Abstract
Peroxiredoxin 6 (Prdx6) is a bifunctional enzyme with peroxidase activity and Ca2+-independent phospholipase A2 (iPLA2) activity. Here, we report that H2O2-induced cellular toxicity acts through Prdx6 hyperoxidation. Under high concentrations of H2O2 (>100 μm), Prdx6, and 2-Cys Prdxs were hyperoxidized. Contrary to hyperoxidation of 2-Cys Prdxs, hyperoxidation of Prdx6 was irreversible in vivo. Surprisingly, H2O2-induced cell cycle arrest at the G2/M transition correlated with hyperoxidation and increased iPLA2 activity of Prdx6. This arrest was also associated with up-regulation of p53 and p21 and with down-regulation of cyclin B1. Furthermore, the H2O2-mediated increase in iPLA2 activity was dramatically abolished in a hyperoxidation mutant (C47A), an iPLA2 mutant (S32A), and a double mutant (C47A/S32A) of Prdx6, demonstrating the essential requirement of Prdx6 C47 hyperoxidation for its iPLA2 activity. Together, our results demonstrate that H2O2-mediated hyperoxidation of Prdx6 induces cell cycle arrest at the G2/M transition through up-regulation of iPLA2 activity.
Peroxiredoxins (Prdxs)2 are a family of peroxidases that reduce mainly hydrogen peroxide (H2O2) and alkyl hydroperoxides to water and alcohol, respectively (1, 2). Prdxs are classified as either 1-Cys or 2-Cys Prdxs, based on whether the protein contains one or two conserved cysteine residues, respectively. In mammals, six members of the Prdx family have been described. Five of these (Prdx1, Prdx2, Prdx3, Prdx4, and Prdx5) are 2-Cys enzymes that use thioredoxin as the electron donor of their catalytic cycle (3, 4). In contrast, Prdx6, the sole mammalian 1-Cys Prdx, does not use thioredoxin as a reductant.
In addition to peroxidase activity, Prdx6 has a Ca2+-independent phospholipase A2 (iPLA2) activity (5). The peroxidase activity of Prdx6 has been widely studied in cells and animal models for its antioxidant properties that provide protection against the harmful consequence of oxidative stress (6-8). However, the iPLA2 activity of Prdx6 remains poorly understood. Considering the many functions of iPLA2 activity, including cell cycle progression, apoptosis, and tumorigenesis (9), it may also play an important role in either H2O2-mediated signaling or H2O2-related cellular events.
Prdxs like Prdx6 use cysteine as a catalytic center, rather than selenocysteine which characterizes the glutathione peroxidases (GPx) (2, 3). The N-terminal conserved cysteines (Cys51 of Prdx1) of Prdx1-5 are selectively oxidized by H2O2 to Cys-SOH (10). The unstable Cys51-SOH reacts with Cys172-SH of another Prdx molecule, creating a homodimer through an intermolecular disulfide bond. The disulfide is then reduced back to the Prdx active thiol form by the thioredoxin-thioredoxin reductase system (11-16). Under oxidative stress condition, however, the sulfenic intermediate is susceptible to hyperoxidation and thus occasionally hyperoxidized by H2O2, leading to the formation of sulfinic acid (Cys-SO2H) or sulfonic acid (Cys-SO3H), which cannot involve disulfide bond formation with the resolving cysteine (14, 16, 17). A recent study suggested that hyperoxidation of 2-Cys Prdx2 may control structural transitions of Prdx2 that correlate with cell cycle arrest and recovery (18), indicating that Prdxs hyperoxidation may activate stress responses that mediate oxidant-induced cell cycle arrest. In contrast, it is poorly understood whether 1-Cys Prdx6 is hyperoxidized upon treatment with H2O2, whether Prdx6 hyperoxidation plays a role in H2O2-induced cellular toxicity, or whether the two enzymatic activities of Prdx6 are functionally linked. In this study, we provide the first evidence that Prdx6 is involved in H2O2-induced cellular toxicity through the hyperoxidation and up-regulation of the iPLA2 activity of Prdx6.
