Abstract
Neurotransmitter binding to Cys-loop receptors promotes a prodigious
transmembrane flux of several million ions/s, but to date, structural
determinants of ion flux have been identified flanking the membrane-spanning
region. Using x-ray crystallography, sequence analysis, and single-channel
recording, we identified a novel determinant of ion conductance near the point
of entry of permeant ions. Co-crystallization of acetylcholine-binding protein
with sulfate anions revealed coordination of
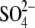 with a ring of lysines at a
position equivalent to 24Å above the lipid membrane in homologous
Cys-loop receptors. Analysis of multiple sequence alignments revealed that
residues equivalent to the ring of lysines are negatively charged in
cation-selective receptors but are positively charged in anion-selective
receptors. Charge reversal of side chains at homologous positions in the
nicotinic receptor from the motor end plate decreases unitary conductance up
to 80%. Selectivity filters stemming from transmembrane α-helices have
similar pore diameters and compositions of amino acids. These findings
establish that when the channel opens under a physiological electrochemical
gradient, permeant ions are initially stabilized within the extracellular
vestibule of Cys-loop receptors, and this stabilization is a major determinant
of ion conductance.
with a ring of lysines at a
position equivalent to 24Å above the lipid membrane in homologous
Cys-loop receptors. Analysis of multiple sequence alignments revealed that
residues equivalent to the ring of lysines are negatively charged in
cation-selective receptors but are positively charged in anion-selective
receptors. Charge reversal of side chains at homologous positions in the
nicotinic receptor from the motor end plate decreases unitary conductance up
to 80%. Selectivity filters stemming from transmembrane α-helices have
similar pore diameters and compositions of amino acids. These findings
establish that when the channel opens under a physiological electrochemical
gradient, permeant ions are initially stabilized within the extracellular
vestibule of Cys-loop receptors, and this stabilization is a major determinant
of ion conductance.
Ion selectivity defines two major classes of Cys-loop receptors. Receptors that selectively translocate cations are excitatory and include vertebrate nAChRs3 and 5-HT3 receptors, whereas receptors that selectively translocate anions are inhibitory and include γ-aminobutyric acid and glycine receptors. A molecular basis for ion selectivity was first proposed based on conserved rings of charged residues and the observation that mutations of these residues in the nAChR influence conductance and selectivity (supplemental Fig. S1) (1). Subsequent studies have focused on reversing selectivity (2, 3) and comparing determinants of ion conductance in nicotinic receptors with those in other Cys-loop receptors (4). These studies described ion selectivity filters in transmembrane-spanning domains using mutagenesis and electrophysiological techniques. More recently, mutations of residues in a channel cytoplasmic region altered conductance in 5-HT3A receptors (5), suggesting that other domains form vestibules leading into the channel that may influence ion conductance and selectivity. In Cys-loop receptors, a large N-terminal domain encloses a vestibule that extends from the constricted ion pore extracellularly by 60 Å. Structural and computational studies have suggested that regions within the N-terminal domain contribute to ion conductance and selectivity (6, 7), but direct experimental evidence is lacking.
Also lacking is a chemical description of ion selectivity and conductance in Cys-loop receptors at the atomic level. Cryo-electron microscopy applied to the nAChR from Torpedo provided structural information at a resolution of 4 Å (6, 8), but single ions and most amino acid side chains could not be resolved. Currently, our understanding of ion translocation through channels comes from studies on voltage-gated ion channels (9–11), where non-hydrated ions are coordinated in a pore lined with partial charges of carbonyl groups of the protein backbone, and single ions pass processionally in a linear chain through the channel. Functional studies suggest a fundamentally different mechanism of ion translocation in Cys-loop receptors. First, hydrophobic α-helices line the pore, and ions remain hydrated as they pass. Second, ions are coordinated by fully charged amino acid side chains in multiple locations along the ion translocation pathway. Third, the diameter of the channel pore is larger in the Cys-loop family of receptors. Herein, we describe a novel ion selectivity filter stemming from the β-sheets of the extracellular ligand-binding domain of nAChRs and provide a high resolution atomic structure of ion coordination in the water-soluble AChBP. Using the low resolution structure of the nAChR transmembrane domain (8), we show spatial and charge similarities between the β-sheet filter and α-helical filters of the transmembrane domain.
