Abstract
Cathepsin S (CatS) is a lysosomal cysteine protease belonging to the papain superfamily. Because of the relatively broad substrate specificity of this family, a specific substrate for CatS is not yet known. Based on a detailed study of the CatS endopeptidase specificity, using six series of internally quenched fluorescent peptides, we were able to design a specific substrate for CatS. The peptide series was based on the sequence GRWHTVGLRWE-Lys(Dnp)-DArg-NH2, which shows only one single cleavage site between Gly and Leu and where every substrate position between P-3 and P-3′ was substituted with up to 15 different amino acids. The endopeptidase specificity of CatS was mainly determined by the P-2, P-1′, and the P-3′ substrate positions. Based on this result, systematically modified substrates were synthesized. Two of these modified substrates, Mca-GRWPPMGLPWE-Lys(Dnp)-DArg-NH2 and Mca-GRWHPMGAPWE-Lys(Dnp)-DArg-NH2, did not react with the purified cysteine proteases cathepsin B (CatB) and cathepsin L (CatL). Using a specific CatS inhibitor, we could further show that these two peptides were not cleaved by endosomal fractions of antigen presenting cells (APCs), when CatS was inhibited and related cysteine proteases cathepsin B, H, L and X were still active. Although aspartic proteases like cathepsin E and cathepsin D were also present, our substrates were suitable to quantify cathepsin S activity specifically in APCs, including B cells, macrophages, and dendritic cells without the use of any protease inhibitor. We find that CatS activity differs significantly not only between the three types of professional APCs but also between endosomal and lysosomal compartments.
Lysosomal cysteine proteases of the papain family were long believed to be exclusively involved in nonspecific proteolysis within the lysosome. However, the use of specific protease inhibitors and the study of gene knock-out mice suggested that some of them are involved in many other processes, such as cellular homeostasis, autophagy, apoptosis, and antigen presentation (1–3). Antigen presentation by MHC2 class II molecules requires the entry of antigens into the endosomal-lysosomal compartment. These antigens are then processed by proteolytic enzymes, of which the lysosomal cysteine proteases of the papain family constitute an important subset. The generated peptides bind to MHC class II molecules, which are then displayed at the surface of professional APCs including macrophages, dendritic cells (DCs), and B cells. The variety of MHC class II peptide products that can activate CD4+ T cells is on the one hand determined by allelic variation in MHC molecule binding specificity and on the other by the identity and level of activity of processing proteases present in APCs. Although CatS is one of the major proteases involved in antigen processing (4–6), specific determination of its activity in antigen presenting cells is currently complicated because a specific substrate is not yet known. One reason for this is that CatB, CatL, and CatS show relatively similar endopeptidase specificities, and CatB and CatL possess a peptidyl-dipeptidase activity of relatively broad specificity as well (7–9). The development of a specific substrate for CatS should be useful to illuminate the complexity of the class II MHC-restricted pathway of antigen presentation, and knowledge of the protease specificity of enzymes involved in antigen processing, for example that of CatS, could help to create software for antigenic peptide prediction.
The mechanism of antigen presentation is regulated not only by antigen processing but also by the degradation of the invariant chain, a surrogate substrate and trafficking chaperone of MHC class II (10, 11). Experiments using human B cells treated with the CatS-specific inhibitor LHVS and the characterization of CatS knock-out mice have elucidated a clear, nonredundant role for CatS, in the late stages of invariant chain degradation (12–14). Blockade of the progressive cleavage of invariant chain causes an accumulation of invariant chain intermediates that occupy the MHC class II peptide-binding groove and prevent normal loading of antigenic peptides. Decreased surface expression of class II MHC products (15) and a reduced humoral immune response are the consequences (13). Furthermore, cathepsin S-deficient mice showed decreased susceptibility to collagen-induced arthritis (14), and rats with adjuvant-induced arthritis displayed significant decreases in inflammation after oral administration of the cathepsin S inhibitor LHVS (16), not only supporting a specific role for CatS in rheumatoid arthritis but also validating CatS as an appropriate drug target in other autoimmune disorders. CatS has recently emerged as an important proteolytic enzyme in cancer development, and CatS inhibitors have been proposed as anticancer agents (17, 18). Compounds targeting CatS in rheumatoid arthritis, bronchial asthma, and psoriasis are already undergoing clinical evaluation, and the development of new CatS inhibitors is still a growing field (19). A specific substrate for CatS could be a useful tool to test whether newly developed inhibitors are able to inhibit CatS specifically in APCs.
In this study we examine the endopeptidase specificity of CatS at the substrate amino acid positions P-3 to P-3′ in detail, using six series of internally quenched fluorescent peptides. Based on this detailed study we were able to design a specific substrate for CatS. The substrate allows a clear differentiation between CatS activity and the activities of enzymatically similar proteases CatB, CatH, CatL, and CatX in APCs. Furthermore, the substrate is suitable for quantification of CatS activity specifically in all three types of professional APCs without the use of any protease inhibitor. Using the specific substrate, we find that CatS activity differs significantly between different types of APCs. Furthermore we show that CatS activity is mainly located in endosomes of the APCs investigated and only to a minor extent in lysosomes.
EXPERIMENTAL PROCEDURES
Peptides—Chemicals were obtained from commercial suppliers and used without further purification. All of the peptides were synthesized by a solid-phase technique, using the Fmoc strategy on a Syro II synthesizer (MultiSynTech, Witten, Germany). Fmoc amino acids were purchased from Novabiochem (San Diego, CA) or from MultiSynTech. Mca was from Sigma. Rink amide resin was purchased from Pep Chem (Reutlingen, Germany). 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetrametyluronium-tetrafluoroborat and 1-hydroxybenzotriazole were from MultiSynTech. The final de-protected peptides were purified by semi-preparative reverse phase HPLC, using a Reprosil C-8 column (5 μm particle size, 150 × 10 mm) from Dr. Maisch GmbH (Tuebingen, Germany) and a two-solvent system: (A) 0,055% (v/v) trifluoroacetic acid in water and (B) 0.05% (v/v) trifluoroacetic acid in 80% (v/v) acetonitrile in water. Purity and molecular mass of the collected fractions was analyzed by MALDI mass spectrometry, using a MALDI time-of-flight system (Reflex IV, Bruker Daltonics, Bremen, Germany). All of the peptides were dissolved in water and stored as 2 mm stock solutions at –20 °C. The synthetic substrate Z-FR-AMC was purchased from Bachem (Weil am Rhein, Germany).
Enzymes—Human recombinant CatS was kindly provided by K. Schilling and B. Wiederanders (Institute of Biochemistry I, Friedrich-Schiller-University, Jena, Germany). CatS was obtained by autocatalytic processing of the zymogene expressed in Escherichia coli as described previously (20). CatL (rat liver) was provided by E. Weber (Institute of Physiological Chemistry, Martin Luther University Halle-Wittenberg, Halle, Germany). CatS and L were quantified by E64 titration, CatS was stored at a concentration of 13 μm, and CatL was stored at a concentration of 18.7 μm at –80 °C. CatB (human liver) was purchased from Calbiochem (Darmstadt, Germany) and stored as a 5.8 μm stock solution at –80 °C without further characterization.
