids were extracted and subjected to ESI-MS (see “Experimental Procedures”). The asterisks indicate the PC peaks that were significantly decreased in the Tg mice compared with control mice. SM, sphingomyelin. B–D, -fold increases in LPC-C16:0 (B), LPC-C18:0 (C), and arachidonic acid (D) from LDL and HDL after a 4-h incubation with 0.2 μg/ml (gray bars) and 1 μg/ml (solid bars) sPLA2s, as evaluated by ESI-MS analyses, are summarized, with areas for individual peaks in control mice being regarded as 1. Averages ± variability of two experiments are shown. E, LDL (top) and HDL (bottom) were separated from sera of PLA2G3 Tg (right) and WT (left) mice and analyzed for LPC contents by ESI-MS. In A and E, representative results of two reproducible experiments are shown.
Increases in LPC species (C16:0 and C18:0) were also prominent when HDL was incubated with 0.2 μg/ml (data not shown) and 1 μg/ml (Fig. 4A) PLA2G3, PLA2G5, or PLA2G10. As revealed by residual PC species, PLA2G10 was apparently most active on HDL, decreasing all PC species markedly; PLA2G5 reduced PC species with C18:1 or C18:2 in preference to those with C20:4; PLA2G3 moderately decreased most PC species; PLA2G2A and PLA2G2E acted only weakly on HDL; and PLA2G2F caused a modest but substantial increase in LPC species, accompanied by a concomitant and preferential decrease in PC species with C20:4 (Fig. 4A).
Hydrolysis of lipoproteins by various sPLA2s in vitro, on the basis of ESI-MS analysis, is summarized in Fig. 4, B–D. In general terms, the rank order of hydrolytic potency, as evaluated by increases in LPC-C16:0 (Fig. 4B) and LPC-C18:0 (Fig. 4C), was PLA2G10 > PLA2G5 > PLA2G3 > PLA2G2F > PLA2G2A and PLA2G2E, for both HDL and LDL. In accordance with the preference of PLA2G10 for arachidonate and of PLA2G5 for oleate/linoleate, as noted above and as described recently (49), the release of arachidonic acid from LDL and HDL by PLA2G10 was prominent, and that by PLA2G3 and PLA2G5 was similar (Fig. 4D).
To ascertain whether phospholipids in plasma lipoproteins could indeed be hydrolyzed by PLA2G3 in vivo, lipoprotein fractions separated by discontinuous centrifugation from PLA2G3 Tg and control mice fed a normal diet were analyzed for their LPC content by ESI-MS analyses. Notably, both LPC-C16:0 and LPC-C18:0 were increased in the LDL and HDL of PLA2G3 Tg mice relative to those of control mice (Fig. 4E), further supporting the hydrolysis of lipoprotein-associated PC by PLA2G3 in the Tg mice (also see Fig. 7).
FIGURE 7.
Enhanced atherosclerosis development in PLA2G3 Tg × apoE-/- mice following a high fat diet. A, PLA2G3 Tg or LNL-PLA2G3 Tg mice were crossed with apoE knock-out (apoE-KO) mice. Blood samples were collected from the tail veins of PLA2G3tg/0 × apoE-/- (III-Tg), LNL-PLA2G3tg/0 × apoE-/- (LNL), and PLA2G3non-Tg × apoE-/- (-) mice as well as WT C57BL/6 (apoE+/+) mice, and plasma cholesterol concentrations were measured. B, PLA2 enzyme activity in the plasma was measured with 1-palmitoyl-2-[14C]linoleoylphosphatidylethanolamine as substrate. C, female mice were fed with a high fat/high cholesterol diet for 10 weeks, and plasma and aortas were collected. Concentrations of LPC in plasma (left) and in LDL (right) were measured using an enzyme-based kit. In A–C, blood samples for individual analyses were randomly chosen, which gave reasonable results in the first round of assays. D, aliquots of LDL fractions (10 μg of protein equivalent) along with size markers (ferritin, 12.2 nm; thyroglobulin, 17 nm; latex beads, 30 nm) were subjected to native PAGE. The gels were stained with Coomassie Brilliant Blue. Aggl, aggregated LDL. E and F, the whole aortas obtained from male and female mice fed a high fat diet for 10–14 weeks were stained with oil red O to visualize atherosclerotic lesions. The percentage of surface lesion area was calculated using Canvas software. Photographs of a typical example of each group are shown in E, and the percentage of surface lesion area in individual mice tested so far as well as the mean ± S.D. in each group is indicated in F. Data were statistically evaluated using one-factor analysis of variance and the Tukey-Kramer or Kruskal-Wallis test. Data with p < 0.05% were regarded as significant.
PLA2G3-treated LDL Facilitates Macrophage Foam Cell Formation—Having established that PLA2G3 is able to modify lipoproteins both in vitro and in PLA2G3 Tg mice, we next examined whether PLA2G3-treated LDL could facilitate lipid accumulation in macrophages (i.e. formation of foam cells). To this end, human LDL was preincubated for 24 h with PLA2G3 as well as with PLA2G5, which was roughly as efficient as PLA2G3 in hydrolyzing LDL-associated PC as assessed by ESI-MS analysis (see above). Quantification of NEFA after overnight incubation showed that there was a massive and quantitatively similar release of NEFA from PLA2G3- or PLA2G5-treated LDL (Fig. 5A). When the PLA2G3- or PLA2G5-treated LDL thus prepared was added to the mouse macrophage cell line J774, cholesteryl ester accumulation in these cells after culture for 24 h was severalfold higher than that in replicate cells after incubation with LDL alone (Fig. 5B). When these cells were stained with oil red O, a marked increase in oil red O-positive lipid droplets was observed in cells incubated with PLA2G3-treated LDL, whereas lipid droplet accumulation was sparse in cells incubated with or without intact LDL (Fig. 5C). Consistent with a previous report (35), PLA2G5-treated LDL or Ac-LDL used as a positive control markedly facilitated the accumulation of oil red O-positive lipid droplets in J774 cells (Fig. 5C).
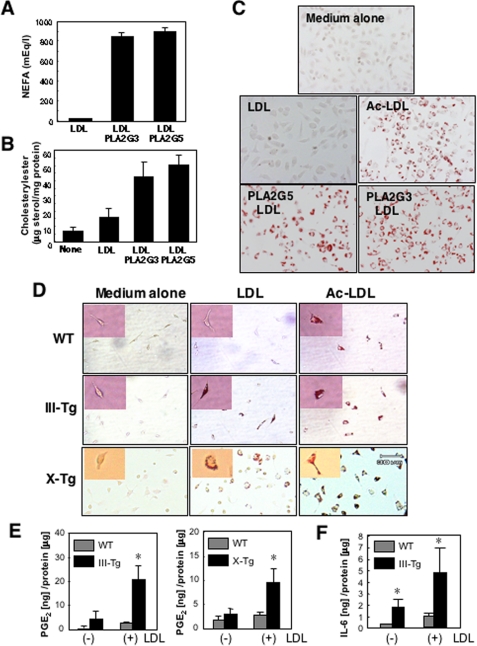
FIGURE 5.