EXPERIMENTAL PROCEDURES
A gene chemically synthesized from oligonucleotides encoding the soluble Ac_AChBP was expressed in HEK293S cells lacking the N-acetylglucosaminyltransferase I gene (GnTI– cells) (12). Ac_AChBP was purified from the media as described previously (13, 14).
Sulfate complexes were formed in 1.26 m
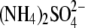 and 0.1 m cacodylate (pH 6.5) with 10–15 mg/ml protein at
room temperature. Crystallization was achieved by vapor diffusion at 18 °C
using a protein-to-well ratio of 1:1 in 0.2-μl sitting drops using a
Douglas Oryx8 robot. 20% glycerol was added to the drop, and the crystals were
flash-cooled in liquid nitrogen. Data were processed with HKL2000
(15), and all further
computing was carried out with the CCP4 Program Suite
(16).
and 0.1 m cacodylate (pH 6.5) with 10–15 mg/ml protein at
room temperature. Crystallization was achieved by vapor diffusion at 18 °C
using a protein-to-well ratio of 1:1 in 0.2-μl sitting drops using a
Douglas Oryx8 robot. 20% glycerol was added to the drop, and the crystals were
flash-cooled in liquid nitrogen. Data were processed with HKL2000
(15), and all further
computing was carried out with the CCP4 Program Suite
(16).
A solution was obtained by molecular replacement with AMoRe (17) using the structure of apo-Ac_AChBP (Protein Data Bank code 2BYN) (13) as a search model. The initial electron density maps were improved considerably by manual adjustment with the graphics program Xtalview Version 4.1 (18). All structures were refined with REFMAC (19) using the maximum likelihood approach and incorporating bulk solvent corrections, anisotropic Fo versus Fc scaling, and TLS refinement with each subunit defining a TLS group.
Electrophysiological studies were performed in BOSC cells (20) using the cell-attached patch-clamp method essentially as described previously (21). For electrophysiological studies, BOSC cells (20), a variant of the HEK293 cell line, were transfected with human wild-type or mutant nAChR subunit cDNAs using calcium phosphate precipitation. A plasmid encoding green fluorescent protein was included in all transfections to allow identification of transfected cells under fluorescence optics. Cells were used for single-channel current measurements 1 or 2 days after transfection. Mutant cDNAs were constructed using the QuikChange site-directed mutagenesis kit (Stratagene) and were confirmed by sequencing the entire coding region. Coexpression of four or five nAChR subunits with Lys substituted for Asp97 greatly reduced the number of nAChRs on the cell surface, as indicated by decreased binding of 125I-α-bungarotoxin and low frequency of acetylcholine-elicited single-channel openings detected by patch clamp. Thus, for receptors with four or five Lys substitutions, we incorporated a Leu-to-Ser mutation at position 9′ of transmembrane domain M2 in the ε-subunit (εL9′S) and found that it enhanced the frequency of channel opening but did not alter the unitary conductance of receptors without D97K mutations.
Single-channel recordings were obtained in the cell-attached patch configuration at 22 °C. The bath and pipette solutions contained 142 mm KCl, 5.4 mm NaCl, 1.8 mm CaCl2, 1.7 mm MgCl2, and 10 mm HEPES (pH 7.4). Acetylcholine (Sigma) was kept as a 100 mm stock solution at –80 °C and added to the pipette solution before recording. Patch pipettes were pulled from 7052 capillary tubes (Garner Glass) and coated with Sylgard (Dow Corning). Single-channel currents were recorded using an Axopatch 200B patch-clamp amplifier (Molecular Devices) and digitized at 2-μs intervals with the PCI-6111E fast data acquisition board (National Instruments) using Acquire software (Bruxton Corp.). Single-channel currents were detected using TAC software (Bruxton Corp.) at a final bandwidth of 10 kHz. Single-channel current amplitudes were determined by fitting a Gaussian function to all-point histograms generated from the digitized current traces. In most cases, two Gaussian functions were needed to describe the all-point histogram from each recording; one Gaussian function corresponded to the closed current level, and the other corresponded to the open current level. The difference between the mean values of the two distributions yielded the single-channel current amplitude.