EXPERIMENTAL PROCEDURES
Cell Culture—HeLa cells (human cervical cancer cells) were obtained from ATCC and maintained in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal calf serum (10% v/v) glutamine and antibiotics. HEK293 cells were grown in minimum essential medium supplemented with 10% (v/v) fetal bovine serum. Both cell lines were maintained at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Apoptosis Assay—Apoptotic cells were identified by staining with Annexin V-FITC (PharMingen) according to the manufacturer's protocols. The percentage of Annexin V-positive cells was analyzed with the FACSCalibur™ system and determined with the CellQuest software.
RNA Interference—shRNA against human Prdx6 (5′-GGGCATGCCTGTGACAGCT-3′) was produced from chemically synthesized DNA oligonucleotides that were cloned into the pSUPER.retro vector according to the manufacturer's instruction (OligoEngine). DNA transfections were performed using FuGENE6 (Roche Applied Sciences) or MP-100 micro-Porator (Digital Bio, Korea) according to the respective manufacturer's instructions.
Immunoblotting—Cell lysates were resolved on SDS-PAGE and electroblotted onto polyvinylidene difluoride membranes. After blocking with 5% skim milk in TBS-T (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, and 0.05% Tween 20), the membranes were probed with antibodies. The antibody-antigen complexes were detected using the ECL detection system (Amersham Biosciences). Antibodies to Prdx1, Prdx2, Prdx3, Prdx6, Prdxs-SO2H/SO3, and Prdx6-SO2H/SO3 were purchased from Lab Frontier (18). Antibodies to GAPDH (Cell Signaling), p53 (Cell Signaling), p21 (Cell Signaling), and cyclin B1 (Cell Signaling) were also used.
Confocal Microscopy—Hela cells were plated on glass coverslips in 100-mm tissue culture dishes, allowed to grow to 60% confluence, treated with 500 mm H2O2 for 20 min, washed with Hank's balanced salt solution (HBSS), and incubated for various amounts of time as indicated. Coverslips were rinsed with PBS. Cells were then fixed with 3% paraformaldehyde for 15 min at room temperature and washed several times with PBS. The cells were permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature. After gentle washing, nonspecific binding was blocked by incubating coverslips for 1 h at room temperature with 10% normal goat serum in PBS. Coverslips were then incubated with either anti-Prdxs-SO2H (Abcam, ab16830), anti-Prdx6-SO2H (Abcam, ab2844), anti-Prdx1 (Abcam, ab16946), anti-Prdx2 (Abcam, ab16749), or anti-Prdx3 (Abcam, 16753) for 30 min at room temperature and then incubated with either goat anti-rabbit or anti-mouse antibodies conjugated with Alexa Fluor 488 or TRICT (Rhodamine) under identical conditions. Finally, coverslips were stained with Hoechst dye to demarcate the nucleus. Coverslips were mounted on slides, and images were generated at room temperature using a confocal scanning laser microscope (LSM510Meta Duoscan, Carlzeiss Microimaging, GmbH, Germany). Digital images were analyzed with the LSM510 Software (Carlzeiss Microimaging, GmbH, Germany).
Cell Cycle Analysis—At 40% confluence, cells were treated with different concentrations of H2O2 for 20 min, washed with HBSS, and incubated for an additional 24 h. Attached cells were harvested by trypsinization, washed twice with PBS, resuspended in a fluorochrome staining solution (3.8 mm sodium citrate, 0.05 mg/ml propidium iodide, 0.1% Triton X-100, and 7 Kunitz unites/ml RNase B) and incubated on ice for 3 h or kept at 4 °C for up to 2 weeks before flow cytometric analysis. The cell cycle was analyzed with the FACSCalibur™ system and determined with the CellQuest software and Modfit LT 3.0 software.
Human Prdx6 cDNA Construct and Site-directed Mutagenesis—The human Prdx6 cDNA (GenBank™ accession number, NM_004905) was cloned from a human cervical cancer cell line, HeLa, into the pCDNA3.0 expression vector. Mutations were made using the MORPH™ plasmid DNA mutagenesis kit supplied by 5′ → 3′ Inc. Cysteine at position 47 was replaced by alanine (C47A) and/or serine at position 32 was substituted by alanine (S32A). Mutagenesis experiments were performed as described previously (5). All mutants were verified by automated DNA sequencing.