Enzyme Assays—The specificity of CatS was analyzed in a qualitative and in a quantitative manner. In the qualitative assay, library peptides at a concentration of 20 μm were processed by 1.3 nm CatS in digestion buffer (50 mm sodium acetate buffer, pH 5.5, containing 4 mm dithiothreitol) at 37 °C. After 30 min the reaction was stopped by adding trifluoroacetic acid to an end concentration of 0.5%. The reaction mixtures were analyzed by analytical reverse phase HPLC, using a binary HPLC system (Shimadzu, Tokyo, Japan) with an SPD-10AV UV-visible detector (Shimadzu), an RF-10Axl fluorescence detector (Shimadzu), a Reprosil C-8 column (5-μm particle size, 150 × 2 mm) from Dr. Maisch GmbH (Tuebingen, Germany), and a two solvent system: (A) 0.055% (v/v) trifluoroacetic acid in water and (B) 0.05% (v/v) trifluoroacetic acid in 80% (v/v) acetonitrile in water. Products were detected by their UV absorbance at 220 nm and by fluorescence emission at 360 nm, following extinction at 280 nm. The percentage of digestion was calculated as 1 minus the UV area ratio of the undigested educt peak of the digestion mixture to the educt peak of an undigested negative control. All of the digestions were conducted in triplicate. The identity of cleavage products was verified by mass spectrometry.
In the quantitative assay of the substrate specificity, kinetic constants of eight peptides for each subsite were determined. The initial velocities of the CatS catalyzed reactions were measured at different substrate concentrations in a stopped enzyme assay. The peptides were subjected to CatS cleavage at pH 5.5 as described above, but this time the reaction was stopped by adding trifluoroacetic acid after 5 min. The product mixture was separated by analytical reverse phase HPLC as described above. The product concentration after 5 min was quantified by fluorescence emission of the N-terminal tryptophan at 360 nm, following extinction at 280 nm. The molar product concentration was calculated by multiplying the fluorescent peak area of the N-terminal fragment with a proportional factor, which was calculated for each peptide individually out of the fluorescent peak area after total substrate turnover. The kinetic parameters Km and Vmax were calculated by nonlinear regression based on the Michaelis-Menten function using the EKI3 software version 1.3 (Wiley-VCH, Weinheim, Germany), which uses the regression analysis described in Ref. 21. The value of kcat was calculated as the Vmax/enzyme concentration ratio.
For the specific detection of CatS activity in APCs, 10 μlof subcellular fractions were added to 80 μl of digestion buffer (50 mm sodium acetate buffer, pH 5.5, containing 4 mm dithiothreitol) and preincubated for 1 h at 37°C. When CatS was inhibited, the buffer additionally contained the specific CatS inhibitor LHVS at a final concentration of 10 nm. The inhibitor was freshly prepared as a 1 mm stock solution in Me2SO and diluted in water. The final Me2SO concentration in the reaction mixture was 0.001%. The reaction was started by the addition of 10 μl of Mca-conjugated substrate solution (0.2 mm in water). The progress of fluorescent product formation was recorded over 2 h, using a fluorimeter (SLT Spectra Fluor, Tecan, Crailsheim, Germany) on kinetic mode at 37 °C (λex = 340 nm, λem = 405 nm). All of the experiments were performed in triplicate.
Cell Culture—The Epstein-Barr virus-transformed human B cell line WT100 was cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% (v/v) heat-inactivated fetal calf serum (Invitrogen), penicillin (final concentration, 100 units/ml; Invitrogen), and streptomycin (final concentration, 0.1 mg/ml; Invitrogen) at 37 °C in tissue culture flasks (Nunc, Wiesbaden, Germany).
DCs and macrophages were generated from peripheral blood mononuclear cells. Peripheral blood mononuclear cells were isolated by Ficoll/Paque (PAA Laboratories, Pasching, Austria) density gradient centrifugation of heparinized blood obtained from buffy coats. Isolated peripheral blood mononuclear cells were plated (DCs: 1 × 108 cells/8 ml/flask; macrophages: 8 × 107 cells/10 ml/flask) into 75-cm2 tissue culture flasks (DCs: Cell Star, Greiner Bio-One GmbH, Frickenhausen, Germany; macrophages: BD Primaria T 75, BD Biosciences, San Jose, CA) in RPMI 1640 (Invitrogen) under the same culture conditions as for WT100. After an incubation of 1.5 h for DCs and 2.5 h for macrophages at 37 °C, nonadherent cells were removed, and adherent cells were cultured in complete culture medium. The macrophage medium was supplemented with granulocyte macrophage-colony-stimulating factor (Leukomax; Sandoz, Basel, Switzerland), the DC medium with granulocyte macrophage-colony-stimulating factor and interleukin-4 (R & D Systems, Minneapolis, MN) for 6 days as described previously (22–24). DCs were harvested, whereas macrophages were cultured for one more week in a specialized macrophage medium, consisting of 60% adoptive immunotherapy medium-V, 30% Iscove's modified Dulbecco's medium (Invitrogen), and 10% human AB+ serum. This resulted in a cell population consisting of 60–70% DCs and 90% macrophages, respectively, as determined by flow cytometry (BD FACSCalibur, Heidelberg, Germany). All of the antibodies for immunophenotyping were obtained commercially (Becton Dickinson, San Diego, CA). DCs were quantified by co-expression of HLA-DR and CD1a. The macrophages were identified by co-expression of HLA-DR and the macrophage mannose receptor CD206.
Subcellular Fractionation and Active Site Labeling—Cell fractionation was performed as previously described (25). The cells were harvested, resuspended in 2.2 ml of fractionation buffer, (10 mm Tris buffer, 250 mm sucrose, pH 7.4), and then homogenized at 4 °C using a cell cracker (HGM Lab Equipments, Heidelberg, Germany). Debris was separated by centrifugation at 8000 × g and 4 °C for 10 min. Endolysosomal fractions were separated by ultra-centrifugation at 100,000 × g for 5 min (Beckman TL100 ultracentrifuge, Palo Alto, CA). Finally, the lysosomes were separated from endosomes by hypotonic lysis with double distilled H2O (approximately 5-fold of the pellet volume) and centrifugation at 100,000 × g for 5 min. Lysosomal material was released into the supernatant, and endosomes remained in the pellet. The pellet was resuspended in 50 mm sodium acetate buffer, pH 5.5, containing 1 mm EDTA. Total protein content was determined according to Bradford (26).
For active site labeling of cysteine proteases in subcellular fractions of macrophages, the compound DCG0N was used. DCG0N, a derivative of DCG04 with identical labeling characteristics, is a biotin-labeled active site-directed probe specific for active papain-like proteases (27, 28). Subcellular fractions, in which protease activity was inhibited, were preincubated for 1 h at 37 °C with either E64 at a concentration of 10 μm or LHVS at a concentration of 10 nm. Subcellular fractions without protease inhibition were treated in the same way. After inhibition, the samples were labeled with 10 μm DCG0N in 50 mm sodium acetate buffer, pH 5.5, for 30 min, separated by SDS-PAGE (0.67 μg of total protein/lane) on a 12% separating gel and transferred onto a polyvinylidene difluoride membrane (Amersham Biosciences). The membranes were then blocked overnight at 4 °C, using TBST (0.15 m NaCl, 10 mm Tris, 0.05% (v/v) Tween 20, pH 8.0), containing 10% (v/v) Roti® Block (Roth, Karlsruhe, Germany). The enzyme bands were stained with a streptavidin-peroxidase reagent (Vectastain, Vector Laboratories, Burlingame, CA) and the ECL detection system (Amersham Biosciences, Little Chalfont, UK).