RESULTS AND DISCUSSION
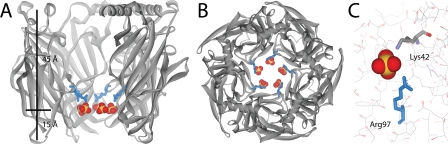
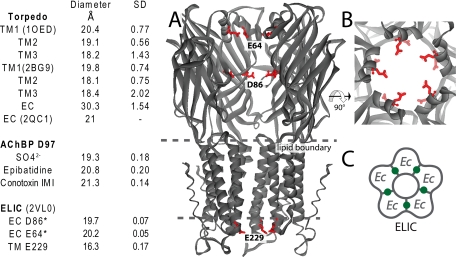
The soluble AChBP from mollusks is an established structural and functional surrogate of the N-terminal ligand-binding domain of Cys-loop receptors amenable to high resolution crystallographic studies (22, 23). We co-crystallized Ac_AChBP in the presence of the anions sulfate and cacodylate. Crystals diffracted to 3.1-Å resolution, and the data were refined to an R/Rfree of 21/25 (supplemental Table S1). The asymmetric subunit contains two pentamers each enclosing a symmetric ring of sulfate ions 13 Å in diameter and orthogonal to the 5-fold symmetry axis located in the vestibule. Arg97 and Lys42 occupy a single conformation in each subunit and coordinate one of five total sulfate ions per pentamer (Fig. 1, A–C). When viewed perpendicular to the central vestibule, the ring of sulfates is located ∼15 Å apical to what would be the outer membrane interface in a full-length receptor (Fig. 1A).
FIGURE 1.
X-ray structure of the ion selectivity filter in Ac_AChBP. A and B, sulfate bound to Ac_AChBP. A shows a side view with one subunit removed. Arg97 is shown in blue; a ring of five sulfates is located in a plane 15 Å above the membrane region (sulfur, orange; and oxygen, red). B shows a view down the 5-fold axis. C, sulfate coordinated between Arg97 and Lys42.
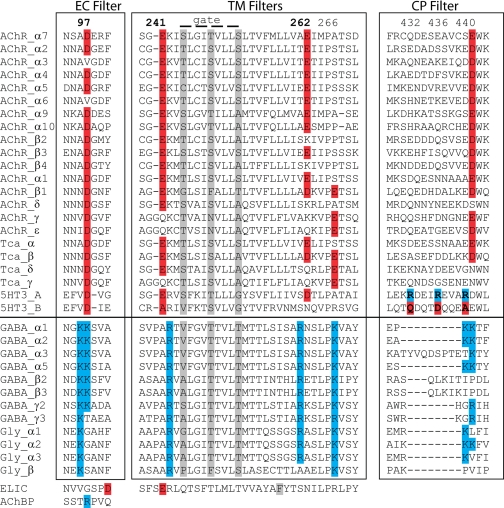
Sequence alignment of human Cys-loop receptors shows that residues at a position equivalent to Arg97 in Ac_AChBP are conserved as Asp in cation-selective receptors, whereas they are conserved as Lys or adjacent Lys residues in anion-selective receptors (Fig. 2); Lys42 is not conserved in the family. In the Torpedo nAChR, Asp97 extends from a loop that forms the narrowest region of the central vestibule of the N-terminal ligand-binding domain. We reasoned that residue 97 may be positioned to filter ions analogous to the selectivity filters that flank the α-helical transmembrane domain or within the cytoplasmic domain.
FIGURE 2.
Sequence alignment of ion selectivity filters: extracellular (EC), transmembrane (TM), and cytoplasmic (CP). Basic residues presumably involved in anion selectivity are shaded blue, and acidic residues involved with cation selectivity are shaded red. Residues implicated in channel gating are shaded gray. Sequences are human except Torpedo californica (Tca), Erwinia chrysanthemi (for ELIC), and A. californica (for AChBP). GABA, γ-aminobutyric acid.
