Abstract
Leucyl-tRNA synthetase (LeuRS) has an insertion domain, called connective peptide 2 (CP2), either directly preceding or following the editing domain (CP1 domain), depending on the species. The global structures of the CP2 domains from all LeuRSs are similar. Although the CP1 domain has been extensively explored to be responsible for hydrolysis of mischarged tRNALeu, the role of the CP2 domain remains undefined. In the present work, deletion of the CP2 domain of Giardia lamblia LeuRS (GlLeuRS) showed that the CP2 domain is indispensable for amino acid activation and post-transfer editing and that it contributes to LeuRS-tRNALeu binding affinity. In addition, its functions are conserved in both eukaryotic/archaeal and prokaryotic LeuRSs from G. lamblia, Pyrococcus horikoshii (PhLeuRS), and Escherichia coli (EcLeuRS). Alanine scanning and site-directed mutagenesis assays of the CP2 domain identified several residues that are crucial for its various functions. Data from the chimeric mutants, which replaced the CP2 domain of GlLeuRS with either PhLeuRS or EcLeuRS, showed that the CP2 domain of PhLeuRS but not that of EcLeuRS can partially restore amino acid activation and post-transfer editing functions, suggesting that the functions of the CP2 domain are dependent on its location in the primary sequence of LeuRS.
Aminoacyl-tRNA synthetases (aaRSs)2 catalyze the esterification of their cognate amino acids at the 3′-end of their cognate tRNAs in a two-step reaction: the synthesis of an aminoacyl-adenylate (aa-AMP) as an activated intermediate from an amino acid and ATP, and the subsequent transfer of the aminoacyl moiety to the 3′ terminus of the cognate tRNA to yield the aminoacyl-tRNA (1). Correctly charged tRNA is transferred to the ribosome, and the attached amino acid is incorporated into the protein.
The family of aaRSs is divided into two structurally distinct and apparently unrelated classes, which are considered to have evolved from two different ancestors, based on completely distinct folds of the aminoacylation domains (2). The class I aaRSs contain two signature peptides, HIGH and KMSKS, located in the characteristic nucleotide binding fold (Rossmann fold) of the active site domain for ATP binding and amino acid activation. The catalytic domain is interrupted by two major inserts, which are designated as connective peptide 1 (CP1) and connective peptide 2 (CP2) (3).
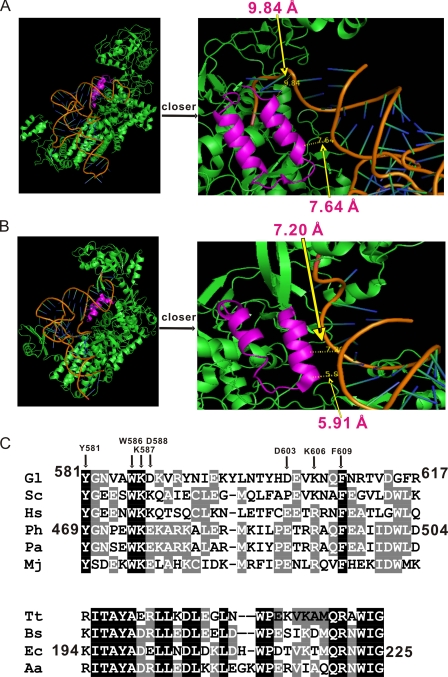
Leucyl-tRNA synthetase (LeuRS) belongs to subclass Ia of the group of aaRSs with cysteinyl-, isoleucyl-, methionyl-, and valinyl-tRNA synthetases (CysRS, IleRS, MetRS, and ValRS, respectively) (2). These aaRSs in class Ia share a common α-helical anticodon-binding domain. LeuRS is responsible for Leu-tRNALeu synthesis and has an aminoacylation catalytic core defined by a Rossmann fold (4). LeuRS, IleRS, and ValRS (LIV-RSs) edit their mistakes via a hydrolytic site within the CP1 domain, which is inserted into the catalytic Rossmann fold (4-7). The CP1 domain hydrolyzes mischarged aminoacyl-tRNAs (post-transfer editing) or misformed aa-AMPs (pre-transfer editing), which is deduced from the inability of LeuRS to effectively distinguish isosteric sets of amino acids that are structurally similar (i.e. Leu, Nov, Ile, and Met) (4-7). The isolated CP1 domains of Aquifex aeolicus LeuRS (AaLeuRS), Escherichia coli IleRS (EcIleRS), and Bacillus stearothermophilus ValRS (BsValRS) all have editing functions (8, 9). In addition, the CP2 domain exists in all three of the above mentioned aaRSs (10). The CP2 domain of prokaryotic and eukaryotic/archaeal LeuRSs consists of 32 and 36 amino acid residues, respectively (10). The CP2 domain of Pyrococcus horikoshii LeuRS (PhLeuRS) is inserted between the second and third β-strands of the CP core and consists of a pair of antiparallel α-helices and a connecting β-strand with the overall shape of “U” (10). Tertiary structure of LeuRS-tRNALeu, IleRS-tRNAIle, and ValRS-tRNAVal complexes in the post-transfer editing conformation suggested that the CP2 domain is spatially close to the acceptor stem of tRNA (Fig. 2, A and B) (6, 11, 12). However, the specific role of CP2 on amino acid activation, aminoacylation, and editing activities of LeuRS remains unknown.
FIGURE 2.
Primary and tertiary structure of the LeuRS CP2 domain. Crystal structures of PhLeuRS-tRNALeu complex in the aminoacylation conformation (A) (22) and TtLeuRS-tRNALeu in the post-transfer editing conformation (B) (11). The CP2 domains are colored in magenta, while the other parts of the LeuRSs are in green. tRNA is shown as an orange ribbon. The right parts of A and B are pictures with a closer look showing the relative positions and distances between the CP2 main chain and tRNALeu phosphate backbone. In A, 7.64 and 9.84 Å are marked, whereas in B, 5.91 and 7.20 Å are marked. C, primary sequence alignment of the CP2 domain from eukaryotic/archaeal and prokaryotic LeuRSs. Conserved and homologous residues are highlighted in black and gray, respectively. The three CP2 domains used in this study are indicated with their location in their respective LeuRSs. The amino acids of GlLeuRS in which site-directed mutagenesis was performed are highlighted by an arrow. The abbreviations of the organisms are as follows: Gl, Giardia lamblia; Sc, Saccharomyces cerevisiae; Hs, Homo sapiens; Ph, Pyrococcus horikoshii; Pa, Pyrococcus abyssi; Mj, Methanococcus jannaschii; Tt, Thurmus thermophilus; Bs, Bacillus subtilis; Ec, Escherichia coli; and Aa, Aquifex aeolicus.
