Abstract
Methylprednisolone (MP), a synthetic glucocorticoid agonist, is widely used for the clinical therapy of white matter diseases in the nervous system, such as spinal cord injury and multiple sclerosis. In addition to its potent anti-inflammatory and antioxidant properties, we recently discovered a selective antiapoptotic effect of MP on oligodendrocytes via the activation of the glucocorticoid receptor (GR) and the upregulation of bcl-XL, a splicing isoform of the bcl-x gene. Based on published findings of the functional interactions between GR and STAT5, a transcription factor from the family of signal transducers and activators of transcription (STAT), we examined whether the glucocorticoid signaling pathway interacts with STAT5 to upregulate bcl-XL and protect oligodendrocytes. We show herein that (1) the GR and STAT5 complex is present on the STAT5-binding site of the bcl-x promoter region in oligodendrocytes; (2) the overexpression of an activated form of STAT5 prevents α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-induced oligodendrocyte cell death; and (3) this prevention is lost when the STAT5 gene is knocked down. Thus, our results provide one molecular mechanism underlying the postinjury protective effects of oligodendrocytes by stress hormones.
Keywords: STAT5, glucocorticoid receptor, methylprednisolone, stress hormone, oligodendrocyte, spinal cord injury
Introduction
To survive in a challenging environment, almost all complex organisms have evolutionarily developed a stress response system. In human and other vertebrates, the adrenal steroid glucocorticoids (GCs), a major component of the stress response system, modulate and control the stress response at a genomic level by regulating gene expression directly (for review, see Kino, 2007; Viegas et al., 2008). Clinically, methylprednisolone (MP), a synthetic GC agonist with potent anti-inflammatory and antioxidant properties, is the mainstay of therapy for a variety of neurological disorders involving white matter injury such as spinal cord injury and multiple sclerosis (Hall and Springer, 2004). Recently, we found that MP selectively inhibits oligodendrocyte (OLG) death via the glucocorticoid receptor (GR) and upregulates the expression of bcl-XL (Lee et al., 2008). However, the subsequent molecular cascades underlying the upregulation of bcl-XL remained unknown.
Stress hormones regulate gene expression mainly although interaction with their intracellular receptors. GR is a ligand regulated transcription factor primarily localized in the cytoplasm. Upon ligand binding, GR dimerizes, translocates into the nucleus, and modulates gene expression by two pathways: direct interactions with the specific GC response elements (GREs) of target genes or interactions with other transcription factors such as STAT5, NF-κB and AP-1 (Beato et al., 1996; Datson et al., 2008). GREs are found in the bcl-X promoter region (Caron-Leslie et al., 1994; Broome et al., 2002); however, the upregulation or downregulation of bcl-XL product after the treatment of GCs occurs in a cell-dependent context, probably due to composite regulatory cross-talks among multiple signaling pathways. For example, in monocytes, macrophages, and T lymphocytes, GCs downregulate the expression level of bcl-XL and induce apoptosis (Amsterdam et al., 2002; Rocha-Viegas et al., 2006), whereas GCs upregulate bcl-XL to protect against apoptosis in many other cell types (Lotem and Sachs, 1995; Schorr and Furth, 2000). In OLGs, it was unclear how MP upregulated the expression of bcl-XL. Previous work shows that after GCs bind to GR, GR binds to STAT5 directly and modifies its transcriptional activity (Stöcklin et al., 1996). Two STAT5 proteins (A and B, which are 96% homologous) can upregulate bcl-x gene expression in certain cell types (Catlett-Falcone et al., 1999; Dumon et al., 1999; Battle and Frank, 2002; Kirito et al., 2002). Putative STAT5-binding sites have been identified on the promoter of the bcl-x gene (Silva et al., 1999; Socolovsky et al., 1999). We show herein for the first time that in OLGs, MP upregulates the expression of bcl-XL via a direct binding of the GR/STAT5 complex on the putative STAT5-binding site.
Materials and Methods
Oligodendrocyte culture.
OLG cultures were prepared as described previously (Lee et al., 2008). Rat brain cortex (postnatal 1–2 d old) was loosely homogenized in DMEM with 10% calf serum, filtered (80 μm nylon mesh), and centrifuged at 350 g for 10 min. The cells were grown in 75 mm flasks (1.5 brains per flask) until confluent, agitated at 180 rpm at 37°C for 6 h to remove microglia, and again for another 18 h to harvest OLGs. The suspension containing OLGs was filtered through a 10 μm nylon mesh, resuspended in a chemically defined medium [DMEM/F-12 in l L containing 5 μg selenium, 2.4 μg biotin, 5 mg insulin, 110 mg pyruvate, 3.6 μg hydrocortisole, 1 g BSA (fraction V), 10% fetal bovine serum, 15 mm HEPES, 10 μg triiodothyronine (T3), 50 μg transferrin iron-saturated supplement] and plated onto 100 mm dishes or 24-well plates. Experiments were performed on OLGs grown for 3–5 d. OLG viability was quantitated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay as described previously (Lee et al., 2008).
