Abstract
A method was developed for assessing ascorbic acid concentration in commercial fruit juice by cyclic voltammetry. The anodic oxidation peak for ascorbic acid occurs at about 490 mV on a Pt disc working electrode (versus SCE). The influence of the potential sweep speed on the peak height was studied. The obtained calibration graph shows a linear dependence between peak height and ascorbic acid concentration in the domain (0.1–10 mmol·L−1). The equation of the calibration graph was y = 6.391x + 0.1903 (where y represents the value of intensity measured for the anodic peak height, expressed as μA and x the analyte concentration, as mmol·L−1, r2 = 0.9995, r.s.d. = 1.14%, n = 10, Cascorbic acid = 2 mmol·L−1). The developed method was applied to ascorbic acid assessment in fruit juice. The ascorbic acid content determined ranged from 0.83 to 1.67 mmol·L−1 for orange juice, from 0.58 to 1.93 mmol·L−1 for lemon juice, and from 0.46 to 1.84 mmol·L−1 for grapefruit juice. Different ascorbic acid concentrations (from standard solutions) were added to the analysed samples, the degree of recovery being comprised between 94.35% and 104%. Ascorbic acid determination results obtained by cyclic voltammetry were compared with those obtained by the volumetric method with dichlorophenol indophenol. The results obtained by the two methods were in good agreement.
1. INTRODUCTION
Ascorbic acid (vitamin C) is a water-soluble vitamin which can be found in many biological systems and foodstuffs (fresh vegetables and fruits, namely, citrus). Ascorbic acid plays an important role in collagen biosynthesis, iron absorption, and immune response activation and is involved in wound healing and osteogenesis. It also acts as a powerful antioxidant which fights against free-radical induced diseases [1–5]. Nevertheless, an ascorbic acid excess can lead to gastric irritation, and the metabolic product of vitamin C (oxalic acid) can cause renal problems [6]. In some cases, excessive quantities of ascorbic acid may result in the inhibition of natural processes occurring in food and can contribute to taste deterioration; added to apple pulp (250 mg/kg), vitamin C inhibits oxidation processes responsible for apple juice aroma [7]. Ascorbic acid is a labile substance, as it is easily degraded by enzymes and atmospheric oxygen. Its oxidation can be accelerated by excessive heat, light, and heavy metal cations [1]. That is why ascorbic acid content of foodstuffs and beverages represents a relevant indicator of quality which has to be carefully monitored, regarding its variation during manufacturing and storage.
Many analytical methods can be used for ascorbic acid determination. Classic (conventional) techniques are represented by volumetric methods—titration with an oxidant solution such as dichlorophenol indophenol (DCPIP) [8, 9], potassium iodate [10], or bromate [11]. Volumetric techniques can suffer from lack of specificity [12] which limits their use to samples not containing other reducing agents.
Güçlü et al. [13] have proposed a spectrophotometric method based on ascorbic acid oxidation to dehydroascorbic acid, by using the Cu(II)-neocuproine complex, which is reduced to Cu(I)-bis(neocuproine), the absorbance of the latter being determined at 450 nm. Other optical methods for vitamin C estimation include spectrophotometrical determination of iodine reacted with ascorbic acid [14] and chemiluminescence [15].
Liquid chromatography is a successful method for vitamin C determination when selectivity and specificity are concerned [16–18]. HPLC with electrochemical detection has turned out to be a selective and sensitive method for ascorbic acid assessment in foodstuffs and biological fluids [19–21].
A potentiometric biosensor [22] for ascorbic acid was made by ascorbate oxidase immobilization in a polymeric matrix, fixed on a graphite-epoxy composite electrode.
Amperometric biosensors were obtained by ascorbate oxidase immobilization on a nylon net [23] or on a collagen membrane, using a Clark oxygen electrode as transducer [24]. Vitamin C analysis was also performed by using a glassy carbon working electrode as transducer incorporated in a flow system [25]. Ascorbic and uric acids were determined by coupling an amperometric technique with flow analysis [26]. Voltammetric and amperometric measurements were performed in a flow cell, using gold microelectrodes on which Pd was electrochemically deposited.
O’Connell et al. [12] developed an amperometric sensor for ascorbic acid determination from foodstuffs and pharmaceutical preparations. This sensor was constructed by aniline electropolymerization on a glassy carbon or a screen-printed working electrode.
Kumar and Narayanan [27] investigated a method for vitamin C assessment based on an amperometric sensor obtained by graphite electrode modification by cobalt ferrocyanide. The decrease of the working potential in these amperometric methods based on electrochemical oxidation of ascorbic acid was possible by using mediators like ferocene [28] or redox couples like ferri/ferrocyanide [29].
Vitamin C determination was also performed in an FIA system with biamperometric detection, based on ascorbic acid reaction with iodine [30].