Total PLA2 and iPLA2 Activity Assay—Total PLA2 and iPLA2 activities were measured according to the manufacturer's recommendations (Cayman Chemicals, Ann Arbor, MI). Cells were scraped into Eppendorf tubes and centrifuged at 1,000 × g for 5 min. Cell pellets were suspended in 200 μl of 50 m m Hepes, pH 7.4, containing 1 mm EDTA, and sonicated at 10% power for 9 s with 9-s intervals for 10 times on ice. Supernatants were obtained by centrifugation at 10,000 × g for 15 min at 4 °C. Supernatants containing 50 μg of protein in a total volume of 45 μl were added to microplate wells containing 5 μl of pf assay buffer with (iPLA2 activity) or without 10 μm BEL (total PLA2 activity). The reaction was initiated by addition of 200 μl of arachidonoyl thiophosphatidylcholine and was incubated at room temperature for 60 min. The reaction was terminated by addition of 10 μl of 25 mm 5,5′-dithio-bis(2-nitrobenzoic acid), and the absorbance was measured at 405 nm in an Emax precision microplate reader (Molecular devices). The iPLA2 activity was calculated according to the manufacturer's instructions.
RESULTS AND DISCUSSION
Antioxidant Effects of Prdx6 against H2O2—To investigate the functional relationship between the peroxidase and iPLA2 activities of Prdx6, we first examined its antioxidant role in defending against oxidative stress. Human Prdx6 was transiently overexpressed in HeLa cells (Fig. 1B), and its protective effects against exogenous H2O2 were evaluated by apoptosis analysis. H2O2-induced apoptosis was attenuated in Prdx6-overexpressing HeLa cells compared with mock-transfected cells after treatment of cells with 500 μm H2O2 (Fig. 1A). To validate the function of endogenous Prdx6, we designed shRNA toward Prdx6 (supplemental Fig. S1) and generated Prdx6-knockdown cells (Fig. 1C). Knockdown cells were more sensitive to H2O2-induced apoptosis than cells transfected with control shRNA (Fig. 1D). In accordance with a previous study (6), Prdx6 protects against H2O2-induced oxidative stress.
FIGURE 1.
Antioxidant effect of Prdx6 against H2O2. A and B, HeLa cells were transiently transfected with human Prdx6 or mock vector. At 36 h after the transfection, cells were treated with different concentrations of H2O2 for 24 h. Cell lysates were subjected to immunoblotting with anti-Prdx6 antibody (A), and cells were stained for Annexin V. The percentage of Annexin V-positive cells was analyzed with the FACSCalibur™ system and determined with the CellQuest software. The results are expressed as mean ± S.D. for triplicate assays. C, HeLa cells were transfected with Prdx6-specific shRNA, control shRNA, or mock vector (pSUPER.retro). At 36 h after transfection, cell lysates were subjected to immunoblotting with anti-Prdx6 antibody. D, HeLa cells were transfected with Prdx6-specific shRNA or control shRNA. At 36 h after transfection, cells were treated with different concentrations of H2O2 for 24 h. Cells were stained for annexin V. The percentage of annexin V-positive cells was analyzed with the FACSCalibur™ system and determined with the CellQuest software. The results are expressed as mean ± S.D. for triplicate assays.