Real-time PCR.
The application of real-time (RT)-PCR to assess gene expression has been described previously (Xu et al., 2001). OLGs were lysed and total RNA was extracted in 1.0 ml of TRI reagent (Molecular Research Center). Total RNA was quantified with spectrophotometry. For RT-PCR, we used the Titan One-tube RT-PCR kit (Roche,1939823). The primer sequences were as follows: bcl-X L: 5-TTGGACAATGGACTGGTTGA-3 and 5-GTAGAGTGGATGGTCAGTG-3; STAT1: 5-AGAGCGACCAGAAACAGGAA-3 and 5-GCTCTCTGCAACAATGGTGA-3; STAT2: 5-TGGTTCAACATGCTCAGCTC-3 and 5-GCTCTCTCGCTTGCTGAAGT-3; STAT3: 5-AGTTCTCGTCCACCACCAAG-3 and 5-CTAGCCAGACCCAGAACGAG-3; STAT6: 5-CTGCCCTCTTCAAGAACCTG-3 and 5-CATGAACGATGACCACCAAG-3; and actin: 5- ATGGTCAACCCCACCGTGT-3 and 5-CGTGTGAAGTCACCACCCT-3.
Western blot analysis.
As described previously (Bao et al., 2004), OLGs were homogenized in Western blot buffer (10 mm Tris-HCl containing 10 mm HEPEs, 1.5 mm MgCl, 10 mm KCl, 0.5 mm DTT, 1 mm PMSF, 0.1 unit/ml protinin, pH 7.9) and centrifuged at 600 g. The pellets were collected for nuclear protein extraction. The supernatant (cytoplasm) was subjected to high-speed (25,000 g) centrifugation. The supernatant was analyzed by Western blot after protein assay. The supernatant of each sample (26 μg protein each) was loaded onto 10–12% polyacrylamide gel depending on protein molecular weight, separated by SDS/PAGE, and transferred to nitrocellulose membranes [polyvinylidene difluoride (PVDF)] by electrophoresis. The membranes were blocked in Tris-buffered saline–Tween 20 (TBST) buffer containing 20 mm Tris-HCl, 5% nonfat milk, 150 mm NaCl, and 0.05% Tween 20, pH 7.5, for 6 h at 22°C. Primary antibodies for each antigen at appropriate dilutions were added to the membrane and incubated at 4°C overnight or 2 h at 22°C. The membrane was washed with TBST three times at 10 min intervals, incubated with the secondary antibody (goat anti-rabbit IgG conjugated with alkaline phosphatase, 1:5000 for polyclonal primary antibody or goat anti-mouse IgG conjugated with alkaline phosphatase, 1:5000) at 22°C for 1 h, then washed three times each at 10 min intervals with TBST and two times each for 2 min with TBS. The Blot AP System (Promega) was used as described for the final color reaction.
Construction of full length of STAT5A, constitutively active STAT5A and bcl-XL gene.
Full-length STAT5A and bcl-X L cDNAs were obtained by RT-PCR using total RNA from rat as template. The following oligonucleotide primer pairs were used for PCRs: 5-AGGTGAACAGCCATGGCGGGC-3 and 5-TCAGGACAAGGAGCTTCTGGC-3 for STAT5A; 5-TTGGACAATGGACTGGTTGA-3 and 5-GTAGAGTGGATGGTCAGTG-3 for bcl-X L. PCR fragments of STAT5A and bcl-X L were cloned into pGEM-T Easy vector (Promega). Constitutively active STAT5A (STAT5Aca) was made by inducing H298R and S710F mutations on full-length STAT5A using site-directed mutagenesis as described previously (Onishi et al., 1998). Mutagenesis was performed using the QuickChange site-directed mutagenesis kit from Stratagene. The oligonucleotides used for mutagenesis are as follows: 5-CGCAGGGCTGAGCGCCTGTGCCAGCAG-3 for H298R, and 5-GTTTGTCAATGCTTTTG CAGATGCTGGAG-3 for S710F (underlines represent mutated bases). DNA sequences were verified.
Generation of adenoviruses expressing STAT5Aca and bcl-XL.