Giardia lamblia is a unicellular eukaryote, among the most ancient eukaryotes, and causes prevalent giardiasis. LeuRS from G. lamblia (GlLeuRS) consists of 1173 amino acid residues and has been obtained by gene expression in our laboratory. Its properties, the function of CP1, and the role of a unique 49-amino acid motif within CP1 have been studied.3 Here, we studied the function of the CP2 domain in various LeuRSs from G. lamblia (eukaryote), E. coli (bacterium), and P. horikoshii (archaebacterium) (GlLeuRS, EcLeuRS, and PhLeuRS) by site-directed mutagenesis and performing leucine activation, aminoacylation, and post-transfer editing assays of these mutants.
EXPERIMENTAL PROCEDURES
Materials—l-Leucine, dithiothreitol (DTT), NTP, 5′-GMP, tetrasodium pyrophosphate, inorganic pyrophosphate, ATP, Tris-HCl, magnesium chloride, sodium chloride, and activated charcoal were purchased from Sigma. [l-3H]Leucine, [l-3H]isoleucine, and tetrasodium [32P]pyrophosphate were obtained from Amersham Biosciences (England). GF/C filters were purchased from Whatman (Germany). Pfu DNA polymerase, a DNA fragment rapid purification kit, and a plasmid extraction kit were purchased from the Biotech Co. T4 ligase and restriction endonucleases were obtained from MBI Fermentas. Ni2+-NTA Superflow was purchased from Qiagen. Pyrobest DNA polymerase and the dNTP mixture were obtained from Takara (Japan). Oligonucleotide primers were synthesized by Invitrogen. The pET28a(+) and pUC19 vectors were acquired from Novagen. T7 RNA polymerase was purified from an overproduction strain in our laboratory (15). E. coli tRNALeu was isolated from an E. coli overproduction strain in our laboratory (16).
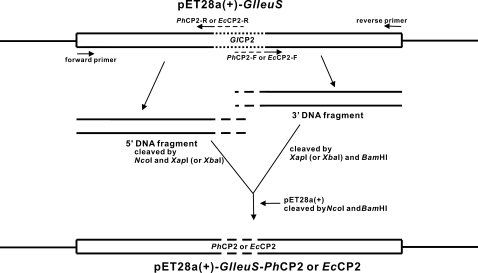
Construction of Various LeuRSs and Their Mutants—The plasmids containing the genes encoding GlLeuRS, EcLeuRS, PhLeuRS, pET28a(+)-glleuS,3 pET30a(+)-ecleuS (7), and pET28a(+)-phleuS (9) were constructed in our laboratory. Deletion and single-point mutants within their CP2 domains were constructed according to the protocol provided by the KOD-Plus-Mutagenesis kit (TOYOBO, Japan). As for the construction of the genes encoding GlLeuRS-PhCP2 and GlLeuRS-EcCP2, four long oligonucleotide primers were designed as follows: for GlLeuRS-PhCP2: PhCP2-F(XapI), 5′-GATGAAAATTTTGCCAGAGACCAGGAGGGCTCAGTTTGAAGCTATAATAGATTGGCTTGACGAATGGGCTGCCTCTCGTTCTTTTGGTTTGGG-3′ and PhCP2-R(XapI), 5′-TGGCAAAATTTTCATCCTTTCAAGTGCTTTTCTTGCTTTTTCTTTCCACTCTGGATTTCCATAATCGAGGTACCACTGATCAGCTGCAGCAACG-3′; for GlLeuRS-EcCP2: EcCP2-F(XbaI), 5′-ACGATCTAGATAAACTGGATCACTGGCCAGACACCGTTAAAACCATGCAGCGTAACTGGATCGGTGAATGGGCTGCCTCTCGTTCTTTTGGTTTGGG-3′ and EcCP2-R(XbaI), 5′-AGTTTATCTAGATCGTTGAGCAGCTCGTCAGCGTAAGCAGTGATTTTATCGAGGTACCACTGATCAGCTGCAGCAACG-3′. GlLeuRS-PhCP2 was constructed as follows (Fig. 1). First, the 5′ DNA fragment encoding GlLeuRS-PhCP2 was obtained by using a primer combination of the forward primer,3 PhCP2-F(XapI), and pET28a(+)-glleuS as the template. The 3′ DNA fragment encoding GlLeuRS-PhCP2 was obtained using the same method with the primer combination of PhCP2-R(XapI), and the reverse primer.3 Second, the 5′ DNA fragment and the 3′ DNA fragment were cleaved by NcoI and XapI, and XapI and BamHI, respectively, and were simultaneously ligated into pET28a(+) cleaved by NcoI and BamHI. GlLeuRS-EcCP2 was constructed according to the same method except that different primers and restriction enzymes were used. All sequences of the recombinant plasmids were confirmed by DNA sequencing.
FIGURE 1.
Representative diagram of pET28a(+)-GlleuS-PhCP2 or pET28a(+)-GlleuS-EcCP2 construction. The CP2 domain of GlLeuRS is represented by a dotted line, while the CP2 domain of PhLeuRS or EcLeuRS is represented by a dashed line.
Preparation of Various tRNALeus—G. lamblia tRNALeu(AAG) was obtained by T7 RNA polymerase transcription as described previously (17). Seven complementary and overlapping oligonucleotides encoding the T7 promoter, the gene, and its complementary chain were chemically synthesized by Invitrogen. Oligonucleotides were phosphorylated by T4 polynucleotide kinase, hybridized, and ligated by T4 DNA ligase into pUC19 between EcoRI and BamHI to produce pUC19-GltRNALeu. The in vitro T7 RNA polymerase transcription was carried out as described previously, and the accepting activity of GltRNALeu was 660 pmol/A260 (17). The P. horikoshii tRNALeu (PhtRNALeu) gene was cloned by the same method, and PhtRNALeu was obtained by in vitro T7 RNA polymerase transcription also with an accepting activity of 510 pmol/A260 (17). E. coli tRNALeu (EctRNALeu) was obtained from an overproduction strain in vivo in our laboratory, and its accepting activity was over 1300 pmol/A260 (16).