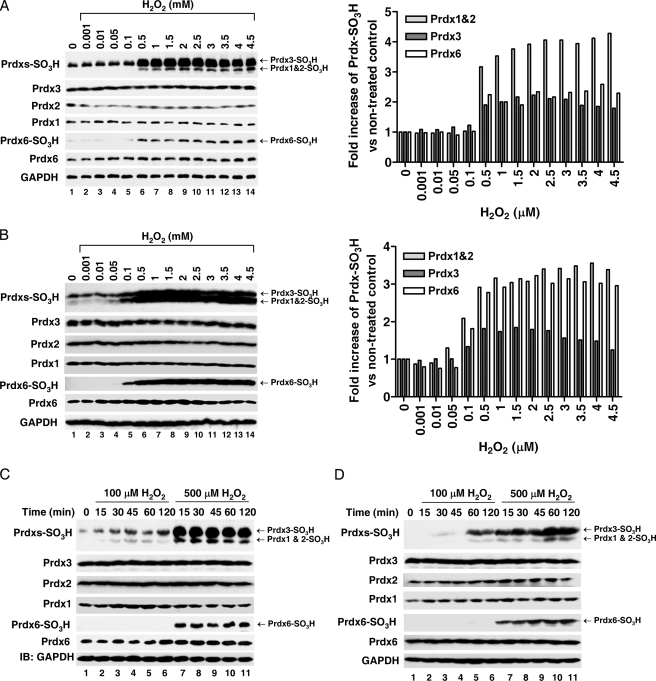
Prdx6 Is Hyperoxidized by High Concentrations of H2O2—Upon oxidative stress generated at greater than normal H2O2 concentrations, intracellular 2-Cys Prdxs such as Prdx2 are hyperoxidized and may induce cytotoxicity through cell cycle arrest (18). Consequently, hyperoxidation of intracellular Prdxs may play a role in H2O2-induced cellular toxicity. However, whether Prdx6 is also hyperoxidized and thereby implicated in cellular toxicity is still unclear. Therefore, HeLa and HEK293 cells were exposed to increasing concentrations of H2O2 (from 0.001 mm to 4.5 mm). Hyperoxidation of Prdxs was monitored by specific antibodies recognizing Cys-SO3H Prdx1, Prdx2, and Prdx3 or Cys-SO3H Prdx6. As shown in Fig. 2, A and B, 2-Cys Prdxs were gradually hyperoxidized, and their hyperoxidation plateaued above 500 μm H2O2. Prdx6 was not hyperoxidized below 100 μm H2O2 but was rapidly hyperoxidized at 500 μm H2O2. To further confirm the requirement of the specified concentration of H2O2 for the hyperoxidation of Prdx6, HeLa and HEK293 cells were exposed to 100 μm or 500 μm H2O2 for different amounts of time. At 100 μm H2O2, 2-Cys Prdxs were gradually hyperoxidized in a time-dependent manner, whereas Prdx6 was not hyperoxidized until 2 h (Fig. 2C, HeLa and Fig. 2D, HEK293). However, both 2-Cys Prdxs and Prdx6 were rapidly hyperoxidized at earlier times, and the levels were quickly saturated at 500 μm H2O2 (Fig. 2, C and D). Thus, the requirement of H2O2 molarity for Prdx6 hyperoxidation is higher than that of 2-Cys Prdxs.
FIGURE 2.
Prdx6 hyperoxidation is dependent on the concentration of H2O2. HeLa (A) and HEK293 (B) cells were treated with different concentrations of H2O2, as indicated, for 20 min. Cell lysates were subjected to immunoblotting with an anti-Prdxs-SO3H antibody that recognizes Prdx1-, Prdx2-, and Prdx3-SO3H, or with anti-Prdx6 SO3H, anti-Prdx1, anit-Prdx2, anti-Prdx3, anti-Prdx6, or anti-GAPDH antibodies. Each graph (left) represents the band intensity quantified by densitometric analysis (ImageJ 1.40 software). HeLa (C) and HEK293 (D) cells were treated with different concentrations of H2O2, as indicated, for different amounts of time. Cell lysates were subjected to immunoblotting as described in A and B.