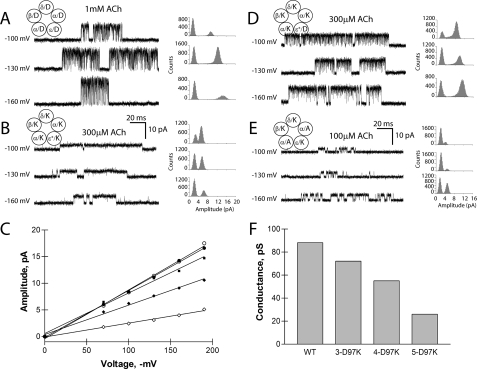
To determine whether the ring of charged residues contributes to ion translocation, we examined Asp97 of the α-subunit and residues at equivalent positions of the β-, δ-, and ε-subunits in the nicotinic receptor from the motor end plate. We reversed the charges of residues in all five subunits, coexpressed the subunits to form heteropentameric receptors, and recorded single-channel currents elicited by acetylcholine (Fig. 3). Compared with the wild-type receptor, the mutant receptor exhibited decreased unitary current amplitude at each test potential (Fig. 3, A and B); a plot of unitary current against transmembrane potential reveals a straight line, the slope of which yields the unitary conductance and shows a decrease of 70% compared with the wild-type nAChR (Fig. 3F). Channel openings of receptors containing five Lys substitutions also appear prolonged; this is likely a consequence of the mutation in the second transmembrane domain needed to enhance expression (see “Experimental Procedures”), which also increases mean channel open time (24). The conductance decrease depends nonlinearly on the number of charge-reversal mutations in the pentamer, showing no change following reversal of the two α-subunits and only a slight decrease with reversal of three subunits (Fig. 3, D and E). However, the net charge of the ring is less important than the side chain substitutions and the locations of the mutant subunits. Introducing four Lys residues and maintaining one Asp residue (net charge of +3) decreased unitary conductance by 55%, whereas introducing three Lys and two Ala residues (net charge of +3) decreased unitary conductance by 80%. The observation that the ring of charge aligned at α-Asp97 affects unitary conductance agrees with predictions from all atom molecular dynamics simulations, which showed that cations pause for extended periods at this location in the course of passing through the channel (7). Thus positioned close to the point where permeant ions enter the channel, this vestibular ring of charge acts to concentrate and select cations for translocation.
FIGURE 3.
Electrostatic contribution of Asp97 in the muscle acetylcholine receptor. A, B, D, and E, single-channel currents are shown at a bandwidth of 10 kHz for the indicated wild-type and mutant receptors. Channel openings are upward deflections. All-point histograms of current amplitude are shown for each test membrane potential and fitted by the sum of two Gaussian functions. C, shown is the current-voltage relationship for receptors with increasing numbers of Lys mutations per pentamer. •, wild-type (WT); ○, εL9′S; ♦, αβδD97K + εL9′S; ⋄, αβδεD97K + εL9′S; ▪, αδD97K. F, shown is a graph of single-channel conductance derived from the slope of the current-voltage relationship in C. In E, for the receptor with three Lys and two Ala substitutions, the current-voltage relationship yields a single-channel conductance of 18 picosiemens (pS).
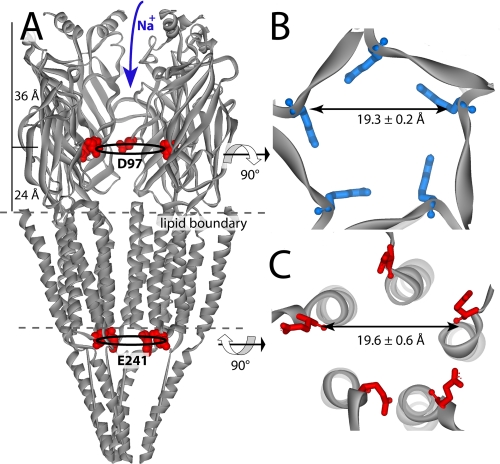
The spacing of the α-carbon atoms that form the selectivity filter in Ac_AChBP is 19.3 Å, whereas the equivalent α-carbon atoms in the Torpedo nAChR at 4-Å resolution (6) show an average spacing of 30.3 ± 1.5 Å, a distance that appears inconsistent with a selectivity filter. However, we overlaid a recent 1.94-Å crystallographic structure of the extracellular domain of the α1-subunit (25) onto the two α-subunits of the 4-Å Torpedo nAChR pentamer and found a ring diameter of 21 Å. The decrease in spacing is due to a distinct conformation in the β4/5 loop, where the tip, containing Asp97, protrudes toward the central vestibule.
