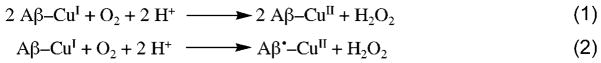Extensive evidence points to oxidative stress as a key event in the pathogenesis and exacerbation of Alzheimer’s Disease (AD). [1] Transition metals, such as Zn, Fe, and Cu, are present in elevated concentrations in AD brain deposits, composed primarily of 40- or 42-mer amyloid beta (Aβ) peptides. The redox-active copper(II) ion binds to the unstructured, hydrophilic N terminus of Aβ; [1g,2] and the ability of copper to promote the formation of reactive oxygen species (ROS) and cause neuronal death by interaction with Aβ has been demonstrated in vitro.[1a,c,3,4] ROS formation is proposed to occur by interaction of reduced CuI–Aβ with O2 or H2O2. However, few direct studies of CuI binding or reactivity with Aβ peptides or fragments have been reported.[5,6]
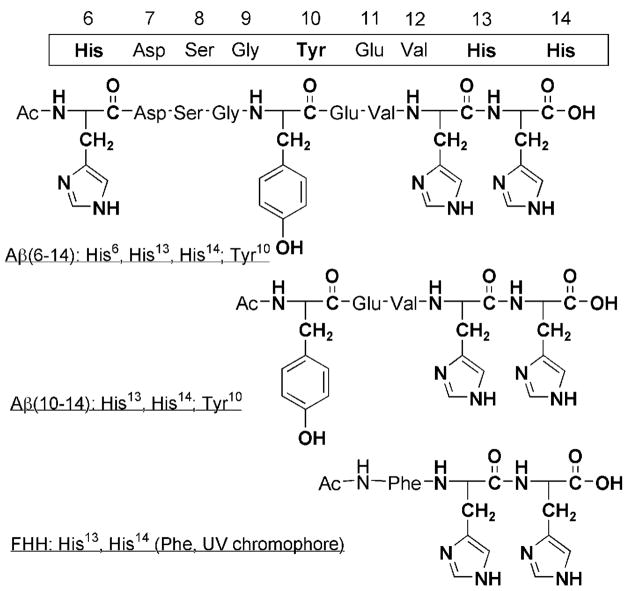
We have studied the interactions of the hydrophilic N-terminal region of the Aβ peptide with CuI. An understanding of the full redox competency of Cu–Aβ, leading to ROS formation and oxidative stress (that is, to cause events associated with the onset of AD), is incomplete without elucidation of the structure/function relationships of the reduced (active) copper(I)–peptide complexes. We report herein studies on the interaction of CuI ions with small portions of the Aβ peptide incorporating specific metal-binding (His6, His13, His14) or potentially redox-active (Tyr10) residues (Figure 1). Of considerable interest are the contiguous His13 and His14 residues. We have previously reported studies on CuI complexes of modified (by end-capping and/or regiospecific Nε- or Nδ-alkylation) His–His dipeptides which, significantly, adopt a two-coordinate, near-linear NHis –CuI–NHis environment.[6] In this report, we demonstrate that CuI complexes of longer Aβ peptide fragments adopt the same apparent two-coordinate structure in the solid state and aqueous solution. Preliminary reactivity investigations, described here, indicate that the His13–CuI–His14 moiety is the active part of the structure, responsible for copper-Aβ reactivity.
Figure 1.
Aβ peptides used for studies with CuI ions.
A range of peptides (Figure 1) were synthesized and purified by reverse-phase (RP) HPLC to a single peak. Their identity and purity were confirmed by ESI mass spectrometry. The peptides were stored either as lyophilized powders or as stock solutions in doubly distilled deionized water, both at −80°C.[7] Copper(I)–peptide complexes were prepared directly from CuI starting materials in the absence of reductants, and their formulation confirmed using ESI-MS. Structural information was obtained by spectroscopic techniques for both solid and solution states (see below). Solid samples of CuI–Aβ(6–14) and CuI–Aβ(10–14) were prepared by incubating stoichiometric amounts of the respective peptides with a [CuI(CH3CN)4]+ salt in DMF and isolated by precipitation with diethyl ether, filtration, and drying under reduced pressure. Their formulation was confirmed using ESI-MS.[8] Mishandled samples turned deep blue, indicating oxidation to CuII, whereas the CuI complexes remained white-to-gray when air was excluded, indicating reduced metal–peptide complexes.