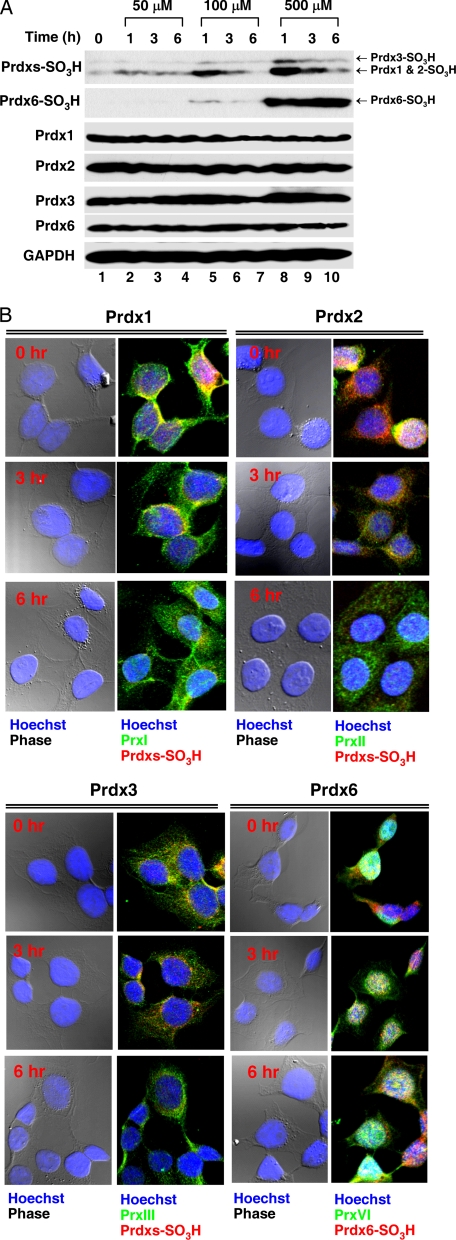
Hyperoxidized Prdx6 Is Irreversible—A recent report demonstrated that hyperoxidized 2-Cys Prdxs can be reduced and can then reenter the redox cycling pathway (18). It is uncertain, however, whether hyperoxidized Prdx6 is also recycled. Thus, HeLa cells were exposed to different concentrations of H2O2 for 20 min, washed with Hanks' balanced salt solution, and incubated for various amounts of time. By Western blot analysis, the degree of Prdx1, Prdx2, and Prdx3 hyperoxidation gradually decreased with time after the removal of H2O2, whereas Prdx6 hyperoxidation remained unchanged during the time course (Fig. 3A). Using immunofluorescence confocal microscopy, we confirmed the two distinct patterns of Prdxs and Prdx6 (Fig. 3B). Interestingly, hyperoxidized Prdx6 was localized predominantly in the nucleus and the intensity was unchanged after the removal of H2O2. These results indicate that hyperoxidized 2-Cys Prdxs are reduced, whereas Prdx6 hyperoxidation is irreversible.
FIGURE 3.
Prdx6 hyperoxidation is irreversible in HeLa cells. A, HeLa cells were treated with different concentrations of H2O2 as indicated for 20 min, washed with HBSS, and incubated for various amounts of time. Cell lysates were subjected to immunoblotting with anti-Prdxs SO3H that recognizes Prdx1-, Prdx2-, and Prdx3-SO3H or with anti-Prdx6 SO3H, anti-Prdx1, anit-Prdx2, anti-Prdx3, anti-Prdx6, or anti-GAPDH antibodies. B, HeLa cells were plated on glass coverslips in 100-mm tissue culture dishes and were allowed to grow to 60% confluence. Cells were treated with 500 μm H2O2 for 20 min, washed with HBSS, and incubated for various amounts of time. Cells were stained with anti-Prdxs SO3H, anti-Prdx1, anit-Prdx2, anti-Prdx3, anti-Prdx6 SO3H, or anti-Prdx6 antibodies as described under “Experimental Procedures” and examined by confocal microscopy.
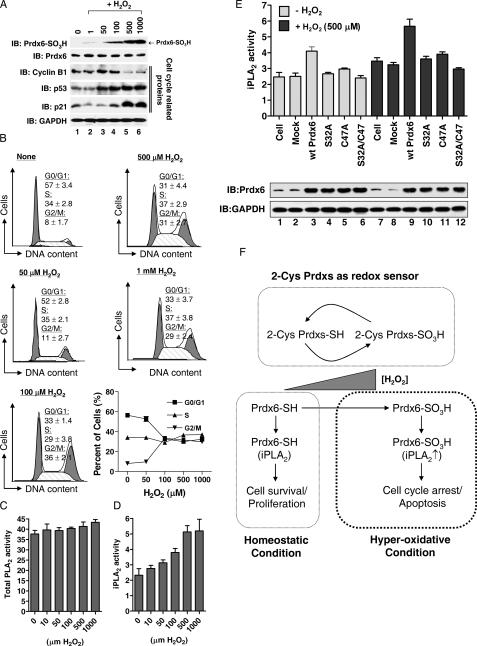
Prdx6 Hyperoxidation Correlates with H2O2-induced Cellular Toxicity—Recently, it has been proposed that hyperoxidized Prdx2 may be directly recognized as a signal of perturbations in oxidant metabolism and, thereby, may contribute to stress responses that mediate oxidant-induced cell cycle arrest (18). In both HeLa and HEK293 cells, we found that the H2O2-induced cell death was initiated at >100 μm H2O2 (supplemental Table S1), ranges that induce Prdx6 hyperoxidation (Fig. 1, A and B), suggesting that Prdx6 hyperoxidation is correlated to H2O2-induced cytotoxicity. We therefore examined whether hyperoxidized Prdx6 plays a role in cellular toxicity. To generate the different oxidation states of Prdx6, HeLa cells were transiently exposed to different concentrations of H2O2 for 20 min, washed with HBSS, and incubated for 18 h. Consistent