Voltammetry is an increasingly popular method applied to the determination of ascorbic acid in real samples [7], because it offers low detection limits, even when compared to more expensive techniques. It requires little or no sample preparation. This technique provides us with the advantage of a fast analysis as well as with the easiness and rapidity of the standard addition method application. Because of the low cost of the required equipment as well as simplicity of the employed procedures necessary to determine vitamin C, voltammetry appears to offer an attractive alternative to the titrimetric or instrumental methods mentioned earlier, in particular in food quality control. It does not require complicated, expensive equipment and well-qualified personnel nor is it laborious or time consuming like the previously mentioned instrumental techniques [7].
Simultanoeus determination of vitamin C and glucose has also been performed using a voltammetric biosensor integrated in an automated SIA system [31].
Recently, the use of various voltammetric techniques has been combined with modified ascorbic acid sensors; square-wave voltammetry was used to determine ascorbic acid based on its oxidation at a zeolite modified carbon paste electrode [32], and the method was applied to ascorbic acid determination in citrus juice. The response of the electrode to ascorbic acid is linear in the range 4 × 10−7–1.2 × 10−3 mol·L−1, with a detection limit of 2 × 10−8 mol·L−1; cyclic and differential pulse voltammetries were used for ascorbic acid electrocatalytical determination at a carbon paste electrode modified with 2,7-bis (ferrocenyl ethynyl) fluoren-9-one [33]. The detection limits (2σ) were determined as 1.8 × 10−5 and 4.2 × 10−6 mol·L−1 by CV and DPV, respectively.
The results reported in literature regarding the determination of ascorbic acid by cyclic voltammetry are not numerous. Nevertheless, cyclic voltammetry has been previously used for antioxidant content assessment, and in particular low-molecular-weight antioxidants, including ascorbic acid; this technique has turned out to be a convenient methodology, validated for the quantification of low-molecular-weight antioxidant capacity of tissue homogenates, blood plasma, or plant extracts [34]. Cyclic voltammetry and spectrophotometry showed good agreement for the antioxidant capacity estimation in buckwheat products after hydrothermal treatment [35]. Ruffien-Ciszak et al. [36] have proposed cyclic voltammetry using a Pt wire as working electrode to assess the total antioxidant capacity of skin, based on the reduction capacity of low-molecular-weight antioxidants. Rapta et al. [37] evaluated the antioxidant capacity of flavonoids by cyclic voltammetry in acetonitrile, by employing a three-electrode cell with Pt working and auxiliary electrodes and a calomel electrode as reference. Zielinska et al. [38] used cyclic voltammetry with glassy carbon working electrode to monitor the total antioxidant capacity and flavonoid content in onions. H. J. Kim and I. K. Kim [39] evaluated ascorbic acid content (after isolation on an anion exclusion column) by amperometric detection at a Pt working electrode operating at 0.6 V (versus Ag/AgCl). The vitamin C content in apple juice has been monitored by cyclic voltammetry by means of a Pt working electrode [7]. Campanella et al. [40] determined the antioxidant capacity of dry vegetal extracts (expressed as mg of ascorbic acid equivalents) by cyclic voltammetry performed at a glassy carbon working electrode.
The aim of this paper was to investigate a method for ascorbic acid determination by cyclic voltammetry, taking into account that the reported data in literature regarding the determination of ascorbic acid by this method are very scarce. The developed method was applied to the determination of ascorbic acid in different fruit juice, and the obtained results were compared with those obtained by a conventional titrimetric method.
2. EXPERIMENTAL
2.1. Reagents and instrumentation
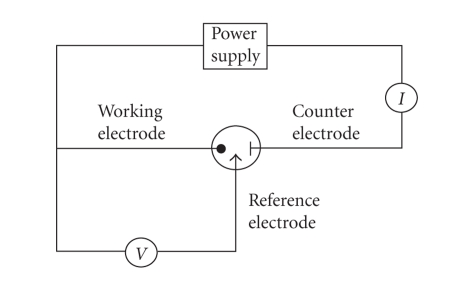
A potentiostat-galvanostat KSP, laboratory made by Slawomir Kalinowski (University Warmia and Mazury, Olsztyn, Poland) was used, as well as the respective soft, Cyclic Voltammetry. A Pt disc electrode (Metrohm, 2 mm diameter) was used as working electrode. The reference electrode was a saturated calomel electrode (SCE). The counter electrode was a Pt strip (30 mm2 surface). Figure 1 provides a schematic representation of the experimental setup; the potentiostat enables control of the potential of the working electrode, with respect to the reference electrode as well as measurement of the current that flows between the working electrode and counter electrode. A stock solution of ascorbic acid, 100 mmol·L−1, was prepared daily by dissolving vitamin C (Merck, Haar, Germany, ACS ISO, biochemical grade) in a 0.34 mol·L−1 KCl solution (Reactivul, Bucharest, Romania) used as supporting electrolyte. Standard solutions of ascorbic acid with concentrations ranging between 0.1 and 10 mmol·L−1 were obtained by diluting the stock solution with the respective volumes of 0.34 mol·L−1 KCl (electrolyte) solution. Standard solutions of glucose (Reactivul Bucuresti), tartaric acid (Merck), citric acid (Reactivul Bucuresti), and sodium benzoate (Sigma-Aldrich, Taufkirchen, Germany) with a concentration of 1 mol·L−1 were prepared by dissolution of the respective amount of reagent in 0.34 mol·L−1 KCl solution.