Foam cell formation from macrophages by PLA2G3, PLA2G5 and PLA2G10. A, LDL (2 mg/ml) was incubated with or without recombinant human PLA2G3 (sPLA2-III) and PLA2G5 (sPLA2-V) for 24 h at 37 °C. Then the NEFA released was quantified (n = 2). B and C, J774 cells were cultured for 24 h in the presence or absence of LDL (200 μg/ml) that had been incubated for 24 h with or without PLA2G3 or PLA2G5. Then the cells were homogenized, and cholesteryl ester contents were quantified (n = 2) (B). Replicate cells were fixed and stained with oil red O (C). Ac-LDL (50 μg/ml) was used as a positive control for foam cell formation. Magnification was ×200. D, peritoneal macrophages obtained from PLA2G3 Tg (III-Tg) mice and PLA2G10 Tg (X-Tg) mice as well as WT mice were placed in RPMI1640 containing 10% lipoprotein-deficient fetal bovine serum and were incubated with or without 200 μg/ml LDL or 50 μg/ml Ac-LDL. After 24 h, the cells were stained with oil red O (magnification, ×200). Magnified views of individual macrophages are shown in the inset. E, PGE2 production by macrophages from PLA2G3 Tg (left) and PLA2G10 Tg (right) mice in comparison with WT mice; macrophages were incubated for 24 h in the presence (+) or absence (-) of 200 μg/ml LDL (mean ± S.E., n = 3–5; *, p < 0.05 versus replicate WT cells). F, interleukin-6 production by macrophages from PLA2G3 Tg and WT mice incubated for 24 h in the presence (+) or absence (-) of 200 μg/ml LDL (mean ± S.E., n = 4–6; *, p < 0.05 versus replicate WT cells).
To confirm the production of atherogenic LDL by PLA2G3, peritoneal macrophages from PLA2G3 Tg mice and control mice were incubated with LDL ex vivo. As shown in Fig. 5D, prominent accumulation of oil red O-positive lipid droplets occurred in macrophages from PLA2G3 Tg mice but not in those from WT mice after incubation for 24 h with LDL (but not with medium alone). We also incubated peritoneal macrophages from PLA2G10 Tg mice (22) with LDL and found that these cells also accumulated oil red O-stained lipid droplets (Fig. 6D), confirming that PLA2G10-exposed LDL facilitates macrophage foam cell formation, as reported previously (23, 31, 34). After treatment with Ac-LDL, formation of foam cells from macrophages of all genotypes was obvious (Fig. 6D). Since it has been reported that foam cell formation by macrophages is accompanied by elevated eicosanoid synthesis (50), we measured PGE2 in the culture supernatants of macrophages from PLA2G3 Tg and PLA2G10 Tg mice relative to that from control mice, with or without 24-h treatment with LDL. Whereas PGE2 generation was increased only modestly in WT macrophages by LDL treatment, a marked augmentation of PGE2 generation by LDL treatment was observed in replicate PLA2G3 Tg or PLA2G10 Tg macrophages (Fig. 5E). Interleukin-6 was also elevated in PLA2G3 Tg macrophages compared with control macrophages after incubation with LDL (Fig. 5F), suggesting that PLA2G3 augments proinflammatory response in macrophages.
FIGURE 6.
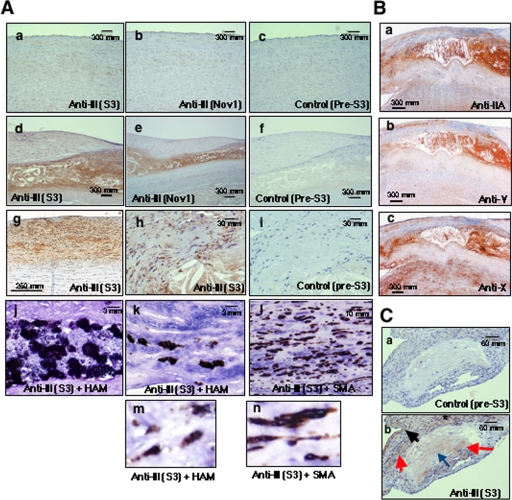
Immunohistochemistry of PLA2G3 and other sPLA2s in human atherosclerosis. A, sections of human aortas with normal histology (a–c) and those with atheroma (d–f and h–l) and fatty streaks (g) were immunostained with two distinct anti-PLA2G3 antibodies (S3 (a, d, g, and h) and Nov1 (b and c)) and with control antibody (c, f, and i). For double immunostaining, the atheroma sections were stained with anti-HAM (j, k, and m) or anti-SMA (l and n) antibody (brown) in combination with anti-PLA2G3 antibody (blue). In lipid cores, staining of PLA2G3 largely overlapped with that of HAM (j). In fibrous caps covering lipid cores, staining of PLA2G3 co-localized with that of HAM (k and m) and SMA (l and n). Magnified images of double immunostaining are shown in m (HAM + PLA2G3) and n (SMA + PLA2G3). B, human atheroma sections were immunostained with anti-PLA2G2A antibody (a), anti-PLA2G5 antibody (b), and anti-PLA2G10 antibody (c). C, sections of the aortic root in apoE-deficient mice fed a high fat diet were immunostained with control (a) and anti-PLA2G3 (b) antisera. Lipid cores (red arrows), foam cells (blue arrows), adventitia (dark arrows), and smooth muscle cells (asterisk) showed PLA2G3 immunoreactivity.
To further evaluate the relationship between sPLA2-mediated LDL hydrolysis and macrophage foam cell formation, peritoneal macrophages were incubated for 24 h with LDL that was pretreated with various concentrations of recombinant PLA2G3 or PLA2G10, and the accumulation of LPC (as an indication of LDL hydrolysis) in medium and the appearance of oil red O-positive cells were examined. Substantial and dose-dependent increases in oil red O-positive cells (Fig. S2A) and LPC (Fig. S2B) were obvious after incubation with >10 ng/ml PLA2G3 or with >1 ng/ml PLA2G10 (Fig. S2B), revealing good correlation with the hydrolysis of LDL and the formation of foam cells by these sPLA2s (with PLA2G10 being 1 order more potent than PLA2G3). Moreover, these experiments suggest that only a few-fold increase of LPC in LDL particles (reaching a few hundred nmol/mg of protein) can facilitate the accumulation of lipid droplets in macrophages.
Localization of Endogenous PLA2G3 in Atherosclerotic Lesions—These observations, together with current reports demonstrating the presence of multiple lipoprotein-active sPLA2s in atherosclerotic lesions (25–36), prompted us to carry out immunohistochemistry with several distinct anti-PLA2G3 antibodies (see “Experimental Procedures”) to assess if endogenous PLA2G3 is present at foci of atherosclerotic lesions. Atherosclerotic lesions are classified into five categories, namely adaptive intimal thickening and types I, II, III, and IV lesions (40). In this study, we examined the immunohistochemical localization of PLA2G3 in normal aorta (without atherosclerosis and fibrocellular thickening), aorta with fatty streaks (type III lesion), and aorta with atheroma (type IV lesion).