with the results presented in Fig. 2C, Prdx6 was rapidly hyperoxidized at high concentrations of H2O2 (>100 μm) and its hyperoxidation was maintained (Fig. 4A, upper blot). Interestingly, cell cycle arrest at the G2/M transition was initiated at 100 μm H2O2 and plateaued above 500 μm H2O2 (Fig. 4B). Thus, the pattern of cell cycle arrest was very similar to the pattern of Prdx6 hyperoxidation (Fig. 4, A and B). To test whether the arrest is associated with change in cell cycle regulatory proteins (19), the protein levels of p53, p21, and cyclin B1 were analyzed. Previous studies had shown that G2/M arrest is paralleled by an accumulation of cyclin B1 (20, 21). In addition, it was previously shown that p53 activation and, thus, induction of Cdk inhibitor p21 are involved in G2/M arrest (21, 22). As shown in Fig. 4A, the levels of p53 and p21 were enhanced markedly, whereas the cyclin B1 levels decreased. Moreover, the arrest was closely related with apoptotic cell death. At 48 h after transient treatment with H2O2, Annexin V-positive cells were markedly appeared at high concentrations of H2O2 (>100 μm) (supplemental Fig. S2). These results strongly suggest that Prdx6 hyperoxidation plays a role in cellular toxicity by regulating the cell cycle.
FIGURE 4.
Prdx6 hyperoxidation plays a role in H2O2-induced cellular toxicity. A, HeLa cells were treated with different concentrations of H2O2 as indicated for 20 min, washed with HBSS, and further incubated for 18 h. Cell lysates were subjected to immunoblotting with anti-Prdx6 SO3H, anti-Prdx6, anti-p53, anti-p21, anti-cyclin B1, or anti-GAPDH antibodies. B, after treatment with different concentrations of H2O2 as described in A, cells were washed with HBSS, further incubated for 18 h, stained with propidium iodide as described under “Experimental Procedures”, analyzed with the FACSCalibur™ system, and then cell cycle distributions were determined with the Modfit LT 3.0 software. The results are expressed the mean ± S.D. for triplicate assays. C and D, HeLa cells were treated with different concentrations of H2O2 for 20 min, scraped into Eppendorf tubes, and centrifuged at 1000 × g for 5 min. Total PLA2 (C) and iPLA2 activities (D) were measured as described under “Experimental Procedures.” The results are expressed as mean ± S.D. for triplicate assays. E, HeLa cells were transiently transfected with wild-type Prdx6, the C47A mutant, the C32A mutant, and the C32A/C47A double mutant of Prdx6. At 36 h after transfection, cells were treated with or without 500 μm H2O2 for 20 min. Cell lysates were subjected to immunoblotting with anti-Prdx6 and anti-GAPDH antibodies, and iPLA2 activity was measured. F, model for the role of Prdx6 hyperoxidation in H2O2-induced cellular toxicity.
