Abstract
The development of polarized hippocampal neurons with a single axon and multiple dendrites depends on the activity of phosphoinositide 3-kinase (PI3K) and the GTPase Rap1B. Here we show that PI3K regulates axon specification and elongation through the GTPase Rheb and its target mammalian target of rapamycin (mTOR). Overexpression of Rheb induces the formation of multiple axons, whereas its suppression by RNA interference blocks axon specification. mTOR is a central regulator of translation that phosphorylates eIF4E-binding proteins like 4E-BP1. Axon formation was suppressed by inhibition of mTOR and expression of mTOR-insensitive 4E-BP1 mutants. Inhibition of PI3K or mTOR reduced the level of Rap1B, which acts downstream of Rheb and mTOR. The ubiquitin E3 ligase Smurf2 mediates the restriction of Rap1B by initiating its degradation. Suppression of Smruf2 by RNA interference is able to compensate the loss of Rheb. These results indicate that the mTOR pathway is required to counteract the Smurf2-initiated degradation of Rap1B during the establishment of neuronal polarity.
The formation of a single axon and multiple dendrites is one of the earliest steps during the differentiation of neurons (1, 2). Primary cultures of dissociated hippocampal neurons are a widely used model system to study the establishment of neuronal polarity and the pathways that direct the specification of axons and dendrites (1-3). During embryonic development, cortical and hippocampal neurons initially form multiple processes after completing their final mitosis before they migrate toward their final position (4-6). Cultured hippocampal neurons extend several processes that are initially equivalent (stage 2 neurons) until one neurite grows faster than the others to become the axon (stage 3) (1-3). The specification of a single neurite as the axon depends on the activity of phosphoinositide 3-kinase (PI3K),3 which acts upstream of the kinase glycogen synthase kinase-3β and the GTPase Rap1B (1, 7-12). Rap1B is necessary and sufficient to specify axonal identity (11). It accumulates in the growth cone of a single neurite before neurons are polarized morphologically. Its restriction to a single neurite is initiated by the ubiquitin E3 ligase Smurf2 and mediated by degradation through the ubiquitin/proteasome system (13). Suppression of Smurf2 results in the persistence of Rap1B in several neurites and the formation of supernumerary axons.
PI3K is one of the major regulators of mRNA translation in response to growth factors, nutrient supply, and other signals (14-17). It stimulates the phosphorylation of the tumor suppressor Tsc2 (also called tuberin) by Akt (18-20) (Fig. 1A). The complex of Tsc1 (also known as hamartin) and Tsc2 acts as a GTPase activating protein for the GTPase Rheb (21-25). Homozygous null mutations of Tsc1 or Tsc2 lead to early embryonic lethality in mice indicating an essential role in development (26). Phosphorylation of Tsc2 by Akt is thought to suppress its Rheb GTPase activating protein activity and increases the level of active Rheb (18, 21-24). Rheb is a direct activator of mTOR (18, 21-23, 27, 28), an evolutionarily conserved protein kinase and central regulator of cell growth and proliferation (14, 16, 26). The rapamycin-sensitive complex of mTOR, Raptor, and mLST8/GβL regulates protein translation (16, 26, 29, 30). The major targets of the PI3K/mTOR pathway are ribosomal S6 protein kinases and 4E-BPs like 4E-BP1 (14, 29, 31). mTOR stimulates translation by directly phosphorylating 4E-BP1. The binding of 4E-BP1 negatively regulates the initiation of cap-dependent translation by sequestering the translation initiation factor eIF4E, which is present in limiting amounts. Sequestration reduces the translation of specific mRNAs that require high levels of eIF4E. Hyperphosphorylation of 4E-BP1 initiated by mTOR stimulates translation by dissociating the 4E-BP1·eIF4E complex (29, 32, 33). PI3K, Akt, Tsc1/2, and Rheb act upstream of mTOR and mediate the activation of mTOR by insulin and other growth factors (14, 31, 34). PI3K/Akt, Tsc1/2, and mTOR have been also implicated in dendritic morphogenesis, axon guidance, synaptic plasticity, and regeneration (35-39).
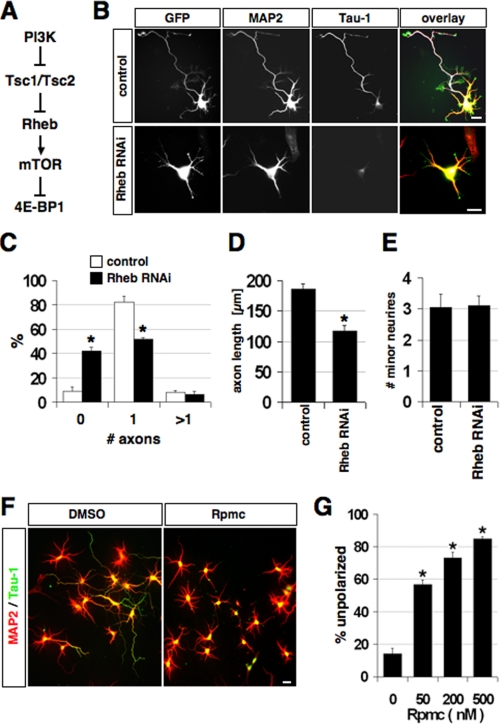
FIGURE 1.
Rheb and mTOR activity are required for neuronal polarity. A, an overview of the PI3K/Rheb/mTOR pathway is shown. PI3K promotes translation by inhibiting the Tsc1·Tsc2 complex that acts as a GTPase activating protein for Rheb (17). B, neurons were transfected on 0 d.i.v. with vectors for EGFP and pSuper (control) or pSb102 (shRNA vector directed against Rheb), and stained on 3 d.i.v. with the Tau-1 (blue) and anti-MAP2 (red) antibodies. C-E, the percentage of neurons without an axon, with a single axon, and with multiple axons (C), the length of axon (D), and the number of minor neurites per neuron (E) is shown (mean ± S.E.; *, p < 0.001 compared with pSuper; n > 100). F, solvent (DMSO) or 200 nm rapamycin (Rpmc) were added to neurons 3-5 h after plating. Neurons were stained at 3 d.i.v. with the Tau-1 (green, axonal marker) and anti-MAP2 antibodies (red, minor neurites). Axons were identified as processes showing Tau-1 immunoreactivity in their distal segments. MAP2-positive neurites longer than one cell diameter were classified as dendrites (mean ± S.E., three independent experiments; *, p < 0.001 compared with control (DMSO); n > 150). G, quantification of polarity defects caused at 3 d.i.v. by rapamycin at different concentrations (n = 200-250 neurons per condition). Neurons, which did not extend any axon, were classified as unpolarized. The scale bar is 20 μm.
Here we investigate the involvement of the PI3K/mTOR pathway in the establishment of neuronal polarity. We show that Rheb and the PI3K/mTOR pathway are required for the specification and elongation of axons and act upstream of Rap1B. Activation of mTOR results in the formation of multiple axons, whereas an inhibition causes the loss of polarity. Our results show that the mTOR pathway, in addition to its well known functions in cell growth, controls neuronal polarity and regulates the expression of Rap1B.