Next, we compared the β-sheet filter in the vestibule of Ac_AChBP with the transmembrane α-helical filters in the 4-Å resolution structure of the Torpedo nAChR (8) transmembrane domain (Fig. 4). The ring diameters for the α-helical filters average 19.6 ± 0.2 Å, very close to that of the β-sheet filter (Fig. 5). At the cytosolic entrance in the Torpedo nAChR, the poreforming α-helices narrow to ∼11 Å. The well conserved selectivity filter containing Glu241 is located in this region; the α-carbons of the cytosolic filter show a filter diameter of 18.4 Å, even though they are situated behind the α-helix on the M1–M2 linker (Fig. 4). Thus, regardless of whether the selectivity filter is formed by α-helices or the tips of a β-sheet loop, the diameter of the filter defined by the corresponding α-carbon atoms is determined to be ∼19 Å (6).
FIGURE 4.
Comparisons of β-sheet and α-helical ion filter dimension. Filter diameters were determined from averaging the distances between α-carbons across the pore. A, side view of the relative filter positions shown on the Torpedo nAChR (with one subunit removed). Additional transmembrane filters (not shown) are located just above the membrane on the extracellular side. B, extracellular β-sheet filter from Ac_AChBP. Arg97 is shown (in blue) looking down the 5-fold axis. C, Torpedo cytosolic transmembrane filter (6) α-Glu241 (Glu–1 transmembrane position) shown in red and viewed from the cytoplasmic side of the 5-fold axis.
FIGURE 5.
The transmembrane (TM) and extracellular (EC) filters are compared in the table on the left. Protein Data Bank codes are in parentheses. Distances are α-carbon distances across the ion permeation pathway. S.D. values were based on five measurements. The asterisks indicate predicted filters based on location and spacing of charge. The bacterial homolog of nAChR from E. chrysanthemi (ELIC) (Protein Data Bank 2VL0) (29) is shown in A–C. A, side view of ELIC with one subunit removed. Side chains of the canonical Glu229 selectivity filter are shown in red. Potential selectivity filters in the extracellular domain are highlighted at Asp86 and Glu64. B, top view looking down the ion channel of ELIC. C, homopentamer of ELIC with five binding sites depicted in green.
Finally, we compared the 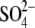 -bound
complex with an Ac_AChBP structure crystallized in the absence of
large anions. The highest resolution structure available is a 1.8-Å
resolution structure of Ac_AChBP complexed with cocaine in the
ligand-binding pocket (26).
The central vestibule is water-filled, and the narrowest region encloses the
extracellular selectivity filter, ∼24 Å from the
“membrane”; two pentameric rings of ordered water stack vertically
near the vestibule wall (supplemental Fig. S1). The vertical position of the
water rings is 9 Å apical to that of the rings that coordinate
-bound
complex with an Ac_AChBP structure crystallized in the absence of
large anions. The highest resolution structure available is a 1.8-Å
resolution structure of Ac_AChBP complexed with cocaine in the
ligand-binding pocket (26).
The central vestibule is water-filled, and the narrowest region encloses the
extracellular selectivity filter, ∼24 Å from the
“membrane”; two pentameric rings of ordered water stack vertically
near the vestibule wall (supplemental Fig. S1). The vertical position of the
water rings is 9 Å apical to that of the rings that coordinate
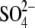 . The lower position of
. The lower position of
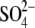 in the vestibule allows it to
occupy a segment with a wider diameter, perhaps reducing electrostatic
repulsion between the internal anions.
in the vestibule allows it to
occupy a segment with a wider diameter, perhaps reducing electrostatic
repulsion between the internal anions.