The pMX-IRES-EGFP vector (Nosaka et al., 1999) was digested with NotI-AfIII to obtain IRES-EGFP, which was then subcloned into NotI-AfIII sites of the p-Shuttle vector (K1650–1, Clontech). The pGEM-T vector carrying STAT5Aca and bcl-X L was digested with NotI-NotI and subcloned into the p-Shuttle vector. After sequence verification, the three p-Shuttle vectors (containing STAT5Aca-IRES-EGFP, bcl-XL-IRES-EGFP and IRES-EGFP) were subcloned into the Adeno-X expression system using PI-SceI and I-Ceu I. The resultant Adeno-X containing STAT5Aca and bcl-X L were transformed into E. coli for amplification. Recombinant Adeno-X viral DNAs were confirmed and amplified. The recombinant Adeno-X DNAs were linearized by digestion with PacI for transfection. The recombinant Adeno-X DNA carrying STAT5Aca-IRES-EGFP, bcl-XL-IRES-EGFP and IRES-EGFP (10 μg each) were used to transfect HEK 293 cells at a density of 2 million per 60 mm dish with calcium phosphate. Transfected cells were monitored with EGFP. The three adenoviruses were collected from the transfected 293 cells 10–14 d after transfection and were amplified for another 10–14 d for high-titer stocks. Adenovirus was concentrated using the BD Adeno-X virus purification kit (K1654–1, Clontech), and titers were determined using Adeno-X rapid titer kits (K1653–1, Clontech). Infectious units (ifu/ml) were calculated for each well as follows: (infected cells/field) × (fields/well)/volume virus (ml) × (dilution factor). OLGs (1 × 105) were infected with virus at a titer of 107 ifu/ml for 36 h, then culture medium was changed with various treatments.
Construction of bcl-x promoter-luciferase reporter gene.
A DNA fragment of rat bcl-x promoter (3.5 kb) was generated by PCR using Platinum TaqDNA Polymerase High Fidelity (Invitrogen) with rat genomic DNA as a template. A forward primer from the 5′-upstream sequence with a SacI site (underlined) (5-CGGAGCTCTGCCTGCTCTCTTTGC-3) and a reverse primer downstream of the initiator methionine codon with a BglII site (underlined) (5-CCACCAGATCTCGGTTGCTCTGAGAAATTTTTA-3) were used. The start codon ATG was mutated to ATT to generate nonfusion expression constructs. This fragment was cloned into pGL3 basic vector (Promega) located upstream of the luciferase reporter gene. A 5′-deleted construct was generated by PCR using the following forward primer with a SacI site (underlined): 2.5 kb (5-GAAGAGCTCGCTCAGTTGTTAAAGGC-3). The constructs were confirmed by sequence analysis.
Luciferase activity assay.
OLGs were transfected in 24-well plates with 0.25 μg of plasmid per well. Transient transfection was performed using FuGene 6 Transfection reagent following the manufacturer's instructions (Roche). The pRL-CMV vector (Promega) was introduced into each transfection and constitutive expression of Renilla luciferase under driven by the CMV promoter served as an internal control. For the siRNA experiment (Fig. 1 B), the GenEclipse control and STAT5A siRNA vector was used (Millipore). Thirty-six hours after transfection, cells were harvested and assayed for reporter gene activity with the Dual-Luciferase Reporter Assay System (Promega).
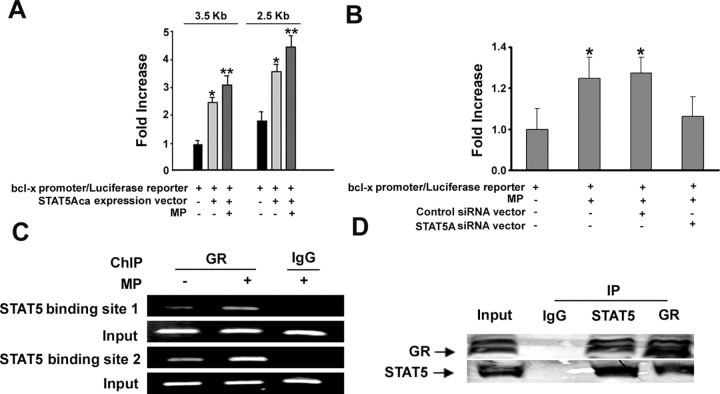
Figure 1.