RESULTS
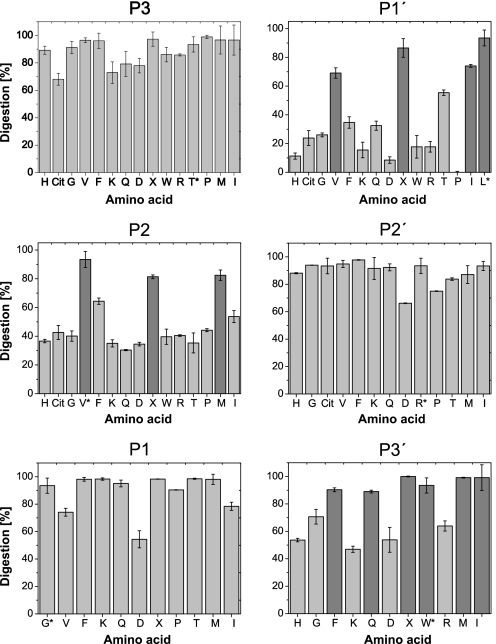
Endopeptidase Specificity of Cathepsin S—Using six series of internally quenched peptides with single amino acid modifications, we analyzed the endopeptidase specificity of CatS at the substrate amino acid positions P-3 to P-3′ in detail. The peptide library was based on the CatS-sensitive substrate GRWHTVGLRWE-Lys(Dnp)-DArg-NH2, which shows only one single cleavage site between Gly and Leu and where the fluorescence of the N-terminal tryptophan is quenched by the Dnp group. Fig. 1 shows the results for all the qualitative analyses of CatS specificity for all substrate positions between P-3 and P-3′. Table 1 shows the kinetic parameters of eight selected peptides per modified substrate position, determined in the quantitative assay. At the P-3 position, all of the 15 tested amino acids were accepted with nearly identical susceptibilities to CatS. Although no special group of amino acids was preferred by CatS at the P-3 position, the negatively charged aspartic acid showed the lowest Vmax and kcat/Km values and was the least well accepted residue in this position. At the P-2 position the hydrophobic branched amino acids valine, norleucine, as well as methionine were clearly preferred by CatS. The substrates with valine and norleucine at the P-2 position showed the highest Vmax values in this series followed by the aromatic phenylalanine. The highest kcat/Vmax values were also obtained by these peptides. Interestingly, the amino acid isoleucine was not favored at the P-2 position. Although all other internally quenched fluorescent peptides with P-2 modifications showed relatively low kcat/Km values (<1 s–1 μm–1), no peptide was completely resistant to the hydrolysis reaction catalyzed by CatS. At the S1 subsite of CatS no clear discrimination was observed. In contrast to the P-2 series, all of the substrates with P-1 variations showed kcat/Km values around 1 s–1 μm–1 or higher. However, peptides with lysine and glutamine at the P-1 position were more susceptible to hydrolysis than the others. They showed the highest Vmax and kcat values not only in the P-1 series, but also among all tested peptides. Substrates with isoleucine and aspartic acid showed the lowest kcat values. Together with valine these were the least well accepted amino acids at this subsite. Among all tested substrates, amino acid permutations at P-1′ affected the hydrolysis reaction most significantly. The substrates containing the hydrophobic branched amino acids valine, leucine, norleucine and isoleucine were hydrolyzed with significantly higher Vmax and kcat/Km values than substrates containing other amino acids at this position. Substrates with charged amino acids at the P-1′ position showed the lowest Vmax values among all of the tested peptides, and the peptide with proline at P-1′ position was the only completely resistant peptide. Together with the qualitative analysis, this indicates that CatS favors hydrophobic branched amino acids at P-1′ position, whereas substrates with charged and aromatic amino acids were hydrolyzed weakly. Because all peptides of the P-2′ series show relatively similar Vmax and Km values, and no peptide was discriminated compared with the basis peptide, the P-2′ position could be occupied by every tested amino acid without affecting hydrolysis. In contrast, the kinetic constants of the P-3′ series peptides differ significantly from each other. Substrates with branched hydrophobic and aromatic amino acids showed the highest Vmax values and the lowest Km values, whereas peptides with charged amino acids were hydrolyzed with the lowest Vmax and highest Km values. Also the glycine peptide was hydrolyzed very weakly, indicating that a side chain at the P-3′ position of the substrate is advantageous. It is noteworthy that the poorly digested peptides of the P-3′ series show relatively high Km values at low or intermediate Vmax values compared with other series. Hydrophobic amino acids at the P-3′ position result in substrates with higher affinity, in contrast to charged amino acids, which result in low affinity substrates. Taken together, these data show that the substrate specificity of CatS was mainly determined by the P-2, P-1′, and P-3′ substrate positions. The amino acid preferences of these positions are summarized in Table 2. The most restrictive position in our assay was the P-1′ substrate position.
FIGURE 1.
Analysis of CatS specificity. Library peptides with single amino acid exchanges and the general sequence GRWHTVGLRWE-Lys(Dnp)-DArg-NH2 were processed at a concentration of 20 μm for 30 min by 1.3 nm CatS. The reaction mixtures were analyzed by HPLC, and UV peak areas were used for quantification of substrate digestion. All of the assays were performed in triplicate. The error bars denote the means ± standard deviation. The percentage of substrate digestion is shown for peptides with P-3, P-2, P-1, P-1′, P-2′, and P-3′ amino acid modifications. The x axis represents the amino acid at the corresponding substrate position, designated by the single-letter code (X, norleucine; Cit, citrulline). The basis peptide of the library experiment is marked with an asterisk. Preferred amino acids at each substrate position are marked dark gray.
TABLE 1.
Kinetic parameters for hydrolysis of six peptide series based on the sequence GRWHTVG ↓ LRWE-Lys(Dnp)-DArg-NH2 by recombinant human cathepsin S for characterization of all subsites from S3 to S3′
The data represent the means ± S.D. of a triplicate experiment. ↓ represents the only cleavage site. X, norleucin; Cit, citrullin.