EXPERIMENTAL PROCEDURES
Reagents, Plasmids, and Antibodies—Insulin (Sigma) was dissolved in 0.01 m hydrochloric acid at 5 mg/ml, rapamycin, and LY294002 (Calbiochem) in DMSO (AppliChem). pCGN-Rheb (67), 4E-BP1-myc, Δ24-4E-BP1-myc (40), HA-4E-BP1, and HA-4E-BP1-AA (33) were kindly provided by Drs. Clark (National Institutes of Health, NCI, Rockville, MD), Proud (University of British Columbia, Vancouver), and Sonenberg (McGill University, Montreal), respectively. The coding sequence for mouse Rheb (Mus musculus) was amplified from E12 spinal cord cDNA and cloned into a modified pEGFP-C1 vector (Clontech) (68).
For small interfering RNA constructs, oligonucleotides (Oligoengine, Seattle) containing the 19-nucleotide targeting sequences of Rheb transcript were cloned into the pSuper vector to generate short hairpin RNAs (69): GATCC CCTAC GATCC AACCA TAGAA ATTCA AGAGATTTCT ATGGT TGGAT CGTAT TTTTC and TCGAG AAAAA TACGA TCCAA CCATA GAAAT CTCTT GAATT TCTAT GGTTG GATCG TAGGG for pSb102; GATCC CCTAA GAAGG ACCTG CATAT GTTCA AGAGA CATAT GCAGG TCCTT CTTAT TTTTC and TCGAG AAAAA TAAGA AGGAC CTGCA TATGT CTCTT GAACA TATGC AGGTC CTTCT TAGGG for pSb357. The underlined target region corresponds to positions 102-120 and 357-375, respectively, of the rat Rheb open reading frame. The shRNA vectors directed against Rap1B and Smurf2 have been described before (11, 13).
The Tau-1 and polyclonal anti-MAP2 antibodies were obtained from Chemicon, antibodies specific for 4E-BP1, 4E-BP2, Tsc2 phosphorylated at Thr1462 (phospho-tuberin Thr1462), and phospho-4E-BP1 (Ser65) from Cell Signaling Technology, anti-RhoA, -Rheb, and -Tsc2 (tuberin) from Santa Cruz Biotechnology, monoclonal anti-MAP2, anti-β-tubulin, and rabbit anti-HA from Sigma, and mouse monoclonal anti-Rap1 from BD Transduction Labs. The Alexa 594-, Alexa 488-, and Alexa 350-conjugated secondary antibodies and phalloidin-rhodamine were obtained from Molecular Probes. The specificity of the anti-phospho-4E-BP1 antibody was confirmed by Western blot (supplemental Fig. S5A).
Cell Culture, Pharmacological Treatment, and Transfection of Hippocampal Neurons—Primary cultures of hippocampal neurons from embryonic day 18 rat (Rattus norvegicus) embryos were prepared as described previously (70). Briefly, dissected hippocampi were incubated with papain (Sigma) for 15 min at 37 °C and dissociated by pipetting in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mm glutamine, and 50 units/ml penicillin/streptomycin (Invitrogen). Cells were seeded onto glass coverslips coated with polyornithine (Sigma) and cultured at 37 °C and 5% CO2. Neurons were plated at a higher density (30-50,000 cells per 14-mm coverslip) for transfection and lower density (10-20,000 cells per coverslip) for protein localization and treatment with rapamycin. After neurons attached to the substrate (around 3-5 h after plating) the medium was changed to Neurobasal medium with B27 supplement, 2 mm glutamine, and 50 units/ml penicillin/streptomycin (Invitrogen). Rapamycin, LY294002, and insulin were added directly to neuronal cultures after the medium was changed to Neurobasal medium. Neurons were transfected by calcium phosphate coprecipitation 6-8 h after plating or by electroporation using the Rat Neuron Nucleofector Kit (Amaxa). For electroporation, 2.5 × 106 cells were resuspended in 100 μl of Nucleofectamine solution, transfected according to the manufacturer's recommendations, and plated at 100,000 cells per coverslip. Cells were fixed with 4% paraformaldehyde and 15% sucrose in phosphate-buffered saline for 20 min on ice.
Immunofluorescence and Data Analysis—Neuronal morphology was analyzed using a Zeiss Axioskop 40 microscope (Carl Zeiss MicroImaging, Inc.) equipped with a Plan-Apochromat ×63/1.4 NA oil objective (Carl Zeiss MicroImaging, Inc.) and a Universal Imaging SPOT CCD camera (SPOT Insight 4; Diagnostic Instruments) and the SPOT Advanced Imaging software 4.1 (Diagnostic Instruments), and Adobe Photoshop. The same settings (exposure time, gain, and binning) and the same post-processing operations were always used to analyze the distribution of a specific protein. During post-processing with Adobe Photoshop, the brightest pixel in a picture was set as white, the darkest pixel as black, and intermediate values were distributed proportionally. The stage of neuronal differentiation was determined following published criteria (3). To analyze the establishment of neuronal polarity, neurons were stained with the Tau-1 (as a marker for axons) and anti-MAP2 antibodies (minor neurites) as described previously (11, 70). Cells were embedded in Fluorescent Mounting Medium (DakoCytomation). Processes showing Tau-1 immunoreactivity in their distal segments were counted as axons, MAP2-positive neurites longer than one cell diameter as dendrites. Neurons, which did not extend any axon, were classified as unpolarized and those that extended a single axon as polarized. The length of neurites was determined using the SPOT Advanced Imaging software, fluorescence intensity with Imagetool. The Student's t test was used to determine statistical significance.
Western Blot—Human embryonic kidney 293T cells and neurons were washed in phosphate-buffered saline and lysed in 2× SDS buffer. Lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Amersham Biosciences). Blots were incubated with primary antibodies overnight at 4 °C and with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Peroxidase activity was visualized by the Enhanced Chemiluminescence Detection system (Pierce).
RESULTS
Rheb and mTOR Activity Are Required for Axon Formation—The activity of mTOR is regulated by the small GTPase Rheb (18-23, 27, 28). PI3K activates Rheb by stimulating the phosphorylation of Tsc2 at Thr1462 (p-Tsc2) by Akt (Fig. 1A). Rheb, Tsc2, and p-Tsc2 were detectable in the cell body and all processes in stage 2 neurons (supplemental Fig. S1). At stage 3, Rheb and p-Tsc2 became enriched in the axon. To test if Rheb is required for the specification of axons, we suppressed endogenous Rheb by RNAi in cultures of hippocampal neurons from E18 rat embryos. The effectiveness of the shRNA vector pSb102 directed against Rheb was confirmed by Western blot after expression in human embryonic kidney 293T cells and immunofluorescence using primary neurons (supplemental Fig. S2, A and B). Suppression of Rheb by RNAi significantly reduced the number of polarized neurons. 42 ± 3% (mean ± S.E.) of the neurons did not extend an axon (control (pSuper): 9 ± 4%) and only 52 ± 2% formed a single axon compared with 82 ± 5% in controls (Fig. 1, B and C). The length of those axons that were formed was significantly reduced to 117 ± 13 μm compared with 186 ± 8 μm in controls (Fig. 1D). Suppression of Rheb had no effect on minor neurites (Fig. 1E). The loss of Rheb could be rescued by expression of mouse Rheb that is resistant to suppression by the RNAi vector pSb102 because of differences to the sequence of rat Rheb (supplemental Fig. S2, C-I). These results show that Rheb is required for the specification and elongation of axons.
