Abstract
We describe the nonenzymatic ligation of RNA oligomers in water. Dimers and tetramers are formed in a time-, pH-, and temperature-dependent reaction. Ligation efficiency depends on oligonucleotide length and sequence and is strongly enhanced by adenine-based nucleotide cofactors. Ligation of short RNA fragments could have liberated the prebiotic polymerization systems from the thermodynamically demanding task of reaching a (pre)genetically meaningful size by stepwise addition of one precursor monomer at the time.
The origin of informational polymers is not understood. Considering only extant nucleic acids and according to the simplest possible logic, the progression of events could have been: synthesis of nucleic bases, formation of nucleosides thereof, activation by phosphorylation, polymerization of short oligonucleotides, their elongation, and eventually their survival through replication and evolution. The bottom-up approach to the origin of (pre)genetic polymers requires a definition of the minimal information. A minimal complexity of two different nucleotides (1–5) was proposed for the ancestral genetic system, major advantages being provided by the more prompt availability of the precursors and by the lower heterogeneity of suitable physical-chemical conditions. Based on the longer half-life of adenine and diamino purine (6) and on the good yield of both compounds from aqueous ammonium cyanide (7, 8), a ribozyme composed of only these two different nucleotides was developed (9). It was reasonably claimed that “without at least two different subunits, there is no information and thus no basis for Darwinian evolution” (9).
The “genetic” meaning of such a minimal molecular information is intrinsically limited. Evolution entails maintenance and at the same time the possibility of accepting variations of the macromolecular information. At some point in Darwinian evolution, nucleic polymers had to arise that were long enough to satisfy these divergent necessities.
The nonenzymatic polymerization process has been studied for decades, the results essentially showing that polymers of several tens can be obtained (reviewed in Ref. 10) from preactivated precursors. The prebiotic validity of a process requiring complex preactivations has been questioned (4, 10, 11). An additional difficulty is to be found in the standard state Gibbs free energy change (12), which essentially states that condensation reactions are extremely inefficient in water.
Considering the crucial step of elongation, the limit to the formation of nucleic polymers endowed of (pre)genetic potential is established by the intrinsic instability of long polymeric forms. If the polymerization process is slow, an equilibrium between synthesis and degradation of the polymer would rapidly be reached, preventing the accumulation of sufficiently long, (pre)genetically meaningful information. Polymer elongation by successive condensation steps adding one monomer at the time is not a likely mechanism for the accumulation of (pre)genetic information.
Focusing on the stability problem, a detailed analysis was performed of the relative stabilities of the key chemical bonds (3′-phosphoesteric, 5′-phosphoesteric, and β-glycosidic) in RNA (13) and in DNA (14). For RNA, this analysis led to the conclusion that conditions exist in which the key bonds are more stable in the polymer than in the precursor monomer. In these conditions (water, temperature between 60 and 90 °C), the polymer is the fittest form (13, 15). The presence of free phosphates (16) and a defined window of pH values (17) further stabilize the polymer and widen the thermodynamic niche in which the accumulation of information is favored.
If a condition is identified in which a ribozyme-like activity is exerted by a sequence-homogeneous oligonucleotide leading to sequence duplication, the basis would be established for the simplest replicatory system eventually able to incorporate mutations and evolve. We focus on the problem: if short RNA polymers form in a simple aqueous environment, does an intrinsic property of RNA exist that allows their spontaneous ligation, thus bypassing the mentioned difficulties?
EXPERIMENTAL PROCEDURES
Materials
Adenine, adenosine, adenosine 5′-monophosphate, adenosine 3′-monophosphate (3′-AMP),3 adenosine 2′-monophosphate (2′-AMP), adenosine 2′,3′-cyclic monophosphate (2′,3′-cAMP), adenosine 3′,5′-cyclic monophosphate (3′,5′-cAMP), ADP, and ATP were from Sigma-Aldrich (analytical grade).
The degradation of RNA was analyzed in water on poly(A)9U, poly(A)14, poly(A)14U, poly(A)23, poly(A)23U, and P1 RNA. P1 is an oligonucleotide with the sequence 5′-GGAAACGUAUCCUUUGGGAG-3′ (18). The oligonucleotides were all purchased from Dharmacon and provided in the standard lyophilized form.
Methods
Commercial distilled water was further purified by tridistillation-deionization with a MilliQ Advantage A10. Pure or Tris-HCl-buffered water was pretreated for 2 h at the temperature of the assay to be performed, a time lapse sufficient to reach and maintain the temperature-specific pH. The temperature-stabilized pH values (determined on a Beckman Ø 40 pH meter) are given throughout.
RNA Labeling and Handling—For RNA 5′ labeling, 300 pmol of the oligonucleotide RNA were labeled with [γ-32P]ATP using polynucleotide kinase (Roche Applied Science). The oligonucleotide was then purified on a 16% denaturing acrylamide (19:1 acrylamide/bisacrylamide, 8 m urea) gel. After elution, the residual polyacrylamide was removed by a NuncTrap Probe purification column (Stratagene). Subsequently the RNA, suspended in STE buffer (100 mm NaCl, 20 mm Tris-HCl, pH 7.5, 10 mm EDTA) was precipitated by the addition of glycogen (20 μg/μl of bidistilled sterile water) and 3 volumes of ethanol, kept overnight at -20 °C, centrifuged, washed once with 70% ethanol/water, and dehydrated (Savant, 13.000 rpm, 10 min, room temperature, environmental atmospheric pressure). The pellet was suspended in H2O, distributed in aliquots, immediately frozen, and conserved at -20 °C. Typically one aliquot was used for each experiment. Each experimental point consisted of 2–4 pmol of RNA (typically 15,000 cpm/pmol).
The Ribo-oligonucleotide Ligation Protocols and Analyses—The basic ligation protocol consisted of the following steps: 20 pmol of 5′-labeled RNA (6 × 105 cpm) were resuspended in 45 μl of Tris-HCl-buffered water at the appropriate pH. This amount of RNA was normally used for six experimental points. A Tris-HCl-buffered solution of 20 mm 3′,5′-cAMP was added to reach a final volume of 90 μl. The sample was divided in six 15-μl aliquots and treated as appropriate for each experimental variable. A sample was immediately precipitated by the addition of 25 μl of MilliQ water, 50 μl of sodium pyrophosphate, 10 μl of 3 m sodium acetate, pH 7.5, 300 μl of 96% ethanol, 1 μl of 20 μg/μl glycogen. Before the addition of alcohol, the sample was thoroughly vortexed. The other samples were precipitated similarly after the appropriate treatments. Variants were tested of this basic protocol, and the oligonucleotides were treated at the temperature, time, and solution conditions indicated where appropriate. After precipitation the samples were suspended in 5 μl of formamide buffer, heated for 3 min at 65 °C, and loaded on a 16% denaturing polyacrylamide gel (19:1 acrylamide/bisacrylamide, 8 m urea). Densitometric analysis was performed with Epson expression 1640 XL Kodak imaging software, and quantification was by OptiQuant 3.10.