| Substrate position | Amino acid | Km | Vmax | kcat/Km | Substrate position | Amino acid | Km | Vmax | kcat/Km |
|---|---|---|---|---|---|---|---|---|---|
| μm | μm/min | 1/(s × μm) | μm | μm/min | 1/(s × μm) | ||||
| P-3 | F | 1.42 ± 0.32 | 0.450 ± 0.004 | 4.05 | P-1′ | F | 4.80 ± 0.44 | 0.206 ± 0.004 | 0.55 |
| K | 1.94 ± 0.29 | 0.349 ± 0.006 | 2.30 | K | 5.35 ± 1.09 | 0.071 ± 0.004 | 0.17 | ||
| V | 2.76 ± 0.02 | 0.752 ± 0.011 | 3.49 | V | 2.37 ± 0.23 | 1.005 ± 0.016 | 5.43 | ||
| G | 5.14 ± 0.12 | 0.626 ± 0.005 | 1.56 | Cit | 1.71 ± 0.38 | 0.156 ± 0.003 | 1.17 | ||
| D | 1.88 ± 0.09 | 0.154 ± 0.001 | 1.05 | X | 0.73 ± 0.05 | 0.644 ± 0.027 | 11.24 | ||
| X | 1.60 ± 0.24 | 0.367 ± 0.005 | 2.95 | R | 2.44 ± 0.26 | 0.082 ± 0.001 | 0.43 | ||
| P | 3.24 ± 0.32 | 0.478 ± 0.01 | 1.89 | I | 1.98 ± 0.22 | 0.579 ± 0.011 | 3.75 | ||
| I | 0.48 ± 0.09 | 0.216 ± 0.006 | 5.82 | La | 2.37 ± 0.17 | 0.616 ± 0.009 | 3.34 | ||
| P-2 | F | 2.01 ± 0.09 | 0.262 ± 0.008 | 1.67 | P-2′ | F | 5.27 ± 0.35 | 0.730 ± 0.014 | 1.78 |
| K | 7.82 ± 0.54 | 0.136 ± 0.006 | 0.22 | K | 1.05 ± 0.12 | 0.484 ± 0.019 | 5.91 | ||
| T | 9.30 ± 1.07 | 0.133 ± 0.004 | 0.18 | H | 3.12 ± 0.01 | 0.419 ± 0.002 | 1.72 | ||
| Va | 2.37 ± 0.17 | 0.616 ± 0.009 | 3.34 | Q | 2.59 ± 0.15 | 0.637 ± 0.012 | 3.16 | ||
| G | 12.86 ± 0.27 | 0.242 ± 0.005 | 0.24 | D | 3.14 ± 0.10 | 0.455 ± 0.002 | 1.86 | ||
| D | 12.37 ± 0.35 | 0.143 ± 0.002 | 0.15 | Ra | 2.37 ± 0.17 | 0.616 ± 0.009 | 3.34 | ||
| X | 4.18 ± 0.16 | 0.277 ± 0.003 | 0.85 | P | 1.31 ± 0.10 | 0.296 ± 0.004 | 2.91 | ||
| P | 5.91 ± 0.29 | 0.231 ± 0.004 | 0.50 | I | 1.50 ± 0.30 | 0.406 ± 0.009 | 3.47 | ||
| I | 3.95 ± 0.44 | 0.174 ± 0.003 | 0.57 | ||||||
| M | 7.79 ± 0.49 | 0.536 ± 0.013 | 0.89 | ||||||
| P-1 | F | 2.58 ± 0.12 | 0.774 ± 0.012 | 3.84 | P-3′ | F | 8.20 ± 0.39 | 0.83 ± 0.010 | 1.30 |
| K | 27.47 ± 1.65 | 2.939 ± 0.150 | 1.37 | K | 37.87 ± 3.16 | 0.551 ± 0.031 | 0.19 | ||
| Q | 24.90 ± 0.89 | 3.834 ± 0.230 | 1.97 | H | 14.93 ± 0.78 | 0.179 ± 0.004 | 0.15 | ||
| T | 5.06 ± 0.11 | 1.083 ± 0.020 | 2.74 | G | 16.48 ± 0.39 | 0.299 ± 0.005 | 0.23 | ||
| D | 2.80 ± 0.10 | 0.265 ± 0.009 | 1.22 | Wa | 2.37 ± 0.17 | 0.616 ± 0.009 | 3.34 | ||
| X | 10.47 ± 0.59 | 1.566 ± 0.039 | 1.92 | D | 16.68 ± 1.91 | 0.353 ± 0.030 | 0.27 | ||
| P | 19.98 ± 0.75 | 1.277 ± 0.027 | 0.82 | R | 11.54 ± 0.24 | 0.400 ± 0.006 | 0.44 | ||
| I | 5.12 ± 0.36 | 0.359 ± 0.003 | 0.90 | I | 8.20 ± 0.69 | 0.897 ± 0.004 | 1.40 | ||
| Ga | 2.37 ± 0.17 | 0.616 ± 0.009 | 3.34 |
The basis peptide of the library experiment.
TABLE 2.
Cathepsin S subsite specificities for cleavage sites
Favorable amino acids are marked +, less favorable are marked 0, and unfavorable are marked –. The ranking reflects the results shown in Fig. 1 and Table 1.
| Side chain | P-2 position | P-1 position | P-1′ position | P-3′ position | |
|---|---|---|---|---|---|
| Hydrophobic | Aromatic | + (only Phe) | - | - | + |
| Aliphatic | + (not Ile) | - | + | + | |
| Methionine | + | + | - | + | |
| Proline | - | + | - | Not tested | |
| Neutral | Amide | - | + | - | 0 |
| Small | - | + | 0 | - | |
| Basic | - | + | - | - | |
| Acidic | - | - | - | - |
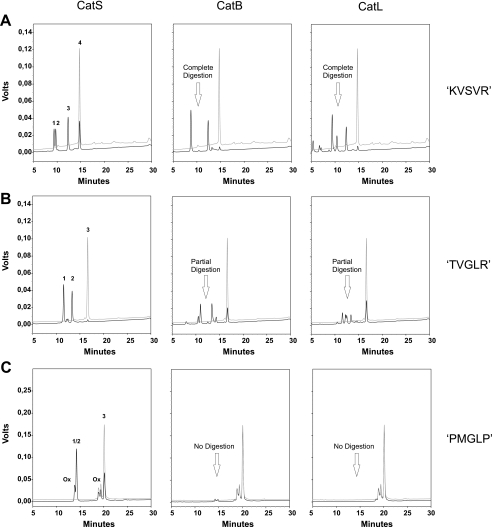
Design of CatS-specific Substrates—Based on the conclusions on preferred CatS cleavage sites shown in Table 2, we synthesized various CatS-sensitive substrates, with multiple amino acid substitutions compared with the original peptide. To identify a peptide that is resistant to digestion by related cysteine proteases, these substrates were additionally subjected to CatB and CatL digestion for 1 h. With regard to a cellular application of a specific substrate, it was important to show that the peptide is not digested even under conditions where CatB and CatL concentrations significantly exceed that of CatS. Therefore we used CatB (11.6 nm) and CatL (13 nm) concentrations that were approximately 10 times higher compared with CatS (1.3 nm) in these assays. Another difficulty in creating a CatS-specific substrate was that CatB as well as CatL show a peptidyl-dipeptidase activity of relatively broad specificity, allowing C-terminal truncations of dipeptides (7, 8). To block this activity, substrates contained a protecting d-Arg residue at the C terminus. Compared with an unspecific substrate Mca-GRWHKVSVRWEK(Dnp)-DArg-NH2 (KVSVR), which was digested equally well by all three enzymes, the peptide Mca-GRWHTVGLRWEK(Dnp)-DArg-NH2 (TVGLR) we used as a basis for the library experiment was not completely digested by CatB and CatL within the given time (Fig. 2). The TVGLR peptide showed only one single CatS cleavage site between the Gly-Leu bond. The same cleavage products were also generated by CatB and CatL digestion, as identified by mass spectrometry of the collected HPLC fractions. In addition to these major cleavage products, CatB and CatL generated two minor cleavage products that were not identical for both enzymes (Fig. 2B). By changing the valine at the P-2 position of the original substrate to a methionine, the cleavage at the major cleavage site was reduced for CatB and L (not shown). However, the modified substrate was still cleaved at the minor cleavage sites. According to the experiments described above (Fig. 1) digestion by CatS was not affected by the valine-methionine exchange at the P-2 position. In these experiments we have also shown that the P-3 P-2′ position could be occupied by every tested amino acid without affecting the hydrolysis catalyzed by CatS. To prevent a CatB- and CatL-catalyzed cleavage at the minor cleavage sites, we chose proline as the new P-4, P-3, and P-2′ residue of the methionine peptide. The P-3 and P-2′ position of the CatS cleavage site are equal to the P-1 positions of the minor cleavage sites, which were generated by CatB and CatL. This modified substrate Mca-GRWPPMGLPWEK(Dnp)DArg-NH2 (PMGLP) was resistant to CatB and CatL digestion within the given time (Fig. 2C). Another peptide substrate Mca-GRWHPMGAPWEK(Dnp)DArg-NH2 (PMGAP) without a third proline at P-4, was also resistant to CatB and CatL digestion (not shown). Oxidation of the methionine residues in either substrate could not be prevented in the reaction mixture. However, when we put the structurally related norleucine instead of methionine at the P-2 position, the substrates were again weakly digested by CatB and CatL. This indicates that the presence of a methionine at the P-2 position is essential for the CatB and CatL resistance of our substrates.
FIGURE 2.