STAT5/GR complex regulates the bcl-x promoter. A, Upregulation of the bcl-x promoter activity by STAT5 and MP. Rat OLGs in 24-well plates were transfected with a bcl-x-luciferase vector with or without a cotransfection of the STAT5Aca expression vector (0.125 μg/well/per each vector). The bcl-x promoter/luciferase reporter was normalized to Renilla Luciferase levels at each transfection. MP treatment (1 μm) was indicated in the figure. Relative fold increase of luciferase activities (bar graphs) is expressed as a ratio of the readout from each group divided by the readout from the control group transfected only with the bcl-x-luciferase reporter. Transfections were performed in triplicate in each experiment, and results are expressed as the means ± SD for four experiments. The single asterisk denotes significant p < 0.05 compared with the control group. **p < 0.05 compared with the group without MP treatment. B, Downregulation of MP-upregulated bcl-x promoter activity by STAT5A siRNA. As the same as the luciferase assay above with the control siRNA or STAT5A siRNA vector (0.125 μg/well/per each vector), we performed the assay in triplicate for each group, and results are expressed as the means ± SD. C, ChIP by an antibody against GR. OLGs were treated without or with MP (1 μm) from 30 min. The cells were fixed with formaldehyde and then immunoprecipitated with anti-GR antibody (2 μg/ml) in combination with protein G Sepharose beads. Input loading controls were without the immunoprecipitation step. The extracted DNA was used for PCR analysis. Nonspecific IgG antibody was used as a control. D, GR/STAT5 is physically associated in the nuclear extract. OLG nuclear protein (500 μg) was incubated with 2 μg of phospho-STAT5A, GR antibody, or IgG antibody. The immunoprecipitated fractions and input (nuclear protein without precipitation) were blocked by either anti-GR or anti-STAT5A antibody.
Immunoprecipitation.
Nuclear protein (500 μg) was incubated with 2 μg of phospho-Stat5 or GR antibodies overnight on ice. Protein A-Sepharose-coupled beads were added for 30 min at 4°C under constant agitation, pelleted and washed with extraction buffer. Immunoprecipitated protein was boiled and subjected to SDS-PAGE (10% polyacrylamide) and electroblotted onto PVDF membranes. Protein detection was performed with specific antisera against GR and phospho-Stat5 as described previously (Cella et al., 1998).
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assay was performed according to the instruction of Upstate Biotechnology with minor modifications. OLGs cultured in 35 mm dishes were incubated with 1% formaldehyde for 15 min for DNA–protein cross-linking, followed by addition of 0.05 ml of 2.5 m glycine and incubated for another 5 min. Cells were washed and harvested in cold phosphate-buffered saline. After brief centrifugation, the pellet was resuspended and homogenized in ice-cold hypotonic buffer (5 mm PIPES, pH 8.0, 0.5% IPGEAL CA-630, 0.5 mm PMSF and protease inhibitor mixture), centrifuged, and the pellet was resuspended in sonication buffer (10 mm EDTA, 50 mm Tris-HCl, pH 8.0, 0.5 mm PMSF, and protease inhibitor mixture). Each sample was sonicated for 20 s at 4°C and centrifuged, and the supernatant was subjected to immunoprecipitation with the appropriate antibodies (mouse anti-GR antibody (Abcam Limited) overnight, followed by incubation with salmon sperm DNA/protein G agarose slurry to immobilize the DNA–protein antibody complex. DNA–protein complexes were then eluted with 200 μl of elution buffer (Tris–EDTA, 1% SDS) for 30 min, and the cross-links were reversed by overnight incubation at 65°C. DNA was purified using a PCR purification kit (Qiagen), and was amplified by PCR for two specific STAT5-binding sites at the bcl-x promoter: STAT5-F1: 5-CGACCTATGATACAAAAGAC-3 and STAT5-R1: 5-CAAATTGAAAGGACCAGCAA-3; STAT5-F2: 5-GGTGGGTGGTTGTAGTAAA-3 and STAT5-R2: 5-GAAGAGAGAGAAAGAGCTTC-3. The PCR products were electrophoresed using a 1.2% agarose gel, and the PCR product at expected size of 200 bp was visualized and quantified using the Image analysis system (Eastman Kodak), and eluted from agarose gel for DNA sequencing to verify the site of amplification. Mock control conditions were performed with a nonspecific IgG.
STAT5 antisense oligonucleotide treatment.
OLGs grown to 70% confluence in serum-free media were treated with 1.4 μm of STAT5 sense and STAT5 antisense oligonucleotides provided by the manufacturer in delivery solution and ethoxylated polyethylenimine for 4 h (according to the manufacturer's instructions, Gene Tools). AMPA at 200 μm was added into the medium with or without MP (1 μm) for 24 h.