Figure 1.
Schematic representation of the experimental setup.
The dichlorophenol indophenol (DCPIP) solution, 5 × 10−4 mol·L−1, was prepared by dissolving 145 mg DCPIP, sodium salt (Merck), in 100 mL hot distilled water and a subsequent addition of 300 mL phosphate buffer, 0.066 mol·L−1, pH = 6.98, previously prepared by mixing the respective volumes of potassium dihydrogen phosphate and sodium monohydrogen phosphate solutions (2/3 ratio). Distilled water was added to the final volume of 1000 mL. After homogenization, the solution was kept in a dark place (protected from light) and filtered [8].
All mentioned solutions were prepared using distilled water which was boiled and chilled until reached room temperature.
2.2. Working procedure
For voltammetric measurements, a three-electrodes cell was used equipped with working, counter, and a reference electrodes [7, 41]. The volume of the analyzed sample was 100 mL, and all measurements were performed at 295.5 K using 0.34 mol·L−1 KCl solution as supporting electrolyte. All voltammograms were recorded for stirred solutions. Before each determination, the Pt working electrode was cleaned mechanically, by polishing it on alumina (Merck, 63–200 μm granularity) and electrochemically, by applying a −1.5 V potential pulse for 3 seconds. For each measurement, the potential was scanned within the range −100 and −1000 mV, with a 50 mV/s scan rate, and the value of the backround current obtained for the KCl 0.34 mol·L−1 solution was substracted from the current corresponding to the analyzed solution/sample. For investigating the potential scan rate influence, this parameter varied from 50 mV/s to 250 mV/s.
3. RESULTS AND DISCUSSIONS
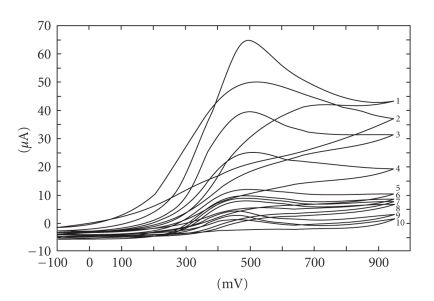
In Figure 2, several voltammograms, obtained for different ascorbic acid concentrations, are presented. The peak that appeared at 490 mV (versus SCE) was attributed to ascorbic acid oxidation. As can be seen from Figure 2, no reduction peak appears for ascorbic acid. This confirms the data reported in literature [42, 43] that electrochemical oxidation of ascorbic acid is an irreversible process.
Figure 2.
Cyclic voltammograms obtained for different ascorbic acid concentrations expressed as mmol·L−1: 0.1 (10), 0.5 (9), 0.75 (8), 1 (7), 1.5 (6), 2 (5), 4 (4), 6 (3), 8 (2), and 10 mmol·L−1 (1).
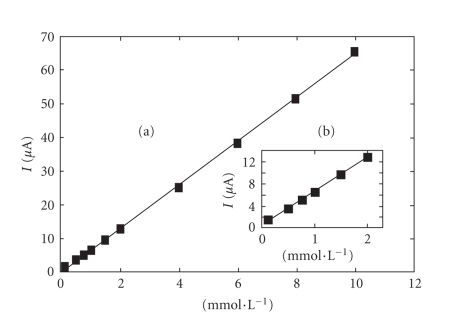
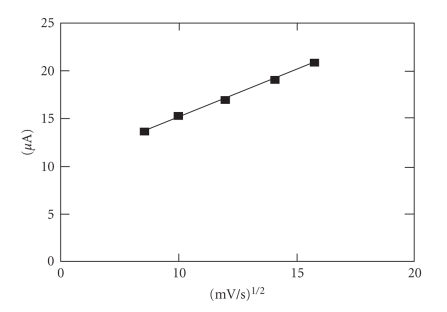
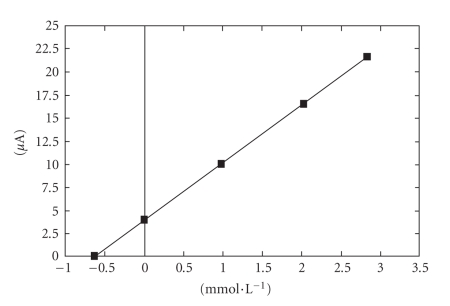
The calibration graph (Figure 3) shows a linear range obtained between 0.1 and 10 mmol·L−1 ascorbic acid (r2 = 0.9995, y = 6.391x + 0.1903). The value calculated for r.s.d. was 1.14%, (c = 2 mmol·L−1 ascorbic acid; n = 10). The influence of the potential scan rate on the anodic peak height was also investigated (Figure 4). The measurements were performed at 2 mmol·L−1 ascorbic acid concentration, and the potential scan rate varied between 50 and 250 mV/s. The anodic peak height corresponding to the analyte oxidation increases with the square root of the potential scan rate and conforms to Randles-Sevcik equation:
| (1) |
where c represents concentration of the electroactive species, v potential scan rate, A electrode surface, D diffusion coefficient of the analyte, and n number of electrons transferred in the redox process.