Design of a CatS-specific substrate. HPLC analysis of three representative substrates at a concentration of 20 μm after incubation with 1.3 nm CatS (left column), 11.6 nm CatB (middle column), and 13 nm CatL (right column) for 1 h in digestion buffer at pH 5.5 and 37 °C. Digestion products were detected by their UV absorbance at 220 nm. The black lines represent the chromatograms of the digestion mixtures, whereas gray lines are chromatograms of the undigested negative controls. A, digestion analysis of the unspecific substrate KVSVR. The substrate is completely digested by CatB and CatL and only partially by CatS within the given time. The fragments Mca-GRWHKVS (peak 1) and VRWE-Lys(Dnp)-DArg-NH2 (peak 3) of Mca-GRWHKVSVRWE-Lys(Dnp)-DArg-NH2 (peak 4) are generated by all three enzymes. CatS additionally generates cleavage products Mca-GRWHKVSVR (peak 2) and WE-Lys(Dnp)-DArg-NH2 (peak 3). B, digestion analysis of the basis peptide TVGLR, which was used in the library experiment. Compared with KVSVR the substrate is now completely digested by CatS and only partially by CatB and CatL. Although the substrate Mca-GRWHTVGLRWE-Lys(Dnp)-DArg-NH2 (peak 3) shows only one major CatS cleavage site and the corresponding fragments Mca-GRWHTVG (peak 1) and LRWE-Lys(Dnp)-DArg-NH2 (peak 2) are present in the reaction mixture of all three enzymes, CatB and CatL generate additional cleavage products. C, digestion analysis of the CatS specific substrate PMGLP. This substrate is strongly digested by CatS and on the other hand completely resistant against CatB and CatL digestion within the given time. CatS cleaves the substrate Mca-GRWPPMGLPWE-Lys(Dnp)-DArg-NH2 (peak 3) between G and L. The generated fragments Mca-GRWPPMG (peak 1) and LPWE-Lys(Dnp)-DArg-NH2 (peak 2) elute at the same retention time. During incubation the methionine residue is oxidized leading to oxidation peaks (Ox).
To further characterize one of the methionine substrates, we determined the kinetic constants for the PMGLP digestion for all three proteases. For this, we increased CatB and CatL concentration until the substrate was again weakly digested. We used CatB (29 nm) and CatL (42.5 nm) concentrations that were ∼30 times higher compared with CatS and measured initial velocities at 11 different substrate concentrations between 1 and 20 μm in a continuous fluorescence assay. Km and Vmax values were determined by nonlinear regression of the Michaelis-Menten curve (Table 3). CatB and CatL showed very low kcat and kcat/Km values, indicating that PMGLP is nearly not digested by both enzymes even under conditions of relatively high CatB and CatL concentrations.
TABLE 3.
Kinetic parameters of PMGLP digestion by cathepsins B, L, and S
The data represent the means ± S.D. of a triplicate experiment.
| Enzyme | Km | kcat | kcat/Km |
|---|---|---|---|
| μm | 1/s | 1/(s × μm) | |
| CatB | 9.94 ± 0.67 | 0.044 ± 0.002 | 0.004 |
| CatL | 3.49 ± 0.79 | 0.112 ± 0.008 | 0.032 |
| CatS | 5.56 ± 1.33 | 5.666 ± 0.478 | 1.018 |
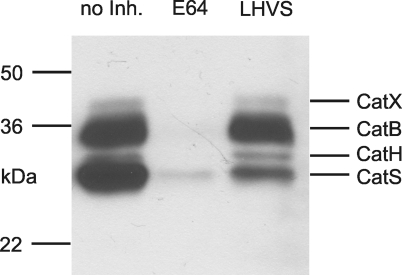
Characterization of Endosomal Fractions of Antigen Presenting Cells—During the process of antigen uptake, endocytic vesicles of antigen presenting cells become enriched with many more proteases than only CatS, CatB, and CatL. Other cysteine proteases like CatH and CatX together with aspartic proteases like CatD and CatE as well as the asparagine-specific endopeptidase are also present (29, 30). To show that even under naturally occurring conditions the two methionine peptides PMGLP and PMGAP are specifically cleaved by CatS, it was important to use endosomal fractions for additional digestion experiments. Therefore, endosomal fractions of dendritic cells, macrophages, and the human B cell line WT100 were isolated, and existing cysteine proteases were characterized by active site labeling as described above. Using this method, the active forms of the enzymes are tagged and visualized (20). Earlier experiments in which we identified polypeptides in the 20–40-kDa range via immunoprecipitation with antibodies directed against individual cathepsins as well as by mass spectrometry-based sequencing after pulldown assay with streptavidin beads (31) allowed us to identify CatB, CatH, CatL, CatS, and CatX. Fig. 3 shows the active site label of the endosomal fraction of macrophages. The endosomal fractions of DCs and the WT100 B cell line showed similar patterns of cysteine proteases (not shown). Earlier experiments had shown that CatL was only visible when the concentration of the labeling compound DCG0N was increased from 10 to 128 μm (21). Preincubation with the cysteine protease inhibitor E64 at a concentration of 10 μm led to an inhibition of all visualized cathepsins. In contrast to E64, the inhibitor LHVS at a concentration of 10 nm inhibited only CatS, whereas other visualized cathepsins remained active (Fig. 3). This indicates that LHVS at a concentration of 10 nm can be used as a specific CatS inhibitor, although it is not impossible that other cathepsins could be inhibited when higher concentrations of LHVS are used.
FIGURE 3.
Cysteine protease characterization in macrophage endosomal compartments. Equal amounts of total protein (0.67 μg) from macrophage endosomal fraction were labeled with the active site binding probe DCG0N. All of the samples were preincubated for 1 h at 37 °C. Left lane, without inhibitor (no Inh.); middle lane, with 10μm of the general cysteine protease inhibitor E64; right lane, with 10 nm of the CatS-specific inhibitor LHVS. After SDS-PAGE and Western blot, proteins were visualized with peroxidase via streptavidin/biotin interaction. The molecular mass markers are indicated on the left, and identified cysteine proteases are on the right. In the right lane only the CatS band intensity is drastically reduced by LHVS, indicating a specific inhibition under these conditions.
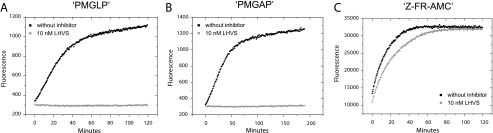
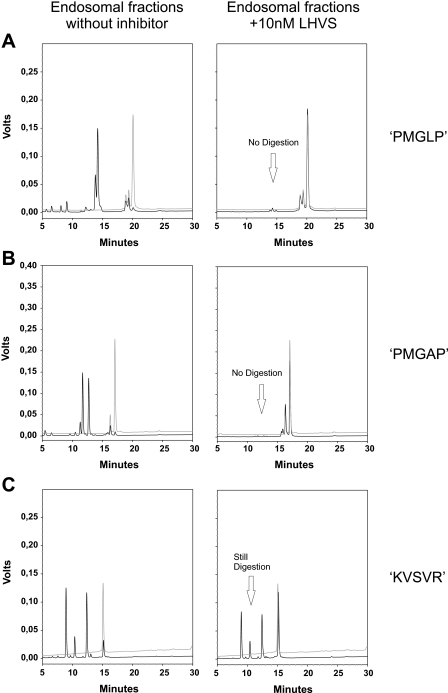
Specific Inhibition of CatS by LHVS Prevents Digestion of Specific Substrates by Endosomal Fractions—As shown above, a mixture of CatS-related cysteine proteases is present in endosomal fractions of antigen presenting cells. These enzymes remain active even when CatS is inhibited by LHVS. If our designed substrates are really specific, which means that they are exclusively cleaved by CatS and simultaneously resistant to other proteases present, a specific inhibition of CatS by LHVS should prevent substrate digestion. In contrast, the digestion of an unspecific substrate such as Z-FR-AMC should not be prevented by LHVS. To perform such digestion experiments, we used endosomal fractions from macrophages in which CatS was still active and others that were subjected to LHVS inhibition for 1 h as described above. Both samples were treated equally. When CatS was active, the two substrates PMGLP and PMGAP were completely digested after 2 h, visualized by an increase of Mca fluorescence intensity to a maximum (Fig. 4, A and B). However, when the preinhibited endosomal fractions were used for the digestion experiment, no increase of Mca fluorescence was detectable. Together with HPLC analysis of the reaction mixtures, obtained after 2 h of incubation with identical endosomal fractions, this indicates that the substrates were stable when CatS was inhibited (Fig. 5, A and B). On the other hand, LHVS was not capable of inhibiting the hydrolysis reaction of the unspecific substrates Z-FR-AMC and KVSVR under similar conditions (Figs. 4C and 5C). It is true that the CatS inhibition reduced the velocity of the reaction, but nevertheless the unspecific substrate Z-FR-AMC was completely hydrolyzed after 2h. In addition to the results of the active site labeling, this indicates that related cysteine proteases remain active and are still capable of cleaving substrates in the presence of LHVS. When DCs and B cell endosomal fractions were used for comparable digestion experiments, similar reaction profiles were obtained (Fig. 6, A and B).