To investigate if Rheb acts through mTOR, we treated hippocampal neurons shortly after plating (0 days in vitro, d.i.v.) with rapamycin, a specific inhibitor of mTOR. Rapamycin blocked the establishment of neuronal polarity and inhibited the specification of axons in a dose-dependent manner (Fig. 1, F and G). Only a minority of neurons extended an axon in the presence of rapamycin, possibly because it was already specified at the time when rapamycin was added to the culture. However, the length of these axons was significantly reduced compared with controls. In the continuous presence of rapamycin, axon formation was blocked even at 5 d.i.v. (supplemental Fig. S2J). The number and length of minor neurites were not changed significantly (data not shown). Treatment with rapamycin did not increase the number of neurons undergoing apoptosis (data not shown). These results show that mTOR activity is required for axon specification and elongation during the establishment of neuronal polarity.
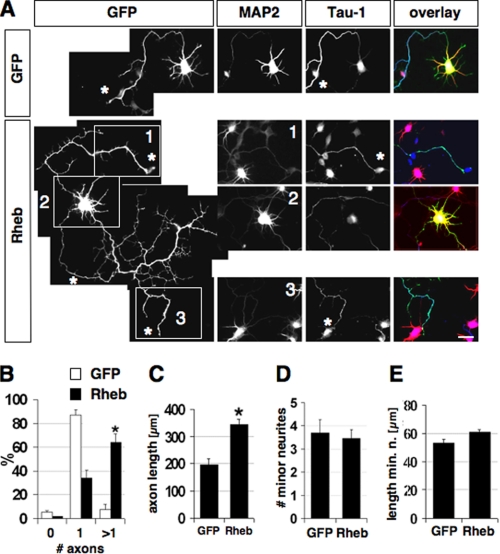
Rheb Acts Downstream of PI3K and Upstream of mTOR to Specify Axons—To test if activation of mTOR has an effect on axon formation, we transfected hippocampal neurons with an expression vector for Rheb. Overexpression of Rheb increased the number of neurons with multiple axons compared with controls (Fig. 2, A and B). In addition, it also enhanced axon extension (Fig. 2C, EGFP, 194 ± 18 μm; Rheb, 344 ± 20 μm). Both the number and length of minor neurites were not changed, showing that the effects of Rheb overexpression are specific for axons and do not result a general stimulation of neurite growth (Fig. 2, D and E).
FIGURE 2.
Rheb expression induces multiple axons. A, neurons were transfected on 0 d.i.v. with plasmids for EGFP or EGFP and Rheb (green) and stained on 3 d.i.v. with the Tau-1 (blue) and anti-MAP2 (red) antibodies. Tau-1-positive axons are marked by asterisks. The cell body (2) and two of the axons (1 and 3) are shown for a representative neuron with multiple axons. B-E, the percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) (B), the length of axons (C), the number of minor neurites (D), and the length of minor neurites (E) after expression of EGFP or Rheb is shown (mean ± S.E.; *, p < 0.001 (B and D) or <0.005 (C) compared with EGFP; n > 90). The scale bar is 20 μm.
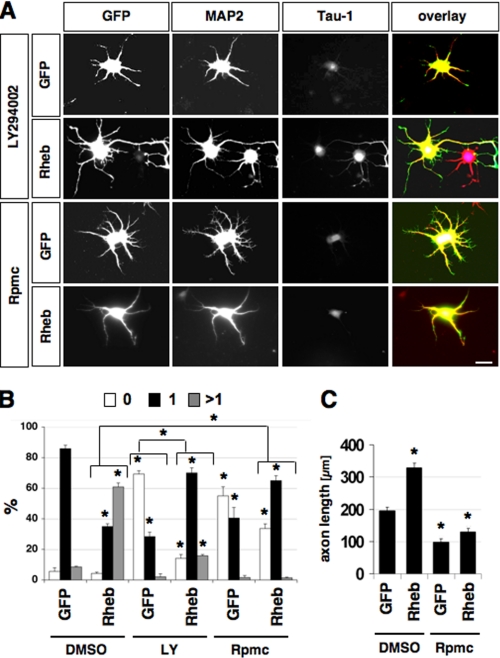
PI3K activity is required for neuronal polarity and the PI3K inhibitor LY294002 blocks axon formation (Fig. 3, A and B) (9, 11). Rheb stimulates cell growth downstream of PI3K and upstream of mTOR (14, 26). To investigate whether the same pathway is active during neuronal polarization, we tested if expression of Rheb can rescue the loss of neuronal polarity observed after treatment with LY294002. In the presence of LY294002, 69 ± 2% of the neurons transfected with a vector for EGFP failed to form an axon (Fig. 3). Expression of Rheb rescued these polarity defects and induced the formation of a single axon in 70 ± 3% of the neurons in the presence of LY294002. The number and length of minor neurites was not affected (data not shown). Rheb induced multiple axons in only 16 ± 1% of the cells when PI3K was inhibited compared with 61 ± 3% in controls (Fig. 3). This effect is similar to that of Rap1BV12 expression in neurons treated with LY294002, which also induces primarily a single axon and requires PI3K to induce supernumerary axons (8, 11, 12).
FIGURE 3.
Rheb acts downstream of PI3K and upstream of mTOR. A, neurons were transfected on 0 d.i.v. with plasmids for EGFP or EGFP and Rheb (green), cultured in the presence of DMSO, 100 μm LY294002 (LY), or 200 nm rapamycin (Rpmc) and stained on 3 d.i.v. with the Tau-1 (blue) and anti-MAP2 (red) antibodies. B and C, the percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) (B) and the length of axons (C) are shown (mean ± S.E.; *, p < 0.001 compared with EGFP or between the values indicated by brackets; n > 70). The scale bar is 20 μm.
To confirm that the effects of Rheb expression are mediated by mTOR, we tested whether they can be blocked by rapamycin. Rapamycin suppressed the induction of multiple axons and the increase in axon length by Rheb (Fig. 3). Although 61 ± 3% of the neurons formed multiple axons after expression of Rheb, only 1 ± 0.4% of the neurons had supernumerary axons when rapamycin was added. The number and length of minor neurites was not affected (data not shown). These results demonstrate that Rheb regulates axon specification and extension downstream of PI3K and upstream of mTOR.
mTOR-insensitive 4E-BP1 Mutants Block Axon Formation and Suppress the Effects of Rheb—One of the targets of mTOR is 4E-BP1 that regulates translation through its interaction with eIF4E (17, 31). Phosphorylation of 4E-BP1 at Thr37 and Thr46 by mTOR serves as a priming event for phosphorylation at other sites such as Ser65. 4E-BP1 hyperphosphorylation results in the stimulation of translation (29, 32, 33). Hippocampal neurons express both 4E-BP1 and 4E-BP2 (supplemental Fig. S3A). At 24 h in culture (early stage 2), 4E-BP1 phosphorylated at Ser65 (p-4E-BP1) was present throughout the cell body and all neurites, but became enriched in the growth cone of a single neurite in the majority of neurons at 36 h (late stage 2) and to the axon tip at stage 3 (supplemental Fig. S3, B-H). The axonal enrichment of p-4E-BP1 in stage 3 neurons was not detectable after inhibition of PI3K or mTOR (supplemental Fig. S4, A and B). An antibody to analyze the distribution of phosphorylated 4E-BP2 by immunofluorescence was not available. Consistent with the function of Rheb as an activator of mTOR, the supernumerary axons induced by Rheb were positive for p-4E-BP1 (data not shown). Thus, 4E-BP1 phosphorylation is stimulated through the PI3K/mTOR pathway during neuronal polarization.