Fragment Size Determination—Given the known difficulty of obtaining precise size markers for large RNA molecules, the determination of the fragment lengths was verified by the linearity of their migration in semi-log plots. Supplemental Fig. S1 shows such linearity in the semi-log plots of molecules 24, 48, and 96 nucleotides long and of their combinations with a 15-mer: 39 (24 + 15), 69 (24 + 15 + 15 + 15), and 78 (24 + 24 + 15 + 15). The electrophoretic image of these data is shown in Fig. 3A. Supplemental Fig. S2 shows the same linearity observed for the molecules resulting from a complex series of ligations, affording fragments of the following sizes: 24, 34, 39, 48, 64, 69, 78, and 96 (data from Fig. 3C).
FIGURE 3.
Multimerization of oligomers with different length. A, the 5′-labeled 5′A23U3′ 24-mer was treated in the usual conditions (60 °C, Tris-HCl-buffered water at pH 6.2) for 6 and 24 h (first and second lanes) in the presence of 10 mm 3′,5′-cAMP. The reaction was also carried out in the additional presence of unlabeled 5′A14U3′ 15-mer (third and fourth lanes). The upper part of the autoradiogram shows exposure (20×). B, scanning profiles of the first and third lanes. Left to right indicates gel position top to bottom. C, scanning profiles of an autoradiogram in which the following ligation reactions were analyzed: Lane 1, 5′A24U3′ 24-mer (5′-labeled); lane 2, 24-mer + 5′A9U3′ 10-mer; lane 3, 24-mer + 5′A14U3′ 15-mer; lane 4, 24 + 10-mer + 15-mer; lane 5, 24-mer + P1 20-mer; lane 6, 24-mer + 10-mer + 20-mer; lane 7, 24-mer + 15-mer + 20-mer; lane 8, 24-mer + 10-mer + 15-mer + 20-mer. All the unlabeled oligonucleotides were added at the same concentration as the labeled 24-mer, namely 3.5 pmol in 15 μl.
Nuclease Treatment—Phosphodiesterase I from Crotalus adamanteus venom (EC 3.1.4.1) (snake venom phosphodiesterase I) from Sigma (in vials of ≥0.4 units, purified, catalog number P3243) is a 5′-exonuclease that hydrolyzes 5′-mononucleotides from 3′-hydroxy-terminated ribo-oligonucleotides. It cleaves both 2′,5′- and 3′,5′ phosphodiester linkages, and it is here typically used at 1 milliunits/assay in 40 mm Tris-HCl, pH 8.4, and 10 mm MgCl2 in 20-μl assays. Stop mix (2 μl/tube) was 50 mm EDTA, pH 8.0, SDS 20%. One unit hydrolyzes 1.0 μmol of bis (p-nitrophenyl)phosphate/min at pH 8.8 at 37 °C.
Nuclease P1 from Penicillium citrinum (EC 3.1.30.1) is from Sigma (catalog number N8630; specific activity, 200 units/mg protein). It catalyzes the sequence nonspecific endonucleolytic cleavage of single-stranded RNA to yield nucleoside 5′-phosphates and 5′-phospho-oligonucleotides. Specific for 3′,5′ phosphodiester linkages, it is here typically used at 1 × 10-3 units/sample in 40 mm Tris-HCl, pH 5.4, 5 mm NaCl, 0.5 mm MgCl2 in 20-μl assays. One unit liberates 1.0 μmol of acid-soluble nucleotides from RNA/min at pH 5.3 at 37 °C.
RESULTS
Multimerization was observed by simply leaving ribo-oligonucleotides in water in the presence of an adenine-based nucleotide cofactor. The reaction is dependent upon time, type of cofactor, oligonucleotide length and sequence, temperature, and pH.
Time
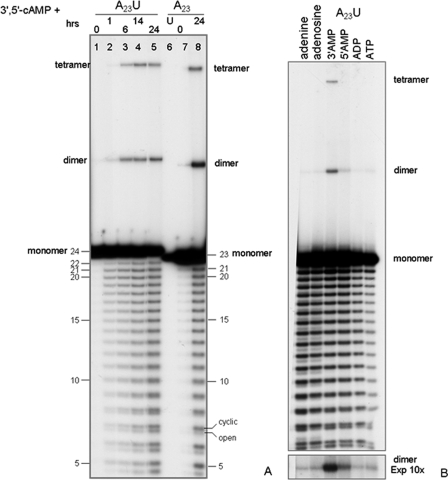
Fig. 1 shows the kinetics of formation of dimer- and tetramer-sized multimers from both a 24-mer ribo-oligonucleotide (5′A23U3′)(first five lanes on the left) and from a 23-mer (5′A233′)(three rightmost lanes) as a function of increasing treatment periods. RNA oligomers were reacted in Tris-HCl-buffered water at 60 °C at pH 6.2. The formation of only even-numbered multimers was observed. The formation of multimers requires the presence of a cofactor (as detailed in Fig. 1B). The formation of the dimer precedes in time that of the tetramer (i.e. compare the relative intensities in Fig. 1A, lane 3). Detailed kinetics are reported in supplemental Fig. S3. The earlier onset of formation of the dimer relative to that of the tetramer is also described in detail (supplemental Fig. S3). The kinetic constants of the reactions leading to the formation of the dimers were determined at 40, 50, and 60 °C, based on kinetic analyses similar to that shown for 60 °C in supplemental Fig. S3: 2 × 10-3 h-1, 2.8 × 10-2 h-1, and 7.1 × 10-2 h-1, respectively. The constants for the reactions leading to tetramers were not determined, because of the complexity of the reaction for their formation (see Fig. 5). The background level was considered as the amount of dimer that is already formed at time 0, because of ligation reactions occurring during the handling of the sample.
FIGURE 1.
Ligation in water. A, gel electrophoretic images of the reaction products of a 5′-labeled 5′A23U3′ (lanes 1–5) and of a 5′A23 3′ (lanes 6–8) oligomer, as indicated. The monomeric oligonucleotides were treated in water, for the periods of time indicated (hours) on top of each lane. The samples were then treated and analyzed as described under “Methods.” For the fragment size determination, see “Methods” and supplemental Figs. S1 and S2. During the permanence in water, the oligomers undergo hydrolysis (affording the ladder of decreasing fragment lengths, as indicated) and ligation (affording the indicated multimers). B, the oligonucleotide A23 was reacted in water for 6 h at 60 °C in the presence of adenine or of the adenosine-based compound indicated on top of the appropriate lane (final concentration, 10 mm). Processing and analysis were as in A. The lowest part of the Panel is an overexposure (10×) of the section of the image containing the dimer.
FIGURE 5.
A model explaining for the observed directionality of the ligation events. Oligomers are idealized as rods whose 5′ (red) and 3′ (black) extremities are indicated throughout by this color-code. Arrow A, 5′ to 5′ ligation brings to the impossibility (×) of continuing the reaction to form the tetramer. Arrow B, 3′ to 3′ ligation: same. Arrow C, the antiparallel interaction of two parallel poly(A) double helices would bring the reactive 5′ phosphate and 3′ hydroxyl group in the correct positioning. For further description, see the text.