Hyperoxidation of Prdx6 Is Functionally Related to Its iPLA2 Activity—We next speculated how Prdx6 hyperoxidation might regulate cellular toxicity. Although Prdx6 hyperoxidation alone can induce toxicity, we focused on Prdx6 iPLA2 activity because several studies have shown that iPLA2 induces cellular toxicity though cell cycle arrest or apoptosis under various conditions (9, 23, 24). We hypothesized that the functional relationship between the peroxidase and iPLA2 activities of Prdx6 may be important for cellular toxicity. We therefore examined whether iPLA2 activity is affected by H2O2. Upon treatment of cells with H2O2, total cellular PLA2 (cPLA2) activity remained unchanged (Fig. 4C), whereas iPLA2 activity gradually increased up to 500 μm H2O2 and was saturated at 1 mm H2O2 (Fig. 4D). To examine whether Prdx6 hyperoxidation is functionally related to its iPLA2 activity, we constructed three mutants, C47A for both the peroxidase activity and the site of hyperoxidation, S32A for iPLA2 activity, and C47A/S32A for both enzymatic activities. After transient transfections, HeLa cells were treated with or without 500 μm H2O2 and the iPLA2 activity was measured. As shown in Fig. 4E, overexpression of wild-type Prdx6 significantly enhanced total iPLA2 activity following treatment with H2O2. iPLA2 activities were also elevated in C47A, S32A, or C47A/S32A double mutants upon H2O2 addition compared with those of without H2O2, indicating intrinsic iPLA2 activities increased by H2O2. The result was similar with Fig. 4D. However, the increased levels of iPLA2 activity were much higher in wild-type Prdx6 than in mutants (Fig. 4E). These results suggest that the hyperoxidation of Prdx6 at C47 by H2O2 is affected on the iPLA2 activity and is correlated with H2O2-induced cellular toxicity.
On the basis of our findings, we propose that Prdx6 serves as a cellular indicator of hyperoxidative stress through the hyperoxidation of Prdx6 C47 and plays a role in cellular toxicity. H2O2-induced apoptosis was initiated above 100 μm H2O2 in both HeLa and HEK293 cells (supplemental Table S1) and was consistent with Prdx6 hyperoxidation (Fig. 1, A and B). Moreover, under moderately increased levels of H2O2 (>100 μm), TNF-α, and LPS (supplemental Fig. S3), Prdx6 was not hyperoxidized, indicating that Prdx6 hyperoxidation is stringently regulated in cells. On the one hand, as depicted in Fig. 4F, 2-Cys Prdxs play key roles in eliminating increased H2O2. Although 2-Cys Prdxs are susceptible to H2O2 and become hyperoxidized, their hyperoxidized forms rapidly reduce into active forms capable of eliminating H2O2. On the other hand, homeostatic Prdx6, having bifunctional activity, iPLA2 and peroxidase, which may play positive roles in cell survival and the protection against H2O2-induced stress, respectively. Under greater than normal H2O2 concentrations, however, Prdx6 is rapidly hyperoxidized, simultaneously increasing its iPLA2 activity and inducing cell cycle arrest, and thereby being involved in H2O2-induced cellular toxicity.
In summary, our findings provide the first demonstration, to our knowledge, that Prdx6 functions both as an antioxidant enzyme and as an inducer of H2O2-induced cellular toxicity. These opposite roles are dramatically dependent on the concentration of H2O2, and may be critical to determine the fate of cells against H2O2-induced oxidative stress.
Supplementary Material
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A080250). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked n“advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S3 and Table S1.
Footnotes
The abbreviations used are: Prdx, peroxiredoxin; iPLA2, Ca2+-independent phospholipase A2; GPx, glutathione peroxidases; cPLA2, cellular PLA2; PBS, phosphate-buffered saline; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