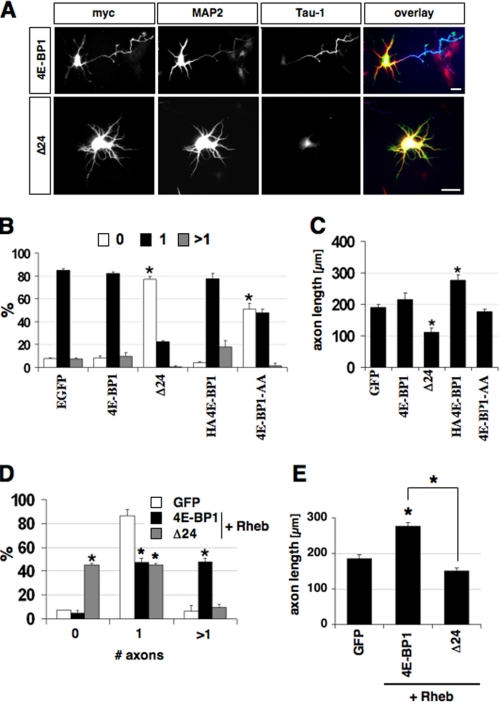
To examine the function of 4E-BP1, two mTOR-insensitive 4E-BP1 mutants were expressed in hippocampal neurons. Δ24-4E-BP1myc lacks the first 24 amino acids and is able to block stimulation of translation by insulin (40). HA4E-BP1-AA contains substitutions of Thr37 and Thr46 by alanine and has been shown to block the effect of constitutive PI3K activity on dendrite complexity (32, 35, 41). Expression of wild-type 4E-BP1myc or HA-4E-BP1 did not change the number of axons or minor neurites per cell but HA-4E-BP1 induced a significant increase in axon length (Fig. 4, A-C, and supplemental Fig. S4, C and D). By contrast, the majority of neurons transfected with a vector for Δ24-4E-BP1myc or HA4E-BP1-AA was unpolarized and did not form an axon. In addition, Δ24-4E-BP1myc reduced axon length (111 ± 15 μm; n = 101). The different magnitude of the effects induced by the 4E-BP1 constructs probably reflects the specific properties of the mutants or differences in their expression levels. None of the constructs had an effect on minor neurites (supplemental Fig. S4D). These results show that the inhibition of the translational regulator 4E-BP1 by mTOR is required for the formation of axons.
FIGURE 4.
Expression of mTOR-insensitive 4E-BP1 mutants blocks axon formation and the effects of Rheb. A, neurons were transfected with vectors for 4E-BP1myc (4E-BP1), Δ24-4E-BP1myc (Δ24), HA4E-BP1, or HA4E-BP1-AA at 0 d.i.v., fixed at 3 d.i.v., and stained with antibodies specific for the myc-tag (green), MAP2 (red), or the Tau-1 antibody (blue). B and C, the percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) (B), and the length of axons (C) are shown (mean ± S.E.; *, p < 0.001 (B) or <0.005 (C) compared with GFP; n > 100). D and E, neurons were transfected with vectors for 4E-BP1myc (4E-BP1) or Δ24-4E-BP1myc (Δ24) on 0 d.i.v., fixed on 3 d.i.v., and stained with antibodies specific for the myc tag, MAP2, or the Tau-1 antibody. The percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) (D), and the length of axons (E) are shown (mean ± S.E.; *, p < 0.001 (B) or <0.005 (C) compared with 4E-BP1; n > 80). The scale bar is 20 μm.
To test whether Rheb regulates neuronal polarity through 4E-BP1, we coexpressed Rheb and 4E-BP1myc or Δ24-4E-BP1myc in hippocampal neurons. Wild-type 4E-BP1myc did not change the effects of Rheb expression. After transfection with vectors for Rheb and 4E-BP1myc, 48 ± 3% of the neurons formed multiple axons compared with 6 ± 5% in controls (Fig. 4, D-E). However, coexpression of Rheb and the mTOR-insensitive 4E-BP1 mutant Δ24-4E-BP1myc blocked the induction of supernumerary axons by Rheb. Only 10 ± 3% of the neurons developed multiple axons and 45 ± 1% of the neurons were unpolarized. Coexpression of Rheb and Δ24-4E-BP1myc also resulted in a reduction of axon length in those neurons that still formed an axon (154 ± 20 μm) in contrast to the stimulation of axon elongation by Rheb (243 ± 11 μm). None of the constructs had an effect on minor neurites (supplemental Fig. S4E). Thus, interfering with the function of the mTOR target 4E-BP1 blocks the effects of Rheb on axon specification and extension.
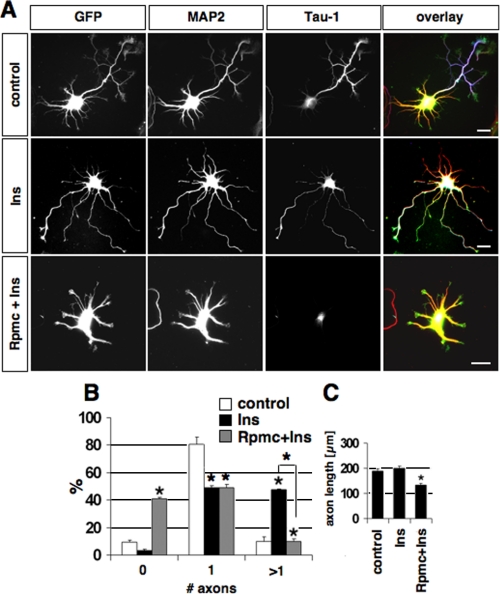
The mTOR Pathway Regulates Rap1B Expression—The ability of 4E-BP1 to block axon formation suggests that the establishment of neuronal polarity depends on the mTOR-mediated regulation of protein synthesis. The expression of proteins whose synthesis is regulated by mTOR should be increased by stimulation of mTOR and suppressed by mTOR or PI3K inhibitors. We first tested whether insulin is able to induce supernumerary axons because it activates the mTOR pathway in many cell types including neurons and its receptor is present in all neurites (18, 21, 42). To stimulate the PI3K/mTOR pathway before neuronal polarity is established, neurons were treated shortly after plating with insulin. Insulin induced the formation of multiple axons in 48 ± 1% (n = 109) of the neurons (compared with 10 ± 4% in control neurons treated with the solvent in 0.01 n HCl) without affecting the number of minor neurites (Fig. 5, and data not shown). The effect of insulin was completely blocked when rapamycin was added 20 min prior to the application of insulin. 41 ± 1% of the neurons did not develop an axon and the remaining neurons developed only short Tau-1 positive neurites under these conditions. Axons induced by insulin were positive for p-4E-BP1 (supplemental Fig. S5A). Thus, activation of the mTOR pathway by insulin induces multiple axons.
FIGURE 5.
Insulin induces the formation of multiple axons in a rapamycin-sensitive manner. A, neurons were transfected with a vector for EGFP to visualize their morphology and treated 4 h after plating with 200 nm insulin (Ins), 200 nm Ins, and 200 nm rapamycin (Rpmc, added 20 min before insulin application), or vehicle and stained on 3 d.i.v. with the Tau-1 (blue) and anti-MAP2 (red) antibodies. B and C, the percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) (B) and the length of axons (C) are shown (mean ± S.E.; *, p < 0.001 compared with control or between the values indicated by brackets; n > 100). The scale bar is 20 μm.