Purification of Proteins—E. coli BL21-Codon Plus (DE3)-RIL cells (Stratagene) were transformed with the plasmids containing the genes encoding GlLeuRS, EcLeuRS, PhLeuRS and their CP2 deletion and single-point mutants to overproduce the above LeuRSs and their mutants, respectively. A single colony of transformants was chosen and cultured in 500 ml of 2×YT medium (8 g of tryptone, 5 g of yeast extract, and 2.5 g of NaCl in 500 ml of sterile water) at 37 °C. When the cells were grown to mid-log phase (A600 = 0.6), isopropyl-1-thio-β-d-galactopyranoside was added to a final concentration of 0.5 mm, and cultivation continued for 6 h at 22 °C. The cells were collected by centrifugation at 5000 rpm for 20 min at 4 °C and washed twice with buffer A (10 mm imidazole, 300 mm NaCl, 10% glycerol, 0.5 mm phenylmethylsulfonyl fluoride, and 50 mm NaH2PO4, pH 8.0). The purification was carried out by affinity chromatography on Ni-NTA Superflow according to the manufacturer's protocol (Qiagen). Wet cells from 500 ml of culture were suspended in 10 ml of buffer A and sonicated on ice. The lysates were centrifuged at 10,000 rpm for 30 min. The supernatant was then ultracentrifuged at 4.6 × 104 rpm for 1 h to remove the debris and insoluble fractions. The supernatant was gently mixed with 1.5 ml of Ni-NTA Superflow resin for 1 h. The mixture was loaded onto a minicolumn for gravity flow chromatography. The resin was then washed with 30 ml of buffer B (20 mm imidazole, 300 mm NaCl, 10% glycerol, 0.5 mm phenylmethylsulfonyl fluoride, 50 mm NaH2PO4, pH 8.0) to remove nonspecific binding contaminants. Then the enzyme was eluted in sequence with 7 ml of buffer C (50 mm imidazole, 300 mm NaCl, 10% glycerol, 0.5 mm phenylmethylsulfonyl fluoride, and 50 mm NaH2PO4, pH 8.0), and 8 ml of buffer D (250 mm imidazole, 300 mm NaCl, 10% glycerol, 0.5 mm phenylmethylsulfonyl fluoride, and 50 mm NaH2PO4, pH 8.0). The eluted fractions were pooled, dialyzed, and concentrated by an Amicon Ultra-15 filter (Millipore, 30-kDa molecular mass cutoff), gently mixed with an equal volume of glycerol, and stored at -20 °C. All purification steps were carried out at 4 °C. All deletion mutants are properly folded and display little change in the secondary structure compared with respective wild-type LeuRS by CD analysis (data not shown). Protein concentrations were determined by the Bradford method (18). The amount of active enzyme in the preparation was ∼90% by active site titration (19).
Preparation of Mischarged tRNALeus—[3H]Ile-tRNALeu from GltRNALeu was prepared by incubating a reaction mixture containing 60 mm Tris-HCl (pH 8.2), 10 mm MgCl2, 2 mm DTT, 4 mm ATP, 20 μm [3H]Ile, 10 μm GltRNALeu, and 4 μm editing-defective GlLeuRS-D444A at 45 °C for 30 min.3 [3H]Ile-tRNALeu from PhtRNALeu was prepared by incubating a reaction mixture containing 60 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 4 mm ATP, 40 μm [3H]Ile, 10 μm PhtRNALeu, and 2 μm editing-defective PhLeuRS-D332A at 65 °C for 30 min (10). [3H]Ile-tRNALeu from EctRNALeu was prepared by incubating a reaction mixture containing 100 mm Tris-HCl (pH 7.8), 30 mm KCl, 12 mm MgCl2, 4 mm ATP, 0.1 mm EDTA, 0.5 mm DTT, 20 μm EctRNALeu, 20 μm [3H]Ile, and 5 μm editing-defective EcLeuRS-T252E at 37 °C for 30 min (20). The mischarged tRNAs were purified by repeated phenol/chloroform extractions, followed by ethanol precipitation.
Assay of ATP-PPi Exchange Reaction—The rate of the first reaction step of various LeuRSs, the formation of aa-AMP, was assayed by the ATP-PPi exchange reaction. The rates of the ATP-PPi exchange reactions catalyzed by GlLeuRS, EcLeuRS, and their mutants were carried out in 65 μl of reaction mixture containing 60 mm Tris-HCl (pH 8.2 or pH 7.8), 10 mm MgCl2, 2 mm DTT, 4 mm ATP, 1 mm Leu, 2 mm tetrasodium [32P]pyrophosphate, and 20 nm enzyme at 45 °C or 37 °C. Those by PhLeuRS and PhLeuRS-ΔCP2 were performed in 65 μl of reaction mixture containing 60 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 2 mm DTT, 4 mm ATP, 1 mm Leu, 2 mm tetrasodium [32P]pyrophosphate, and 50 nm enzymes at 65 °C. A 15-μl aliquot of reaction mixture was removed every 5 min and added to 200 μl of quenching solution containing 2% activated charcoal, 3.5% HClO4, and 50 mm tetrasodium pyrophosphate, and then mixed by vortex for 20 s. The solution was filtered through a Whatman GF/C filter, followed by washing with 20 ml of 10 mm tetrasodium pyrophosphate solution and 10 ml of 100% ethanol. The filters were dried and [32P]ATP was counted by a scintillation counter (Beckman Coulter).
Determination of Aminoacylation and Misaminoacylation—Leucylation of tRNALeu or isoleucylation of tRNALeu was carried out in 60 μl of reaction mixture containing 60 mm Tris-HCl (pH 8.2), 10 mm MgCl2, 2 mm DTT, 4 mm ATP, 20 μm [3H]Leu or [3H]Ile, 40 μm tRNALeu, and 50 nm GlLeuRS or its editing-defective mutants at 45 °C. Aliquots of 11 μl of reaction solution were removed at specific time points, quenched on What-man filter pads, and equilibrated with 5% trichloroacetic acid. The pads were washed three times for 15 min each with cold 5% trichloroacetic acid and then three times for 10 min each with 100% ethanol. The pads were dried under a heat lamp. The radioactivities of the precipitates were quantified by a scintillation counter (Beckman Coulter).
Hydrolytic Editing Assay—The [3H]Ile-tRNALeu deacylation assays were carried out at 37 °C in 60 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 2 mm DTT, 2 μm [3H]Ile-tRNALeu (from Gl-, Ec-, and PhtRNALeu, respectively, as indicated), and 20 nm each LeuRS or mutant, separately. Aliquots of 11 μl of reaction solution were removed at specific time points, quenched on What-man filter pads, washed, and analyzed as described above.