Figure 3.
Calibration graph for the determination of ascorbic acid by cyclic voltammetry within (a) the domain 0.1–10 mmol·L−1 and (b) the domain 0.1–2 mmol·L−1.
Figure 4.
The influence of the square root of the potential scan rate on the anodic peak current; cascorbic acid = 2 mmol·L−1.
3.1. Specificity and interferences
Previously published studies have proved that compounds commonly found in foodstuffs and juice (citric acid, tartaric acid, phenylalanine, glutamic acid, aminoacetic acid, and glucose) do not interfere in the ascorbic acid determination by cyclic voltammetry performed on glassy carbon working electrodes [44]. A study of interference for ascorbic acid determination was performed, also at a glassy carbon working electrode modified with nickel(II) macrocycle containing dianionic tetraazaannulene ligand [45]. When the permitted relative deviation is less than ±5%, no interference is observed from citric acid, malic acid, tartaric acid, and glucose, at a ratio substance/L-ascorbic acid (w/w) of 250 [45].
Table 1 presents the results obtained at the determination of ascorbic acid by cyclic voltammetry, in the presence of some common substances usually accompanying ascorbic acid in citrus juice, namely, glucose, tartaric acid, citric acid, and benzoate anion. All the determinations were performed by using the reported working procedure. The studied interferent was added to the analyzed sample as a concentrated solution, and the final volume of the analyzed sample was of 100 mL. The ascorbic acid concentration was 2 mmol·L−1. The values presented in Table 1 represent the average of three determinations.
Table 1.
Study of interference performed on some common chemical species found in citrus juice.
| Interferent | Interferent/analyte molar ratio | Influence on the analytical peak current |
|---|---|---|
| Glucose | 200 | less than 1% |
| Citric acid | 150 | less than 1% |
| 200 | 2.26% decrease | |
| Tartaric acid | 200 | less than 1% |
| Benzoate anion | 150 | less than 1% |
| 200 | 4.84% decrease |
As can be seen from Table 1, glucose and tartaric acid do not influence the ascorbic acid analytical signal in concentrations up to 200 times greater than that of vitamin C. Benzoate anion does not influence the ascorbic acid analytical signal in concentrations up to 150 times greater than that of vitamin C. Concentrations of benzoate anion 200 times greater than that of the analyte produce a decrease of the analytical signal of 4.84%. Interference tests have proved that citric acid, in concentrations up to 150 times greater than that of the analyte, has no influence on the analytical peak current. A citric acid concentration 200 times greater than that of vitamin C produces a decrease of the ascorbic acid peak current of 2.26%.
Therefore, citric acid, tartaric acid, and benzoate anion do not interfere at ascorbic acid determination (error of determination <5%), in concentrations commonly found in fresh or commercial fruit juice, for these organic interferents.
3.2. Analysis of real samples
Natural orange juice and lemon juice were obtained by fruit pressing. To this purpose, five average-sized fruits were peeled and the juice was obtained by using a centrifugal device. Then, the obtained juice was centrifugated until a clear sample was obtained, which was subsequently analyzed.
Commercial juice containing fruit pulp (Santal, Tymbark) were centrifugated before analysis, and the obtained clear sample was analyzed. Solid KCl was added as supporting electrolyte into the clear fruit juice (without a previous dilution) in order to obtain a concentration of 0.34 mol·L−1 KCl. The working procedure for the standard ascorbic acid solutions was applied to fruit juice analysis. The ascorbic acid content was calculated by measuring the peak current and by using the calibration graph presented in Figure 2. The obtained results are presented in Table 2 together with those obtained by a volumetric technique which uses a dichlorophenol indophenol (DCPIP) 5 × 10−4 mol·L−1 solution as titrating agent [8, 9].
Table 2.