Although immunoreactivity for PLA2G3 was sparse in sections of normal human aorta (Fig. 6A, a and b), staining was prominent in atheroma core and fibrous cap covering the core (Fig. 6A, d and e). Moderate staining for PLA2G3 was detected in intimal cells and stroma with fatty streak lesions (Fig. 6A, g), an initial stage of atherosclerosis, suggesting that expression of PLA2G3 is increased concomitantly with the development of atherosclerosis. Higher magnification of the atheromatous areas revealed marked staining of lipid cores and scattered staining of neighboring cells in fibrous caps (Fig. 6A, h). In the lipid cores, most PLA2G3-positive cells were also stained with anti-HAM56 antibody (Fig. 6A, j), indicating the localization of PLA2G3 in macrophages. PLA2G3-positive signals in the fibrous caps covering lipid cores overlapped with signals for either anti-HAM56 antibody (macrophages) or anti-SMA antibody (smooth muscle cells), with the latter being more dominant (Fig. 6A, k and l). Magnified views of double immunostaining verified the coexistence of brown (macrophages or smooth muscle cells) and blue (PLA2G3) signals in the same cells (Fig. 6A, m and n). Staining of normal and atherosclerotic aortas with control antibody was negative (Fig. 6A, c, f, and i). In comparison, signals for PLA2G2A (Fig. 6B, a), PLA2G5 (Fig. 6B, b), and PLA2G10 (Fig. 6B, c) were also intense in the atheroma lesions of human aortas, in agreement with previous reports (25–36).
We also examined the localization of PLA2G3 immunoreactivity in atherosclerotic plaques in the aortas of apoE-deficient mice fed an atherogenic diet. As in the case of human atherosclerotic lesions (Fig. 6A), PLA2G3 immunoreactivity was located in atheromatous lipid cores and nearby foam cells in the aortic root of these mice (Fig. 6C, b). The adventitia as well as smooth muscle cells of the media just beneath the atheroma were also stained for PLA2G3, whereas control antibody yielded no obvious staining throughout the aortic root sections (Fig. 6C, a and b). None of the anti-PLA2G3 antibodies tested stained normal mouse aorta sections (data not shown).
Development of Atherosclerosis in PLA2G3 Tg Mice in Vivo—In an effort to evaluate the pathological relevance of PLA2G3 in atherosclerosis in vivo, PLA2G3 Tg mice (C57BL/6 background) were fed an atherogenic, high fat/high cholesterol diet. After 14 weeks, total plasma cholesterol was increased ∼3-fold, whereas HDL cholesterol was decreased by 30–60%, in mice fed a high fat diet compared with those fed a normal diet in both genotypes (Fig. S3). Under each diet condition, total and HDL cholesterol levels were consistently lower in PLA2G3 Tg mice than in replicate WT mice. Plasma levels of phospholipids and LPC were minimally affected by feeding conditions, with trends that phospholipids levels were slightly lower and LPC levels were higher in the Tg mice than in replicate WT mice (Fig. S3). Although the development of atherosclerosis in these mice was evaluated by staining the aortic sections with oil red O, accumulation of lipid-rich plaques was barely seen even under high fat-fed conditions (data not shown).
To circumvent this limitation, we next crossed PLA2G3 Tg mice with apoE-/- mice, a mouse line that is known to be highly susceptible to atherosclerosis. PLA2G3tg/0 × apoE+/- mice of the F1 generation were mated again with apoE-/- mice, and the two types of offspring, PLA2G3tg/0 × apoE-/- and PLA2G3non-Tg × apoE-/-, were used in subsequent experiments. As another control, LNL-PLA2G3 Tg mice (in which the PLA2G3 transgene was silent) were mated with apoE-/- mice to obtain LNL-PLA2G3tg/0 × apoE-/- mice. Under normal diet conditions, plasma cholesterol concentrations of all of these mice reached >350 mg/dl, whereas that of apoE+/+ mice was <100 mg/dl (Fig. 7A), indicating that introducing the PLA2G3 or LNL-PLA2G3 transgene into apoE-/- mice did not significantly change plasma cholesterol levels. However, fractionation of plasma lipoproteins on gel filtration revealed that there was an increase of VLDL/LDL and a reciprocal decrease of HDL in apoE-/- mice, as compared with apoE+/- mice and that Tg expression of PLA2G3 in these mice reduced HDL cholesterol levels (Fig. S4). Plasma PLA2 enzyme activity in PLA2G3tg/0 × apoE-/- mice was >20 times higher than that in PLA2G3non-Tg × apoE-/- or LNL-PLA2G3tg/0 × apoE-/- mice (Fig. 7B).
After consumption of a high fat/high cholesterol diet for 10 weeks, apoE-/- mice exhibited hypercholesterolemia, with total cholesterol levels in the plasma reaching 2000–3000 mg/dl regardless of PLA2G3 genotype (data not shown). Concentrations of LPC in plasma (Fig. 7C, left) and in LDL (Fig. 7C, right) were increased 2–3-fold in PLA2G3tg/0 × apoE-/- mice compared with those in PLA2G3non-Tg × apoE-/- or LNL-PLA2G3tg/0 × apoE-/- mice, further implying that introduced PLA2G3 is active on LDL phospholipids under in vivo conditions. Since the formation of aggregated LDL as a result of phospholipid hydrolysis by sPLA2s has been proposed to be a causal factor for atherosclerosis (31, 36), LDL fractions separated from these mice by ultracentrifugation were analyzed on native PAGE to assess their particle sizes. Normally, LDL has a particle diameter around 25 nm (as seen in PLA2G3non-Tg × apoE-/- mice), whereas LDL from PLA2G3tg/0 × apoE-/- mice had a slightly larger diameter and an additional, very large, particle with a diameter of ∼45 nm (Fig. 7D). These results suggest that, in PLA2G3tg/0 × apoE-/- mice, increased LPC levels might cause aggregation of LDL to form large particles.
Atherosclerotic lesion formation following a high fat diet was evaluated by measuring the surface lesion area of the aorta (aortic root to abdominal aorta) after staining with oil red O. Average surface lesion area in the absence of active Tg PLA2G3 (i.e. in both PLA2G3non-Tg × apoE-/- and LNL-PLA2G3tg/0 × apoE-/- mice) was about 20% (Fig. 7, E and F). Importantly, the atherosclerotic lesion area was increased about 2-fold on average in PLA2G3tg/0 × apoE-/- mice compared with that in the controls (Fig. 7, E and F), implying that Tg PLA2G3 was able to exacerbate atherosclerosis in vivo. Under a normal diet, oil red O-positive lesions were scarce in apoE-/- mice and were not influenced by the overexpression of PLA2G3 (data not shown).
