Abstract
A iron-dinitrosyl species (6L)Fe(NO)2 (2), generated from nitrogen monoxide (•NO) binding to its related iron(II)-mononitrosyl complex (6L)Fe(NO) (1), efficiently effects reductive coupling of two •NO molecules to release nitrous oxide (N2O), when Cu+ ion and two equiv acid are added; the heme/Cu product is [(6L)FeIII…CuII(D)]3+ (D = H2O or MeCN). In a control experiment where only (6L)Fe(NO)2 (2) is exposed to two equiv acid, no UV-vis change is observed; •NO(g) is released and (6L)Fe(NO) is reformed. The copper ion complex within the 6L ligand framework is required for the •NO coupling chemistry. In a further control experiment Cu+ ion is added to (6L)Fe(NO)2 without acid present, [(6L)Fe(NO)…CuII(NO2−)]+ is obtained, with the amount of N2O(g) released fitting with copper(I) ion promoted disproportionation chemistry, 3 •NO + ligand-CuI →N2O + ligand-CuII(NO2−). The chemical system described represents a (stoichiometric) functional model for heme/Cu protein nitric oxide reductase activity.
The subject of the interaction of nitrogen monoxide (•NO) with hemes is very important in the biology of this highly reactive diatomic molecule. As a signaling agent, heme proteins are involved in its biosynthesis and detection.1 In aerobic microorganisms, heme cofactors are key in the oxidative response to toxic concentrations of •NO.2 In anaerobic denitrifiers with the biochemical pathway NO3− → NO2− → •NO → N2O → N2, •NO reductases (NORs) possess a heme/non-heme diiron active site which couples two •NO molecules giving nitrous oxide(N2O).3,4 In addition to their role as a mediator for the reduction of O2 to water accompanied by membrane proton translocation, heme-copper oxidases (HCOs) including cytochrome c oxidases (CcOs) effect this same reductive coupling reaction,3a,b,5 (diagram). HCO active sites contain a high-spin heme/CuB dinuclear center;6 HCOs and NORs are evolutionarily related.5,7 The NO coupling mechanism in HCOs has received considerable recent attention via biophysical and computational studies,3a,8 and in a designed small protein model study.9 Yet, we are far from having a sufficient understanding of the underlying metal/NO and metal ion mediated NO-NO coupling chemistry.
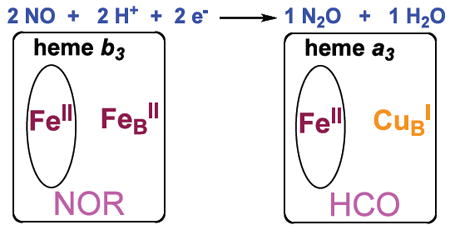
While there is extensive recent activity in the design and study of discrete heme/copper synthetic complexes which resemble HCO active sites and/or effect dioxygen binding and reduction chemistry,6,10 there are no cases where a synthetic small molecule heme/Cu complex reacts with •NO to effect reductive coupling leading to nitrous oxide. In this report, we describe such a system, employing the binucleating ligand 6L and its iron and heme-copper derivatives which have been previously used in our investigations involving O2-chemistry.10a The starting point is the reductive nitrosylation of (6L)FeIII(OH)11 to straightforwardly give the iron(II)-nitrosyl compound (6L)Fe(NO) (1) (Scheme 1), possessing the expected three-line hyperfine split EPR spectrum12 (Fig. 1a).13 As has been reported previously,14 such complexes may react with additional •NO(g) to form a dinitrosyl species. This occurs here and evidence in support of this formulation, (6L)Fe(NO)2 are as follows: (a) As monitored by UV-vis spectroscopy in acetone (−80 °C), 1 reversibly binds •NO(g) to form (6L)Fe(NO)2 (2); 2 is stable, but loses •NO(g) upon warming to RT. (b) With formation of 2 at −80 °C, the EPR signal due to (6L)Fe-(NO) (1) disappears, as would be expected for 2, having an even number of electrons. (c) Titration of a −80 °C solution of (6L)Fe(NO)2 (2) with one equiv (F8)FeII {F8 = tetrakis(2,6-difluorophenyl)porphyrinate(2-)} leads to a clean conversion to (6L)Fe(NO) (1) plus (F8)Fe(NO) (X-Ray structure determined); 2 possesses two equiv of bound •NO.13
Scheme 1.