Results obtained for vitamin C determination in fruit juice and for the calculation of the degree of recovery of ascorbic acid added in analyzed samples by using the titrimetric method with DCPIP and the voltammetric method. The values obtained by the titrimetric method represent the average of three determinations, whereas those obtained by cyclic voltammetry represent the average of five determinations.
| Juice and producers | Ascorbic acid concentration—DCPIP method | Ascorbic acid concentration— cyclic voltammetry | Ascorbic acid concentration after 35.2 mg (2 mL standard solution, 0.1 mol·L−1, added to 100 mL juice) vitamin C addition | Recovery% | Ascorbic acid concentration after 70.4 mg (4 mL standard solution, 0.1 mol·L−1, added to 100 mL juice) vitamin C addition | Recovery% | |
|---|---|---|---|---|---|---|---|
| mg/100 mL juice | mmol·xL−1 | mg/100 mL juice | mg/100 mL juice | mg/100 mL juice | |||
| Orange juice (Greece) obtained by fruit pressing | 30.48 | 1.67 | 29.39 | 64.35 | 103.01 | 95.61 | 99.50 |
| Lemon juice (Greece) obtained by fruit pressing | 35.20 | 1.93 | 33.97 | 68.31 | 101.48 | 98.32 | 97 |
| Cappy grapefruit (Coca Cola HBC Romania) | 8.21 | 0.46 | 8.09 | 40.67 | 94.90 | 75.30 | 99.75 |
| Fanta lemon (Coca Cola) | 10.79 | 0.58 | 10.21 | 42.91 | 95.36 | 79.37 | 102.75 |
| Tymbark orange (mw La Festa Int’l Romania) | 22.29 | 1.23 | 21.65 | 56.41 | 102 | 88.53 | 100.03 |
| Frutti fresh Tutti frutti (European Drinks Romania) | 14.37 | 0.83 | 14.61 | 46.87 | 94.35 | 79.88 | 97.25 |
| Prigat orange (Sigat Beverage Company Romania) | 14.96 | 0.83 | 14.61 | 47.58 | 96.40 | 81.07 | 99.01 |
| Prigat Peach (Sigat Beverage Company Romania) | 12.32 | 0.73 | 12.85 | 46.82 | 99.19 | 82.75 | 104 |
| Fruttia orange (European Drinks Romania) | 21.12 | 1.24 | 21.82 | 55.44 | 98.69 | 89.61 | 101.39 |
| Santal grapefruit (Parmalat Romania) | 31.68 | 1.84 | 32.38 | 64.42 | 94.72 | 100.35 | 102.26 |
3.3. Determination of the degree of recovery of vitamin C added to analyzed juice samples
All measurements were performed following the working procedure detailed for the standard ascorbic acid solutions. To 100 mL clear fruit juice, solid KCl was added to obtain a concentration of 0.34 mol·L−1 electrolyte. Then, 2 mL (35.2 mg) and 4 mL (70.4 mg), respectively, from a concentrated (100 mmol·L−1) ascorbic acid solution containing 0.34 mol·L−1 KCl, were added to the sample. The obtained analytical signal was corrected by taking into account the sample dilution originating from the addition of standard ascorbic acid solution. For each addition, the degree of recovery was calculated as follows:
| (2) |
where QDET represents mg determined ascorbic acid in 100 mL juice, QP represents mg ascorbic acid previously present in 100 mL juice, and QADD represents mg added ascorbic acid in 100 mL juice.
The obtained results are presented in Table 2. As can be seen from Table 2, the degree of recovery of ascorbic acid varies between 94.35% and 104%, which indicates a good recovery of the added ascorbic acid amounts.
In order to verify the accuracy of the developed method for ascorbic acid determination in fruit juice, the standard addition method was applied for an analyzed sample, namely, Fruttia orange. The following procedure was employed: to four 100 mL volumetric flasks, 50 mL sample (Fruttia orange) was added. Then, known amounts from the standard 0.1 mol·L−1 ascorbic acid solution were added in each flask as follows: (1) 0 mL, (2) 2 mL, (3) 4 mL, and (4) 6 mL. Solid KCl was added in each volumetric flask, as to reach a 0.34 mol·L−1 final electrolyte concentration. Double-distilled water was added to the final 100 mL volume, followed by homogenization. The ascorbic acid content for each flask was determined, and the obtained results are presented in Figure 5. The determined concentration in Fruttia was 0.625 mmol·L−1 (11 mg/100 mL) ascorbic acid. Taking into account the dilution degree (1/1), this corresponds to a concentration of 1.25 mmol·L−1 ascorbic acid in the undiluted juice (Fruttia). The obtained result is in accordance with the one presented in Table 2, which indicates the absence of matrix effects at ascorbic acid determination by the proposed method.
Figure 5.
Application of the standard addition method for determination of ascorbic acid in Fruttia orange. The working procedure described in Section 2 was used.