Determination of KD by Tryptophan Fluorescence Quenching—Equilibrium titrations were performed at room temperature with 0.1 μm of enzyme or the mutant in 60 mm Tris-HCl (pH 8.2), 10 mm MgCl2, and 2 mm DTT. Tryptophan fluorescence was excited at 295 nm. An emission wavelength of 338 nm was used to quantify binding after correction for dilution and for the inner filter effect. Control solutions of bovine serum albumin or tryptophan were performed to show that there was no fluorescence response to tRNA. The KD values were determined by fitting fluorescence intensity change data versus tRNA concentration using Originpro 7.5 software.
RESULTS
Deletion of CP2 Domain Abolished Leucine Activation and Post-transfer Editing—G. lamblia contains a eukaryotic/archaeal LeuRS with 1173 amino acid residues.3 The tertiary structures of PhLeuRS-tRNALeu and TtLeuRS-tRNALeu complexes show that the CP2 domains are very close to the acceptor stem of tRNALeu (Fig. 2, A and B). For example, The distance between PhLeuRS-CP2 main chain and tRNALeu acceptor stem phosphate backbone is ∼9 Å (7.64 and 9.84 Å marked in Fig. 2A); that of TtLeuRS-CP2 main chain and tRNALeu acceptor stem phosphate backbone is ∼7 Å (5.91 and 7.20 Å marked in Fig. 2B). By sequence alignment of various LeuRSs, GlLeuRS is 39.2% homologous to PhLeuRS and 25.4% homologous to TtLeuRS. The CP2 domain of GlLeuRS (GlLeuRS-CP2) contains 37 amino acid residues from Tyr-581 to Arg-617, and that of EcLeuRS (EcLeuRS-CP2) contains 32 residues from Lys-194 to Gly-225, according to the structures of PhLeuRS and TtLeuRS (Fig. 2C).
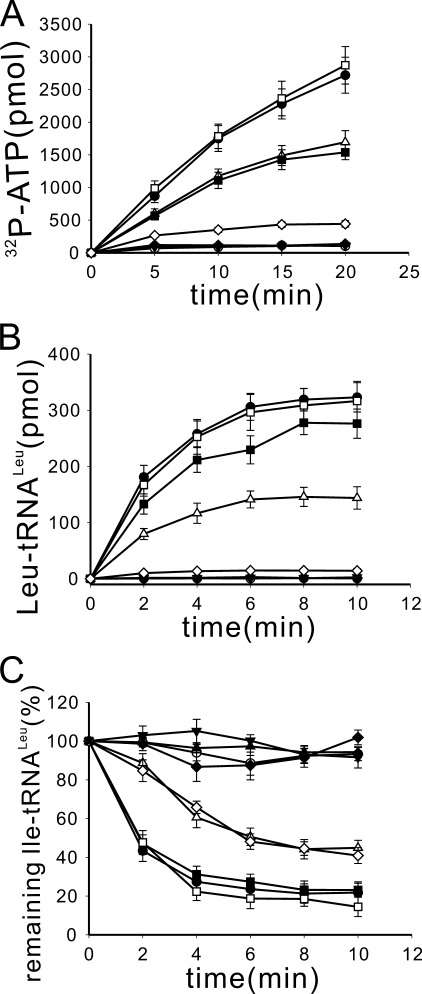
To investigate the effect of GlLeuRS-CP2 on various activities, the mutant GlLeuRS-ΔCP2, which replaced GlLeuRS-CP2 with three Ala residues, was constructed. Based on the crystal structure of PhLeuRS (10), three Ala residues would function as a linker to maintain the approximate distance between the second and the third β-strands of the CP core. GlLeuRS-ΔCP2 with an N-terminal 6-His tag was stably produced in an E. coli strain containing its gene and purified to over 90% homogeneity by Ni-NTA affinity chromatography (data not shown). The activation, aminoacylation, and post-transfer editing activities of GlLeuRS-ΔCP2 were assayed. GlLeuRS-ΔCP2 did not activate cognate leucine at all in the ATP-PPi exchange assay (supplemental Fig. S1A), indicating that the CP2 domain plays an indispensable role in amino acid activation. The mutant could not leucylate tRNALeu, as expected (data not shown). Compared with the post-transfer editing ability of GlLeuRS, the mutant could not catalyze Ile-tRNALeu hydrolysis either, and its editing activity was absolutely lost (supplemental Fig. S1B).
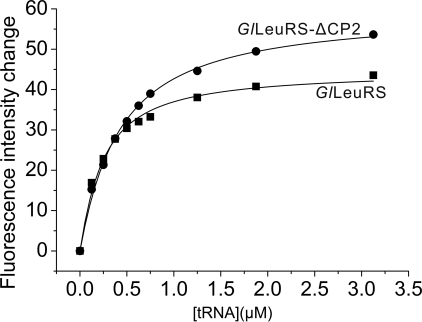
The CP2 Domain Contributed to LeuRS-tRNALeu Binding Affinity—Because of the inability of GlLeuRS-ΔCP2 to activate leucine, the contribution of GlLeuRS-CP2 to the binding of tRNALeu could not be measured by aminoacylation kinetics. Intrinsic tryptophan equilibrium fluorescence was carried out to test the role of GlLeuRS-CP2 in the binding affinity for tRNALeu. The excitation and emission wavelengths were 295 nm and 338 nm, respectively. Fluorescence quenching of the enzyme by tRNALeu titration was measured, and the KD values of GlLeuRS and its mutant with tRNALeu were calculated. For GlLeuRS and GlLeuRS-ΔCP2, the KD values were 0.19 μm (±0.02 μm) and 0.37 μm (±0.02 μm), respectively, indicating that the GlLeuRS-ΔCP2 has looser binding with tRNALeu and GlLeuRS-CP2 should contribute to optimal tRNALeu binding (Fig. 3).
FIGURE 3.
Quenching of intrinsic tryptophan fluorescence of the wild-type GlLeuRS (▪) and its CP2 deletion mutant (•) through tRNALeu titration. The concentration of tRNA was varied from 0 to 3 μm. The fluorescence intensity is given as an arbitrary unit, and the emission at 338 nm was quantified. Analysis of the wild-type GlLeuRS and its CP2 deletion mutant fluorescence intensity change versus tRNA concentration gave the KD values of 0.19 ± 0.02 μm and 0.37 ± 0.02 μm, respectively.