The extracellular β-sheet filter appears late in prokaryote development and is maintained in all eukaryotes (27). Ac_AChBP contains an Arg at position 97 (Fig. 1) (14), and anion-conducting nAChRs are found for Aplysia (28), suggesting that Ac_AChBP evolved from an anion channel. Recently, the first high resolution structure of a nicotinic receptor homolog called ELIC was solved from a bacterial species (29). Asp86 from ELIC occupies a position near Asp97 in the nAChR, and the diameter defined by α-carbon atoms is 19.1 Å. ELIC may also have an even more apical selectivity filter at Glu64, where an α-helix narrows the extracellular vestibule to a diameter of 20 Å, and the aspartate carboxylates are tilted inward for hydrogen bonding (Fig. 5).
The molecular basis of ion selectivity correlates well with the
charge-selective nature of Cys-loop receptors. Filters are located at
relatively wide regions of the extracellular domain, where a flexible side
chain points into the channel lumen. The channel is selective for charge but
limited in its selectivity for size or valence. Ions are thought to be
hydrated at the level of the filter
(30), and bound
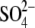 coordinates several waters.
Coincidently, a pentameric ring forms angles of 108°, an angle amenable to
water and ion coordination. Potentially, these geometrical constraints and the
need to translocate hydrated ions may have contributed to the rise of
pentameric ion channels with an elongated vestibule for ion entry.
coordinates several waters.
Coincidently, a pentameric ring forms angles of 108°, an angle amenable to
water and ion coordination. Potentially, these geometrical constraints and the
need to translocate hydrated ions may have contributed to the rise of
pentameric ion channels with an elongated vestibule for ion entry.
Characterization of ion selectivity filters in the N-terminal domain and a structural description of ion translocation are fundamental to understanding how Cys-loop receptors function. We have shown for the first time that pentameric β-sheets form a vestibular structure capable of filtering ions as they flow through the open channel. These data demonstrate that the structure of the extracellular domain plays a function role beyond that of ligand binding and its linkage to allosteric gating. The location of the filter is also significant; when nicotinic receptor channels open, sodium ions flow from outside the cell to the cytoplasm and are thus first exposed to the most extracellular selectivity filter. As a region of conserved structure and critical sequence positions, the vestibule could serve as a site for non-competitive modulators.
Supplementary Material
Acknowledgments
We thank Zoran Radic and Ryan Hibbs for helpful discussion and Cory Ralston and the staff at Advanced Light Source beamline 8.2.2 for data collection.
This work was supported by United States Public Health Service Grants R37-GM18360/UO1-DA019372 (to P. T.) and R37-NS031744 (to S. M. S). Beamline 8.2.2 is supported by the Howard Hughes Medical Institute. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and Table S1.
Footnotes
The abbreviations used are: nAChR, nicotinic acetylcholine receptor; AChBP, acetylcholine-binding protein; Ac, Aplysia californica.