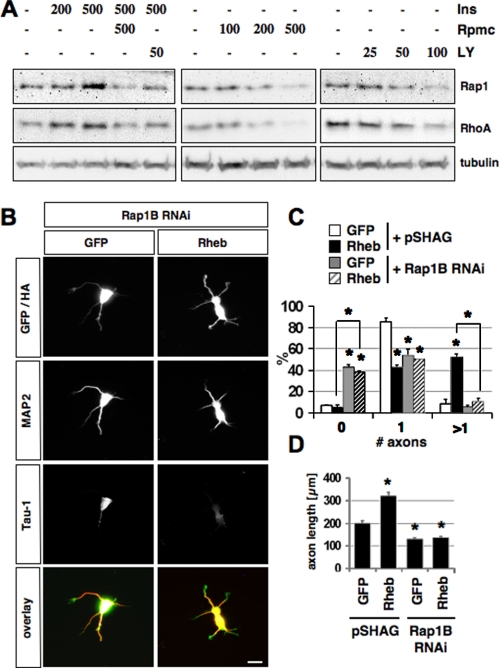
To identify possible targets of mTOR, we investigated if the expression of Rap1B or RhoA is affected by insulin or inhibitors of PI3K and mTOR. Rap1B is a central regulator of neuronal polarity and is necessary and sufficient to specify axonal identity (11). The ability of insulin to induce multiple axons suggested an involvement of Rap1B, which has a similar effect when expressed as a constitutively active mutant. RhoA was used as a control because its translation is regulated by mTOR (43). Polarized hippocampal neurons were incubated with insulin, rapamycin, or LY294002 for 6-10 h and the level of endogenous Rap1B and RhoA was analyzed by Western blot at 2.5 d.i.v. (Fig. 6A). Insulin reproducibly increased the expression of both Rap1B and RhoA in a dose-dependent manner, whereas no effect was observed on tubulin expression. Inhibition of mTOR or PI3K had the opposite effect, reducing the expression of Rap1B and RhoA and blocking the effect of insulin. The same effects were observed when unpolarized neurons were treated for 2 days and the cells lysed at 2.5 d.i.v. (supplemental Fig. S5B). When polarized neurons were treated with rapamycin for 10 h before they were stained on 2.5 d.i.v. with an anti-Rap1 antibody, Rap1B was no longer detectable in the axon (supplemental Fig. S5C). The loss of Rap1B from axons after their specification confirms that the reduction of Rap1B expression by rapamycin and PI3K does not result from a loss of axons. These results show that mTOR activity is continuously required also after the axon is specified. Thus, the PI3K/mTOR pathway regulates the expression of Rap1B in neurons during and after the specification of axons.
FIGURE 6.
The effect of the PI3K/mTOR pathway depends on Rap1B. A, polarized neurons were incubated with the indicated concentrations of insulin (Ins, nm), rapamycin (nm, Rpmc), or LY294002 (μm, LY) for 10 h before the expression of Rap1B and RhoA was analyzed at 2.5 d.i.v. by Western blot. The loading of comparable amounts of protein was confirmed by staining with an anti-tubulin antibody. B, neurons were transfected on 0 d.i.v. with a vector for EGFP or HA-tagged Rheb (green) and the pSHAG RNAi vector (control) or an shRNA vector directed against Rap1B, and stained on 3 d.i.v. with the Tau-1 (blue) and anti-MAP2 (red) antibodies. C and D, the percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) (C) and the length of axons (D) are shown (mean±S.E.; *, p < 0.001 compared with control or between the values indicated by brackets; n > 100). The scale bar is 20 μm.
mTOR and Rheb Act Upstream of Rap1B—The regulation of its expression by the PI3K/mTOR pathway suggests that Rap1B could be one of the mTOR targets. To test this possibility, we investigated if the induction of multiple axons by Rheb requires Rap1B. Neurons were transfected with expression vectors for Rheb and an shRNA to suppress Rap1B by RNAi (11). In controls, Rheb expression induced multiple axons and promoted axon growth (Fig. 6, B-D). This effect was completely blocked after suppression of Rap1B. Only 11 ± 3% of the neurons had multiple axons and 38 ± 1% were unpolarized. Suppression of Rap1B also blocked the increase in axon length by Rheb (Fig. 6D).
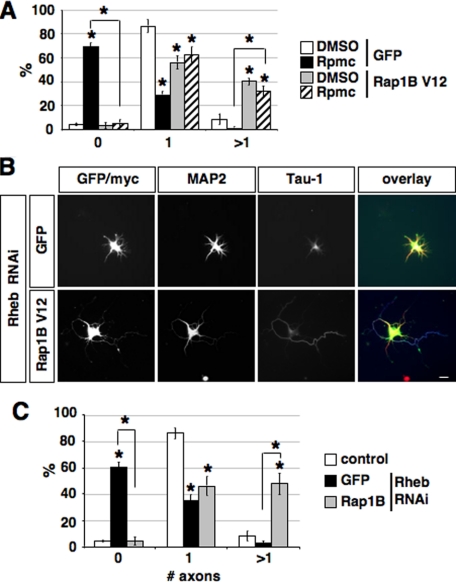
If Rap1B acts downstream of Rheb and mTOR, expression of Rap1BV12 should rescue the loss of axons after administration of rapamycin or suppression of Rheb. Expression of Rap1BV12 was able to restore the ability to form axons after treatment with rapamycin or after suppression of Rheb by RNAi (Fig. 7 and supplemental Fig. S6). Expression of Rap1B did not increase the length of axons compared with controls (supplemental Fig. S6, B and E). The number and length of minor neurites were not affected (data not shown). Treatment with rapamycin reduced the number of neurons with a single axon and increased the percentage of unpolarized cells (Fig. 7A). Expression of Rap1BV12 restored the ability of neurons to form axons in the presence of rapamycin. Suppression of Rheb by RNAi resulted in a loss of axons in the majority of neurons (Fig. 7, B and C). Coexpression of Rap1BV12 together with the shRNA directed against Rheb rescued axon formation and reduced the percentage of unpolarized to that of controls. These data show that Rap1B acts downstream of Rheb and is required for the function of Rheb during the establishment of neuronal polarity.
FIGURE 7.
Rap1B acts downstream of mTOR and Rheb. A, neurons were transfected on 0 d.i.v. with a vector for EGFP or myc-tagged Rap1BV12, treated with solvent (DMSO) or rapamycin (Rpmc) after transfection, and stained on 3 d.i.v. with the Tau-1 and an anti-MAP2 antibody. The percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) (B) is shown (mean ± S.E.; *, p < 0.001 compared with solvent (DMSO) or between the values indicated by brackets; n > 100). B, neurons were transfected on 0 d.i.v. with a vector for EGFP (control) or myc-tagged Rap1BV12 (green) and the pSHAG RNAi vector (control) or an shRNA vector directed against Rheb (Rheb RNAi) as indicated, and stained on 3 d.i.v. with the Tau-1 (blue) and an anti-MAP2 antibody (red). C, the percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) is shown (mean ± S.E.; *, p < 0.001 compared with control or between the values indicated by brackets; n > 100). The scale bar is 20 μm.