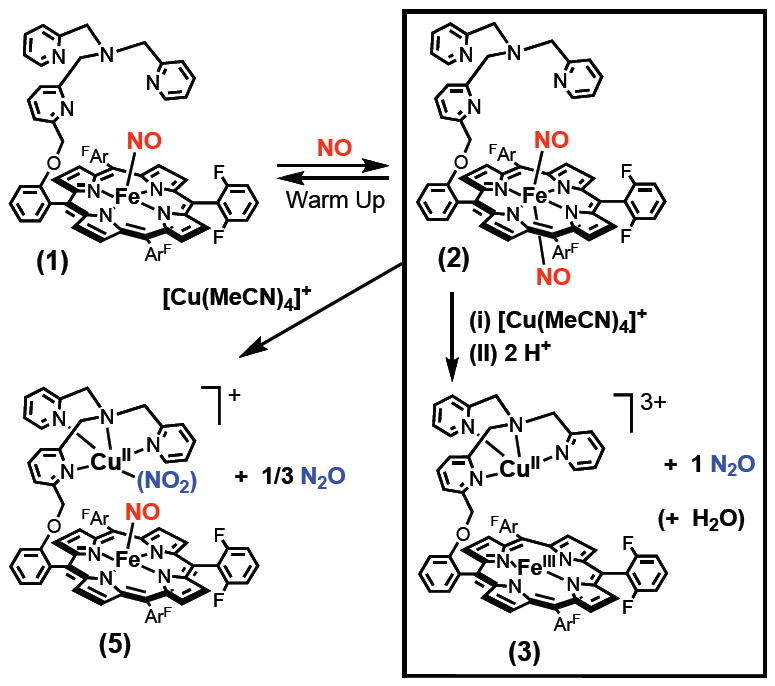
Figure 1.

EPR spectra of species formed in the chemistry described by Scheme 1: (a) (6L)Fe(NO) (1), (b) [(6L)FeIII…CuII(D)]3+ (3), with high-spin FeIII (g ~ 6) and CuII (g∥ = 2.26, g┴ = 2.05, A∥ = 150 G, A┴ = 36 G) centers; the spectrum matches a synthetically prepared 1:1 mixture of [(F8)FeIII]SbF6 and CuII complex, (c) [(6L)Fe(NO)…CuII(NO2−)]+ (5); the spectrum matches a 1:1 mixture of (F8)Fe(NO) and [CuII(NO2−)]+ complex. See text.
The existence of (6L)Fe(NO)2 (2) is very useful for the present case.15 The system is simplified and a stoichiometric reaction (e.g., one enzyme turnover, by analogy) can now be studied; exactly two •NO molecules are present for each heme or heme/Cu assembly. Thus, a copper ion source, [CuI(MeCN)4]B(C6F5)4, was added to (6L)Fe(NO)2 (2) at −80 °C in acetone/MeCN (3:1) along with two equiv acid in the form of H(Et2O)2[B(C6F5)4]. Upon warming to RT, identification of the products reveals that an NOR type reaction has taken place: (i) Nitrous oxide is produced in very good yield (80 % ave; best run gave 87%), as determined by GC analysis of the reaction vessel headspace. (ii) The product heme complex is formulated as [(6L)FeIII…CuII(D)]3+ (3) (D = MeCN or H2O) (Scheme 1). This is based on the observed UV-vis spectrum, appearing as a single species with λmax = 396, 515 nm in THF, matching that of a typical (porphyrinate)FeIII-X high-spin complex (X = a non-coordinating anion like PF6− or here B(C6F5)4−). Further supporting this formulation, an EPR spectrum (Fig. 1b) reveals that a high-spin heme-FeIII along with a CuII (tetragonal complex) are both present.13,16
In order to rule out the possibility that only (6L)Fe(NO)2 (2) itself mediates the •NO reductive coupling observed, its reaction with two equiv HBArF was investigated. No UV-Vis change was observed and even following warming from −80 °C, the iron(II) nitrosyl complex (6L)Fe(NO) (1) was produced; •NO(g) was released as determined by GC analysis.13 Copper ion has a critical role in the NOR chemistry described here.