3.4. Working procedure for vitamin C titrimetric determination with DCPIP
A sample of 2.5 mL clear juice was diluted with distilled water to a final volume of 10 mL. Then, it was titrated with the DCPIP 5 × 10−4 mol·L−1 solution until a pink tint appears that persists for about 30 seconds. The obtained results are presented in Table 2.
4. CONCLUSIONS
The developed method has proved its accuracy in vitamin C determination in fruit juice, having the value of the recovery of known quantities of ascorbic acid ranging between 94.35% and 104%. The highest values for ascorbic acid were obtained for natural juice made by fruit squeezing.
The detection limit of the method was of 9 × 10−5 mol·L−1 (calculated as 3x, the standard deviation of the blank signal), and the limit of quantification was 3 × 10−4 mol·L−1 (calculated as 10x, the standard deviation of the blank signal).
Although other voltammetric methods (e.g., differential pulse voltammetry or linear sweep voltammetry) are more sensitive than cyclic voltammetry for determining ascorbic acid, cyclic voltammetry can be used with very good results to analyze ascorbic acid in fruit juice.
The concentrations of ascorbic acid in fruit juice determined by cyclic voltammetry are in good agreement with the data obtained by a classical volumetric method (Table 2). The obtained results are also in good agreement with the data reported in literature regarding the content of ascorbic acid in citrus fruits. Thus, the reported values for lemon are 44.5 mg/100 mL juice [24] or 48 mg/100 g fruit [46]. Other results indicate a vitamin C content of 33–50 mg/100 mL for orange juice (Valencia) obtained by squeezing the fruits [46]. For the grapefruit juice (Florida), also obtained by fruit pressing, the ascorbic acid content varies between 38 and 56 mg/100 mL [47]. These values are in accordance with those we obtained for orange and lemon juice (fruit squeezing), 30.48 mg/100 mL and 35.2 mg/100 mL, respectively, as well as those obtained for grapefruit juice (Santal), 31.68 mg/100 mL.
The results obtained in this study show that cyclic voltammetry can be successfully used as part of quality management in food industry, for assessing the vitamin C content in natural fruit juice and soft drinks. The results prove why, recently, this technique has been more and more preferred to the previously applied methods, as it is characterized by accuracy, rapidity, good specificity, and sensitivity, and also by the simplicity of the required equipment and procedure.
ACKNOWLEDGMENT
This work was performed with the support of the Romanian National Agency for Research, in the frame of the Research Project no. 183, CNCSIS code TD-387.
References
- 1.Bhagavan NV. Medical Biochemistry. 4th edition. Amsterdam, The Netherlands: Elsevier; 2001. [Google Scholar]
- 2.Cathcart RF. A unique function for ascorbate. Medical Hypotheses. 1991;35(1):32–37. doi: 10.1016/0306-9877(91)90080-i. [DOI] [PubMed] [Google Scholar]
- 3.Mohora M. Biochimie Medicală. Bucuresti, Romania: Editura Niculescu; 2006. [Google Scholar]
- 4.Şerban M, Tămaş V, Cotruţ V. Biochimie Medicală Veterinară. Bucuresti, Romania: Editura Didactica si Pedagogica; 1981. [Google Scholar]
- 5.Fox BA, Cameron AG. Food Science, Nutrition and Health. 5th edition. London, UK: Edward Arnold; 1989. [Google Scholar]
- 6.Hodgkinson A. Oxalic Acids in Biology and Medicine. London, UK: Academic Press; 1977. [Google Scholar]
- 7.Wawrzyniak J, Ryniecki A, Zembrzuski W. Application of voltammetry to determine vitamin C in apple juices. Acta Scientiarum Polonorum-Technologia Alimentaria. 2005;42(2):5–16. [Google Scholar]
- 8.Şerban M, Câmpeanu Gh, Ionescu E. Metode de Laborator în Biochimia Animală. Bucuresti, Romania: Editura Didactica si Pedagogica; 1993. [Google Scholar]
- 9.Papuc C, Pop A, Şerban M. Metode Analitice in Biochimia Veterinara. Bucuresti, Romania: Editura Printech; 2001. [Google Scholar]
- 10.Balan D, Pele M, Artimon M, Luta G. Bioactive compounds in sea buckthorn fruits and in some products obtained by their processing. Revue de Cytologie et de Biologie Végétales-Le Botaniste. 2005;28:364–368. [Google Scholar]
- 11.Matei N, Magearu V, Birghilă S, Dobrinaş S. The determination of vitamin C from sweet cherries and cherries. Revista de Chimie. 2004;55(5):294–296. [Google Scholar]