Results
Based on previous studies (Stöcklin et al., 1996; Kabotyanski et al., 2006), we examined whether activated STAT5 could upregulate the gene expression of bcl-x in OLGs. Two luciferase reporters were made: one driven by a long bcl-x promoter (3.5 kb) and the other driven by a short promoter but still containing the P4 element (2.5 kb) (Viegas et al., 2004). Luciferase activity increased over 56-fold after transient transfection with either one of the two reporter vectors into OLGs (data not shown). Addition of STAT5Aca, a constitutively activated form of STAT5A (Onishi et al., 1998), increased the luciferase activity an additional 2.4-fold for both promoter constructs (Fig. 1 A) indicating that activation of STAT5 could upregulate the expression of bcl-XL. MP treatment further significantly increased the luciferase activity for both promoters (Fig. 1 A). Since we have known that MP upregulates bcl-XL expression (Lee et al., 2008), we tested whether MP alone, without STAT5Aca, could activate the bcl-x promoter in oligodendrocytes expressing endogenous STAT5. As predicated, MP significantly increased the luciferase activity for the bcl-x promoter (3.5 kb), and this increase was inhibited after cotransfection with a STAT5 siRNA vector (Fig. 1 B). These data strongly suggested that the increase in bcl-XL expression by MP was mediated solely by STAT5. We next examined a possible direct interaction of GR to STAT5 on the bcl-x promoter with the ChIP assays. Using OLG nuclear extracts, we found that GR was weakly present at both STAT5-binding sites on the bcl-x promoter in untreated cells, and an increase in GR recruitment was observed 30 min after MP treatment (Fig. 1 C). To examine whether GR and STAT5 form a direct physical complex in OLGs, we performed coimmunoprecipitation assays. GR was coimmunoprecipitated by an antibody against phospho-STAT5, and STAT5 was coimmunoprecipitated by an antibody against GR (Fig. 1 D). In addition, computer modeling predicted the interaction of GR and STAT5 via the interaction of GR's DNA binding domain with a dimer region of STAT5A at amino acids 404–416 (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
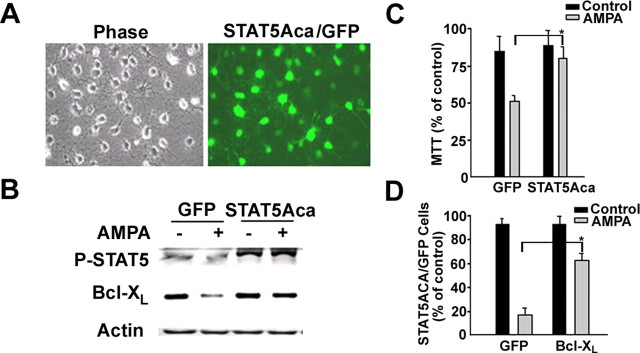
If the GR-signaling pathway acts via STAT5 to prevent AMPA-induced OLG death, the active form of STAT5, STAT5Aca, should also upregulate bcl-XL and protect OLGs. Adenovirus vectors carrying GFP or STAT5Aca (STAT5Aca/GFP) were used to transduce OLGs (an example of the transduction with STAT5Aca shown in Fig. 2 A). OLGs overexpressed with only GFP exhibited a decrease in phospho-STAT5 and bcl-XL expression after AMPA treatment. OLGs overexpressed with STAT5Aca did not increase bcl-XL expression, possibly due to a saturated basal level of expression, but did maintain the levels of phospho-STAT5 and Bcl-xL after AMPA treatment (Fig. 2 B). OLGs overexpressed with STAT5Aca were also resistant to AMPA-induced cell death. Using the MTT assay, we found a significant difference in the amount of OLG survival between OLG cultures infected with GFP (43%) versus STAT5Aca (75%) after AMPA treatment (Fig. 2 C) (p < 0.01, t test). A similar result was obtained for bcl-XL-infected OLGs by counting live transfected cells: 20% of survival for GFP-infected cells versus 63% of survival for bcl-XL-infected cells (Fig. 2 D) (p < 0.01, t test). Thus, one molecular mechanism underlying the resistance to AMPA-induced OLG death is the upregulation of bcl-XL by STAT5Aca.
Figure 2.
An activated form of STAT5 prevents AMPA-induced OLG cell death. A, Primary cultures of OLGs were transduced with an adenovirus expressing a constitutively activated STAT5A and GFP (STAT5Aca/GFP). B, Western blot analysis of phospho-STAT5 and bcl--XL in the cultured OLGs transduced with adenoviruses expressing GFP only or STAT5Aca with or without AMPA treatment for 24 h. A representative blot selected from three independent experiments was shown here. C, OLGs transduced with 107 ifu/ml of adenoviruses expressing GFP only or STAT4ca for 36 h and then treated with AMPA for 24 h. The cell survival was determined by MTT (n = 3). D, Bcl-xL protects AMPA-induced cell death in OLGs. The OLGs transduced with an adenovirus expressing bcl--XL (107 ifu/ml) for 36 h and then treated with AMPA for 24 h. GFP-positive cells were counted in control EGFP and Bcl-xL transduced cells (n = 3). The single asterisk denotes significant p < 0.05.