In the experiment reported in Fig. 1, 3′,5′-cAMP was present. The dependence of ligation upon the concentration of cofactors is reported in Table 1. A detailed titration of the 3′,5′-cAMP enhancing activity is in supplemental Fig. S4.
TABLE 1.
Quantitative analysis of ligation test-system results
| Self-ligating polymer | Cofactor | Ligation ratea | Half maxb |
|---|---|---|---|
| mm | |||
| A23U | Adenine | 200 ± 50 | 3.0 |
| Adenosine | 200 ± 50 | 3.0 | |
| 2′-AMP | 3150 ± 580 | 3.0 | |
| 3′-AMP | 1900 ± 210 | 3.0 | |
| 5′-AMP | 800 ± 100 | 3.0 | |
| 2′,3′-cAMP | 0 | ||
| 3′,5′-cAMP | 10,000 ± 1500 | 3.0 | |
| ADP | 200 ± 50 | 3.0 | |
| ATP | 400 ± 120 | 6.0 |
The ligation rates were determined based upon densitometry measurements of autoradiograms of gel electrophoretic analysis of ligation reactions and have been normalized with respect to the ligation rate of A23U in the best observed conditions (24 h, 60 °C, pH 6.2, 6 mm 3′,5′-cAMP), which has been scaled to 10,000. The ligation products are considered collectively (dimers plus tetramers). The relative yield of tetramers versus dimers as a function of the oligonucleotide used is in Table II. The differential kinetics of formation of dimers versus tetramers is in supplemental Fig. S3. Dimer formation precedes tetramer
Half max indicates the concentration of cofactor at which the rate of product yield is one-half of the maximum ligation rate
Poly(A) oligomers ending with a terminal uridine were routinely studied in parallel to the homogeneous poly(A)s to verify whether two terminal purine bases are a prerequisite for the ligation events to occur. The results (i.e. Fig. 1A, lanes 6–8) show that this is not the case and that ligation occurs for both types of extremities. A relatively higher efficiency was observed for the homogeneous A-stretch (compare in Fig. 1A, lanes 1–5 versus 6–8, and Table 2). An additional reason for the use of oligonucleotides with a U at the 3′ extremity is the empirically observed higher stability in water of the 3′-terminal ApU step (relative to the ApA steps) (not detailed). In a ladder of fragments produced by hydrolysis, this feature decreases the presence of the (n - 1) oligomer, thus facilitating, in kinetic analyses, the quantitative evaluation of full-sized molecules. In addition to ligation, during the treatment in water, the oligomers also undergo hydrolytic degradation, as shown by the ladder of bands below the monomer-sized 24-mer fragment.
TABLE 2.
Quantitative analysis of ligation test system results
| Self-ligating polymer | Ligation ratea | Tetramer vs. dimerb |
|---|---|---|
| % | ||
| A23U | 10,000 ± 900 | 38.4 ± 2.5 |
| A9U | 3430 ± 250 | 0 |
| A14U | 8200 ± 550 | 29.8 ± 1.8 |
| A23 | 15,040 ± 1100 | 32.1 ± 2.1 |
| P1 | 0 |
Given is the ligation rate in the condition that proved optimal for A23U, which has been scaled to 10,000 (see Table I)
Tetramer formation is observed only in the presence of the cofactors 2′-AMP, 3′-AMP, and 3′,5′-cAMP. The data reported here refer to the ligation in the presence of 10 mm 3′,5′-cAMP and at the kinetic plateau
The mechanism of RNA hydrolysis is fully characterized (19, 20). The cleavage of the phosphoester chain normally requires participation of the 2′-OH group as an internal nucleophile (20) in two “nucleophilic cleavage” events: the transesterification and hydrolysis reactions. During transesterification, the 2′-OH nucleophile attacks the tetrahedral phosphorus to afford a 2′,3′-cyclic monophosphate. This species is then hydrolyzed into a mixture of 3′- and 2′-phosphate monoesters. Both steps are catalyzed by protons, hydroxide, nitrogen derivatives, and metal ions. The degradation profile characteristically yields a double-banded profile, because of a first cleavage of the 5′ phosphodiester bond leaving a 2′,3′-cyclic phosphate extremity (20), which is successively opened resulting in a 2′ or 3′ phosphate extremity (as indicated in the right side of Fig. 1A). The stability at high temperature of oligonucleotide A and of other oligonucleotides in water as a function of pH is characterized in detail (17). Oligonucleotide As are particularly stable between pH 5.0 and 6.5.
Ligation and hydrolytic degradation occur simultaneously (Fig. 1). No indication of reciprocal interference by the two reactions was observed, aside from the fact that hydrolysis slowly depletes the pool of full-length molecules undergoing ligation. The half-life of the full-length oligomers was calculated measuring the decrease of the monomer-sized molecules (for details of this simple procedure see Ref. 17). At 60 °C the half-life of the 24-mer is 65 ± 7 h. The kinetics of ligation are much faster, as described above.
Cofactors
The nature of the cofactor favoring ligation was explored (Fig. 1B). In addition to 3′,5′-cAMP (Fig. 1A), adenine, adenosine, 2′-AMP, 3′-AMP, adenosine 5′-monophosphate, 2′,3′-cAMP, ADP, and ATP were analyzed in the same conditions as the analysis for di- and tetramerization reported in Fig. 1A. Active ligation was favored by 2′-AMP (not shown), 3′-AMP, and 5′-AMP. Adenine, adenosine, ADP, and ATP only showed minor stimulation of the reaction. 2′,3′-cAMP was not active at all (not shown). The relative half max values of all cofactors analyzed are reported in Table 1.
Treatment for longer periods did not modify the pattern. In the moderate reaction conditions used (namely, 6 h, 60 °C, pH 6.2), the nucleoside and nucleotide forms are stable, and no transphosphorylation occurs (13, 21). Thus, the observed effect can be attributed to the primary chemical forms tested, excluding the involvement of molecular species deriving from degradation or transphosphorylation.
The formation of dimers and tetramers but not of trimers and pentamers (nor of higher forms) entails a ligation reaction preceded by the coupling of the reacting molecules two by two and excludes a tandem-wise polymerization mechanism. The two coupled molecules may undergo covalent linking only if the reacting phosphate and hydroxyl groups are in the necessary close proximity and in the appropriate relative position. These two conditions are satisfied only by matching the oligomers into a double strand encompassing the whole length of the molecules in the case of equal-sized molecules or by matching them starting from one extremity in the case of molecules differing in size. If matching is not complete, and the double strand is slipped with its ends protruding, the terminal phosphate and hydroxyl groups will not be in the necessary reactive proximity. Presumptive circular forms were also observed (see supplemental Fig. S5). This model is mentioned here to allow the interpretation of the experiments that follow and is described in more detail below.