The acid (proton) source present is key to the chemistry observed, as is also known for •NO reduction catalyzed by HCOs.3a,8b In a further control experiment, copper(I) ion was added to (6L)Fe(NO)2 (2) but in the absence of acid (Scheme 1). The product obtained is formulated as the heme-nitrosyl..CuII-nitrite complex [(6L)Fe(NO)…CuII(NO2−)]+ (5), Scheme 1. A UV-Vis spectrum reveals bands associated with (6L)Fe(NO) (1) {λmax (THF) = 411, 547 nm} and IR spectroscopy gives νNO = 1687 cm-1. An EPR spectrum of the product mixture (Fig. 1c) matches to a mixture of typical (porphyrinate)FeII-NO and CuII species, consistent with our proposed course of reaction.13
Thus, without added acid, one of the two nitrogen monoxide molecules (per heme/Cu assembly) remains in the product. Our expectation was that any remaining •NO released could be disproportionated to nitrite and N2O, according to the well established chemistry:3c
| (eq. 1) |
In fact, this seems to be the case. GC analysis reveals that N2O is produced in 90 % yield according to eq. 1. This is far less than should be or is produced by our NOR chemistry with acid present, Scheme 1. In further support of the chemistry outlined here, Ion Chromatography analysis of an aqueous solution derived from a reaction mixture indicates nitrite is present.13 In separate experiments, we have observed that a copper(I) complex of the tris(2-pyridylmethyl)amine moiety found in 6L does react with •NO(g) according to eq. 1. To reiterate, without added acid, heme-copper mediated NOR chemistry does not occur and [(6L)Fe(NO)…CuII(NO2−)]+ (5) is instead produced, Scheme 1.17
In summary, we have shown that the heme-copper assembly described here efficiently effects reductive coupling of nitrogen monoxide molecules in the same stoichiometry as is known for HCOs. Two equiv •NO,18 the heme, the juxtaposed copper ion and protons are all required, [(6L)FeII..CuI]+ + 2 NO(g) + 2 H+ → [(6L)FeIII..CuII]3+ + N2O(g) + H2O. Collman et al.19 recently reported on stoichiometric functional model systems for heme/non-heme diiron NORs, and the continued interrogation of well-designed synthetic systems20 will be important in the elucidation of fundamental aspects of heme/NOx 12,21 and heme/M/•NO (M = Cu, Fe))7 interactions and their reductive (or oxidative)2,22 chemistries. We note that a heme and/or copper coordinated hyponitrite (N2O22−) moiety23 has been postulated (or even detected)8a in protein chemistry.8 For this present system, further studies will be directed to mechanistic analyses and attempts to generate or find this or other intermediates that may form.
Supplementary Material
Details concerning synthesis, spectroscopy and reactivity and CIF file. This material is available free of charge on the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported NIH grant GM 60353 (K.D.K.).
References
- 1.(a) Poulos TL. Curr Opin Struct Biol. 2006;16:736–743. doi: 10.1016/j.sbi.2006.09.006. [DOI] [PubMed] [Google Scholar]; (b) Zhu Y, Silverman RB. Biochemistry. 2008;47:2231–2243. doi: 10.1021/bi7023817. [DOI] [PubMed] [Google Scholar]
- 2.(a) Poole RK. Biochem Soc Trans. 2005;33:176–180. doi: 10.1042/BST0330176. [DOI] [PubMed] [Google Scholar]; (b) Gardner PR. J Inorg Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.(a) Pinakoulaki E, Varotsis C. J Inorg Biochem. 2008;102:1277–1287. doi: 10.1016/j.jinorgbio.2008.01.014. [DOI] [PubMed] [Google Scholar]; (b) Moenne-Loccoz P. Natural Product Reports. 2007;24:610–620. doi: 10.1039/b604194a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wasser IM, de Vries S, Moënne-Loccoz P, Schröder I, Karlin KD. Chem Rev. 2002;102:1201–1234. doi: 10.1021/cr0006627. [DOI] [PubMed] [Google Scholar]
- 4.A number of non-heme diiron proteins effect the reductive coupling of nitrogen monoxide to N2O. Kurtz DM., Jr Dalton Trans. 2007:4115–4121.Coufal DE, Tavares P, Pereira AS, Hyunh BH, Lippard SJ. Biochemistry. 1999;38:4504–4513. doi: 10.1021/bi9823378.Haskin CJ, Ravi N, Lynch JB, Münck E, Que JL. Biochemistry. 1995;34:11090–11098. doi: 10.1021/bi00035a014.
- 5.Zumft WG. J Inorg Biochem. 2005;99:194–215. doi: 10.1016/j.jinorgbio.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Kim E, Chufán EE, Kamaraj K, Karlin KD. Chem Rev. 2004;104:1077–1133. doi: 10.1021/cr0206162. [DOI] [PubMed] [Google Scholar]
- 7.Based on sequence analyses, certain NOR families in fact appear to be heme-copper enzymes. Personal communication, R.B. Gennis, U. Illinois.