To further confirm the involvement of STAT5 in the protection of OLGs against apoptosis by the GR-signaling pathway, we transfected a vector containing either a scramble siRNA control or a STAT5 siRNA into OLGs. STAT5 siRNA inhibited STAT5 expression at both the mRNA level as determined by RT-PCR and the protein level as determined by Western blots (Fig. 3 A), while the scramble siRNA control and vehicle control did not. We also examined the expression of other STATs, and found no dramatic changes of their expression after STAT5 siRNA transfection (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). As predicted, STAT5 siRNA significantly inhibited the OLG protection by MP, whereas the scramble siRNA control did not show any effect on MP protection (Fig. 3 B). An ANOVA analysis for all pairwise multiple comparison showed a significant difference for AMPA/MP treated control cultures, scrambled siRNA cultures, versus STAT5 siRNA cultures (Fig. 3 B) (p < 0.001, Tukey test). A similar result was obtained with an antisense oligonucleotide targeting STAT5 (Fig. 3 C) (p < 0.001, Tukey test). In addition, even without AMPA treatment, the ANOVA analysis revealed a significant decrease in OLG viability after the transfection of STAT5 siRNA or antisense STAT5, which strongly suggested an important role for STAT5 in regulating OLG survival. Together, these data clearly demonstrate that STAT5 plays a key role in mediating the protection of OLGs by the MP/GR-signaling pathway.
Figure 3.
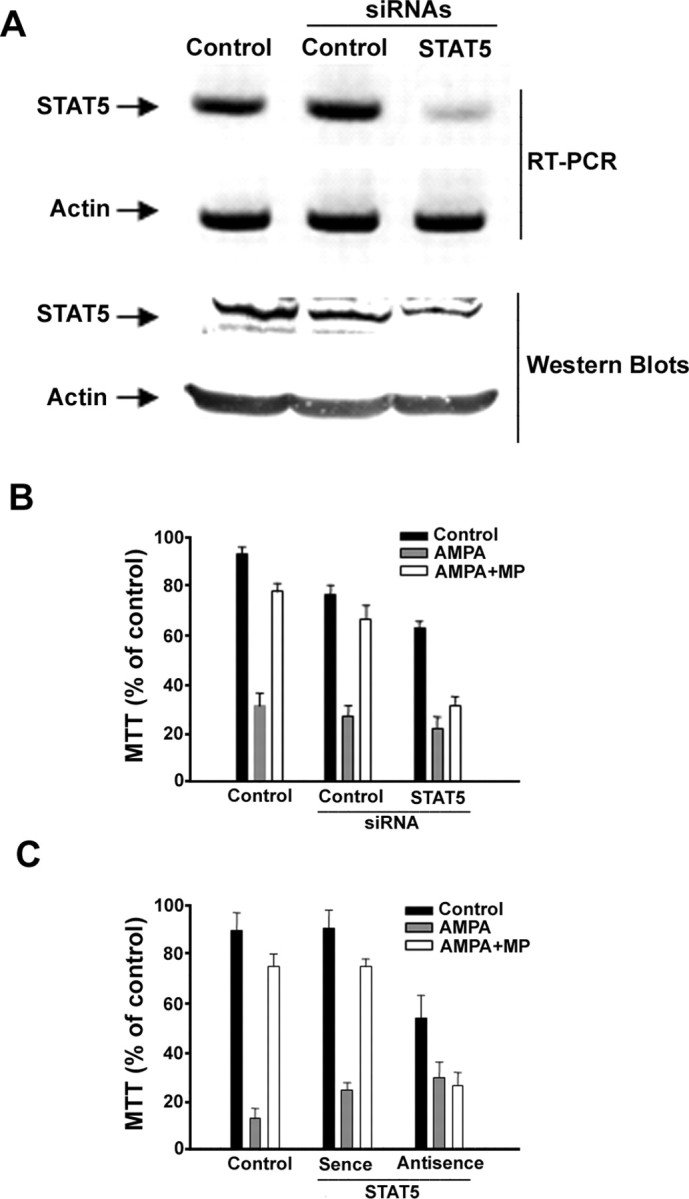
Downregulation of STAT5 reduce the protection of MP on OLGs. A, B, OLGs transfected with vehicle, scramble siRNA and STAT5 siRNA (50 nm each) for 24 h were treated with AMPA (200 μm) for another 24 h with or without pretreatment of MP (1 μm) for 2 h. STAT5 siRNA transfection reduced STAT5 at its mRNA and protein level. Actin served as control for equal amount of RNA and protein (A). Cell survival assay was determined by MTT assay (B). C, OLGs transfected with vehicle, sense, and antisense oligonucleotide complementary to STAT5A (1.4 μm each) were treated with AMPA (200 μm) for 24 h with or without pretreatment of MP (1 μm). Cell survival assay was determined by MTT assay.