The Effect of Fragment Size
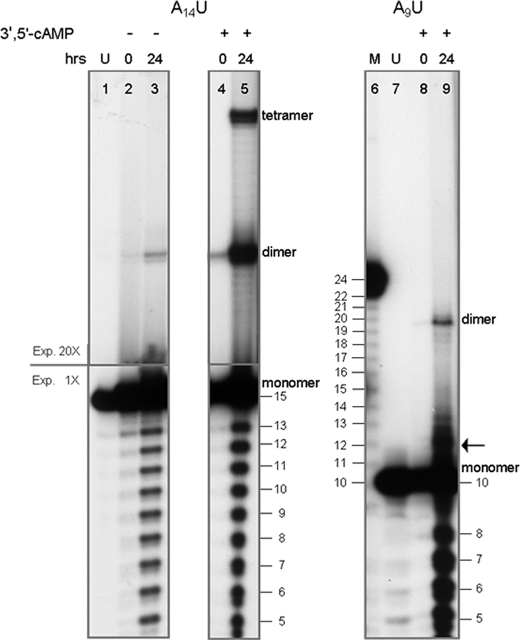
Fig. 2 shows the results of the same analysis performed on similar but shorter oligomers: 5′A9U3′ and 5′A14U3′. The behavior of the 15-mer (Fig. 2, lanes 1–5) is similar to that of the 24-mer (Fig. 1A). The formation of dimers still takes place with the 10-mer, although at a reduced rate (lane 9). This behavior is consistent with the observation (22) that fragment size is the limiting factor for the formation of poly(A) double strands at acidic pH values. The effect of the pH in the ligation mechanism is described below.
FIGURE 2.
Ligation in water of shorter oligonucleotides. The ligation reaction was carried out with the 5′A14U3′ (lanes 1–5) and the 5′A9U3′ (lanes 6–9) oligomers in the same conditions described for Fig. 1 except for the amount of RNA, in this case 7 pmol. The reactions were carried out in the absence (lanes 2 and 3) and in the presence (lanes 4, 5, 8, and 9) of 10 mm 3′,5′-cAMP. To show clearly the difference in abundance of the resulting products, the experiment carried on the A14U 15-mer is shown at two different exposures of the autoradiograms: 20× and 1×, as indicated on the left side of lane 1. U, untreated; M, marker lane, fragments obtained by hydrolysis of 5′-labeled A23U molecules. The arrow on the right side of lane 9 points to the presumptive (see supplemental Fig. S4) circularization product.
The ligation of the A14U 15-mer was tested in the absence and in the presence of 3′,5′-cAMP, as indicated where appropriate (Fig. 2, lanes 2 and 3 and lanes 4 and 5, respectively). In the absence of the cofactor, the ligation of the monomer to yield dimer is marginal (Fig. 2, compare lane 3 with lane 5), and the tetramer is not formed at all. However, the very fact that ligation may occur in the absence of an externally added molecular species (Fig. 2, lane 3) is in principle relevant per se.
The A9U 10-mer undergoes less efficient dimerization in water (Fig. 2, lane 9). In this case no ligation is evident in the absence of cofactor (not shown), no tetramer is formed, and the dimer formation does not exceed 3.0% of the total input. Molecular forms with molecular size slightly higher than the monomer size are also formed (arrow on the right side of lane 9). One form is particularly evident that is presumably the product of self-ligation leading to a presumptive circular molecule (supplemental Fig. S5).
Despite the strong increase in the multimerization efficiency brought about by a cofactor (i.e. 3′,5′-cAMP or 2′-AMP), the products formed in its absence are of identical size (Fig. 2, lanes 1–5). In the 10-mer the size of the dimer formed is exactly 20, as shown by its correspondence (Fig. 2, lane 9) with the 20-mer in a degradation ladder obtained by hydrolysis of a 24-mer (Fig. 2, lane 6 versus lane 9). This shows that (i) 3′,5′-cAMP is not retained in the final product (unless one hypothesizes an unlikely and unnecessary intermediate nucleotide substitution step, for which no experimental indication was obtained) and (ii) its function is that of a typical catalysis cofactor, favoring a reaction that would occur anyway.
This conclusion is strengthened by the observation that 3′,5′-cAMP favors aggregation of RNA oligomers, as shown by the following analysis: 5′-labeled A14U RNA was left in water in the usual conditions (60 °C, 6 h) in the presence of increasing concentrations of 3′,5′-cAMP. The samples were centrifuged (13,000 rpm, 20 min in Eppendorf apparatus), and the supernatant was then separated from the precipitate. Both were measured by Cherenkov, the results showing that starting at 10 mm 3′,5′-cyclic AMP, the RNA oligomer markedly decreases its solubility (data not shown). Solubility of cyclic AMP is known to be limited, and a concentrated solution (>50 mm) tends to precipitate at room temperature. In addition, the solubility of double-stranded RNA is also known to be limited, as initially reported by Holcomb and Tinoco (23), who describe a “large aggregation of the double strand helices” of polyribonadenylic acid. Both phenomena (low solubility of 3′,5′-cAMP and decreased solubility of RNA upon formation of the double strand) are concurrent in decreasing the solubility of RNA and in concentrating it. The aggregates can be dissolved by resuspending the precipitate obtained in 0.5 mm sodium pyrophosphate (see “Methods”).
In summary, RNA oligomers in water undergo dimerization and tetramerization. The order of increasing efficiency is 10-mer < 15-mer < 23-mer < 24-mer (Table 2). Cofactors 3′,5′-cAMP, 2′-AMP, and 3′-AMP stimulate the reaction by at least 2 orders of magnitude. Tetramerization only occurs with the 15-mer and 23- or 24-mer in the presence of 3′,5′-cAMP, 2′-AMP, and 3′-AMP.
Ligation of Oligomers with Different Lengths
Two-species Ligation—Fig. 3 shows the multimerization reaction performed with mixtures of oligomers. In A a reaction is shown in which the oligomers were 5′ A23U3′ and 5′ A14U3′. The two oligonucleotides were present in water at the same concentration (3.5 pmol each in 15 μl), with only the A23U 24-mer being labeled at the 5′ extremity. Thus, only the products of ligation encompassing at least one 24-mer should be observed in the autoradiogram. As indicated on the top of Fig. 3A, the two lanes on the left show the ligation reactions of the A23U 24-mer when present alone, resulting in the formation of the dimer and of the tetramer. When unlabeled A14U 15-mer is included (shown in the two lanes on the right), additional fragments are observed, corresponding to the combinations expected in the case of heterogeneous ligation events. The numerical combinations are indicated at the right side of the figure. The linearity of the semilog plot of these values as a function of the migration confirms the attributed values (supplemental Fig. S1).
Fig. 3B shows a scanning profile of the upper part of the gel containing the ligation products (from an underexposed auto-radiogram). The profile shows that length-homogeneous new species (i.e. 24-mer + 24-mer) form more abundantly than heterogeneous ones (i.e. 24-mer + 15-mer), and as observed for homogeneous ligations, shorter combinations are more abundant than longer ones (i.e. dimers > tetramers, both in homo- and in heterogeneous species).
The ladder generated by hydrolysis of the tetramer in the upper part of the second lane (Fig. 3A) is noteworthy. The distance between the bands is twice as wide as in the “each band cleavage” pattern, indicating that the hydrolytic cleavage occurs every second residue, not every single one (scanning pattern not shown).