- 12.O'Connell PJ, Gormally C, Pravda M, Guilbault GG. Development of an amperometric L-ascorbic acid (vitamin C) sensor based on electropolymerised aniline for pharmaceutical and food analysis. Analytica Chimica Acta. 2001;431(2):239–247. [Google Scholar]
- 13.Güçlü K, Sözgen K, Tütem E, Özyürek M, Apak R. Spectrophotometric determination of ascorbic acid using copper(II)-neocuproine reagent in beverages and pharmaceuticals. Talanta. 2005;65(5):1226–1232. doi: 10.1016/j.talanta.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 14.Danet AF, David V, Oancea M. Dispozitiv de analiza in flux cu injectare hidrodinamica. Determinarea vitaminei C. Revista de Chimie. 1994;45(11):1000–1006. [Google Scholar]
- 15.Danet AF, Badea M, Aboul-Enein HY. Flow injection system with chemiluminometric detection for enzymatic determination of ascorbic acid. Luminescence. 2000;15(5):305–309. doi: 10.1002/1522-7243(200009/10)15:5<305::AID-BIO599>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira EJ, Watson DG. Chromatographic techniques for the determination of putative dietary anticancer compounds in biological fluids. Journal of Chromatography B. 2001;764(1-2):3–25. doi: 10.1016/s0378-4347(01)00401-7. [DOI] [PubMed] [Google Scholar]
- 17.Kall MA, Andersen C. Improved method for simultaneous determination of ascorbic acid and dehydroascorbic acid, isoascorbic acid and dehydroisoascorbic acid in food and biological samples. Journal of Chromatography B. 1999;730(1):101–111. doi: 10.1016/s0378-4347(99)00193-0. [DOI] [PubMed] [Google Scholar]
- 18.Iwase H, Ono I. Determination of ascorbic acid in food by column liquid chromatography with electrochemical detection using eluent for pre-run sample stabilization. Journal of Chromatography A. 1998;806(2):361–364. doi: 10.1016/s0021-9673(98)00062-4. [DOI] [PubMed] [Google Scholar]
- 19.Iwase H. Use of nucleic acids in the mobile phase for the determination of ascorbic acid in foods by high-performance liquid chromatography with electrochemical detection. Journal of Chromatography A. 2000;881(1-2):327–330. doi: 10.1016/s0021-9673(00)00057-1. [DOI] [PubMed] [Google Scholar]
- 20.Rizzolo A, Brambilla A, Valsecchi S, Eccher-Zerbini P. Evaluation of sampling and extraction procedures for the analysis of ascorbic acid from pear fruit tissue. Food Chemistry. 2002;77(2):257–262. [Google Scholar]
- 21.Rodríguez-Comesaña M, García-Falcón MS, Simal-Gándara J. Control of nutritional labels in beverages with added vitamins: screening of β-carotene and ascorbic acid contents. Food Chemistry. 2002;79(2):141–144. [Google Scholar]
- 22.Fernandes JCB, Kubota LT, de Oliveira Neto G. Potentiometric biosensor for L-ascorbic acid based on ascorbate oxidase of natural source immobilized on ethylene-vinylacetate membrane. Analytica Chimica Acta. 1999;385(1–3):3–12. [Google Scholar]
- 23.Tomita IN, Manzoli A, Fertonani FL, Yamanaka H. Amperometric biosensor for ascorbic acid. Eclética Química. 2005;30(2):37–43. [Google Scholar]
- 24.Matsumoto K, Yamada K, Osajima Y. Ascorbate electrode for determination of L-ascorbic acid in food. Analytical Chemistry. 1981;53(13):1974–1979. doi: 10.1021/ac00236a006. [DOI] [PubMed] [Google Scholar]
- 25.Greenway GM, Ongomo P. Determination of L-ascorbic acid in fruit and vegetable juices by flow injection with immobilised ascorbate oxidase. Analyst. 1990;115(10):1297–1299. doi: 10.1039/an9901501297. [DOI] [PubMed] [Google Scholar]
- 26.Matos RC, Augelli MA, Lago CL, Angnes L. Flow injection analysis-amperometric determination of ascorbic and uric acids in urine using arrays of gold microelectrodes modified by electrodeposition of palladium. Analytica Chimica Acta. 2000;404(1):151–157. [Google Scholar]
- 27.Kumar SS, Narayanan SS. Amperometric sensor for the determination of ascorbic acid based on cobalt hexacyanoferrate modified electrode fabricated through a new route. Chemical & Pharmaceutical Bulletin. 2006;54(7):963–967. doi: 10.1248/cpb.54.963. [DOI] [PubMed] [Google Scholar]
- 28.Fernández L, Carrero H. Electrochemical evaluation of ferrocene carboxylic acids confined on surfactant-clay modified glassy carbon electrodes: oxidation of ascorbic acid and uric acid. Electrochimica Acta. 2005;50(5):1233–1240. [Google Scholar]
- 29.Zen J-M, Tsai D-M, Kumar AS. Flow injection analysis of ascorbic acid in real samples using a highly stable chemically modified screen-printed electrode. Electroanalysis. 2003;15(14):1171–1176. [Google Scholar]