Since it has been reported that treatment of LDL with PLA2G2A renders the particle more susceptible to oxidative modification (51), we measured the plasma concentrations of oxidized LDL in these mice. However, there was no substantial difference in plasma oxidized LDL levels between PLA2G3tg/0 × apoE-/- and PLA2G3non-Tg × apoE-/- mice (0.030 ± 0.018 and 0.029 ± 0.019 ng/mg of LDL, respectively; n = 6), suggesting that the atherogenicity of PLA2G3 is not a result of formation of oxidized LDL. The blood pressure (49.8 ± 8.4 to 95.2 ± 12.6 and 47.6 ± 7.4 to 89.1 ± 10.4 mm Hg, respectively (mean ± S.D.; n = 7–8)) and the ventricular rate (538.0 ± 60.0 and 548.9 ± 75.9 min-1, respectively (mean ± S.D.; n = 7–8)) did not differ significantly between PLA2G3non-Tg × apoE-/- and PLA2G3tg/0 × apoE-/- mice fed a high cholesterol diet. Finally, measurement of plasma eicosanoid levels revealed that thromboxane A2 (TXA2), a cyclooxygenase product, and 12-hydoxyeicosaenic acid, a 12-lipoxygenase product, were increased robustly in Tg mice over control mice, whereas other eicosanoids were minimally detected in both genotypes (Fig. S5). These results suggest more pronounced activation of platelets in Tg mice than in control mice, in agreement with the notion that development of atherosclerosis is associated with increased TXA2 production by platelets (52–54). Detailed plasma lipid profiling (PC and LPC species) of high fat-fed PLA2G3 Tg and control mice is shown in Fig. S6, providing further confirmation that various unsaturated fatty acids are released nonselectively from PC in the plasma of PLA2G3 Tg mice.
DISCUSSION
In our ongoing effort to gain new insights into the in vivo functions of sPLA2 enzymes, in this study we established and analyzed Tg mice for PLA2G3, an atypical mammalian sPLA2 structurally more similar to bee venom PLA2 than to other mammalian sPLA2s. PLA2G3 Tg mice did not display noticeable phenotypic changes in the skin, which is profoundly affected in PLA2G2A mice (24), or lung, whose architecture is disrupted in PLA2G5 Tg mice (22) and macrophage-specific PLA2G10 Tg mice (23), suggesting that phospholipids in mouse skin and lung surfactant may not be main targets for PLA2G3 in vivo. However, we have obtained evidence that PLA2G3 can target phospholipids in plasma lipoproteins in vivo, leading to a decrease in HDL and an increase in modified and aggregated LDL, which are risk factors for atherosclerosis. The action of PLA2G3 on lipoproteins observed in this study is reminiscent of that of its homolog, bee venom PLA2, which also has potent hydrolytic activity against lipoprotein-associated phospholipids in vitro (55). Our finding that the lipolytic action of PLA2G3 renders the LDL particle smaller in size (Fig. 2E) is important, since the smaller size of the LDL particle, the higher its atherogenic potential (31). In addition to the in vitro lipoprotein hydrolysis, we found that PLA2G3, as in the case of PLA2G5 and PLA2G10 (31, 34, 36), induces the accumulation of lipid droplets in LDL-loaded macrophages ex vivo (Fig. 5). Thus, these lipoprotein-acting sPLA2s commonly cause LDL particles to be more atherogenic. Moreover, after an atherogenic diet, PLA2G3 Tg mice crossed with apoE-/- mice developed more severe aortic atherosclerotic lesions than did control mice (Fig. 7), further supporting an unexplored atherosclerotic property of PLA2G3 in vivo.
Oxidation of LDL is believed to occur in the subendothelial space, where circulating antioxidant defenses are less effective. Oxidized LDL becomes a ligand for the scavenger receptors (e.g. scavenger receptor A and CD36) that contribute to foam cell formation by facilitating uptake of lipoprotein particles (56, 57). Current evidence suggests that sPLA2-mediated modification of lipoproteins also plays a role in the development of atherosclerosis (25–36). Hydrolysis of PC in lipoproteins by sPLA2 produces NEFA and LPC, which can trigger vasoactive and proinflammatory actions leading to the acceleration of atherosclerosis (see below). Hydrolysis of LDL by sPLA2 correlates with production of the more atherogenic, small-dense, modified LDL with increased net negative charge, whereas hydrolysis of HDL reduces the capacity of this antiatherogenic particle to promote cholesterol efflux from lipid-rich foam cells (58). Modified LDL retained in atherosclerotic lesions contains less PC and more LPC than does circulating LDL, suggesting that arterial LDL undergoes lipolytic modification by sPLA2 at lesion sites. Further, clinical analyses have shown that elevated plasma PLA2 activity is an independent risk factor for cardiovascular disease (59), and a low content of surface phospholipids often characterizes the small-dense LDL and HDL subclasses (60). Over the past few years, attention initially focused on the potential role of PLA2G2A in atherosclerosis, because this enzyme is enriched in human atherosclerotic plaques and because PLA2G2A Tg mice are susceptible to development of atherosclerosis (25–30). However, the recent discovery of PLA2G5 and PLA2G10 in atherosclerotic foci, plus the fact that these two enzymes hydrolyze lipoprotein phospholipids more readily than does PLA2G2A, has raised the question of which sPLA2 types are the true contributors to atherosclerosis (31–36). The recent finding that overexpression of PLA2G5 by retrovirus-mediated gene transfer leads to increased lesion area, whereas mice deficient in bone marrow-derived PLA2G5 have reduced lesion area, indicates that PLA2G5 does play a role in atherosclerosis in vivo (33). A tagging single nucleotide polymorphism analysis demonstrating an association of the human PLA2G5 gene haplotype with LDL and oxidized LDL supports this view (61). PLA2G10 also renders lipoprotein particles more proatherogenic to promote macrophage foam cell formation in vitro (31, 34). Taking these understandings together, our results support the idea that PLA2G3 is another sPLA2 enzyme that is capable of producing atherogenic lipoprotein particles and thereby has the potential to promote the development of atherosclerosis.
The lipolytic action of PLA2G3 on LDL and HDL is much superior to that of PLA2G2A and is similar to that of PLA2G5, albeit weaker than that of PLA2G10. The overall modes of sPLA2 action on phospholipids in lipoproteins are PLA2G10 > PLA2G5 ≥ PLA2G3 > PLA2G2F > PLA2G2A and PLA2G2E (Figs. 4, 5, 6), an order that appears broadly consistent with their ability to interact with PC-rich vesicles and with PC-rich cellular plasma membranes (1, 2). Although our present study failed to detect appreciable lipoprotein hydrolysis by PLA2GA, a number of previous studies have shown that PLA2G2A can do so after incubation for longer periods at higher concentrations, after oxidative modification of lipoproteins, or after binding to matrix proteoglycans (25–30). On the basis of our ESI-MS analyses of lipoproteins, PLA2G3 does not discriminate appreciably between fatty acids at the sn-2-position, whereas a trend of preference for oleic and linoleic acids over arachidonic and docosahexaenoic acids is observed for PLA2G5, and vice versa for PLA2G10 (Figs. 3 and 4). This fatty acid selectivity may be relevant in vivo, since PC species containing sn-2 saturated and monounsaturated fatty acids (C16:0-C16:0 and C16:0-C16:1) are robustly hydrolyzed in the lungs of PLA2G5 Tg mice (22) and since nonselective release of unsaturated fatty acids from plasma PC was observed in PLA2G3 Tg mice (Fig. S6). Although the physiological importance of these observations is unclear at present, similar fatty acid preferences of each sPLA2 have also been reported in in vitro enzymatic assays (31, 62, 64) and cellular fatty acid release assays (65), and Pruzanski et al. (49) have also reported a similar fatty acid selectivity of PLA2G5 and PLA2G10 toward lipoprotein PC.