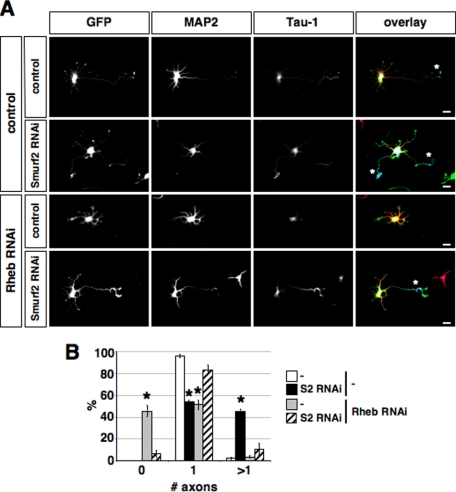
Rheb Is Required to Balance the Smurf2-dependent Loss of Rap1B—We have shown previously that Rap1B is ubiquitinated by the E3 ligase Smurf2 and degraded by the proteasome (13). The degradation of Rap1B is responsible for restricting the GTPase to a single neurite and required for preventing the formation of supernumerary axons. To test if the mTOR pathway antagonizes the destruction of Rap1B through Smurf2-initiated degradation, we investigated if the loss of Rheb can be compensated by suppressing in addition also Smurf2. Suppression of Smurf2 induced the formation of supernumerary axons in 46 ± 1.5% of the cells (Fig. 8) as shown before (13), whereas the loss of Rheb resulted in the development of unpolarized neurons. Suppression of Smurf2 was able to rescue the loss of Rheb. When both Smurf2 and Rheb were suppressed, the majority of neurons extended a single axon. Thus, the block of axon formation after inhibition of the mTOR pathway can be rescued by preventing Rap1B degradation. This result shows that the mTOR pathway has an effect opposite to that of Smurf2-initiated degradation.
FIGURE 8.
Suppression of Smurf2 rescues the loss of Rheb. A and B, neurons were transfected on 0 d.i.v. with a vector for EGFP (green) and shRNAs directed against Rheb (Rheb RNAi, RB), Smurf2 (Smurf2 RNAi, S2) as indicated. As controls, vectors for shRNAs containing mismatches to the target sequence that do not suppress Rheb (Rheb RNAi mut, control in A and B) or Smurf2 (Smurf2 RNAi mut, control in A and in B) were used. Neurons were stained on 3 d.i.v. with the Tau-1 (blue) and an anti-MAP2 antibody (red). Axons are marked by asterisks. B, the percentage of neurons without an axon (0), with a single axon (1), and with multiple axons (>1) is shown (mean ± S.E.; *, p < 0.001 compared with control (Rheb RNAi mut + Smurf2 RNAi mut)).
DISCUSSION
Our results show that Rheb and mTOR pathways are required for the establishment of neuronal polarity and act through Rap1B. Rheb acts downstream of PI3K and upstream of mTOR to promote axon formation and stimulate axon growth. Inhibition of PI3K, Rheb, or mTOR blocked axon specification and elongation, whereas activation of mTOR by Rheb overexpression or treatment with insulin induced the formation of supernumerary axons and increased axon length. We could also show that Rheb and mTOR act upstream of Rap1B. This conclusion is based on the following observations. The amount of Rap1B was increased in neurons by activating the mTOR pathway. Suppression of Rap1B by RNAi blocked the induction of supernumerary axons by Rheb, whereas expression of Rap1BV12 compensated the suppression of Rheb or inhibition of mTOR and induced the formation of axons. Unlike Rheb, expression of Rap1BV12 does not increase the length of axons, indicating that, whereas Rap1B is sufficient to mediate the function of Rheb/mTOR to initiate axon formation, additional mTOR targets are involved in stimulating neurite growth.
Rap1B initially is present at the tip of all neurites but becomes restricted to a single neurite in late stage 2 neurons and to the axon at stage 3 (11). This restriction to one neurite is mediated by proteolytic degradation and, because Rap1B is sufficient to induce axon formation, ensures that neurons form only a single axon. Inactive Rap1B is ubiquitinated and degraded by the proteasome, whereas active Rap1B is protected from degradation (13). The E3 ubiquitin ligase Smurf2 can be detected in all neurites at stage 2 and stage 3 and initiates the degradation of GDP-bound Rap1B (13, 44). Active Rap1B is concentrated in a single neurite at stage 2 and the axon at stage 3. Therefore, Rap1B is retained in a single neurite but proteolytically degraded in the remaining processes. Our data show that Rap1B is a target not only for the ubiquitin/proteasome system but also for mTOR, which acts antagonistically to Smurf2. Because GTPase function involves the cycling between the GTP- and GDP-bound states, Rap1B is not completely protected from degradation even in the axon. Rap1B is susceptible to modification by Smurf2 when it is in the GDP-bound state and the resulting low rate of degradation would lead to a constant loss of Rap1B also when it is activated. Consistent with this idea, treatment of stage 3 neurons with rapamycin leads to the loss of Rap1B from the axon. The loss of Smurf2 increases the amount of Rap1B and induces the extension of multiple axons. Interfering with PI3K or mTOR activity has the opposite effect, reducing the amount of Rap1B and preventing axon formation. However, the loss of Rheb can be compensated by suppression of Smurf2, which restores the ability to form an axon. Thus, the mTOR pathway counteracts Smurf2 activity and the resulting degradation of Rap1B by the proteasome.
There are two distinct complexes containing mTOR in mammals, TORC1 and TORC2 (45, 46). Although TORC1 is a crucial regulator of translation, TORC2 has been implicated in the activation of Akt and the regulation of the cytoskeleton (25). Unlike TORC1, however, TORC2 is not inhibited by rapamycin. Prolonged treatment with rapamycin can reduce the level of TORC2 in some cell lines indirectly by blocking its assembly (47). The rapamycin-sensitive induction of multiple axons by Rheb argues against a major contribution of TORC2 to the function of the PI3K/Rheb/mTOR pathway in the establishment of neuronal polarity, because Rheb specifically activates mammalian TORC1 but has no effect on TORC2 (48). Thus, it is unlikely that the rapamycin-sensitive effects described here result from changes in the cytoskeleton.
TORC2 is the major kinase that phosphorylates the hydrophobic motif of Akt (25). Because Akt has been implicated in axon extension, a loss of TORC2 after prolonged treatment with rapamycin could also block axon formation indirectly by a reduction in Akt activity (9, 49). Active Akt is restricted to the axon and absent from minor neurites at stage 3. Thus, both an indirect effect on Akt activity as a consequence of mTOR inhibition by rapamycin and a defect in axon specification result in the absence of P-Akt in all neurites. However, suppression of Rheb by RNAi results in a loss of axons like the inhibition of mTOR, supporting the conclusion that TORC1 is required for axon extension. This result suggests that TORC2 activity is not compromised or that phosphorylation of Akt by TORC2 is not required under these conditions. Experiments in Drosophila also suggest that TORC2 phosphorylation of Akt is required in vivo mainly to facilitate a broad range of Akt signaling (25, 50).
The conclusion that mTOR promotes axon formation by stimulating translation is also supported by the analysis of the mTOR target 4E-BP1. The phosphorylation of 4E-BP1 in neurites depends on the PI3K/mTOR pathway. Expression of mTOR-insensitive 4E-BP1 mutants caused polarity defects and prevented the induction of supernumerary axons by Rheb. 4E-BP1 regulates the availability of the translation initiation factor eIF4 that is present at low abundance (17, 31). The amount of 4E-BP1 strongly affects the translation of specific mRNAs that are particularly sensitive to the level of eIF4. mTOR initiates hyperphosphorylation of 4E-BP1 and blocks its inhibitory effect on translation by dissociating the eIF4·4E-BP1 complex (29, 32, 33). Inactivation of the genes encoding 4E-BP1 or 4E-BP2 in mice results in metabolic defects and deficits in synaptic plasticity, respectively (51-53). However, no abnormalities in brain morphology have been reported for Eif4ebp1 or Eif4ebp2 mutants. An absence of 4E-BP1 should lead to the formation of supernumerary axons as a consequence of an increase in the amount of Rap1B. The normal development of Eif4ebp1 knock-out mice could reflect a compensatory effect of 4E-BP2. Supernumerary axons may also be formed transiently in the mutant but could be lost during the maturation of the nervous system by pruning (54, 55).