- 30.Cheregi M, Danet AF. Flow injection determination of L-ascorbic acid in natural juice with biamperometric detection. Analytical Letters. 1997;30(14):2625–2640. [Google Scholar]
- 31.Gutés A, Ibáñez AB, del Valle M, Céspedes F. Automated SIA e-tongue employing a voltammetric biosensor array for the simultaneous determination of glucose and ascorbic acid. Electroanalysis. 2006;18(1):82–88. [Google Scholar]
- 32.Nezamzadeh A, Amini MK, Faghihian H. Square-wave voltametric determination of ascorbic acid based on its electrocatalytic oxidation at zeolite-modified carbon-paste electrodes. International Journal of Electrochemical Science. 2007;2:583–594. [Google Scholar]
- 33.Raoof JB, Ojani R, Beitollahi H. Electrocatalytic determination of ascorbic acid at chemically modified carbon paste electrode with 2, 7-bis (ferrocenyl ethynyl) fluoren-9-one. International Journal of Electrochemical Science. 2007;2(7):534–548. [Google Scholar]
- 34.Chevion S, Roberts MA, Chevion M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radical Biology and Medicine. 2000;28(6):860–870. doi: 10.1016/s0891-5849(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 35.Zielinska D, Szawara-Nowak D, Zielinski H. Comparison of spectrophotometric and electrochemical methods for the evaluation of the antioxidant capacity of buckwheat products after hydrothermal treatment. Journal of Agricultural and Food Chemistry. 2007;55(15):6124–6131. doi: 10.1021/jf071046f. [DOI] [PubMed] [Google Scholar]
- 36.Ruffien-Ciszak A, Gros P, Comtat M, Schmitt A-M, Questel E, Casas C, Redoules D. Exploration of the global antioxidant capacity of the stratum corneum by cyclic voltammetry. Journal of Pharmaceutical and Biomedical Analysis. 2006;40(1):162–167. doi: 10.1016/j.jpba.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 37.Rapta P, Misik V, Stasko A, Vrabel I. Redox intermediates of flavonoids and caffeic acid esters from propolis: an EPR spectroscopy and cyclic voltammetry study. Free Radical Biology and Medicine. 1995;18(5):901–908. doi: 10.1016/0891-5849(94)00232-9. [DOI] [PubMed] [Google Scholar]
- 38.Zielinska D, Wiczkowski W, Piskula MK. Determination of the relative contribution of quercetin and its glucosides to the antioxidant capacity of onion by cyclic voltammetry and spectrophotometric methods. Journal of Agricultural and Food Chemistry. 2008;56(10):3524–3531. doi: 10.1021/jf073521f. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Kim IK. Analysis of ascorbic acid by ion exclusion chromatography with electrochemical detection. Journal of Food Science. 1988;53(5):1525–1527. [Google Scholar]
- 40.Campanella L, Martini E, Rita G, Tomassetti M. Antioxidant capacity of dry vegetal extracts checked by voltammetric method. Journal of Food, Agriculture & Environment. 2006;4(1):135–144. [Google Scholar]
- 41.Shi C, Xie S, Jia J. The study of a new method to determine copper ion by square-wave voltammetry-extraction iodometry at the liquid/liquid interfaces. Journal of Automated Methods and Management in Chemistry. 2008;2008:5 pages. doi: 10.1155/2008/453429. Article ID 453429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams RN. Probing brain chemistry with electroanalytical techniques. Analytical Chemistry. 1976;48(14):1126A–1138A. doi: 10.1021/ac50008a001. [DOI] [PubMed] [Google Scholar]
- 43.Wen X-L, Han Z-X, Rieker A, Liu Z-L. Significant micellar effect on the oxidative electrochemistry of ascorbic acid. Journal of Chemical Research Part S. 1997;(3):108–109. [Google Scholar]
- 44.Sun H, Lian K, Liang S, Liu Z. Preparation of activated rough electrode in KMnO4 solutions and its application for the electrocatalytic oxidation of ascorbic acid. Chemical Journal on Internet. 2004;6(10):p. 65. [Google Scholar]
- 45.Khorasani-Motlagh M, Noroozifar M. Electrocatalytic determination of ascorbic acid using glassy carbon modified with nickel(II) macrocycle containing dianionic tetraazaannulene ligand. Turkish Journal of Chemistry. 2004;28(3):369–378. [Google Scholar]
- 46.Aydoğmuş Z, Çetin SM, Özgür MÜ. Determination of ascorbic acid in vegetables by derivative spectrophotometry. Turkish Journal of Chemistry. 2002;26(5):697–704. [Google Scholar]
- 47.French RB, Abbott OD. Investigation of the vitamin C content of Florida fruits and vegetables. I. Effects of maturation and of cold storage on the vitamin C potency of oranges and grapefruit. Journal of Nutrition. 1940;19(3):223–232. [Google Scholar]