- 8.(a) Varotsis C, Ohta T, Kitagawa T, Soulimane T, Pinakoulaki E. Angew Chem Int Ed. 2007;46:2210–2214. doi: 10.1002/anie.200602963. [DOI] [PubMed] [Google Scholar]; (b) Blomberg LM, Blomberg MRA, Siegbahn PEM. Biochim Biophys Acta. 2006;1757:31–46. doi: 10.1016/j.bbabio.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, Yeung N, Russell BS, Garner DK, Lu Y. J Am Chem Soc. 2006;128:6766–6767. doi: 10.1021/ja058822p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Chufán EE, Puiu SC, Karlin KD. Acc Chem Res. 2007;40:563–572. doi: 10.1021/ar700031t. [DOI] [PubMed] [Google Scholar]; (b) Collman JP, Devaraj NK, Decreau RA, Yang Y, Yan Y-L, Ebina W, Eberspacher TA, Chidsey CED. Science. 2007;315:1565–1568. doi: 10.1126/science.1135844. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Collman JP, Decreau RA, Yan Y, Yoon J, Solomon EI. J Am Chem Soc. 2007;129:5794–5795. doi: 10.1021/ja0690969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obias HV, van Strijdonck GPF, Lee D-H, Ralle M, Blackburn NJ, Karlin KD. J Am Chem Soc. 1998;120:9696–9697. [Google Scholar]
- 12.Cheng L, Richter-Addo GB. Porphyrin Handbook. 2000;4:219–291. [Google Scholar]
- 13.See Supporting Information.
- 14.(a) Wayland BB, Olson LW. J Am Chem Soc. 1974;96:6037–6041. doi: 10.1021/ja00826a013. [DOI] [PubMed] [Google Scholar]; (b) Lorkovic I, Ford PC. J Am Chem Soc. 2000;122:6516–6517. [Google Scholar]; (c) Ghosh A. Acc Chem Res. 2005;38:943–954. doi: 10.1021/ar050121+. [DOI] [PubMed] [Google Scholar]
- 15.The dinitrosyl complex 2 is inherently interesting and will be the subject of separate future reports.
- 16.ESI-MS analysis of the reaction mixture gives only the well known structurally characterized μ-oxo complex [(6L)FeIII-O-CuII]+ (4) (m/z = 1173, matching a spectrum calculated for the structure which includes the distinctive pattern due to the 63,65Cu isotopes). In fact, solutions of 3 are cleanly converted to 4 with addition of Et3N/H2O as indicated by the change to the known UV-vis spectrum with red-shifted Soret band, λmax = 440, 557 nm.
- 17.The same product 5 is observed if [(6L)FeII..CuI]+ is exposed to excess •NO(g), unpublished observations.
- 18.To date, a heme dinitrosyl species such as 2 has not been observed as an intermediate in the enzyme reaction.
- 19.(a) Collman JP, Yang Y, Dey A, Decréau RA, Ghosh S, Ohta T, Solomon EI. Proc Nat Acad Sci. 2008;105:15660–15665. doi: 10.1073/pnas.0808606105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Collman JP, Dey A, Yang Y, Decréau RA, Ohta T, Solomon EI. J Am Chem Soc. 2008;130:16498–16499. doi: 10.1021/ja807700n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A diruthenium complex exhibits novel NOR activity, the reservible addition of NO to give N2O, see Arikawa Y, Asayama T, Moriguchi Y, Agari S, Onishi M. J Am Chem Soc. 2007;129:14160–14161. doi: 10.1021/ja0763504.
- 21.(a) Wyllie GRA, Scheidt WR. Chem Rev. 2002;102:1067–1089. doi: 10.1021/cr000080p. [DOI] [PubMed] [Google Scholar]; (b) Kurtikyan TS, Ford PC. Coord Chem Rev. 2008;252:1486–1496. [Google Scholar]; (c) Ford PC. Coord Chem Rev. 2005;249:391–403. [Google Scholar]; (d) Praneeth VKK, Paulat F, Berto TC, George SD, Näther C, Sulok CD, Lehnert N. J Am Chem Soc. 2008;130:15288–15303. doi: 10.1021/ja801860u. [DOI] [PubMed] [Google Scholar]
- 22.Cooper CE, Mason MG, Nicholls P. Biochim Biophys Acta. 2008;1777:867–876. doi: 10.1016/j.bbabio.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Arulsamy N, Bohle DS, Imonigie JA, Moore RC. Polyhedron. 2007;26:4737–4745. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details concerning synthesis, spectroscopy and reactivity and CIF file. This material is available free of charge on the Internet at http://pubs.acs.org.