Four-species Ligation—A ligation assay was performed on a mixture of four different oligomers. The 5′-labeled 5′A23U3′ 24-mer was added with equal amounts of unlabeled 5′A14U3′ 15-mer + 5′A9U3′ 10-mer + mixed sequence P1 20-mer. Also in this case only molecules containing at least one labeled 24-mer could be observed. The result revealed that a complex ensemble of ligation events takes place. The scanning profiles of the relevant samples are given in Fig. 3C, showing that the efficiency of the ligation events pertaining to the shortest oligonucleotide (10-mer) is low (trace 2); that the ligation with the 15-mer is markedly higher (trace 3); that when present together, the 10-mer partially competes off the 15-mer (trace 4); that the heterogeneous P1 sequence does not participate in the ligation events (traces 5–7); and that a complex mixture of fragments is actually obtained (trace 8).
In summary, mixtures of RNA oligomers in water undergo dimerization and tetramerization according to a mechanism that does not require complete matching of the whole molecule but that nevertheless depends on the sequence involved.
pH Effects
The single-strand helix of stacked bases is the stable form of poly(A) at neutral pH and room temperature and also at acidic pH and higher temperature. The conformation of poly(A) at low temperature and acidic pH is a double-strand helix. The equilibrium between the two helical forms is established by modifying temperature, pH, and fragment size (22–25).
Thus, we tested whether the ligation reaction depends on the pH values indicated by these early studies as discriminatory between the single- and double-stranded forms. A series of Tris-HCl-buffered waters was used as solvent medium: 2.46, 2.77, 4.16, 5.36, 6.10, 6.43, 6.78, 7.29, and 8.36, and the ligation reactions were carried out at 45, 60, and 75 °C. Fig. 4 shows that the correspondence with the data reported (23, 25) is complete. Above the pH value 6.10 ligation occurs at the lower temperature but not at the higher one (see arrows in B and C). At these pH values at 60 °C, single strands prevail (see Fig. 3 in Ref. 23).
FIGURE 4.
Ligation as a function of pH. A shows the yield of the dimers and tetramers of the 5′A14U3′ 15-mer as a function of the pH values 2.46 (lane 1), 2.77 (lane 2), 4.16 (lane 3), 5.36 (lane 4), 6.10 (lane 5), 6.43 (lane 6), 6.78 (lane 7), 7.29 (lane 8), 8.36 (lane 9). The reactions were carried out at 45, 60 and 75 °C for 24 h. At 75 °C only traces of multimers formed (data not shown). The yields were quantified by scanning densitometry relative to the unreacted monomer and are reported as percentages values in B (reactions at 45 °C) and C (reactions at 60 °C). The arrow localizes the pH values at which ligations occur at 45 but not at 60 °C.
We have verified that multimerization also occurs in pure water and that no specific or peculiar effects are exerted by the Tris molecules used for buffering purpose (data not shown). Tris was used to avoid the minor pH variations associated with the use of pure water. The need for a careful control on the pH in this experimental set-up is evident.
Orientation of the Multimers
Nonenzymatically driven formation of phosphoester bonds may occur in numerous orientations. A vast literature, pioneered by decades of studies by Khorana and co-workers (26–28), has detailed the versatility of phosphate ester bonds chemistry. Fig. 5 schematically shows the alternatives that may be conceived in our system. Starting from the consideration that poly(A) forms parallel-stranded double helices (see “Discussion”), the two strands might connect through their 5′ extremities (Fig. 5, arrow A) or their 3′ extremities (Fig. 5, arrow B). In addition, or alternatively, ligation could occur between the 5′ and 3′ extremities of two stacked double helices (Fig. 5, arrow C).
The Two Less Likely Possibilities
Two 5′P-(N)-OH3′ oligomers might in principle react through their phosphates and ligate with a 5′P-P5′ diphosphoester bond, resulting in a 3′-OH-(N)-5′P-P5′-(N)-3′-OH divergently oriented molecule. Specifically, the ligation could occur at the 3′-OH extremities, cAMP-mediated or otherwise, resulting in the 5′P-(N)-3′-OH-OH3′-(N)-5′P also divergent molecule. In this case the absence of a phosphate bridge makes this alternative highly improbable, and the phosphate should derive from an external source. The experimental observations reported above exclude the covalent implication and persistence of adenine nucleotide cofactors and the involvement of internal sites of the oligomers (i.e. of the 2′,3′-cyclic phosphate bridges obtained in the first step of the hydrolytic degradation).
Both mechanisms are not in agreement with the results obtained (Figs. 1, 2, 3, 4). They can be operationally discarded also based on the fact that in both cases the reactive extremities are located, already in the first reactions (Fig. 5, A and B), in the center of the molecule. This prevents additional ligation steps based on the same type of ligation reaction. Neither of these two possible reactions explains the active formation of tetramers.
The Plausible Mechanism
The asymmetric 3′ to 5′ ligation between 5′P-(N)-3′-OH and 5′P-(N)-3′-OH molecules characterizing the extant enzyme-driven ligation reactions remains the only plausible alternative. This could occur between two double strands coupled in the appropriate antiparallel orientation (Fig. 5, arrow C). In this case the proximity of the reactive phosphate and hydroxyl groups would be secured by the interaction between the two macromolecules, with the high temperature providing the energy necessary for the bond formation. The three 3′-5′ phosphodiester bonds thus formed (green arrows in Fig. 5B) would yield a linear tetramer-sized molecule, which is actually observed in the gels. Arrow 3 indicates a bond whose size is enlarged for reasons of graphical clarity. The implications of this type of events are (i) the resulting uninemic linearity (verified as mentioned above) and (ii) the constant direction of the 3′-5′ (or 2′-5′) phosphodiester bonds.
Two poly(A) oligomers are easily fully matched. In the case of equal sized molecules both extremities will coincide, whereas only one extremity will do so in the case of different length molecules. The observed absence of trimers excludes the head-to-tail interaction of a third molecule at one of the two available extremities of the double strand and/or the formation of triple strands. Tetramers are at the contrary efficiently formed (Figs. 1, 2, 3, 4). For their formation side-by-side columnar interaction of two double strands is necessary, followed by ligation. This type of interaction has been recently described for DNA double strands of both short (29) and long molecules (30). A similar type of interaction has not been described for double-stranded RNA but, given its helical configuration in solution (31), there is no reason to exclude it a priori. Ligation would thus occur between correctly (i.e. 3′ close to 5′) positioned extremities (arrows in Fig. 5B). This type of interaction is in principle favored by the high concentration of the reactants.
The model provides a coherent mechanism explaining the mechanics of the observed ligations. However, it should not be considered as the unique possibility. One could note the strong similarity of the model with the four-stranded structure actually demonstrated for telomeric DNA (32).