The enrichment of phosphorylated 4E-BP1 in a single neurite of stage 2 neurons indicates the activation of mTOR in the axon. The stimulation of mTOR in the axon could be mediated by the insulin-like growth factor 1 receptor, which is required for neuronal polarity (42, 56, 57). An important function for the PI3K/mTOR pathway and the rapamycin-sensitive local regulation of mRNA translation in axons and dendrites is well established for axon guidance, neuronal plasticity and regeneration, learning, and memory (36, 38, 43, 58-61). A local translation in the growth cone has been demonstrated for β-actin and RhoA (43, 62, 63). Future experiments will show if the mTOR pathway regulates also Rap1B translation locally.
In summary, we show that Rheb and mTOR are required for the establishment of neuronal polarity and act through Rap1B to oppose the activity of Smurf2. PI3K and Akt are essential for the establishment of neuronal polarity (9, 49, 64). One pathway downstream of Akt acts by inhibiting glycogen synthase kinase-3β (8, 12, 65), although recent results suggest that phosphorylation of glycogen synthase kinase-3β by Akt may not be required for neuronal polarity (66). Our results show that, in addition, PI3K acts in parallel through Rheb and mTOR.
Supplementary Material
Acknowledgments
We are grateful to Drs. Clark, Proud, and Sonenberg for plasmids, and Drs. J. Schwamborn, V. Gerke, and C. Klämbt for helpful comments on the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft Grants SPP1111 and SFB 629 (to A. W. P.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S6.
Footnotes
The abbreviations used are: PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; Tsc, tuberous sclerosis complex; TORC, mammalian target of rapamycin complex; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1; Smurf2, Smad ubiquitination regulatory factor-2; shRNA, short hairpin RNA; RNAi, RNA interference; d.i.v., days in vitro; DMSO, dimethyl sulfoxide; HA, hemagglutinin; MAP, mitogen activated protein; EGFP, enhanced green fluorescent protein.
References
- 1.Arimura, N., and Kaibuchi, K. (2007) Nat. Rev. Neurosci. 8 194-205 [DOI] [PubMed] [Google Scholar]
- 2.Da Silva, J. S., and Dotti, C. G. (2002) Nat. Rev. Neurosci. 3 694-704 [DOI] [PubMed] [Google Scholar]
- 3.Dotti, C. G., Sullivan, C. A., and Banker, G. A. (1988) J. Neurosci. 8 1454-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakahira, E., and Yuasa, S. (2005) J. Comp. Neurol. 483 329-340 [DOI] [PubMed] [Google Scholar]
- 5.Noctor, S. C., Martinez-Cerdeno, V., Ivic, L., and Kriegstein, A. R. (2004) Nat. Neurosci. 7 136-144 [DOI] [PubMed] [Google Scholar]
- 6.Tabata, H., and Nakajima, K. (2003) J. Neurosci. 23 9996-10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horiguchi, K., Hanada, T., Fukui, Y., and Chishti, A. H. (2006) J. Cell Biol. 174 425-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang, H., Guo, W., Liang, X., and Rao, Y. (2005) Cell 120 123-135 [DOI] [PubMed] [Google Scholar]
- 9.Shi, S. H., Jan, L. Y., and Jan, Y. N. (2003) Cell 112 63-75 [DOI] [PubMed] [Google Scholar]
- 10.Menager, C., Arimura, N., Fukata, Y., and Kaibuchi, K. (2004) J. Neurochem. 89 109-118 [DOI] [PubMed] [Google Scholar]
- 11.Schwamborn, J. C., and Püschel, A. W. (2004) Nat. Neurosci. 7 923-929 [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura, T., Kawano, Y., Arimura, N., Kawabata, S., Kikuchi, A., and Kaibuchi, K. (2005) Cell 120 137-149 [DOI] [PubMed] [Google Scholar]
- 13.Schwamborn, J. C., Muller, M., Becker, A. H., and Puschel, A. W. (2007) EMBO J. 26 1410-1422 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Hay, N., and Sonenberg, N. (2004) Genes Dev. 18 1926-1945 [DOI] [PubMed] [Google Scholar]
- 15.Inoki, K., Corradetti, M. N., and Guan, K. L. (2005) Nat. Genet. 37 19-24 [DOI] [PubMed] [Google Scholar]
- 16.Martin, D. E., and Hall, M. N. (2005) Curr. Opin. Cell Biol. 17 158-166 [DOI] [PubMed] [Google Scholar]
- 17.Ruggero, D., and Sonenberg, N. (2005) Oncogene 24 7426-7434 [DOI] [PubMed] [Google Scholar]
- 18.Inoki, K., Li, Y., Zhu, T., Wu, J., and Guan, K. L. (2002) Nat. Cell Biol. 4 648-657 [DOI] [PubMed] [Google Scholar]
- 19.Manning, B. D., Tee, A. R., Logsdon, M. N., Blenis, J., and Cantley, L. C. (2002) Mol. Cell 10 151-162 [DOI] [PubMed] [Google Scholar]
- 20.Potter, C. J., Pedraza, L. G., and Xu, T. (2002) Nat. Cell Biol. 4 658-665 [DOI] [PubMed] [Google Scholar]
- 21.Garami, A., Zwartkruis, F. J., Nobukuni, T., Joaquin, M., Roccio, M., Stocker, H., Kozma, S. C., Hafen, E., Bos, J. L., and Thomas, G. (2003) Mol. Cell 11 1457-1466 [DOI] [PubMed] [Google Scholar]
- 22.Inoki, K., Li, Y., Xu, T., and Guan, K. L. (2003) Genes Dev. 17 1829-1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tee, A. R., Manning, B. D., Roux, P. P., Cantley, L. C., and Blenis, J. (2003) Curr. Biol. 13 1259-1268 [DOI] [PubMed] [Google Scholar]
- 24.Zhang, Y., Gao, X., Saucedo, L. J., Ru, B., Edgar, B. A., and Pan, D. (2003) Nat. Cell Biol. 5 578-581 [DOI] [PubMed] [Google Scholar]
- 25.Bhaskar, P. T., and Hay, N. (2007) Dev. Cell 12 487-502 [DOI] [PubMed] [Google Scholar]
- 26.Inoki, K., Ouyang, H., Li, Y., and Guan, K. L. (2005) Microbiol. Mol. Biol. Rev. 69 79-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long, X., Lin, Y., Ortiz-Vega, S., Yonezawa, K., and Avruch, J. (2005) Curr. Biol. 15 702-713 [DOI] [PubMed] [Google Scholar]
- 28.Saucedo, L. J., Gao, X., Chiarelli, D. A., Li, L., Pan, D., and Edgar, B. A. (2003) Nat. Cell Biol. 5 566-571 [DOI] [PubMed] [Google Scholar]
- 29.Gingras, A. C., Raught, B., and Sonenberg, N. (2001) Genes Dev. 15 807-826 [DOI] [PubMed] [Google Scholar]
- 30.Sarbassov, D. D., Ali, S. M., and Sabatini, D. M. (2005) Curr. Opin. Cell Biol. 17 596-603 [DOI] [PubMed] [Google Scholar]
- 31.Richter, J. D., and Sonenberg, N. (2005) Nature 433 477-480 [DOI] [PubMed] [Google Scholar]