Hydrolysis of lipoprotein-bound phospholipids by sPLA2s gives rise to the two proatherogenic and proinflammatory lipid products, lysophospholipids and fatty acids. Lysophospholipids function as extracellular signaling molecules in multiple biological processes and also affect insulin metabolism (66) and adipocyte growth and function (67). LPC, the most predominant lysophospholipid, modulates the expression of a number of proteins, such as growth factors, leukocyte adhesion molecules, inducible nitric-oxide synthase, and cyclooxygenase-2 (68). LPC plays an etiologic role in atherosclerosis, is a major constituent of atherogenic lipoproteins (69), and exhibits proinflammatory functions, including activation of macrophages and expression of chemotactic factors and adhesion molecules in endothelial cells (70). Lysophosphatidic acid, an autotaxinhydrolyzed product of LPC that elicits numerous effects on cells of the cardiovascular system, induces the formation of arterial neointima lesions, a prelude to atherosclerosis, through a PPARγ-dependent mechanism (71). Lysophosphatidic acid accumulates in the lipid-rich core of human carotid atherosclerotic plaques (72). Arachidonate-oxygenated lipid mediators, including prostaglandins and leukotrienes, also have diverse effects on atherosclerosis, as evidenced by studies employing knock-out mice for their receptors or biosynthetic enzymes. For instance, gene ablation of TXA2 receptor or PGE2 synthase ameliorates, whereas that of PGI2 receptor or PGD2 synthase exacerbates, atherosclerosis in apoE-/- or LDLR-/- mice (54, 73, 74). Null mice for 5- or 12/15-lipoxygenase are also partially protected from the development of atherosclerosis (75, 76). In this study, a marked decrease in PC and an increase in LPC, which reflect increased lipoprotein hydrolysis, were observed in the plasma and in LDL of PLA2G3 Tg mice fed an atherogenic diet (Fig. 7). Furthermore, we found striking increases in two particular eicosanoids, TXA2 and 12-hydoxyeicosaenic acid, in the plasma of PLA2G3 Tg mice (Fig. S5), implying that the development of atherosclerosis in these mice is accompanied by accelerated platelet activation. Activated platelets are found in the blood of patients with atherosclerosis (52) and hypercholesterolemia (53), and platelets of apoE-/- × TP-/- mice, which are highly susceptible to atherosclerosis, have lower reactivity than do those of apoE-/- mice (54). Thus, increased LPC and TXA2 in the circulation could also account for the atherosclerotic phenotype of PLA2G3 Tg mice.
Our results showed that only a few-fold increase in LDL-associated LPC by sPLA2s could promote the formation of foam cells from macrophages in vitro (Fig. S2). The plasma concentration of PLA2G3 in PLA2G3 Tg mice was estimated to be 10–100 ng/ml, a level above the threshold of inducing macrophage foam cell formation by this enzyme in vitro. In fact, the levels of LPC in plasma and LDL of high fat-fed PLA2G3 Tg × apoE-null mice were severalfold higher than those of control apoE-null mice (Fig. 7C), agreeing with the development of atherosclerosis in the PLA2G3 Tg × apoE-null mice. However, unlike PLA2G2A, whose plasma levels are highly elevated during inflammation and cardiovascular disease (1, 2), there is no current evidence that either PLA2G3, PLA2G5, or PLA2G10 is present in the circulation. It is thus conceivable that, in pathophysiological settings, the modification of lipoproteins by these sPLA2s may proceed at local foci of atherosclerosis, where the enzymes are enriched and can interact with matrix-captured lipoprotein particles. This speculation may be true for PLA2G5 and PLA2G10, since the expression of these enzymes are up-regulated at local sites of inflammation (e.g. alveolar macrophages and bronchial epithelial cells in asthma) (11, 12), since atherosclerosis is characterized by inflammation in the arterial walls where lipid-loaded activated macrophages accumulate (77, 78) and since the expression of PLA2G5 is in fact increased in atherosclerotic lesions in mice fed a high cholesterol diet and in human subjects (32, 36). The observations that transplantation of bone marrow cells overexpressing PLA2G5 (33) and PLA2G2A (27) into recipient LDL receptor-null mice results in increased atherosclerosis in the absence of alteration in systemic lipoprotein metabolism after a high fat feeding also suggest that increased sPLA2 activity in macrophages within the vessel wall is sufficient to promote atherosclerosis. In this study, we found that endogenous PLA2G3 is also expressed in macrophages and vascular smooth muscle cells of atherosclerotic lesions but only weakly in normal aorta (Fig. 6). Consistent with our immunohistochemical results, detection of PLA2G3 mRNA in macrophages and vascular smooth muscle cells of human atherosclerotic lesions by means of in situ hybridization has been described recently (40), indicating that PLA2G3 is synthesized in the aortic walls. These observations raise the possibility that PLA2G3 may also act on lipoproteins within the subendothelium at the atherosclerotic foci. It is noteworthy that the immunoreactive, endogenous PLA2G3 is localized in the microvascular endothelium of various tissues (19). Thus, hydrolysis of circulating lipoproteins by PLA2G3 might occur preferentially on the vascular walls, thereby influencing vascular lipid homeostasis.
As an associated finding, we show that PLA2G2F is also capable of substantial hydrolysis of PC in LDL and HDL in vitro, with a preferential reduction in PC species containing arachidonic acid (Figs. 4 and 5). Reportedly, PLA2G2F shows a ∼2-fold preference for arachidonic acid over linoleic acid in vitro (63), and its ability to hydrolyze PC is similar to that of PLA2G3 both in vitro and in cultured cells (18). Although the pathophysiological relevance of these observations is still unclear, a recent study has shown the inducible expression of PLA2G2F (in addition to several other sPLA2s) in human atherosclerotic plaques (40). Therefore, it will be interesting to examine whether alterations in plasma lipoproteins and in the process of atherosclerosis also take place in mice with Tg expression or gene ablation of this unique group II sPLA2 enzyme, of which the behavior and function in vivo are scarcely understood.