Important Residues of GlLeuRS-CP2 Were Identified by Alanine Scanning—Sequence alignment from several eukaryotic/archaeal LeuRSs showed that residues in GlLeuRS-CP2 are not strictly conserved (Fig. 2C). To find the crucial residues for the functions of GlLeuRS-CP2, some conserved and (or) semi-conserved amino acid residues (Tyr-581, Trp-586, Lys-587, Asp-588, Asp-603, Lys-606, and Phe-609) were selected and mutated to Ala (Fig. 2C). These single-point mutants were named as GlLeuRS-Y581A, GlLeuRS-W586A, GlLeuRS-K587A, GlLeuRS-D588A, GlLeuRS-D603A, GlLeuRS-K606A, and GlLeuRS-F609A, respectively. Their amino acid activation, aminoacylation, and post-transfer editing activities were assayed and are shown in Fig. 4. Compared with GlLeuRS, GlLeuRS-D603A had similar amino acid activation, aminoacylation, and post-transfer editing activities, indicating that Asp-603 is not a critical residue for the functions of the enzyme. These results were consistent with the fact that some other eukaryotic/archaeal LeuRSs have this aspartic acid replaced with glutamic acid or proline (Fig. 2C). GlLeuRS-K587A and GlLeuRS-D588A displayed lower amino acid activation and aminoacylation activities than GlLeuRS. In addition, GlLeuRS-K587A had a decreased post-transfer editing activity; however, GlLeuRS-D588A had a similar editing activity as GlLeuRS. The kinetic parameters of the ATP-PPi exchange reaction showed that GlLeuRS-K587A and GlLeuRS-D588A displayed similar Km values for both leucine and ATP, but the kcat was about half of that of GlLeuRS, so the relative catalytic efficiency (kcat/Km) was about half of that of GlLeuRS (Table 1 and supplemental Table S1). These results suggest that these two residues are important but not indispensable for GlLeuRS-CP2 (Fig. 4, A-C). GlLeuRS-Y581A, GlLeuRS-W586A, and GlLeuRS-K606A lost their amino acid activation, aminoacylation, and post-transfer editing activities completely. GlLeuRS-F609A lost its synthetic activity, and its post-transfer editing activity decreased drastically (Fig. 4, A-C). These data indicated that Tyr-581, Trp-586, Lys-606, and Phe-609 are the crucial residues for both the synthetic and editing functions of GlLeuRS.
FIGURE 4.
Enzymatic activities of wild-type GlLeuRS and its mutants in the alanine scanning assay. A, the ATP-PPi exchange reaction was carried out using 1 mm Leu and 20 nm enzyme. B, the aminoacylation assay was carried out using 50 nm GlLeuRS or its mutants. C, post-transfer editing was performed using 2 μm [3H]Ile-tRNALeu from GltRNALeu and 20 nm GlLeuRS or its mutants. Symbols are GlLeuRS (•), GlLeuRS-Y581A (○), GlLeuRS-W586A (▾), GlLeuRS-K587A (▵), GlLeuRS-D588A (▪), GlLeuRS-D603A (□), GlLeuRS-K606A (♦), and GlLeuRS-F609A (⋄). Control (spontaneous hydrolysis) (▴) was performed in the absence of enzyme.
TABLE 1.
Relative kcat/Km of various mutants from G/LeuRS in ATP-PPi exchange reaction
| Substrate | GlLeuRS | K587A | D588A | D603A | K606R | K606E | K606L | K606D | |
|---|---|---|---|---|---|---|---|---|---|
| kcat/Km | |||||||||
| ATP | 1 | 0.43 | 0.57 | 0.91 | 0.98 | 0.90 | 0.85 | 0.86 | |
| Leu | 1 | 0.42 | 0.37 | 0.94 | 0.99 | 0.99 | 0.98 | 0.83 | |
Functional Analysis of Pivotal Residues Was Performed by Site-directed Mutagenesis—The effect of side chains of these crucial residues on the activities was analyzed by further site-directed mutagenesis studies. The side chain of Tyr-581 is composed of a hydroxyl group attached to a benzene ring. We mutated it into serine and phenylalanine to keep either the hydroxyl group or the benzene ring of the side chain, respectively. Tyr-581 was also mutated to glutamic acid to see the effect of a negative charge and to lysine to see the effect of a positive charge. GlLeuRS-Y581S, GlLeuRS-Y581E, and GlLeuRS-Y581K were all unable to activate leucine by the ATP-PPi exchange assay, and they all had no post-transfer editing activity. Although GlLeuRS-Y581F had some detectable but significantly reduced leucine activation activity, its post-transfer editing activity was similar to that of GlLeuRS (Fig. 5, A and B). Two hydrophobic and absolutely conserved residues, Trp-586 and Phe-609, in the CP2 domains of eukaryotic/archaeal LeuRSs were mutated to glutamic acid and lysine, respectively. All four mutants lost leucine activation activity (Fig. 5, C and G). Their post-transfer editing activities were either lost or were very weak (Fig. 5, D and H). These results indicate that both Trp-586 and Phe-609 play crucial roles in interacting with mischarged tRNA in the post-transfer editing complex formation. This role may be accompanied by a hydrophobic interaction; all of the mutants with glutamic acid, lysine, and alanine at these residues abolished or impaired editing activity (Figs. 4C, 5D, and 5H). Lys-606 is a semi-conserved residue and is replaced with arginine in other eukaryotic/archaeal LeuRSs (Fig. 2C). Its conservation might indicate that its positive charge is necessary for its function. The two mutants GlLeuRS-K606R and GlLeuRS-K606E, which replaced Lys-606 with positively charged arginine and negatively charged glutamic acid, had no difference in leucine activation and post-transfer editing activities compared with GlLeuRS (Fig. 5, E and F). In addition, the mutant GlLeuRS-K606L with a large side chain and the mutant GlLeuRS-K606D with a negative charge had similar leucine activation and post-transfer editing activities as GlLeuRS (Fig. 5, E and F). Likewise, their kcat and Km values for both ATP and leucine, and the relative kcat/Km in the ATP-PPi exchange reaction were similar to those of GlLeuRS (Table 1 and supplemental Table S1). Because the mutant GlLeuRS-K606A with the smallest side chain was inactive, the above results suggest that a given size but not electric charge of the side chain of the residue at position 606 may be important for leucine activation and post-transfer editing activities.
FIGURE 5.