References
- 1.Imoto, K., Busch, C., Sakmann, B., Mishina, M., Konno, T., Nakai, J., Bujo, H., Mori, Y., Fukuda, K., and Numa, S. (1988) Nature 335 645–648 [DOI] [PubMed] [Google Scholar]
- 2.Galzi, J. L., Devillers-Thiery, A., Hussy, N., Bertrand, S., Changeux, J. P., and Bertrand, D. (1992) Nature 359 500–505 [DOI] [PubMed] [Google Scholar]
- 3.Corringer, P. J., Bertrand, S., Galzi, J. L., Devillers-Thiery, A., Changeux, J. P., and Bertrand, D. (1999) Neuron 22 831–843 [DOI] [PubMed] [Google Scholar]
- 4.Keramidas, A., Moorhouse, A. J., Schofield, P. R., and Barry, P. H. (2004) Prog. Biophys. Mol. Biol. 86 161–204 [DOI] [PubMed] [Google Scholar]
- 5.Kelley, S. P., Dunlop, J. I., Kirkness, E. F., Lambert, J. J., and Peters, J. A. (2003) Nature 424 321–324 [DOI] [PubMed] [Google Scholar]
- 6.Unwin, N. (2005) J. Mol. Biol. 346 967–989 [DOI] [PubMed] [Google Scholar]
- 7.Wang, H.-L., Cheng, X., Taylor, P., McCammon, J. A., and Sine, S. M. (2008) PLoS Comput. Biol. 4 e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazawa, A., Fujiyoshi, Y., and Unwin, N. (2003) Nature 423 949–955 [DOI] [PubMed] [Google Scholar]
- 9.Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T., and MacKinnon, R. (2002) Nature 415 287–294 [DOI] [PubMed] [Google Scholar]
- 10.Zhou, Y., Morais-Cabral, J. H., Kaufman, A., and MacKinnon, R. (2001) Nature 414 43–48 [DOI] [PubMed] [Google Scholar]
- 11.Doyle, D. A., Morais Cabral, J., Pfuetzner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T., and MacKinnon, R. (1998) Science 280 69–77 [DOI] [PubMed] [Google Scholar]
- 12.Reeves, P. J., Callewaert, N., Contreras, R., and Khorana, H. G. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 13419–13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen, S. B., Sulzenbacher, G., Huxford, T., Marchot, P., Taylor, P., and Bourne, Y. (2005) EMBO J. 24 3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen, S. B., Talley, T. T., Radic, Z., and Taylor, P. (2004) J. Biol. Chem. 279 24197–24202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otwinowski, Z., and Minor, W. (1997) Methods Enzymol. 276 307–326 [DOI] [PubMed] [Google Scholar]
- 16.Collaborative Computational Project Number 4 (1994) Acta Crystallogr. Sect. D Biol. Crystallogr. 50 760–76315299374 [Google Scholar]
- 17.Navaza, J. (1994) Acta Crystallogr. Sect. A 50 157–163 [Google Scholar]
- 18.McRee, D. (1992) J. Mol. Graph. 10 44–46 [Google Scholar]
- 19.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. Sect. D Biol. Crystallogr. 53 240–255 [DOI] [PubMed] [Google Scholar]
- 20.Pear, W. S., Nolan, G. P., Scott, M. L., and Baltimore, D. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, H.-L., Milone, M., Ohno, K., Shen, X. M., Tsujino, A., Batocchi, A. P., Tonali, P., Brengman, J., Engel, A. G., and Sine, S. M. (1999) Nat. Neurosci. 2 226–233 [DOI] [PubMed] [Google Scholar]
- 22.Bouzat, C., Gumilar, F., Spitzmaul, G., Wang, H.-L., Rayes, D., Hansen, S. B., Taylor, P., and Sine, S. M. (2004) Nature 430 896–900 [DOI] [PubMed] [Google Scholar]
- 23.Brejc, K., van Dijk, W. J., Klaassen, R. V., Schuurmans, M., van der Oost, J., Smit, A. B., and Sixma, T. K. (2001) Nature 411 269–276 [DOI] [PubMed] [Google Scholar]
- 24.Labarca, C., Nowak, M. W., Zhang, H., Tang, L., Deshpande, P., and Lester, H. A. (1995) Nature 376 514–516 [DOI] [PubMed] [Google Scholar]
- 25.Dellisanti, C. D., Yao, Y., Stroud, J. C., Wang, Z. Z., and Chen, L. (2007) Nat. Neurosci. 10 953–962 [DOI] [PubMed] [Google Scholar]
- 26.Hansen, S. B., and Taylor, P. (2007) J. Mol. Biol. 369 895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tasneem, A., Iyer, L. M., Jakobsson, E., and Aravind, L. (2005) Genome Biol. 6 R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kehoe, J., and McIntosh, J. M. (1998) J. Neurosci. 18 8198–8213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilf, R. J., and Dutzler, R. (2008) Nature 452 375–379 [DOI] [PubMed] [Google Scholar]
- 30.Lewis, C. A., and Stevens, C. F. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 6110–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.