Since the oxidation hypothesis of atherosclerosis still remains inconclusive and oxidation alone may not explain the accumulation of LPC in atherosclerotic lesions (34), it is plausible that, in addition to oxidative modification of LDL, lipolytic modification of LDL may represent an alternative pathway for the progression of atherosclerosis. In the arterial wall, multiple sPLA2 isoforms may exert proatherogenic actions by inducing the release of lipid mediators, such as LPC and TXA2, and by lipolytic modification of lipoprotein particles independently of oxidation. Understanding of the roles of sPLA2s in atherosclerosis is thus emerging, and the efficacy of a specific inhibitor of PLA2G2A, PLA2G5, and PLA2G10 (valespladib) in human cardiovascular disease is now the subject of a clinical trial (available on the World Wide Web). Our findings about the atherosclerotic properties of PLA2G3, including lipoprotein modification, macrophage foam cell formation, and aortic lipid-rich plaque formation, are novel and point to this structurally unique sPLA2 as a potential target for the development of a novel antiatherosclerotic drug. Nonetheless, because of the limitations of the Tg expression strategy, it will be necessary to investigate the true in vivo actions, expression, and dynamics of PLA2G3, including the biological mechanisms involved, by conducting studies in mice with targeted disruption of the pla2g3 gene.
Supplementary Material
Acknowledgments
We thank Drs. M. H. Gelb (University of Washington, Seattle, WA) and G. Lambeau (CNRS-UPR 411, Sophia Antipolis, France) for providing cDNAs, recombinant proteins, and antibodies for sPLA2s. We thank Drs. I. Saito (University of Tokyo, Tokyo, Japan) and J. Miyazaki (Osaka University, Suita, Japan) for kind provision of pCALNL5 plasmid and CAG-Cre mice, respectively.
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Culture, Sports and Technology of Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains upplemental Table S1 and Figs. S1–S6.
Footnotes
The abbreviations used are: sPLA2, secretory PLA2; PLA2, phospholipase A2; ESI-MS, electrospray ionization-mass spectrometry; HDL, high density lipoprotein; HPLC, high performance liquid chromatography; LDL, low density lipoprotein; Ac-LDL, acetyl-LDL; LPC, lysophosphatidylcholine; NEFA, nonesterified fatty acid(s); PBS, phosphate-buffered saline; PC, phosphatidylcholine; PGE2, prostaglandin E2; Tg, transgenic; TXA2, thromboxane A2; VLDL, very low density lipoprotein; WT, wild-type; SMA, smooth muscle actin.
References
- 1.Murakami, M., and Kudo, I. (2001) Adv. Immunol. 77 163-194 [DOI] [PubMed] [Google Scholar]
- 2.Kudo, I., and Murakami, M. (2002) Prostaglandins Other Lipid Mediat. 68/69, 3-58 [DOI] [PubMed] [Google Scholar]
- 3.Huggins, K. W., Boileau, A. C., and Hui, D. Y. (2002) Am. J. Physiol. 283 E994-E1001 [DOI] [PubMed] [Google Scholar]
- 4.Labonte, E. D., Kirby, R. J., Schildmeyer, N. M., Cannon, A. M., Huggins, K. W., and Hui, D. Y. (2006) Diabetes 55 935-941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacPhee, M., Chepenik, K. P., Liddell, R. A., Nelson, K. K., Siracusa, L. D., and Buchberg, A. M. (1995) Cell 81 957-966 [DOI] [PubMed] [Google Scholar]
- 6.Yanaru-Fujisawa, R., Matsumoto, T., Kukita, Y., Nakamura, S., Yao, T., Hayashi, K., and Iida, M. (2007) Dis. Colon Rectum 50 223-231 [DOI] [PubMed] [Google Scholar]
- 7.Weinrauch, Y., Elsbach, P., Madsen, L. M., Foreman, A., and Weiss, J. (1996) J. Clin. Invest. 97 250-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koduri, R. S., Gronroos, J. O., Laine, V. J., Le Calvez, C., Lambeau, G., Nevalainen, T. J., and Gelb, M. H. (2002) J. Biol. Chem. 277 5849-5857 [DOI] [PubMed] [Google Scholar]
- 9.Satake, Y., Diaz, B. L., Balestrieri, B., Lam, B. K., Kanaoka, Y., Grusby, M. J., and Arm, J. P. (2004) J. Biol. Chem. 279 16488-16494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balestrieri, B., Hsu, V. W., Gilbert, H., Leslie, C. C., Han, W. K., Bonventre, J. V., and Arm, J. P. (2006) J. Biol. Chem. 281 6691-6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñoz, N. M., Meliton, A. Y., Arm, J. P., Bonventre, J. V., Cho, W., and Leff, A. R. (2007) J. Immunol. 179 4800-4807 [DOI] [PubMed] [Google Scholar]
- 12.Henderson, W. R., Jr., Chi, E. Y., Bollinger, J. G., Tien, Y. T., Ye, X., Castelli, L., Rubtsov, Y. P., Singer, A. G., Chiang, G. K., Nevalainen, T., Rudensky, A. Y., and Gelb, M. H. (2007) J. Exp. Med. 204 865-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujioka, D., Saito, Y., Kobayashi, T., Yano, T., Tezuka, H., Ishimoto, Y., Suzuki, N., Yokota, Y., Nakamura, T., Obata, J. E., Kanazawa, M., Kawabata, K. I., Hanasaki, K., and Kugiyama, K. (2008) Circulation 117 2977-2985 [DOI] [PubMed] [Google Scholar]
- 14.Uozumi, N., Kume, K., Nagase, T., Nakatani, N., Ishii, S., Tashiro, F., Komagata, Y., Maki, K., Ikuta, K., Ouchi, Y., Miyazaki, J., and Shimizu, T. (1997) Nature 390 618-622 [DOI] [PubMed] [Google Scholar]
- 15.Murakami, M., Koduri, R. S., Enomoto, A., Shimbara, S., Seki, M., Yoshihara, K., Singer, A., Valentin, E., Ghomashchi, F., Lambeau, G., Gelb, M. H., and Kudo, I. (2001) J. Biol. Chem. 276 10083-10096 [DOI] [PubMed] [Google Scholar]
- 16.Hanasaki, K., Ono, T., Saiga, A., Morioka, Y., Ikeda, M., Kawamoto, K., Higashino, K., Nakano, K., Yamada, K., Ishizaki, J., and Arita, H. (1999) J. Biol. Chem. 274 34203-34211 [DOI] [PubMed] [Google Scholar]