To obtain information on the type of phosphate bond formed we have performed enzymatic analyses with snake venom phosphodiesterase I and with P1 endonuclease. Snake venom phosphodiesterase is a 5′-exonuclease cleaving 3′-5′ and 2′-5′ phoshodiester bonds from the 3′ extremity in a nonprocessive manner. Treatment of a sample of A23U 24-mer containing dimers and tetramers with 1 milliunit of SVDP in the appropriate buffer (see “Methods”) for 20 min at 37 °C showed that the multimeric forms are completely susceptible to digestion by the enzyme. The control treatment with the buffer without enzyme in the same conditions only resulted in 10% degradation. Thus, the bonds formed are canonical phosphodiesteric bonds of the 3′-5′ or 2′-5′ species. This result excludes the possibility that bonds other than ester bonds were involved in the ligation reaction.
The regioselectivity (2′-5′ or 3′-5′) of the phosphodiester bonds was determined by P1 nuclease treatment of an RNA population that had undergone ligation in the standard conditions (18 h, 60 °C, poly(A)23U). P1 nuclease is a 3′-5′-specific riboendonuclease.
The determination was based on the following data: (i) The population of molecules was analyzed and resulted to be composed of 3.7% tetramers, 8.9% dimers, 87.4% monomers. (ii) The tetramer contains four labeled phosphates, three of which are internal, located at the 5′ extremity of the oligonucleotides that have undergone ligation. The fourth is at the 5′ extremity of the tetramer and is the only labeled phosphate that is not part of a newly formed bond. Thus, three-quarters of the label-containing phosphodiester bonds of the tetramer may be of the 2′-5′ or of the 3′-5′ type (3/4 of 3.7% = 2.77%). (iii) The dimer contains two labeled positions: one internal in the newly formed bond and one 5′-terminal. Thus, one-half of 8.9% = 4.45%. (iv) All the label in the monomer is at the unreacted 5′ extremity.
The amount of the digestion-resistant label-containing dinucleotides remaining upon extensive treatment with P1 determines the amount of 2′-5′ (resistant) relative to that of 3′-5′ (sensitive) bond. In our case 2.77% (from tetramers) + 4.45% (from dimers) = 7.22% resistant dinucleotides would indicate 100% of 2′-5′ bonds. The value experimentally observed is 4.8%, thus indicating that 66.5% of the bonds formed in the ligation reaction are of the 3′-5′ type.
DISCUSSION
We describe the spontaneous ligation of preformed polyriboadenylate in water at moderate temperature. This is possibly the least demanding molecular sequence amplification reaction yet identified, provides RNA with a simple way-out from the short-size constraint in its possible evolutionary path, and is of potential prebiotic interest.
The reaction was analyzed for An and AnU ribo-oligomers and takes place at acidic pH levels. Lowering the temperature (to 45 °C) increases by about 1 pH unit the permissive pH value. Increasing the temperature to 70 °C abolishes the reaction. This behavior is diagnostic of the formation of a double-stranded RNA, as established in pioneering studies (22–25), and is in full agreement with the physical-chemical setting in which those early studies were performed. In the conditions analyzed here the reaction is complete in 14 h and affords dimeric and tetrameric molecules with a yield that directly depends on the length of the oligonucleotide: 10 < 15 < 23 < 24 nucleotides. In particular, this behavior is in agreement with the studies by Brahms et al. (22), who first described the direct relationship between poly(A) length and double strand formation.
What is new in the present study is that the RNA double strand formed in these conditions promptly ligates with the help of an adenine-based cofactor to yield longer molecular species.
The new molecules are exclusively dimers and tetramers, and an odd number of components was never observed in any of the tested fragment combinations. This corroborates the formation of a double-stranded structure as the initial step of the ligation reaction.
10 nucleotides appears to be the lower size limit, in agreement with the minimal size indicated for the formation of RNA double strands (22). At this fragment size dimers are still formed but with marginal efficiency, and bona fide circular forms indicate that the preferred reaction is intramolecular. The analysis of the ligation products obtained from mixtures of differently sized fragments shows that the combinations involving short fragments are less favored, as expected from the lower stability of shorter double-stranded molecules.
Thus, the ligation starts with the coupling of RNA oligomers two by two. This coupling increases the local concentration of the two reacting groups (the hydroxyl and the phosphate) and allows consideration of the formation of the double-strand as the means through which the reaction-promoting proximity is obtained. Proximity and thermal energy explain the occurrence of the ligation reaction.
In conclusion, the formation of even-numbered multimers is efficient for the first two multiplicatory ligation steps, beyond which efficiency drops. Multimerization depends on fragment size, the discriminatory limit passing between 10 and 15 units (Table 2). Regardless of the exact mechanism involved, the previous formation of correctly positioned multimolecular complexes and acidic pH are required.
The presence of 2′-AMP, 3′-AMP and of 3′,5′-cAMP strongly stimulates multimerization (Fig. 1 and Table 1). The very fact that dimers are formed in their absence whose size is identical to the size of the dimers formed in their presence (Fig. 2) shows that the function of the nucleotides is cofactorial in its nature. In this respect, it is worth mentioning that adenine-dependent self-association of hairpin ribozymes was described (33), not followed by covalent linking. The report dealt with ribozymes that are dependent on adenine for their reversible self-cleavage (see also Refs. 34 and 35). The present findings are in agreement with the observation that RNA can use exogenous reactive molecules to enhance its own catalytic activity.
The Poly(A) Double Strand and Its Formation by Stacking Interactions—Poly(A) was the focus of intense studies, and several of the properties underlying the process leading to its terminal intermolecular ligation are known. Stacking interactions are particularly relevant (31).
The stacked states were found to have 2–6 Kcal/mol lower free energy than the unstacked states for purine dimers; for pyrimidine-pyrimidine dimers no barrier or a very small one was obtained (36). The stacking-unstacking process has also been shown to be temperature-dependent, and the transition barrier was shown to be lower at higher temperatures (37).
Although vertical stacking of bases, or of mono nucleotides, in aqueous solution seems to be noncooperative (38) clear cooperativity was reported for the formation of double helices (Ref. 22; reviewed in Ref. 39). Cooperativity depends on pH, length of the oligomer, and ionic environment and temperature (22, 23, 37). In particular, it was shown (22) that although at neutral pH, poly(A) and oligonucleotide A exist in a single-strand conformation stabilized by base stacking, at pH levels lower than 5, a double-strand conformation is assumed by oligomers larger than the heptamer; the melting of these structures depends on the chain length and has the characteristics of a cooperative process. The Tm analysis of oligoadenylics of various lengths at pH 4.9, 4.5, and 4.0 shows that a substantial fraction of oligomers longer than 10 nucleotides is in double-stranded form. These observations are in agreement with the analysis by Holcomb and Tinoco (23), who established the correlation between the transition of the single- to the double-stranded form in RNA and the pH/temperature of the system. The higher the temperature, the lower is the pH allowing the double-stranded structure. Similar plot was presented by T'so et al. (25), who also found a linear relationship of the same slope but displaced to lower pH at higher salt concentration.
The phenomenon we report only occurs in water, not in organic solvents like formamide (data not shown). This is considered as a confirmation of the interpretation given, because stacking phenomena are sensitive to the dielectric constant of the solvents (40, 41).