Enzymatic activities of mutants on Tyr-581, Trp-586, Lys-606, and Phe-609. The ATP-PPi exchange reaction was carried out using 1 mm Leu, and 20 nm mutated enzyme on Tyr-581 (A), Trp-586 (C), Lys-606 (E), and Phe-609 (G); post-transfer editing was performed using 2 μm [3H]Ile-tRNALeu from GltRNALeu, and 20 nm mutated enzyme on Tyr-581 (B), Trp-586 (D), Lys-606 (F), and Phe-609 (H). In A and B, symbols are GlLeuRS-Y581F (○), GlLeuRS-Y581S (▾), GlLeuRS-Y581E (▵), GlLeuRS-Y581K (▪), and hydrolytic control (□); in C and D, symbols are GlLeuRS-W586E (○), GlLeuRS-W586K (▾), and hydrolytic control (▵); in E and F, symbols are GlLeuRS-K606E (○), GlLeuRS-K606R (▾), GlLeuRS-K606L (▵), GlLeuRS-K606D (▪), and hydrolytic control (□); in G and H, symbols are GlLeuRS-F609E (○), GlLeuRS-F6096K (▾), and hydrolytic control (▵). Wild-type GlLeuRS is represented by • in all figures.
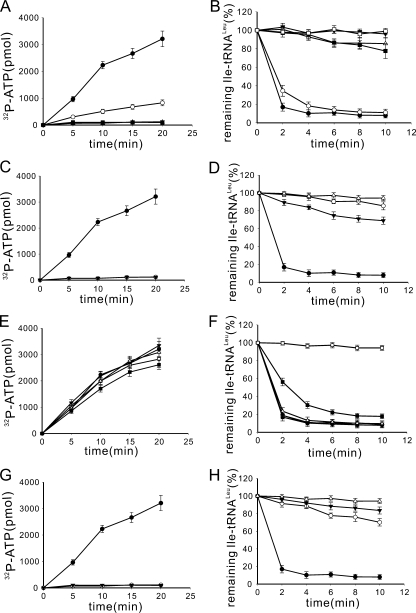
Role of CP2 Domain Was Conserved in LeuRSs from Different Species—The CP2 domain has a different insertion point in LeuRSs from prokaryotes (before the CP1 domain) and eukaryotes/archaea (after the CP1 domain) to adapt to different tRNA recognition modes (10). Its sequence is more conserved in prokaryotic LeuRSs than in eukaryotic LeuRSs, albeit they show similar global structures (Fig. 2). Additionally, the CP2 domains from prokaryotic LeuRSs display significant sequence differences from those from eukaryotic LeuRSs (Fig. 2C). Whether the function of the CP2 domain is conserved despite its insertion point and sequence difference was studied. Based on the CP2 domain crystal structures of TtLeuRS and PhLeuRS (4, 10), EcLeuRS-ΔCP2, which replaced the CP2 domain of EcLeuRS (prokaryotic) by five Ala residues, and PhLeuRS-ΔCP2, which substituted three Ala residues for the CP2 domain of PhLeuRS (archaeal), were constructed. The distance of the two ends of the CP2 domain of TtLeuRS is 16.22 Å, whereas that of PhLeuRS is 8.22 Å; we hypothesized that these Ala linker peptides would maintain the approximate distances between the two ends. These proteins were purified to over 90% homogeneity by affinity chromatography using an N-terminal 6-His tag. The leucine activation, aminoacylation, and hydrolysis of mischarged tRNALeu activities of the two native LeuRSs and the above deletion mutants were measured and are shown in supplemental Fig. S2. As compared with EcLeuRS and PhLeuRS, EcLeuRS-ΔCP2 (supplemental Fig. S2, A and B) and PhLeuRS-ΔCP2 (supplemental Fig. S2, C and D), like GlLeuRS-ΔCP2, lost their leucine activation, aminoacylation (data not shown), and post-transfer editing activities. The relatively lower post-transfer editing activity of PhLeuRS was assayed at 37 °C, which is not the optimal temperature for PhLeuRS (10). These results show that the role of the CP2 domain in amino acid activation and post-transfer editing is conserved among various LeuRSs from different species, although the CP2 domains are located at various positions and have different sequences among prokaryotic and eukaryotic/archaeal LeuRSs.
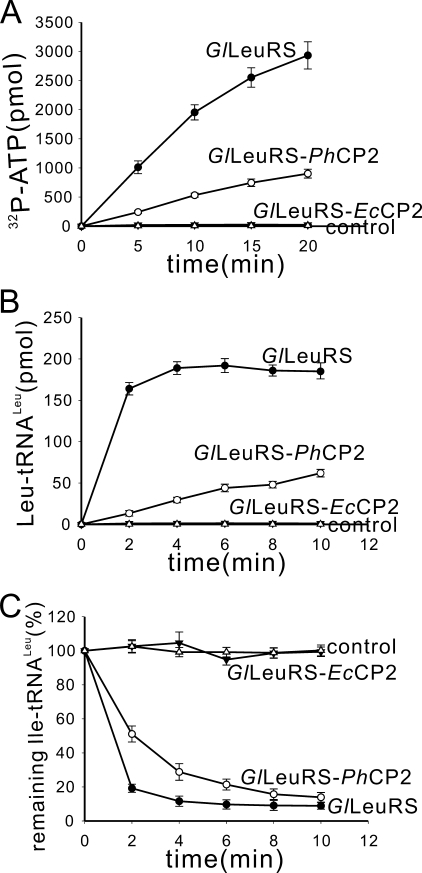
The CP2 Domain from PhLeuRS but Not EcLeuRS Can Substitute That of GlLeuRS—To understand whether the role of the CP2 domain is dependent on its conserved conformation or divergent location in the primary sequence of different species of LeuRSs, the CP2 domain of GlLeuRS was substituted by the CP2 domains of PhLeuRS (from Tyr-469 to Asp-504) (10) and EcLeuRS (from Lys-194 to Gly-225), and the chimeric mutants were named GlLeuRS-PhCP2 and GlLeuRS-EcCP2, respectively. Both proteins were overproduced stably and efficiently in E. coli transformants containing their genes and purified by N-terminal six-His affinity chromatography.
The ATP-PPi exchange, aminoacylation, and hydrolysis of Ile-tRNALeu data showed that GlLeuRS-PhCP2 had these three activities (Fig. 6, A-C), although the three activities of the chimeric GlLeuRS with the CP2 domain of PhLeuRS decreased significantly as compared with those of GlLeuRS. However, GlLeuRS-EcCP2 lost amino acid activation, aminoacylation, and post-transfer editing activities (Fig. 6, A-C). These results suggest that Leu-AMP formation and enzyme-tRNA interaction are possibly very sensitive to the different position of the CP2 domain rather than its similar global domain structure. The different location of EcLeuRS-CP2 in the chimeric GlLeuRS-EcCP2 from its natural position in the EcLeuRS may account for the functional absence of GlLeuRS-EcCP2.