- 17.Valentin, E., Ghomashchi, F., Gelb, M. H., Lazdunski, M., and Lambeau, G. (2000) J. Biol. Chem. 275 7492-7496 [DOI] [PubMed] [Google Scholar]
- 18.Murakami, M., Masuda, S., Shimbara, S., Bezzine, S., Ladzunski, M., Lambeau, G., Gelb, M. H., Matsukura, S., Kokubu, F., Adachi, M., and Kudo, I. (2003) J. Biol. Chem. 278 10657-10667 [DOI] [PubMed] [Google Scholar]
- 19.Murakami, M., Masuda, S., Shimbara, S., Ishikawa, Y., Ishii, T., and Kudo, I. (2005) J. Biol. Chem. 280 24987-24998 [DOI] [PubMed] [Google Scholar]
- 20.Masuda, S., Yamamoto, K., Hirabayashi, T., Ishikawa, Y., Ishii, T., Kudo, I., and Murakami, M. (2008) Biochem. J. 409 429-438 [DOI] [PubMed] [Google Scholar]
- 21.Mounier, C. M., Wendum, D., Greenspan, E., Fléjou, J. F., Rosenberg, D. W., and Lambeau, G. (2008) Br. J. Cancer 98 587-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohtsuki, M., Taketomi, Y., Arata, S., Masuda, S., Ishikawa, Y., Ishii, T., Takanezawa, Y., Aoki, J., Arai, H., Yamamoto, K., Kudo, I., and Murakami. M. (2006) J. Biol. Chem. 281 36420-36433 [DOI] [PubMed] [Google Scholar]
- 23.Curfs, D. M., Ghesquiere, S. A., Vergouwe, M. N., van der Made, I., Gijbels, M. J., Greaves, D. R., Verbeek, J. S., Hofker, M. H., and de Winther, M. P. (2008) J. Biol. Chem. 283 21640-21648 [DOI] [PubMed] [Google Scholar]
- 24.Grass, D. S., Felkner, R. H., Chiang, M. Y., Wallace, R. E., Nevalainen, T. J., Bennett, C. F., and Swanson, M. E. (1996) J. Clin. Invest. 97 2233-2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurt-Camejo, E., Camejo, G., Peilot, H., Oorni, K., and Kovanen, P. (2001) Circ. Res. 89 298-304 [DOI] [PubMed] [Google Scholar]
- 26.Romano, M., Romano, E., Bjorkerud, S., and Hurt-Camejo, E. (1998) Arterioscler. Thromb. Vasc. Biol. 18 519-525 [DOI] [PubMed] [Google Scholar]
- 27.Webb, N. R., Bostrom, M. A., Szilvassy, S. J., van der Westhuyzen, D. R., Daugherty, A., and de Beer, F. C. (2003) Arterioscler. Thromb. Vasc. Biol. 23 263-268 [DOI] [PubMed] [Google Scholar]
- 28.Ivandic, B., Castellani, L. W., Wang, X. P., Qiao, J. H., Mehrabian, M., Navab, M., Fogelman, A. M., Grass, D. S., Swanson, M. E., de Beer, M. C., de Beer, F., and Lusis, A. J. (1999) Arterioscler. Thromb. Vasc. Biol. 19 1284-1290 [DOI] [PubMed] [Google Scholar]
- 29.Sartipy, P., Johansen, B., Gasvik, K., and Hurt-Camejo, E. (2000) Circ. Res. 86 707-714 [DOI] [PubMed] [Google Scholar]
- 30.Sartipy, P., Johansen, B., Camejo, G., Rosengren, B., Bondjers, G., and Hurt-Camejo, E. (1996) J. Biol. Chem. 271 26307-26314 [DOI] [PubMed] [Google Scholar]
- 31.Hanasaki, K., Yamada, K., Yamamoto, S., Ishimoto, Y., Saiga, A., Ono, T., Ikeda, M., Notoya, M., Kamitani, S., and Arita, H. (2002) J. Biol. Chem. 277 29116-29124 [DOI] [PubMed] [Google Scholar]
- 32.Rosengren, B., Peilot, H., Umaerus, M., Jonsson-Rylander, A. C., Mattsson-Hulten, L., Hallberg, C., Cronet, P., Rodriguez-Lee, M., and Hurt-Camejo, E. (2006) Arterioscler. Thromb. Vasc. Biol. 26 1579-1585 [DOI] [PubMed] [Google Scholar]
- 33.Bostrom, M. A., Boyanovsky, B. B., Jordan, C. T., Wadsworth, M. P., Taatjes, D. J., de Beer, F. C., and Webb, N. R. (2007) Arterioscler. Thromb. Vasc. Biol. 27 600-606 [DOI] [PubMed] [Google Scholar]
- 34.Karabina, S. A., Brocheriou, I., Le Naour, G., Agrapart, M., Durand, H., Gelb, M., Lambeau, G., and Ninio, E. (2006) FASEB J. 20 2547-2549 [DOI] [PubMed] [Google Scholar]
- 35.Boyanovsky, B. B., van der Westhuyzen, D. R., and Webb, N. R. (2005) J. Biol. Chem. 280 32746-32752 [DOI] [PubMed] [Google Scholar]
- 36.Wooton-Kee, C. R., Boyanovsky, B. B., Nasser, M. S., de Villiers, W. J., and Webb, N. R. (2004) Arterioscler. Thromb. Vasc. Biol. 24 762-767 [DOI] [PubMed] [Google Scholar]
- 37.Kanegae, Y., Takamori, K., Sato, Y., Lee, G., Nakai, M., and Saito, I. (1996) Gene (Amst.) 181 207-212 [DOI] [PubMed] [Google Scholar]
- 38.Sakai, K., and Miyazaki, J. (1997) Biochem. Biophys. Res. Commun. 237 318-324 [DOI] [PubMed] [Google Scholar]
- 39.Niwa, H., Yamamura, K., and Miyazaki, J. (1991) Gene (Amst.) 108 193-199 [DOI] [PubMed] [Google Scholar]
- 40.Kimura-Matsumoto, M., Ishikawa, Y., Komiyama, K., Tsuruta, T., Murakami, M., Masuda, S., Akasaka, Y., Ito, K., Ishiguro, S., Morita, H., Sato, S., and Ishii, T. (2008) Atherosclerosis 196 81-91 [DOI] [PubMed] [Google Scholar]
- 41.Heider, J. G., and Boyett, R. L. (1978) J. Lipid Res. 19 514-518 [PubMed] [Google Scholar]
- 42.Hatch, F. T. (1968) Adv. Lipid Res. 6 1-68 [PubMed] [Google Scholar]
- 43.Bligh, E. G., and Dyer, W. J. (1959) Can. J. Biochem. Physiol. 37 911-917 [DOI] [PubMed] [Google Scholar]
- 44.Taguchi, R., Houjou, T., Nakanishi, H., Yamazaki, T., Ishida, M., Imagawa, M., and Shimizu, T. (2005) J. Chromatogr. B 823 26-36 [DOI] [PubMed] [Google Scholar]
- 45.Houjou, T., Yamatani, K., Nakanishi, H., Imagawa, M., Shimizu, T., and Taguchi, R. (2004) Rapid Commun. Mass Spectrom. 18 3123-3130 [DOI] [PubMed] [Google Scholar]
- 46.Itabe, H., Takeshima, E., Iwasaki, H., Kimura, J., Yoshida, Y., Imanaka, T., and Takano, T. (1994) J. Biol. Chem. 269 15274-15279 [PubMed] [Google Scholar]
- 47.Nichols, A. V., Krauss, R. M., and Musliner, T. A. (1986) Methods Enzymol. 128 417-431 [DOI] [PubMed] [Google Scholar]
- 48.Fox, N., Song, M., Schrementi, J., Sharp, J. D., White, D. L., Snyder, D. W., Hartley, L. W., Carlson, D. G., Bach, N. J., Dillard, R. D., Draheim, S. E., Bobbitt, J. L., Fisher, L., and Mihelich, E. D. (1996) Eur. J. Pharmacol. 308 195-203 [DOI] [PubMed] [Google Scholar]