The formation of two helical forms of polyriboadenilic acid and the pH-dependent transition between them was also shown by crystallographic analysis by Finch and Klug (24). The transition is possible within the fiber. The chains on the double helix formed at pH levels between 5 and 6, consistent with a protonated state of the adenine residue. A detailed analysis of the structure of the trinucleoside diphosphate adenylyl-(3′,5′)-adenylyl-(3′,5′)-adenosine hexahydrate was reported (42). This and related studies by the same group (reviewed in Ref. 39) establish the exact structure of the extremities and the proximities of the terminal groups in protonation conditions. More recently, this overall picture was confirmed by the structural transitions in polyriboadenilic acid induced by the changes in pH and temperature analyzed by vibrational circular dichroism (43). The precise definition of the mechanistic aspects of the reaction leading to the ligation events is beyond the scope of the present report. Nevertheless, the solid body of information available for polypurines, and poly(A) in particular, is expected to facilitate this task.
A Possible Role for the Adenine-based Cofactors—The adenosine phosphate molecules enhance the ligation reaction by more than 1 order of magnitude (Table 1) and are not retained in the final products of ligation, thus playing a standard cofactorial role. The following observation may provide an indication of their function; the pattern of bands produced by hydrolytic cleavage of the tetramer occurs every second residue (Fig. 3 and the related text). The nearest neighbor intercalation avoidance is a well known phenomenon (44–46). Assuming that the adenine-based cofactors intercalate in the multimeric structures and do so in every second position because of the nearest-neighbor avoidance principle, their alternate presence would alternately protect from hydrolysis, thus affording the actually observed second residue cleavage pattern. A direct consequence of this model is that the cofactorial action is exerted by the adenine-related molecules by intercalation, presumably through the additional stabilization of the multimers. An evolutionarily appealing corollary of this model is that the size of the oligomers is expanded by a ligation reaction stimulated by the very same monomeric precursors from which they are polymerized. This model will be matter for further studies.
The Possible Reconciliation of Unfavorable Gibbs Free Energy Change—It has been pointed out (12) that condensation reactions are not thermodynamically spontaneous in dilute aqueous solution or even at moderate water activities. One way around this problem is the conversion of the precursor monomers to their phosphorylated derivatives and the use of the favorable free energy of phosphate hydrolysis to drive the reaction. This does not seem to be the case in our system; the phosphate at 5′ of the starting oligomer is not hydrolyzed, and ADP and ATP are not better cofactors than 2′-AMP or 3′-AMP.
Concentration of the Reactive Groups—The other way of evading the unfavorable ΔG°′ value is by concentrating the reactants or reducing the water activity (12). The following consideration alleviates the ΔG°′ problem through a local concentration-related property. The strands to be ligated are brought together by stacking interactions and are presumably further stabilized by adenine cofactors intercalation. The positioning so obtained for the phosphate and the hydroxyl groups is the correct one to favor the reaction (as functionally a posteriori verified by the very fact that these groups react and ligate). In this system the RNA structure has a function similar to that of an enzyme, positioning correctly the reactive species, increasing their local concentration, and reducing local water activity. Thus, the thermodynamic foundation of the system is that of the poly(A) interaction described above.
Concentrating the reactants at the limit of their solubility may further alleviate the unfavorable Gibbs free energy change. We describe an experiment that correlates the decreased solubility of 3′,5′-cAMP starting before 10 mm (as already observed by Holcomb and Tinoco in Ref. 23) with the ligation efficiency (supplemental Fig. S4), that is highest in the same concentration range.
The Possible Evolutionary Meaning of Nonenzymatic Ligations—The ligation reaction described has remarkable potential in prebiotic terms: (i) It helps to solve the problem of the thermodynamically uphill one-by-one polymerization needed to reach a (pre)genetic meaningful size. (ii) It has the intrinsic potential of favoring the evolution of complex sequences. If we suppose the insertion of bases different from As in the oligonucleotides just described, the molecules with matching complementarities could use not only base stacking but also base pairing as means to favor dimerization. This would widen the range of temperatures in which ligation could occur, and at higher temperatures ligation would be faster. An experimentally verifiable positive Darwinian cycle would thus be allowed. (iii) We have observed (17) that molecular complexity favors the evolution of ribopolymers based on the higher stability of complex sequences in defined pH ranges. The present observations describe yet another intriguing phenotype of RNA, suggesting its intrinsic evolutionary capacity. (iv) An RNA ligase was evolved to a form that contains only two different nucleotide subunits (47), the minimum requirement for the specificity of genetic information. This enzyme was evolved to construct a self-replicating ribozyme that operates by RNA-catalyzed ligation of preformed RNA substrates to form additional copies of itself (48). This process was dubbed as replication, involving a single joining reaction to form a new molecule identical to the previous one. As pointed out (48), this system is not capable of undergoing Darwinian evolution because it does not allow heritable mutations.
The system described here is fit for evolution. The requirement for the transmission of genetic information is that the two strands do so through a pairing mechanism. Strand pairing by stacking can be obtained with a number of sequence combinations and allows for internal sequence defects. As long as the two reactive extremities are matched in the correct reacting position, the strands could evolve into the appropriate although different sequence combinations.
Even though the term “replication” introduced by Joyce and co-workers (47, 48) to describe the reported reproduction of a catalytic RNA sequence might appear extreme from a genetic point of view, we think that it actually describes the molecular function under consideration. The nonenzymatic ligation of poly(A) oligonucleotide monomers into dimers allowing the formation of other poly(A) dimers falls into the same category of processes, yet simpler and closer to plausible initial prebiotic events. The process described here is based on base stacking rather than base pairing. Stacking is notoriously a less sequence-dependent mechanism. The suggestion that the initial replicatory events were based on stacking is tempting.
Supplementary Material
This work was supported by the Italian Space Agency Molecules-to-Man (MoMa) Project, by Agenzia Spaziale Italiana - Istituto Nazionale di Astrofisica (ASI-INAF) Grant I/015/07/0 Esplorazione del Sistema Solare, and by the National Science Foundation Chemical Bond Center (CBC) Program. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
Footnotes
The abbreviations used are: 3′-AMP, adenosine 3′-monophosphate; 2′-AMP, adenosine 2′-monophosphate; 2′,3′-cAMP, adenosine 2′,3′-cyclic monophosphate; 3′,5′-cAMP, adenosine 3′,5′-cyclic monophosphate; U, uridine.