FIGURE 6.
Enzymatic activities of two chimeric enzymes, GlLeuRS-PhCP2 and GlLeuRS-EcCP2. A, the ATP-PPi exchange reaction was carried out using 1 mm Leu and 20 nm enzyme. B, the aminoacylation assay was carried out using 50 nm enzyme. C, post-transfer editing was performed using 2 μm [3H]Ile-tRNALeu from GltRNALeu and 20 nm enzyme. Wild-type GlLeuRS, GlLeuRS-PhCP2, and GlLeuRS-EcCP2 are represented by •, ○, and ▾. Controls without leucine in A, without enzyme in B, and of spontaneous hydrolysis in C are represented by ▵.
DISCUSSION
The presence of CP2 domains in LIV-RSs with similar lengths and conformations indicates that the CP2 domain may have emerged before the divergence of these three related aaRSs. The CP2 domain is composed of two antiparallel α-helices and a connecting β-strand, but its sequence between bacteria and eukaryotic/archaeal LeuRSs is not conserved. Meanwhile, the CP2 domains of LeuRSs from different groups have different insertion points into the aminoacylation active site, which is derived from a distinct editing domain (the CP1 domain) insertion point and orientation patterns (10).
Until now, the function of the CP2 domain of class I aaRSs was only studied in Acidithiobacillus ferrooxidans tryptophanyl-tRNA synthetase (TrpRS) (21). A deletion mutant in the CP2 domain of A. ferrooxidans TrpRS retained in vitro and in vivo activity and showed no effect on amino acid activation, suggesting that the conformation of the activation domain of TrpRS was not distorted. However, the CP2 domain deletion mutant of A. ferrooxidans TrpRS had a higher Km value for cognate tRNATrp, showing that the CP2 domain is involved in tRNA binding (21). In the LIV-RSs system, although x-ray structures of T. thermophilus ValRS-tRNAVal (6), T. thermophilus LeuRS-tRNALeu (11), and S. aureus IleRS-tRNAIle (12) complexes in the post-transfer editing conformation and P. horikoshii LeuRS-tRNALeu (22) in the aminoacylation conformation all show that the CP2 domain and the tRNA acceptor stem are close to each other (a little less than 10 Å between the CP2 main chain and tRNA acceptor stem phosphate backbone in PhLeuRS-tRNALeu and TtLeuRS-tRNALeu complexes) (Fig. 2, A and B), the direct interaction between CP2 and the acceptor stem of tRNA was not indicated, and the specific role of CP2 has not been reported.
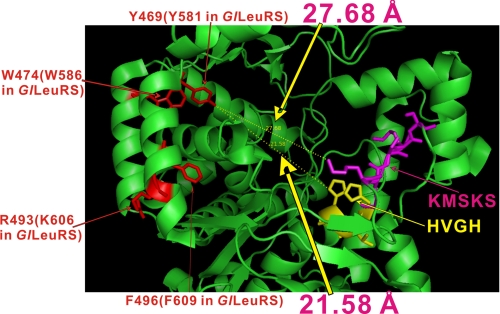
Our results showed that the CP2 domain of GlLeuRS, PhLeuRS, or EcLeuRS significantly affects amino acid activation and post-transfer editing. This is consistent with the fact that this domain in LeuRSs is located close to the Rossmann fold, and its connecting β-strand forms a β-sheet with the other four β-strands in the Rossmann fold (10). By analyzing the effect of alanine scanning and single-point mutations within CP2 on amino acid activation and post-transfer editing activities, the crucial residues were identified that played multiple roles during the entire precise tRNALeu-charging process. Based on the structure of the archaeal PhLeuRS (10), the CP2 domain is at the opposite side of the two signature sequences of class I aaRSs, HIGH and KMSKS, in the Rossmann fold, which have been shown to be responsible for ATP binding and stabilization of the transition state during catalysis (1) (Fig. 7). The crucial residues Tyr-581, Trp-586, Lys-606, and Phe-609 of GlLeuRS (Tyr-469, Trp-474, Arg-493, and Phe-496 in PhLeuRS) all penetrated into the catalytic core; however, it is a long distance from these residues to HIGH and KMSKS (for example, the side hydroxyl group of Tyr-469 of PhLeuRS is 21.58 and 27.68 Å from the NH group of the second histidine of “HVGH” and the ε-NH3 group of the first lysine of “KMSKS,” respectively) (Fig. 7). Thus, it is unlikely that these residues in the CP2 domain directly bind leucine and ATP during leucine activation. This is consistent with our kinetic data that the activity-retaining mutants on Lys-587, Asp-588, Asp-603, and Lys-606 displayed no difference in Km values for leucine and ATP from those of GlLeuRS (supplemental Table S1). Consistently, the crystal structure of TtLeuRS complexed with a Leu-adenylate analogue (Leu-AMS) also showed that the residues in the CP2 domain are not in direct contact with Leu-AMS (4). Therefore, we suggest that the CP2 domain plays its pivotal role in leucine activation by modulation of the exact conformation of the active site through indirect residue interaction between the CP2 domain and active site.
FIGURE 7.
Crystal structure of Rossmann fold of PhLeuRS (10) showing positions of the crucial residues in the CP2 domain relative to the two class I aaRS signature sequences, HVGH and KMSKS (1). The four residues corresponding to Tyr-581, Trp-586, Lys-606, and Phe-609 of GlLeuRS (Tyr-469, Trp-474, Arg-493, and Phe-496 in PhLeuRS) are colored in red, whereas HVGH and KMSKS are highlighted in yellow and magenta, respectively. The distances between the side hydroxyl group of Tyr-469 and the NH group of the second histidine of “HVGH” (21.58 Å) and the ε-NH3 group of the first lysine of “KMSKS” (27.68 Å) are marked. Other parts of PhLeuRS, including the anticodon binding domain, the CP1 domain, and the C-terminal domain, etc. were omitted for clarity.