Discussion
Recently, we found that MP selectively reduces AMPA-induced OLG apoptosis in culture and OLG death induced by spinal cord injury in vivo, while MP does not protect neurons. Under both in vitro and in vivo conditions, MP upregulates the expression of bcl-XL in OLGs. RU486, an antagonist of GR, can block the antiapoptotic action of MP indicating that MP acts via GR in regulation of OLG apoptosis (Lee et al., 2008). The GR regulation of the expression of bcl-XL is complicated. In certain cell types, GR downregulates bcl-XL and promotes apoptosis, while in other cells, GR upregulates bcl-XL and prevents apoptosis, which may explain why MP selectively protect OLGs but not neurons. Previous studies suggest that this cell-context dependent GR regulation of apoptosis is due to the interaction of GR with other transcription factors (Lotem and Sachs, 1995; Schorr and Furth, 2000; Amsterdam et al., 2002; Rocha-Viegas et al., 2006; Viegas et al., 2008). Our results demonstrate that the GR/STAT5/bcl-XL molecular cascade is a novel pathway underlying the antiapoptotic effects of MP on OLGs.
Two major issues remain to be further explored. First, in most of our studies, we focused on the role of the STAT5A gene in mediating MP antiapoptotic functions. STAT5A and STAT5B (collectively called STAT5) are 96% conserved at the protein level. The highest degree of divergence is found in the C-terminal transaction domain (Hennighausen and Robinson, 2008). Because GR interacts with STAT5 most likely at the N-terminal, STAT5B should be able to interact with GR. However, STAT5A and STAT5B are encoded by two different genes, and a difference in their functional phenotypes is observed in their “knock-out” mutant mice. A more detailed examination of the role STAT5B has in the antiapoptotic effects of MP is needed. The second issue is whether a similar MP/GR/STAT5A/bcl-XL molecular cascade exists in human OLGs. The underlying purpose of our study is to identify down-stream molecular targets for the protective effects of MP on OLGs so that new drugs with fewer side-effects can be developed to treat human neurological disorders involving white matter injury. Although we have identified STAT5 as one major down-stream molecular target in the MP antiapoptotic pathway in rat OLGs, and the stress response system should be conserved evolutionally, the possible existence of the same molecular pathway in human OLGs should be examined, especially now that human OLGs can be derived from human embryonic stem cells (Brüstle et al., 1999; Keirstead et al., 2005).
In summary, we have shown that MP prevents the death of OLGs treated with AMPA. This protection is through the binding of GR with STAT5, which subsequently binds to the promoter region of bcl-x, and activates the expression of bcl-XL. The evidence for the physical interaction between STAT5 and GR are demonstrated by the luciferase assays, chromatin immunoprecipitation, coimmunoprecipitation, and computational molecular modeling. Activated STAT5A can regulate the expression of bcl-XL and protect against the AMPA-induced death of OLGs. Therefore, MP protects OLGs via this STAT5A-mediated molecular cascade. Our findings have important implications in the treatment of neurological disorders such as spinal cord injury and multiple sclerosis.
Footnotes
This work was supported by National Institutes of Health Grants AG024250 and DC004665. We thank Kevin Ohlemiller for analyzing the data.