References
- 1.Rich, A. (1962) Horizons in Biochemistry (Kasha, M., and Pullman, B., eds) pp. 103-126, Academic Press, New York
- 2.Crick, F. H. C. (1968) J. Mol. Biol. 38 367-379 [DOI] [PubMed] [Google Scholar]
- 3.Orgel, L. E. (1968) J. Mol. Biol. 38 381-393 [DOI] [PubMed] [Google Scholar]
- 4.Wächtershäuser, G. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 1134-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubay, G. (1991) Chemtracts 2 439-442 [Google Scholar]
- 6.Levy, M., and Miller S. L. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 7933-7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orò, J. (1961) Nature 191 1193-1194 [DOI] [PubMed] [Google Scholar]
- 8.Sanchez, R. A., Ferris, J. P., and Orgel, L. E. (1968) J. Mol. Biol. 38 121-128 [DOI] [PubMed] [Google Scholar]
- 9.Reader, J. S., and Joyce, G. F. (2002) Nature 420 841-844 [DOI] [PubMed] [Google Scholar]
- 10.Orgel, L. E. (2004) Crit. Rev. Biochem. Mol. Biol. 39 99-123 [DOI] [PubMed] [Google Scholar]
- 11.Orgel, L. E. (1998) Trends in Biochem. Sci. 23 491-495 [DOI] [PubMed] [Google Scholar]
- 12.van Holde, K. (1980) The Origins of Life and Evolution (Halvorson, H. O., and van Holde, K. E., eds) p. 31, Alan R. Liss, Inc., New York
- 13.Saladino, R., Crestini, C., Ciciriello, F., Di Mauro, E., and Costanzo, G. (2006) J. Biol. Chem. 281 5790-5796 [DOI] [PubMed] [Google Scholar]
- 14.Saladino, R., Crestini, C., Busiello, V., Ciciriello, F., Costanzo, G., and Di Mauro, E. (2005) J. Biol. Chem. 280 35658-35669 [DOI] [PubMed] [Google Scholar]
- 15.Saladino, R., Crestini, C., Ciciriello, F., Costanzo, G., and Di Mauro, E. (2007) Helv. Chim. Acta 4 694-720 [DOI] [PubMed] [Google Scholar]
- 16.Saladino, R., Crestini, C., Neri, V., Ciciriello, F., Costanzo, G., and Di Mauro, E. (2006) ChemBioChem 7 1707-1714 [DOI] [PubMed] [Google Scholar]
- 17.Ciciriello, F., Costanzo, G. Pino, S., Crestini, C., Saladino, R., and Di Mauro, E. (2008) Biochemistry 47, 2732-2742 [DOI] [PubMed] [Google Scholar]
- 18.La Neve, P., Altieri, F., Fiori, M. E., Scaloni, A., Bozzoni, I., and Caffarelli, E. (2003) J. Biol. Chem. 278 13026-13032 [DOI] [PubMed] [Google Scholar]
- 19.Morrow, J. R., Aures, K., and Epstein, D. (1995) J. Chem. Soc. Chem. Commun. 23 2431-2432 [Google Scholar]
- 20.Soukup, G., and Breaker, R. (1999) RNA 5 1308-1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costanzo, G., Saladino, R., Crestini, C., Ciciriello, F., and Di Mauro, E. (2007) J. Biol. Chem. 282 16729-16735 [DOI] [PubMed] [Google Scholar]
- 22.Brahms, J., Michelson, A. M., and Van Holde, K. E. (1966) J. Mol. Biol. 15 467-488 [DOI] [PubMed] [Google Scholar]
- 23.Holcomb, D. N., and Tinoco, I. (1965) Biopolymers 3 121-133 [Google Scholar]
- 24.Finch, J. T., and Klug, A. (1969) J. Mol. Biol. 46 597-598 [DOI] [PubMed] [Google Scholar]
- 25.T'so, P. O. P., Helmkamp, G. K., and Sander, C. (1962) Proc Natl Acad. Sci. U. S. A. 48 686-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekker, C. A., and Khorana, H. G. (1954) J. Am. Chem. Soc., 76 3522-3527 [Google Scholar]
- 27.Gilham, P. T., and Khorana, H. G. (1958) J. Am. Chem. Soc. 80 6212-6222 [Google Scholar]
- 28.Fritz, H. J., Belagaje, R., Brown, E. L., Fritz, R. H., Jones, R. A., Lees, R. G., and Khorana, H. G. (1978) Biochemistry 17 1257-1267 [DOI] [PubMed] [Google Scholar]
- 29.Nakata, M., Zanchetta, G., Chapman, B. D., Jones, C. D., Cross, J. O., Pindak, R., Bellini, T., and Clark, N. A. (2007) Science 318 1276-1279 [DOI] [PubMed] [Google Scholar]
- 30.Baldwin, G. S., Brooks. N. J., Robson, R. E., Wynveen, A., Goldar, A., Leikin, S., Seddon, J. M., and Kornyshev, A. A. (2008) J. Phys. Chem. B. 112 1060-1064 [DOI] [PubMed] [Google Scholar]
- 31.Olson, W. K. (1975) Nucleic Acids Res. 2 2055-2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neidle, S., and Parkinson, G. N. (2003) Curr. Opin. Struct. Biol. 13 275-283 [DOI] [PubMed] [Google Scholar]
- 33.Li, Y. L., Maurel, M. C., Ebel, C., Vergne, J., Pipich, V., and Zaccai, G. (2008) Eur. Biophys. J. 37 173-182 [DOI] [PubMed] [Google Scholar]
- 34.Meli, M., Vergne, J., Décout, J. L., and Maurel, M. C. (2002) J. Biol. Chem. 277 2104-2111 [DOI] [PubMed] [Google Scholar]
- 35.Meli, M., Vergne, J., and Maurel, M. C. (2003) J. Biol. Chem. 278 9835-9842 [DOI] [PubMed] [Google Scholar]
- 36.Norberg, J., and Nilsson, L. (1995) J. Am. Chem. Soc. 117 10832-10840 [Google Scholar]
- 37.Norberg, J., and Nilsson, L. (1995) J. Phys Chem. 99 13056-13058 [Google Scholar]
- 38.Norberg, J., and Nilsson, L. (1996) Biopolymers 39 765-768 [DOI] [PubMed] [Google Scholar]
- 39.Saenger, W. (1984) Principles of Nucleic Acid Structure, pp. 116-158, Springer Verlag, New York
- 40.Norberg, J., and Nilsson, L. (1998) Biophys. J. 74 394-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinder, J. C., Staynov, D. Z., and Gratzer, W. B. (1974) Biochemistry 13 5367-5373 [DOI] [PubMed] [Google Scholar]
- 42.Suck, D., Manor, P. C., and Saenger, W. (1976) Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 32 1727-1737 [Google Scholar]
- 43.Petrovic, A. G., and Polavarapu, P. L. (2005) J. Phys. Chem. B. 109 23698-23705 [DOI] [PubMed] [Google Scholar]
- 44.Lerman, L. S. (1961) J. Mol. Biol., 3 18-30 [DOI] [PubMed] [Google Scholar]
- 45.von Hippel, P. H., and McGhee, J. D. (1972) Annu. Rev. Biochem. 41 231-300 [DOI] [PubMed] [Google Scholar]
- 46.Jain, S. S., Anet, F. A. L., Stahle, C. J., and Hud, N. V. (2004) Angew. Chem. Int. Ed. Eng. 43 2004-2008 [DOI] [PubMed] [Google Scholar]
- 47.Reader, J. S., and Joyce, G. F. (2001) RNA 7 395-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul, N., and Joyce, G. F. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 12733-12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.