FIGURE 4.
Specific inhibition of CatS prevents digestion of designed substrates by
endosomal fractions; comparison with an unspecific substrate.
Time-progress curves of peptide substrate hydrolysis reactions catalyzed by
macrophages endosomal fractions followed by fluorescence emission. 0.67
μgof total protein from macrophage endosomal fractions were preincubated
for 1 h at 37 °C: ▪, without inhibitor;
 , with 10 nm
LHVS. All of the assays were performed in triplicate at 37 °C in 50
mm sodium acetate buffer (pH 5.5). A, hydrolysis of the
internally quenched fluorescent peptide
Mca-GRWPPMGLPWE-Lys(Dnp)-DArg-NH2 (PMGLP) at a concentration of 20
μm. Progress of product formation was recorded over 2 h by
fluorescence emission of the N-terminal Mca group at 405 nm, following
extinction at 340 nm. LHVS prevents the hydrolysis. B, hydrolysis of
the internally quenched fluorescent peptide
Mca-GRWHPMGAPWE-Lys(Dnp)-DArg-NH2 (PMGAP) at a concentration of 20
μm. Progress of product formation was recorded over 3 h
(λex = 340 nm, λem = 405 nm). LHVS
prevents the hydrolysis. C, hydrolysis of the unspecific fluorogenic
peptide Z-FR-AMC at a concentration of 100μm. Progress of
product formation was recorded over 2 h by fluorescence emission of the AMC
group (λex = 360 nm, λem = 465 nm). LHVS
reduces the initial velocity of the hydrolysis reaction but does not prevent
it.
, with 10 nm
LHVS. All of the assays were performed in triplicate at 37 °C in 50
mm sodium acetate buffer (pH 5.5). A, hydrolysis of the
internally quenched fluorescent peptide
Mca-GRWPPMGLPWE-Lys(Dnp)-DArg-NH2 (PMGLP) at a concentration of 20
μm. Progress of product formation was recorded over 2 h by
fluorescence emission of the N-terminal Mca group at 405 nm, following
extinction at 340 nm. LHVS prevents the hydrolysis. B, hydrolysis of
the internally quenched fluorescent peptide
Mca-GRWHPMGAPWE-Lys(Dnp)-DArg-NH2 (PMGAP) at a concentration of 20
μm. Progress of product formation was recorded over 3 h
(λex = 340 nm, λem = 405 nm). LHVS
prevents the hydrolysis. C, hydrolysis of the unspecific fluorogenic
peptide Z-FR-AMC at a concentration of 100μm. Progress of
product formation was recorded over 2 h by fluorescence emission of the AMC
group (λex = 360 nm, λem = 465 nm). LHVS
reduces the initial velocity of the hydrolysis reaction but does not prevent
it.
FIGURE 5.
HPLC analysis of endosomal reaction mixtures treated with the CatS inhibitor LHVS. Chromatograms of three substrates PMGLP (A), PMGAP (B), and KVSVR (C) at a concentration of 20 μm after incubation with endosomal fractions from macrophages (0.67 μg of total protein) for 2 h in digestion buffer at pH 5.5 and 37 °C. Endosomal fractions were preincubated for 1 h at 37 °C: Left column, without inhibitor; right column, with 10 nm LHVS. Digestion products were detected by their UV absorbance at 220 nm. The black lines represent chromatograms of the digestion mixtures, whereas the gray lines are chromatograms of the undigested negative controls. LHVS prevents the digestion of the two substrates PMGLP and PMGAP, whereas the unspecific substrate KVSVR is still digested under similar conditions.
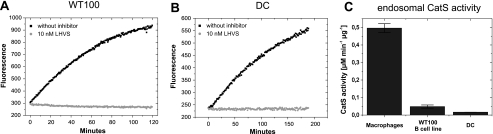
FIGURE 6.
Determination of CatS activity in antigen presenting cells.
Hydrolysis of the internally quenched fluorescent peptide PMGLP at a
concentration of 20μm catalyzed by endosomal extracts from WT100
(A) and DCs (B). Endosomal fractions containing 2.75 μg
(WT100) and 2.88 μg (DC) of total protein were preincubated for 1 h at 37
°C: ▪, without inhibitor;
 , with 10 nm
LHVS. Product formation was recorded by fluorescence emission
(λex = 340 nm, λem = 405 nm). All of the
assays were performed in triplicate at 37 °C in 50 mm sodium
acetate buffer (pH 5.5). C, determination of endosomal CatS activity
in antigen presenting cells. The initial velocities of the hydrolysis
reactions were determined and referred to total protein amount in endosomal
fractions. The heights of the bars denote endosomal CatS activity in
μm min–1 μg–1. The results
are representative of at least three independent experiments. The error
bars indicate standard deviation.
, with 10 nm
LHVS. Product formation was recorded by fluorescence emission
(λex = 340 nm, λem = 405 nm). All of the
assays were performed in triplicate at 37 °C in 50 mm sodium
acetate buffer (pH 5.5). C, determination of endosomal CatS activity
in antigen presenting cells. The initial velocities of the hydrolysis
reactions were determined and referred to total protein amount in endosomal
fractions. The heights of the bars denote endosomal CatS activity in
μm min–1 μg–1. The results
are representative of at least three independent experiments. The error
bars indicate standard deviation.