References
- Amsterdam A, Tajima K, Sasson R. Cell-specific regulation of apoptosis by glucocorticoids: implication to their anti-inflammatory action. Biochem Pharmacol. 2002;64:843–850. doi: 10.1016/s0006-2952(02)01147-4. [DOI] [PubMed] [Google Scholar]
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2002;2:381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- Beato M, Truss M, Chávez S. Control of transcription by steroid hormones. Ann N Y Acad Sci. 1996;784:93–123. doi: 10.1111/j.1749-6632.1996.tb16231.x. [DOI] [PubMed] [Google Scholar]
- Broome HE, Yu AL, Diccianni M, Camitta BM, Monia BP, Dean NM. Inhibition of Bcl-xL expression sensitizes T-cell acute lymphoblastic leukemia cells to chemotherapeutic drugs. Leuk Res. 2002;26:311–316. doi: 10.1016/s0145-2126(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Brüstle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Caron-Leslie LA, Evans RB, Cidlowski JA. Bcl-2 inhibits glucocorticoid-induced apoptosis but only partially blocks calcium ionophore or cycloheximide-regulated apoptosis in S49 cells. FASEB J. 1994;8:639–645. doi: 10.1096/fasebj.8.9.8005391. [DOI] [PubMed] [Google Scholar]
- Catlett-Falcone R, Dalton WS, Jove R. STAT proteins as novel targets for cancer therapy. Signal transducer an activator of transcription. Curr Opin Oncol. 1999;11:490–496. doi: 10.1097/00001622-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Cella N, Groner B, Hynes NE. Characterization of Stat5a and Stat5b homodimers and heterodimers and their association with the glucocortiocoid receptor in mammary cells. Mol Cell Biol. 1998;18:1783–1792. doi: 10.1128/mcb.18.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson NA, Morsink MC, Meijer OC, de Kloet ER. Central corticosteroid actions: search for gene targets. Eur J Pharmacol. 2008;583:272–289. doi: 10.1016/j.ejphar.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Dumon S, Santos SC, Debierre-Grockiego F, Gouilleux-Gruart V, Cocault L, Boucheron C, Mollat P, Gisselbrecht S, Gouilleux F. L-3 dependent regulation of Bcl-xL gene expression by STAT5 in a bone marrow derived cell line. Oncogene. 1999;18:4191–4199. doi: 10.1038/sj.onc.1202796. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabotyanski EB, Huetter M, Xian W, Rijnkels M, Rosen JM. Integration of prolactin and glucocorticoid signaling at the beta-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers. Mol Endocrinol. 2006;20:2355–2368. doi: 10.1210/me.2006-0160. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T. Tissue glucocorticoid sensitivity: beyond stochastic regulation on the diverse actions of glucocorticoids. Horm Metab Res. 2007;39:420–424. doi: 10.1055/s-2007-980193. [DOI] [PubMed] [Google Scholar]
- Kirito K, Watanabe T, Sawada K, Endo H, Ozawa K, Komatsu N. Thrombopoietin regulates Bcl-xL gene expression through Stat5 and phosphatidylinositol 3-kinase activation pathways. J Biol Chem. 2002;277:8329–8337. doi: 10.1074/jbc.M109824200. [DOI] [PubMed] [Google Scholar]
- Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, Xu J. Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J Neurosci. 2008;28:3141–3149. doi: 10.1523/JNEUROSCI.5547-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J, Sachs L. Regulation of bcl-2, bcl-XL and bax in the control of apoptosis by hematopoietic cytokines and dexamethasone. Cell Growth Differ. 1995;6:647–653. [PubMed] [Google Scholar]
- Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui AL, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M, Nosaka T, Misawa K, Mui AL, Gorman D, McMahon M, Miyajima A, Kitamura T. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Viegas L, Vicent GP, Barañao JL, Beato M, Pecci A. Glucocorticoids repress bcl-X expression in lymphoid cells by recruiting STAT5B to the P4 promoter. J Biol Chem. 2006;281:33959–33970. doi: 10.1074/jbc.M602408200. [DOI] [PubMed] [Google Scholar]
- Schorr K, Furth PA. Induction of bcl-xL expression in mammary epithelial cells is glucocorticoid-dependent but not signal transducer and activator of transcription 5-dependent. Cancer Res. 2000;60:5950–5953. [PubMed] [Google Scholar]
- Silva M, Benito A, Sanz C, Prosper F, Ekhterae D, Nuñez G, Fernandez-Luna JL. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J Biol Chem. 1999;274:22165–22169. doi: 10.1074/jbc.274.32.22165. [DOI] [PubMed] [Google Scholar]
- Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Stöcklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- Viegas LR, Vicent GP, Barañao JL, Beato M, Pecci A. Steroid hormones induce bcl-X gene expression through direct activation of distal promoter P4. J Biol Chem. 2004;279:9831–9839. doi: 10.1074/jbc.M312402200. [DOI] [PubMed] [Google Scholar]
- Viegas A, Brás NF, Cerqueira NM, Fernandes PA, Prates JA, Fontes CM, Bruix M, Romão MJ, Carvalho AL, Ramos MJ, Macedo AL, Cabrita EJ. Molecular determinants of ligand specificity in family 11 carbohydrate binding modules - an NMR, X-ray crystallography and computational chemistry approach. FEBS J. 2008;275:2524–2535. doi: 10.1111/j.1742-4658.2008.06401.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Kim GM, Ahmed SH, Xu J, Yan P, Xu XM, Hsu CY. Glucocorticoid receptor-mediated suppression of activator protein-1 activation and matrix metalloproteinase expression after spinal cord injury. J Neurosci. 2001;21:92–97. doi: 10.1523/JNEUROSCI.21-01-00092.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]