- 49.Pruzanski, W., Lambeau, G., Lazdunsky, M., Cho, W., Kopilov, J., and Kuksis, A. (2005) Biochim. Biophys. Acta 1736 38-50 [DOI] [PubMed] [Google Scholar]
- 50.Yokode, M., Kita, T., Kikawa, Y., Ogorochi, T., Narumiya, S., and Kawai, C. (1988) J. Clin. Invest. 81 720-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuzil, J., Upston, J. M., Witting, P. K., Scott, K. F., and Stocker, R. (1998) Biochemistry 37 9203-9210 [DOI] [PubMed] [Google Scholar]
- 52.van Zanten, G. H., de Graaf, S., Slootweg, P. J., Heijnen, H. F., Connolly, T. M., de Groot, P. G., and Sixma, J. J. (1994) J. Clin. Invest. 93 615-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poston, R. N., Haskard, D. O., Coucher, J. R., Gall, N. P., and Johnson-Tidey, R. R. (1992) Am. J. Pathol. 140 665-673 [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi, T., Tahara, Y., Matsumoto, M., Iguchi, M., Sano, H., Murayama, T., Arai, H., Oida, H., Yurugi-Kobayashi, T., Yamashita, J. K., Katagiri, H., Majima, M., Yokode, M., Kita, T., and Narumiya, S. (2004) J. Clin. Invest. 114 784-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deregnaucourt, C., and Schrevel, J. (2000) J. Biol. Chem. 275 39973-39980 [DOI] [PubMed] [Google Scholar]
- 56.Brown, M. S., and Goldstein, J. L. (1983) Annu. Rev. Biochem. 52 223-261 [DOI] [PubMed] [Google Scholar]
- 57.Ross, R. (1993) Nature 362 801-809 [DOI] [PubMed] [Google Scholar]
- 58.Ishimoto, Y., Yamada, K., Yamamoto, S., Ono, T., Notoya, M., and Hanasaki, K. (2003) Biochim. Biophys. Acta 1642 129-138 [DOI] [PubMed] [Google Scholar]
- 59.Kugiyama, K., Ota, Y., Takazoe, K., Moriyama, Y., Kawano, H., Miyao, Y., Sakamoto, T., Soejima, H., Ogawa, H., Doi, H., Sugiyama, S., and Yasue, H. (1999) Circulation 100 1280-1284 [DOI] [PubMed] [Google Scholar]
- 60.Hurt-Camejo, E., Paredes, S., Masana, L., Camejo, G., Sartipy, P., Rosengren, B., Pedreno, J., Vallve, J. C., Benito, P., and Wiklund, O. (2001) Arthritis Rheum. 44 2761-2767 [DOI] [PubMed] [Google Scholar]
- 61.Wootton, P. T., Arora, N. L., Drenos, F., Thompson, S. R., Cooper, J. A., Stephens, J. W., Hurel, S. J., Hurt-Camejo, E., Wiklund, O., Humphries, S. E., and Talmud, P. J. (2007) Hum. Mol. Genet. 16 1437-1444 [DOI] [PubMed] [Google Scholar]
- 62.Murakami, M., Shimbara, S., Kambe, T., Kuwata, H., Winstead, M. V., Tischfield, J. A., and Kudo, I. T. (1998) J. Biol. Chem. 273 14411-14423 [DOI] [PubMed] [Google Scholar]
- 63.Murakami, M., Yoshihara, K., Shimbara, S., Lambeau, G., Gelb, M. H., Singer, A. G., Sawada, M., Inagaki, N., Nagai, H., Ishihara, M., Ishikawa, Y., Ishii, T., and Kudo, I. (2002) J. Biol. Chem. 277 19145-19155 [DOI] [PubMed] [Google Scholar]
- 64.Chen, Y., and Dennis, E. A. (1998) Biochim. Biophys. Acta. 1394 57-64 [DOI] [PubMed] [Google Scholar]
- 65.Mitsuishi, M., Masuda, S., Kudo, I., and Murakami, M. (2007) Biochim. Biophys. Acta 1771 1389-1389 [DOI] [PubMed] [Google Scholar]
- 66.Soga, T., Ohishi, T., Matsui, T., Saito, T., Matsumoto, M., Takasaki, J., Matsumoto, S., Kamohara, M., Hiyama, H., Yoshida, S., Momose, K., Ueda, Y., Matsushime, H., Kobori, M., and Furuichi, K. (2005) Biochem. Biophys. Res. Commun. 326 744-751 [DOI] [PubMed] [Google Scholar]
- 67.Ferry, G., Tellier, E., Try, A., Gres, S., Naime, I., Simon, M. F., Rodriguez, M., Boucher, J., Tack, I., Gesta, S., Chomarat, P., Dieu, M., Raes, M., Galizzi, J. P., Valet, P., Boutin, J. A., and Saulnier-Blache, J. S. (2003) J. Biol. Chem. 278 18162-18169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang, A., and Dennis, E. A. (1999) Biochim. Biophys. Acta 1439 1-16 [DOI] [PubMed] [Google Scholar]
- 69.Kume, N., Cybulsky, M. I., and Gimbrone, M. A., Jr. (1992) J. Clin. Invest. 90 1138-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murugesan, G., Sandhya Rani, M. R., Gerber, C. E., Mukhopadhyay, C., Ransohoff, R. M., Chisolm, G. M., and Kottke-Marchant, K. (2003) J. Mol. Cell Cardiol. 35 1375-1384 [DOI] [PubMed] [Google Scholar]
- 71.Zhang, C., Baker, D. L., Yasuda, S., Makarova, N., Balazs, L., Johnson, L. R., Marathe, G. K., McIntyre, T. M., Xu, Y., Prestwich, G. D., Byun, H. S., Bittman, R., and Tigyi, G. (2004) J. Exp. Med. 199 763-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rother, E., Brandl, R., Baker, D. L., Goyal, P., Gebhard, H., Tigyi, G., and Siess, W. (2003) Circulation 108 741-747 [DOI] [PubMed] [Google Scholar]
- 73.Wang, M., Zukas, A. M., Hui, Y., Ricciotti, E., Puré, E., and FitzGerald, G. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 14507-14512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ragolia, L., Palaia, T., Hall, C. E., Maesaka, J. K., Eguchi, N., and Urade, Y. (2005) J. Biol. Chem. 280 29946-29955 [DOI] [PubMed] [Google Scholar]
- 75.Mehrabian, M., Allayee, H., Wong, J., Shi, W., Wang, X. P., Shaposhnik, Z., Funk, C. D., and Lusis, A. J. (2002) Circ. Res. 91 120-126 [DOI] [PubMed] [Google Scholar]
- 76.Cyrus, T., Witztum, J. L., Rader, D. J., Tangirala, R., Fazio, S., Linton, M. F., and Funk, C. D. (1999) J. Clin. Invest. 103 1597-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stoll, G., and Bendszus, M. (2006) Stroke 37 1923-1932 [DOI] [PubMed] [Google Scholar]
- 78.Yan, Z. Q., and Hansson, G. K. (2007) Immunol. Rev. 219 187-120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.