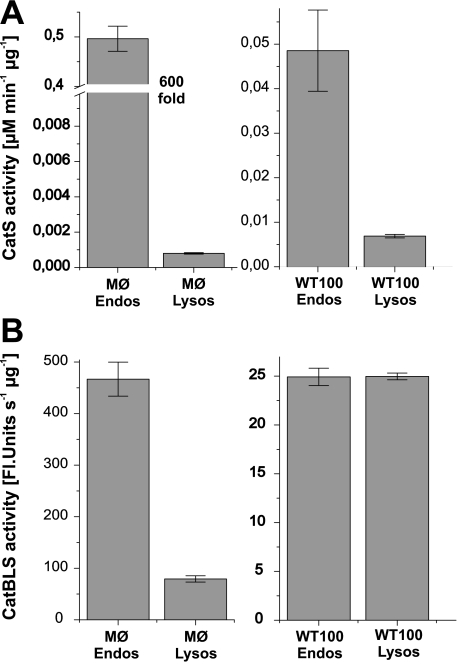
Quantification and Subcellular Distribution of CatS in Antigen Presenting Cells—Taken together, the above data shows that our designed substrates PMGLP and PMGAP were exclusively cleaved by CatS in subcellular fractions of antigen presenting cells and not by other present proteases. Therefore, they can be designated CatS-specific. Using one of these specific substrates, we quantified CatS activity in all three types of professional APCs (Fig. 6C). DCs and B cells showed significantly lower endosomal CatS activity compared with macrophages. The CatS activity in endosomal extracts from macrophages was ∼10 times higher than in the corresponding extracts from the B cell line and even 30 times higher than in endosomal fractions from DCs. CatS activity differed not only between the different types of APCs but also between endosomal and lysosomal compartments of one cell type. We localized CatS activity mainly in endosomal compartments and only to a minor extent in lysosmal extracts (Fig. 7A). In WT100 B cells the endosomal CatS activity was ∼7 times higher than the lysosomal CatS activity. On the other hand, the total activity of CatB, L, and S together, measured by digestion of the unspecific substrate Z-FR-AMC, was equally distributed between endosomes and lysosomes (Fig. 7B), indicating that CatS is more an endosomal than a lysosomal enzyme in APCs. Macrophage endosomal extracts even showed a 600-fold higher CatS activity compared with lysosomal extracts. Because of this high CatS activity in endosomal fractions of macrophages, it is not surprising that also the total activity of CatB, L, and S is higher in endosomes than in lysosomes. However, compared with the difference in CatS activity between the subcellular compartments the total CatBLS activity in endosomes was only five times higher than in lysosomes. These results confirm that CatS is mainly located in endosomes of APCs and show also the great advantage of a CatS-specific substrate in quantifying specifically the CatS activity compared with an unspecific substrate.
FIGURE 7.
Comparison of endosomal and lysosmal cysteine protease activities. Cysteine protease activities in endosomal (Endos) and lysosomal (Lysos) extracts of macrophages (MØ) and WT100 B cells were determined using the CatS specific substrate PMGLP (A) or the unspecific substrate Z-FR-AMC (B), which is hydrolyzed by CatB, CatL, and CatS. Initial velocities of the hydrolysis reactions were determined at 37 °C in 50 mm sodium acetate buffer (pH 5.5) and normalized to total protein amount inμg. The heights of the bars denote CatS activity in μm min–1 μg–1 for the specific substrate PMGLP and total activity of CatB, CatL, and CatS together in relative fluorescence units s–1 μg–1 for the unspecific substrate Z-FR-AMC. The results are representative of at least three independent experiments. The error bars indicate standard deviation.
DISCUSSION
The specificity profile of CatS that we obtained from the library experiment highlighted clear amino acid preferences at the P-2, P-1′, and P-3′ substrate positions. According to previously reported studies, the specificity of CatS is mainly determined by the amino acid at the P-2 position (9, 32–34). In those studies, conclusions on the CatS specificity were often drawn from the cleavage of fluorogenic substrates containing the fluorophore at the P-1′ position. These substrates are relatively short and do not contain amino acids at the P′ positions. In our strategy we used longer peptides and avoided the effects of a non-amino acid residue at the P-1′ position. Although we have also seen a clear preference of branched hydrophobic amino acids at the P-2 position, we find that the P-1′ position is even more restrictive than the P-2 position. Compared with studies with shorter substrates, no peptide with P-2 variations was completely resistant to CatS digestion. We have also seen that reduction of the peptide length results in a decrease of CatS activity (not shown), indicating that the CatS activity is not only dependent on amino acid composition of a substrate but also on its length.
The diversity of autoantigenic or tumor-specific MHC class II presented peptides is on the one hand determined by allelic variations of MHC molecule binding specificity and on the other by the substrate specificity of proteases involved in antigen processing. Therefore it would be useful to predict the MHC-presented peptides generated or destroyed by a given protease, such as CatS. The kinetic constants we determined for substrates with a distinct amino acid composition could be advantageous in predicting cleavage sites from the primary sequence of an antigen protein and could help to improve software for antigenic peptide prediction (35–37).
The substrates we designed on the basis of the specificity profile of CatS were resistant to CatB and CatL digestion under time-limited conditions when CatB and CatL concentrations were approximately 10 times higher than the CatS concentration. However, we cannot exclude that the substrates would be cleaved at higher CatB and CatL concentrations. Nevertheless, we have clearly shown that they are exclusively cleaved by CatS in subcellular fractions of professional APCs and not by other proteases present. Therefore they can be used as CatS-specific substrates in APCs.
These novel substrates offer new possibilities to study CatS-related physiologies and pathologies. For example, the role of CatS in cross-presentation of exogenous antigens on MHC class I molecules (6) or in the development of autoimmune disorders remains largely unclear. As described above, CatS is an appropriate drug target in cancer and autoimmune disorders. New compounds targeting CatS are currently being developed. Because therapeutic inhibition of CatS is linked to chronic diseases, irreversible inhibitors are less suitable as therapeutic drugs. Noncovalent and covalent reversible inhibitors, mimicking the substrate, should be more suitable because of their higher selectivity and the lower probability of an adverse immune response (19). The sequence of the developed substrate is highly selective toward CatS and could be helpful not only to create new inhibitors but also to test already existing inhibitors, as to whether they are able to inhibit CatS in APCs specifically. Further applications of the internally quenched specific substrates, for example in fluorescence microscopy, are also possible.
Using one of these specific substrates, we have found a significant difference in activity levels of CatS between the different types of APCs. Whereas macrophages contained high levels of CatS, DCs showed significantly lower CatS activity. It is now well established that DCs are the most effective antigen presenting cells (38). In general DCs capture antigens in peripheral tissues, receive activatory signals, and migrate to the local lymph nodes, where they can activate T cells. During that maturation process antigens were captured long before the DCs encounter their specific T cells, and a rapid degradation of antigens by CatS and other lysosomal proteases might prevent an effective antigen presentation (39). The low activity level of CatS in DCs compared with macrophages might be one reason for their capacity to cross-present exogenous antigens on MHC class I by allowing them a greater chance to exit endosomal compartments to the cytosol. DC maturation is largely regulated by cytokines. Using our specific substrate, the influence of these cytokines on CatS activity during DC maturation can easily be measured. The finding that CatS is mainly located in endosomal compartments of antigen presenting cells is only one useful piece of information that can help to illuminate the complexity of CatS-related physiology.
Acknowledgments
We gratefully acknowledge Florian Kramer and Jürgen Beck for their technical assistance. We thank T. Rückrich and C. Driessen (Department of Medicine II, University of Tuebingen, Tuebingen, Germany) for kindly providing DCG0N.
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 685. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: MHC, major histocompatibility complex; APC, antigen presenting cell; Cat, cathepsin; DC, dendritic cell; LHVS, morpholinurea-leucine-homophenylalanin-vinyl sulfone-phenyl; Dnp, 2,4-dinitrophenyl; Mca, (7-methoxycoumarin-4-yl)-acetyl; Fmoc, N-(9-fluorenyl)methoxycarbonyl; HPLC, high pressure liquid chromatography; MALDI, matrix-assisted laser desorption ionization; Z, benzyloxycarbonyl; AMC, 7-amino-4-methylcoumarin.