Tyr-581 is of great importance for amino acid activation and post-transfer editing. When Tyr was changed to Phe, the mutant had significantly decreased amino acid activation activity but similar post-transfer editing activity compared with wild-type GlLeuRS. However, when Tyr was replaced with Ser, Glu, and Lys, the amino acid activation and editing activities of the mutants were abolished completely. Therefore, we suggest that the hydroxyl group of Tyr-581 is crucial for amino acid activation. The benzene ring may function as a linker to support the terminal hydroxyl group to reach into the catalytic core during amino acid activation; because the mutant GlLeuRS-Y581S, with the hydroxyl group and without the benzene ring, is unable to activate amino acid. Meanwhile, the benzene ring of Tyr-581 is of great importance for post-transfer editing. We speculate that the benzene ring might insert between two stacking base pairs of the acceptor stem or form hydrophobic interactions with bases of the acceptor stem, based on the proximity of the CP2 domain and the acceptor stem revealed by the cocrystal structure of TtValRS-tRNAVal (6), TtLeuRS-tRNALeu (11), SaIle-tRNAIle (12), and PhLeuRS-tRNALeu (22). Trp-586 and Phe-609 might function through hydrophobic interactions with other residues of the activation domain and mischarged tRNA. As for Lys-606, it was surprisingly found that it could be replaced by four amino acid residues with a large side chain of varying polarity (Leu, Glu, Arg, and Asp). However, when Lys-606 was substituted with Ala, the mutant lost its synthetic and editing activities completely. The size of the side chain of residue 606 may be important to its activities; however, the detailed chemical mechanism remains unclear.
After removal of the CP2 domain, the dissociation constant of GlLeuRS-ΔCP2-tRNALeu was elevated 2-fold, indicating that the CP2 domain contributed to LeuRS-tRNALeu binding affinity. This is consistent with the CP2 domain of A. ferrooxidans TrpRS (21). The deletion mutant was also unable to hydrolyze mischarged tRNA. Combined with a series of co-crystal structures of TtLeuRS-tRNALeu, SaIleRS-tRNAIle, and TtValRS-tRNAVal in the post-transfer editing conformation (6, 11, 12), our results suggested that GlLeuRS-ΔCP2 may be defective in orienting the 3′-end of the mischarged tRNALeu into the editing active site located in the CP1 domain, because the interaction between the acceptor stem of mischarged tRNA and LeuRS is disturbed.
Supplementary Material
This work was funded by the National Key Basic Research Foundation of China (Grant 2006CB910301), the Natural Science Foundation of China (Grant 30670463), and the Committee of Science and Technology in Shanghai (Grant 06JC14076). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Table S1.
Footnotes
The abbreviations used are: aaRS, aminoacyl-tRNA synthetase; CP1, connective peptide 1; CP2, connective peptide 2; IleRS, isoleucyl-tRNA synthetase; LeuRS, leucyl-tRNA synthetase; TrpRS, tryptophanyl-tRNA synthetase; ValRS, valyl-tRNA synthetase; aa-AMP, aminoacyl-adenylate; DTT, dithiothreitol; Ni-NTA, nickel-nitrilotriacetic acid.
X.-L. Zhou, P. Yao, L.-L. Ruan, B. Zhu, J. Luo, L.-H. Qu, and E.-D. Wang, submitted for publication.
References
- 1.Ibba, M., and Söll, D. (2000) Annu. Rev. Biochem. 69 617-650 [DOI] [PubMed] [Google Scholar]
- 2.Eriani, G., Delarue, M., Poch, O., Gangloff, J., and Moras, D. (1990) Nature 347 203-206 [DOI] [PubMed] [Google Scholar]
- 3.Starzyk, R. M. Webster, T. A., and Schimmel, P. (1987) Science 237 1614-1618 [DOI] [PubMed] [Google Scholar]
- 4.Cusack, S., Yaremchuk, A., and Tukalo, M. (2000) EMBO J. 19 2351-2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nureki, O., Vassylyev, D., Tateno, M., Shimada, A., Nakama, T., Fukai, S., Konno, M., Hendrickson, T., Schimmel, P., and Yokoyama, S. (1998) Science 280 578-582 [DOI] [PubMed] [Google Scholar]
- 6.Fukai, S., Nureki, O., Sekine, S., Shimada, A., Tao, J., Vassylyev, D. G., and Yokoyama, S. (2000) Cell 103 793-803 [DOI] [PubMed] [Google Scholar]
- 7.Chen, J. F., Guo, N. N., Li, T., Wang, E. D., and Wang, Y. L. (2000) Biochemistry 39 6726-6731 [DOI] [PubMed] [Google Scholar]
- 8.Lin, L., Hale, S. P., and Schimmel, P. (1996) Nature 384 33-34 [DOI] [PubMed] [Google Scholar]
- 9.Zhao, M. W., Zhu, B., Hao, R., Xu, M. G., Eriani, G., and Wang, E. D. (2005) EMBO J. 24 1430-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukunaga, R., and Yokoyama, S. (2005) J. Mol. Biol. 346 57-71 [DOI] [PubMed] [Google Scholar]
- 11.Tukalo, M., Yaremchuk, A., Fukunaga, R., Yokoyama, S., and Cusack, S. (2005) Nat. Struct. Mol. Biol. 12 923-930 [DOI] [PubMed] [Google Scholar]
- 12.Silvian, L. F., Wang, J., and Steitz, T. A. (1999) Science 285 1074-1077 [PubMed] [Google Scholar]
- 13.Deleted in proof
- 14.Deleted in proof
- 15.Li, Y., Wang, E. D., and Wang, Y. L. (1998) Protein Expr. Purif. 16 355-358 [DOI] [PubMed] [Google Scholar]
- 16.Li, Y., Wang, E. D., and Wang, Y. L. (1998) Sci. China (Ser C) 41 225-231 [Google Scholar]
- 17.Li, Y., Chen, J. F., Wang, E. D., and Wang, Y. L. (1999) Sci China (Ser C) 42 185-190 [DOI] [PubMed] [Google Scholar]
- 18.Bradford, M. M. (1976) Anal. Biochem. 72 248-254 [DOI] [PubMed] [Google Scholar]
- 19.Xu, M. G., Chen, J. F., Martin, F., Zhao, M. W., Eriani, G., and Wang, E. D. (2002) J. Biol. Chem. 277 41590-41596 [DOI] [PubMed] [Google Scholar]
- 20.Xu, M. G., Li, J., Du, X., and Wang, E. D. (2004) Biochem. Biophys. Res. Commun. 318 11-16 [DOI] [PubMed] [Google Scholar]
- 21.Zúñiga, R., Salazar, J., Canales, M., and Orellana, O. (2002) FEBS Lett. 532 387-390 [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga, R., and Yokoyama, S. (2005) Nat. Struct. Mol. Biol. 12 915-922 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.