References
- 1.Rhee, S. G., Kang, S. W., Chang, T. S., Jeong, W., and Kim, K. (2001) IUBMB Life 52 35-41 [DOI] [PubMed] [Google Scholar]
- 2.Woo, H. A., Chae, H. Z., Hwang, S. C., Yang, K. S., Kang, S. W., Kim, K., and Rhee, S. G. (2003) Science 300 653-656 [DOI] [PubMed] [Google Scholar]
- 3.Rhee, S. G., Chae, H. Z., and Kim, K. (2005) Free. Radic. Biol. Med. 38 1543-1552 [DOI] [PubMed] [Google Scholar]
- 4.Wood, Z. A., Poole, L. B., and Karplus, P. A. (2003) Science 300 650-653 [DOI] [PubMed] [Google Scholar]
- 5.Chen, J. W., Dodia, C., Feinstein, S. I., Jain, M. K., and Fisher, A. B. (2000) J. Biol. Chem. 275 28421-28427 [DOI] [PubMed] [Google Scholar]
- 6.Wang, Y., Feinstein, S. I., and Fisher, A. B. (2008) J. Cell. Biochem. 104 1274-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, Y., Manevich, Y., Feinstein, S. I., and Fisher, A. B. (2004) Am. J. Physiol. Lung. Cell. Mol. Physiol. 286 L1188-L1193 [DOI] [PubMed] [Google Scholar]
- 8.Wang, Y., Phelan, S. A., Manevich, Y., Feinstein, S. I., and Fisher, A. B. (2006) Am. J. Respir. Cell Mol. Biol. 34 481-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song, Y., Wilkins, P., Hu, W., Murthy, K. S., Chen, J., Lee, Z., Oyesanya, R., Wu, J., Barbour, S. E., and Fang, X. (2007) Biochem. J. 406 427-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schröder, E., Littlechild, J. A., Lebedev, A. A., Errington, N., Vagin, A. A., and Isupov, M. N. (2000) Structure 8 605-615 [DOI] [PubMed] [Google Scholar]
- 11.Hofmann, B., Hecht, H. J., and Flohé, L. (2002) Biol. Chem. 383 347-364 [DOI] [PubMed] [Google Scholar]
- 12.Alphey, M. S., Bond, C. S., Tetaud, E., Fairlamb, A. H., and Hunter, W. N. (2000) J. Mol. Biol. 300 903-916 [DOI] [PubMed] [Google Scholar]
- 13.Wood, Z. A., Poole, L. B., Hantgan, R. R., and Karplus, P. A. (2002) Biochemistry 41 5493-5504 [DOI] [PubMed] [Google Scholar]
- 14.Woo, H. A., Kang, S. W., Kim, H. K., Yang, K. S., Chae, H. Z., and Rhee, S. G. (2003) J. Biol. Chem. 278 47361-47364 [DOI] [PubMed] [Google Scholar]
- 15.Rabilloud, T., Heller, M., Gasnier, F., Luche, S., Rey, C., Aebersold, R., Benahmed, M., Louisot, P., and Lunardi, J. (2002) J. Biol. Chem. 277 19396-19401 [DOI] [PubMed] [Google Scholar]
- 16.Yang, K. S., Kang, S. W., Woo, H. A., Hwang, S. C., Chae, H. Z., Kim, K., and Rhee, S. G. (2002) J. Biol. Chem. 277 38029-38036 [DOI] [PubMed] [Google Scholar]
- 17.Wagner, E., Luche, S., Penna, L., Chevallet, M., Van Dorsselaer, A., Leize-Wagner, E., and Rabilloud, T. (2002) Biochem. J. 366 777-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phalen, T. J., Weirather, K., Deming, P. B., Anathy, V., Howe, A. K., Van der Vliet, A., Jönsson, T. J., Poole, L. B., and Heintz, N. H. (2006) J. Cell Biol. 175 779-789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saigusa, K., Imoto, I., Tanikawa, C., Aoyagi, M., Ohno, K., Nakamura, Y., and Inazawa, J. (2007) Oncogene 26 1110-1121 [DOI] [PubMed] [Google Scholar]
- 20.Donà, F., Prosperi, E., Savio, M., Coppa, T., Scovassi, A. I., and Mondello, C. (2008) Int. J. Oncol. 33 613-621 [PubMed] [Google Scholar]
- 21.Lee, S. M., Kwon, J. I., Choi, Y. H., Eom, H. S., and Chi, G. Y. (2008) Phytother. Res. 22 752-758 [DOI] [PubMed] [Google Scholar]
- 22.Taylor, W. R., and Stark, G. R. (2001) Oncogene 20 1803-1815 [DOI] [PubMed] [Google Scholar]
- 23.Lauber, K., Bohn, E., Kröber, S. M., Xiao, Y. J., Blumenthal, S. G., Lindemann, R. K., Marini, P., Wiedig, C., Zobywalski, A., Baksh, S., Xu, Y., Autenrieth, I. B., Schulze-Osthoff, K., Belka, C., Stuhler, G., and Wesselborg, S. (2003) Cell 113 717-730 [DOI] [PubMed] [Google Scholar]
- 24.Pérez, R., Balboa, M. A., and Balsinde, J. (2006) J. Immunol. 176 2555-2561 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.