References
- 1.Hsing, L. C., and Rudensky, A. Y. (2005) Immunol. Rev. 207 229–241 [DOI] [PubMed] [Google Scholar]
- 2.Villadangos, J. A., Bryant, R. A., Deussing, J., Driessen, C., Lennon-Dumenil, A. M., Riese, R. J., Roth, W., Saftig, P., Shi, G. P., Chapman, H. A., Peters, C., and Ploegh, H. L. (1999) Immunol. Rev. 172 109–120 [DOI] [PubMed] [Google Scholar]
- 3.Cirman, T., Oresic, K., Droga, M. G., Turk, V., Reed, J. C., and Myers, R. M. (2004) J. Biol. Chem. 279 3578–3587 [DOI] [PubMed] [Google Scholar]
- 4.Beck, H., Schwarz, G., Schroter, C. J., Deeg, M., Baier, D., Stevanovic, S., Weber, E., Driessen, C., and Kalbacher, H. (2001) Eur. J. Immunol. 31 3726–3736 [DOI] [PubMed] [Google Scholar]
- 5.Pluger, E. B., Boes, M., Alfonso, C., Schroter, C. J., Kalbacher, H., Ploegh, H. L., and Driessen, C. (2002) Eur. J. Immunol. 32 467–476 [DOI] [PubMed] [Google Scholar]
- 6.Shen, L., Sigal, L. J., Boes, M., and Rock, K. L. (2004) Immunity 21 155–165 [DOI] [PubMed] [Google Scholar]
- 7.Cezari, M. H., Puzer, L., Juliano, M. A., Carmona, A. K., and Juliano, L. (2002) Biochem. J. 368 365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagler, D. K., Tam, W., Storer, A. C., Krupa, J. C., Mort, J. S., and Menard, R. (1999) Biochemistry 38 4868–4874 [DOI] [PubMed] [Google Scholar]
- 9.Choe, Y., Leonetti, F., Greenbaum, D. C., Lecaille, F., Bogyo, M., Bromme, D., Ellman, J. A., and Craik, C. S. (2006) J. Biol. Chem. 281 12824–12832 [DOI] [PubMed] [Google Scholar]
- 10.Driessen, C., Bryant, R. A., Lennon-Dumenil, A. M., Villadangos, J. A., Bryant, P. W., Shi, G. P., Chapman, H. A., and Ploegh, H. L. (1999) J. Cell Biol. 147 775–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brachet, V., Raposo, G., Amigorena, S., and Mellman, I. (1997) J. Cell Biol. 137 51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riese, R. J., Wolf, P. R., Bromme, D., Natkin, L. R., Villadangos, J. A., Ploegh, H. L., and Chapman, H. A. (1996) Immunity 4 357–366 [DOI] [PubMed] [Google Scholar]
- 13.Shi, G. P., Villadangos, J. A., Dranoff, G., Small, C., Gu, L., Haley, K. J., Riese, R., Ploegh, H. L., and Chapman, H. A. (1999) Immunity 10 197–206 [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa, T. Y., Brissette, W. H., Lira, P. D., Griffiths, R. J., Petrushova, N., Stock, J., McNeish, J. D., Eastman, S. E., Howard, E. D., Clarke, S. R., Rosloniec, E. F., Elliott, E. A., and Rudensky, A. Y. (1999) Immunity 10 207–217 [DOI] [PubMed] [Google Scholar]
- 15.Neefjes, J. J., and Ploegh, H. L. (1992) EMBO J. 11 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biroc, S. L., Gay, S., Hummel, K., Magill, C., Palmer, J. T., Spencer, D. R., Sa, S., Klaus, J. L., Michel, B. A., Rasnick, D., and Gay, R. E. (2001) Arthritis Rheum. 44 703–711 [DOI] [PubMed] [Google Scholar]
- 17.Wang, B., Sun, J., Kitamoto, S., Yang, M., Grubb, A., and Chapman, H. A. (2006) J. Biol. Chem. 281 6020–6029 [DOI] [PubMed] [Google Scholar]
- 18.Palermo, C., and Joyce, J. A. (2008) Trends Pharmacol. Sci. 29 22–28 [DOI] [PubMed] [Google Scholar]
- 19.Vasiljeva, O., Reinheckel, T., Peters, C., Turk, D., Turk, V., and Turk, B. (2007) Curr. Pharm. Des. 13 387–403 [DOI] [PubMed] [Google Scholar]
- 20.Kramer, G., Paul, A., Kreusch, A., Schuler, S., Wiederanders, B., and Schilling, K. (2007) Protein Expression Purif. 54 147–156 [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson, G. N. (1961) Biochem. J. 80 324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lautwein, A., Burster, T., Lennon-Dumenil, A. M., Overkleeft, H. S., Weber, E., Kalbacher, H., and Driessen, C. (2002) Eur. J. Immunol. 32 3348–3357 [DOI] [PubMed] [Google Scholar]
- 23.Sinzger, C., Eberhardt, K., Cavignac, Y., Weinstock, C., Kessler, T., Jahn, G., and Davignon, J. L. (2006) J. Gen. Virol. 87 1853–1862 [DOI] [PubMed] [Google Scholar]
- 24.Sallusto, F., and Lanzavecchia, A. (1994) J. Exp. Med. 179 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroter, C. J., Braun, M., Englert, J., Beck, H., Schmid, H., and Kalbacher, H. (1999) J. Immunol. Methods 227 161–168 [DOI] [PubMed] [Google Scholar]
- 26.Bradford, M. M. (1976) Anal. Biochem. 72 248–254 [DOI] [PubMed] [Google Scholar]
- 27.Greenbaum, D., Medzihradszky, K. F., Burlingame, A., and Bogyo, M. (2000) Chem. Biol. 7 569–581 [DOI] [PubMed] [Google Scholar]
- 28.Reich, M., van Swieten, P. F., Sommandas, V., Kraus, M., Fischer, R., Weber, E., Kalbacher, H., Overkleeft, H. S., and Driessen, C. (2007) J. Leukocyte Biol. 81 990–1001 [DOI] [PubMed] [Google Scholar]
- 29.Chain, B. M., Free, P., Medd, P., Swetman, C., Tabor, A. B., and Terrazzini, N. (2005) J. Immunol. 174 1791–1800 [DOI] [PubMed] [Google Scholar]
- 30.Zaidi, N., Herrmann, T., Baechle, D., Schleicher, S., Gogel, J., Driessen, C., Voelter, W., and Kalbacher, H. (2007) FEBS J. 274 3138–3149 [DOI] [PubMed] [Google Scholar]
- 31.Lautwein, A., Kraus, M., Reich, M., Burster, T., Brandenburg, J., Overkleeft, H. S., Schwarz, G., Kammer, W., Weber, E., Kalbacher, H., Nordheim, A., and Driessen, C. (2004) J. Leukocyte Biol. 75 844–855 [DOI] [PubMed] [Google Scholar]
- 32.Bromme, D., Steinert, A., Friebe, S., Fittkau, S., Wiederanders, B., and Kirschke, H. (1989) Biochem. J. 264 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bromme, D., Bonneau, P. R., Lachance, P., and Storer, A. C. (1994) J. Biol. Chem. 269 30238–30242 [PubMed] [Google Scholar]
- 34.Ruckrich, T., Brandenburg, J., Cansier, A., Muller, M., Stevanovic, S., Schilling, K., Wiederanders, B., Beck, A., Melms, A., Reich, M., Driessen, C., and Kalbacher, H. (2006) Biol. Chem. 387 1503–1511 [DOI] [PubMed] [Google Scholar]
- 35.Schuler, M. M., Nastke, M. D., and Stevanovic, S. (2007) Methods Mol. Biol. 409 75–93 [DOI] [PubMed] [Google Scholar]
- 36.Singh, H., and Raghava, G. P. (2001) Bioinformatics 17 1236–1237 [DOI] [PubMed] [Google Scholar]
- 37.Guan, P., Doytchinova, I. A., Zygouri, C., and Flower, D. R. (2003) Nucleic Acids Res. 31 3621–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villadangos, J. A., Schnorrer, P., and Wilson, N. S. (2005) Immunol. Rev. 207 191–205 [DOI] [PubMed] [Google Scholar]
- 39.Delamarre, L., Pack, M., Chang, H., Mellman, I., and Trombetta, E. S. (2005) Science 307 1630–1634 [DOI] [PubMed] [Google Scholar]