- 32.Gingras, A. C., Kennedy, S. G., O'Leary, M. A., Sonenberg, N., and Hay, N. (1998) Genes Dev. 12 502-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gingras, A. C., Raught, B., Gygi, S. P., Niedzwiecka, A., Miron, M., Burley, S. K., Polakiewicz, R. D., Wyslouch-Cieszynska, A., Aebersold, R., and Sonenberg, N. (2001) Genes Dev. 15 2852-2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fingar, D. C., and Blenis, J. (2004) Oncogene 23 3151-3171 [DOI] [PubMed] [Google Scholar]
- 35.Jaworski, J., Spangler, S., Seeburg, D. P., Hoogenraad, C. C., and Sheng, M. (2005) J. Neurosci. 25 11300-11312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelleher, R. J., 3rd, Govindarajan, A., and Tonegawa, S. (2004) Neuron 44 59-73 [DOI] [PubMed] [Google Scholar]
- 37.Kumar, V., Zhang, M. X., Swank, M. W., Kunz, J., and Wu, G. Y. (2005) J. Neurosci. 25 11288-11299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piper, M., and Holt, C. (2004) Annu. Rev. Cell Dev. Biol. 20 505-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavazoie, S. F., Alvarez, V. A., Ridenour, D. A., Kwiatkowski, D. J., and Sabatini, B. L. (2005) Nat. Neurosci. 8 1727-1734 [DOI] [PubMed] [Google Scholar]
- 40.Tee, A. R., and Proud, C. G. (2002) Mol. Cell. Biol. 22 1674-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fingar, D. C., Richardson, C. J., Tee, A. R., Cheatham, L., Tsou, C., and Blenis, J. (2004) Mol. Cell. Biol. 24 200-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sosa, L., Dupraz, S., Laurino, L., Bollati, F., Bisbal, M., Caceres, A., Pfenninger, K. H., and Quiroga, S. (2006) Nat. Neurosci. 9 993-995 [DOI] [PubMed] [Google Scholar]
- 43.Wu, K. Y., Hengst, U., Cox, L. J., Macosko, E. Z., Jeromin, A., Urquhart, E. R., and Jaffrey, S. R. (2005) Nature 436 1020-1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwamborn, J. C., Khazaei, M. R., and Puschel, A. W. (2007) J. Biol. Chem. 282 35259-35268 [DOI] [PubMed] [Google Scholar]
- 45.Jacinto, E., Loewith, R., Schmidt, A., Lin, S., Ruegg, M. A., Hall, A., and Hall, M. N. (2004) Nat. Cell Biol. 6 1122-1128 [DOI] [PubMed] [Google Scholar]
- 46.Sarbassov, D. D., Ali, S. M., Kim, D. H., Guertin, D. A., Latek, R. R., Erdjument-Bromage, H., Tempst, P., and Sabatini, D. M. (2004) Curr. Biol. 14 1296-1302 [DOI] [PubMed] [Google Scholar]
- 47.Sarbassov, D. D., Ali, S. M., Sengupta, S., Sheen, J. H., Hsu, P. P., Bagley, A. F., Markhard, A. L., and Sabatini, D. M. (2006) Mol. Cell 22 159-168 [DOI] [PubMed] [Google Scholar]
- 48.Yang, Q., Inoki, K., Kim, E., and Guan, K. L. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 6811-6816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan, D., Guo, L., and Wang, Y. (2006) J. Cell Biol. 174 415-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hietakangas, V., and Cohen, S. M. (2007) Genes Dev. 21 632-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banko, J. L., Poulin, F., Hou, L., DeMaria, C. T., Sonenberg, N., and Klann, E. (2005) J. Neurosci. 25 9581-9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blackshear, P. J., Stumpo, D. J., Carballo, E., and Lawrence, J. C., Jr. (1997) J. Biol. Chem. 272 31510-31514 [DOI] [PubMed] [Google Scholar]
- 53.Tsukiyama-Kohara, K., Poulin, F., Kohara, M., DeMaria, C. T., Cheng, A., Wu, Z., Gingras, A. C., Katsume, A., Elchebly, M., Spiegelman, B. M., Harper, M. E., Tremblay, M. L., and Sonenberg, N. (2001) Nat. Med. 7 1128-1132 [DOI] [PubMed] [Google Scholar]
- 54.Catalano, S. M., Messersmith, E. K., Goodman, C. S., Shatz, C. J., and Chedotal, A. (1998) Mol. Cell. Neurosci. 11 173-182 [DOI] [PubMed] [Google Scholar]
- 55.White, F. A., and Behar, O. (2000) Dev. Biol. 225 79-86 [DOI] [PubMed] [Google Scholar]
- 56.Avruch, J., Hara, K., Lin, Y., Liu, M., Long, X., Ortiz-Vega, S., and Yonezawa, K. (2006) Oncogene 25 6361-6372 [DOI] [PubMed] [Google Scholar]
- 57.Rommel, C., Bodine, S. C., Clarke, B. A., Rossman, R., Nunez, L., Stitt, T. N., Yancopoulos, G. D., and Glass, D. J. (2001) Nat. Cell Biol. 3 1009-1013 [DOI] [PubMed] [Google Scholar]
- 58.Huang, Y. S., and Richter, J. D. (2004) Curr. Opin. Cell Biol. 16 308-313 [DOI] [PubMed] [Google Scholar]
- 59.Klann, E., and Dever, T. E. (2004) Nat. Rev. Neurosci. 5 931-942 [DOI] [PubMed] [Google Scholar]
- 60.Lyles, V., Zhao, Y., and Martin, K. C. (2006) Neuron 49 349-356 [DOI] [PubMed] [Google Scholar]
- 61.Verma, P., Chierzi, S., Codd, A. M., Campbell, D. S., Meyer, R. L., Holt, C. E., and Fawcett, J. W. (2005) J. Neurosci. 25 331-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leung, K. M., van Horck, F. P., Lin, A. C., Allison, R., Standart, N., and Holt, C. E. (2006) Nat. Neurosci. 9 1247-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao, J., Sasaki, Y., Wen, Z., Bassell, G. J., and Zheng, J. Q. (2006) Nat. Neurosci. 9 1265-1273 [DOI] [PubMed] [Google Scholar]
- 64.Oinuma, I., Katoh, H., and Negishi, M. (2007) J. Biol. Chem. 282 303-318 [DOI] [PubMed] [Google Scholar]
- 65.Kim, W. Y., Zhou, F. Q., Zhou, J., Yokota, Y., Wang, Y. M., Yoshimura, T., Kaibuchi, K., Woodgett, J. R., Anton, E. S., and Snider, W. D. (2006) Neuron 52 981-996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gartner, A., Huang, X., and Hall, A. (2006) J. Cell Sci. 119 3927-3934 [DOI] [PubMed] [Google Scholar]
- 67.Clark, G. J., Kinch, M. S., Rogers-Graham, K., Sebti, S. M., Hamilton, A. D., and Der, C. J. (1997) J. Biol. Chem. 272 10608-10615 [DOI] [PubMed] [Google Scholar]
- 68.Rohm, B., Rahim, B., Kleiber, B., Hovatta, I., and Püschel, A. W. (2000) FEBS Lett. 486 68-72 [DOI] [PubMed] [Google Scholar]
- 69.Brummelkamp, T. R., Bernards, R., and Agami, R. (2002) Science 296 550-553 [DOI] [PubMed] [Google Scholar]
- 70.Schwamborn, J. C., Li, Y., and Püschel, A. W. (2006) Methods Enzymol. 406 715-727 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.