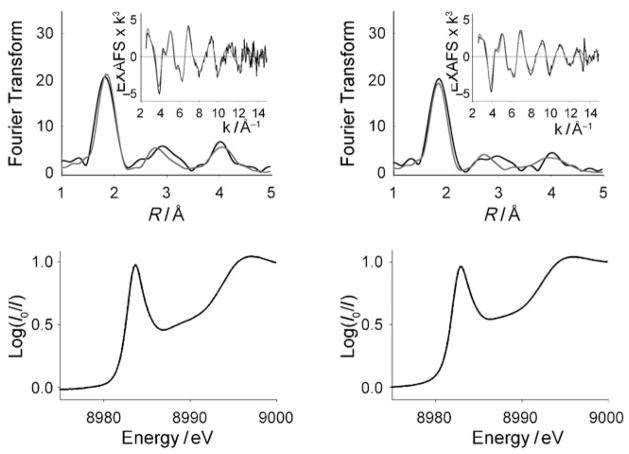
For these solid samples, X-ray absorption spectroscopy (XAS) was used as a powerful (yet unexploited, in the case of Cu–Aβ complexes) tool for the determination of oxidation state, coordination environment, and bond lengths in the derived metal complexes.[9,10] For both Aβ(6–14) and Aβ(10–14) complexes, the occurrence of the 1s→4p transition at 8983–84 eV (Figure 2) definitively indicated that copper was in the +1 oxidation state. Extended X-ray absorption fine structure (EXAFS) spectroscopic data fits for the CuI–Aβ(10–14) complex, with only two histidine residues (Figure 1), indicated two nitrogen ligands from imidazole donors, as further supported by back-scattering from the ring carbons and nitrogen. The data were consistent with these donors being the only ligands bound to the CuI ion. The intensity of the pre-edge (1s→4p) feature (Figure 2) was further indicative of two-coordination, to the exclusion of other (i.e., three-coordinate) geometries.[9,10] In addition, the short Cu–N bond lengths—at 1.878 Å—are characteristic of linear, two-coordinate geometry in copper(I)–nitrogen ligand complexes, by comparison to crystallographically characterized synthetic copper(I) complexes.[11] The data also conform to the structures identified previously in our CuI(His)2 dipeptide complexes, in which intramolecular binding of the imidazole moieties of the dipeptide affords tight, linear Cu–N two-coordinate geometry.[6]
Figure 2.
EXAFS (top, including insets) and XANES (bottom) spectroscopic data for CuI–Aβ (6–14) (left) and CuI–Aβ (10–14) (right). Fourier transforms: black, fits: gray.
Further results obtained for CuI–Aβ (6–14) firmly demonstrate the propensity for CuI to adopt near-linear two-coordinate geometry: EXAFS spectroscopic analysis of solid CuI–Aβ (6–14) indicated formation of the same structure, despite the presence of a third potential histidine ligand. For CuI–Aβ (6–14), the Fourier Transform with fit is shown in Figure 2. The data could only be fit to two N/O scatterers, thus indicating the presence of only two ligands at the CuI center; these were identified unambiguously as His nitrogen atoms by backscattering. The Cu–NHis bond lengths of 1.876 Å and the X-ray absorption near-edge structure (XANES) absorption intensity clearly indicate two-coordination (and three-coordination).
Binding of CO to CuI was used as a probe of solution structure. Results indicated that the 2Nimid structure persists in solution, even for the three-His-containing complex CuI–Aβ (6–14). CO complexes were formed for each of the three peptides [Figure 1: Aβ (6–14) and Aβ (10–14), discussed above, and the tripeptide FHH, discussed in more detail below] in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered (pH 7.4) D2O and characterized using FTIR spectroscopy. The stretching frequency of copper(I)-bound CO is diagnostic for the overall coordination number in cationic copper(I) species,[6,11e,12] and has been noted in cuprous enzymes.[13] All three complexes had stretching frequencies greater than 2110 cm−1, varying by no more than 2 cm−1 (Table 1). The high frequency is clearly indicative of the presence of only two N donors coordinating to the CuI ion. The results recalled our previous finding that His–His dipeptide moieties strongly favor near-linear two-coordination (Table 1).[6]
Table 1.
Structural data for CuI complexes of His-containing peptides.
| Complex | Donorsa | Cu– NImid [Å] | υCOb [cm−1] |
|---|---|---|---|
| [CuI Lδ]+c | 2 His | 1.876 | 2110d |
| [CuI LH]+c | 2 His | 1.869 | 2105e |
| [CuIAβ (6–14)]+ | 3 His | 1.876 | 2110f |
| [CuIAβ (10–14)]+ | 2 His | 1.878 | 2112f |
| [CuIFHH]+ | 2 His | N/A | 2110f |
| [CuI Lδ(MeImid)]+c | 2 His | 1.896 | 2075 |
| 1 Imid | 2.008g |
N-Donor ligands available for coordination to CuI.
For corresponding peptide–CuI–CO complex.
Copper(I) complexes of His–His dipeptides; Lδ contains two trityl-protected imidazole ε nitrogen atoms, whereas LH incorporates two unprotected imidazole moieties. See Ref. [6].
Dichloromethane solution.
Methanol solution.
With HEPES buffer, pH 7.4, D2O.
Two Cu–NHis bonds 1.896 Å, Cu–→NImid bond 2.008 Å.
The similarity in structure deduced for these complexes, [CuI–Aβ(6–14) and CuI–Aβ(10–14), by EXAFS and IR spectroscopy (CO binding); and also CuI(FHH), by IR] strongly suggests that His13 and His14 constitute the two N-donor ligands to the CuI center. Whereas the unique redox properties of a CuI ion in a linear, two-coordinate environment have been noted in model complexes[11a,e,14] and the structure has been proposed to be important in some copper-enzyme active sites,[15] the possibility of a CuI(His)2 site involved in Aβ chemistry has been overlooked.
With our structural results in mind, we have begun studying the redox reactivity of these systems. Preliminary experiments on the ability of CuI–Aβ fragment complexes to produce ROS have been carried out. The first step in Cu–Aβ ROS production has been proposed to be CuII reduction followed by reaction with O2 to produce H2O2.[16] Hydrogen peroxide has been formed in vitro from Cu–Aβ complexes, but only in the presence of very large excesses of reducing agents, such as ascorbate,[16,17] or by electrochemical reduction of CuII.[4] Direct reactivity of CuI–Aβ with O2, by way of the reactions shown in Scheme 1, has not been studied, until now.
Scheme 1.
Potential reactions of Cu–Aβ with dioxygen to form H2O2.
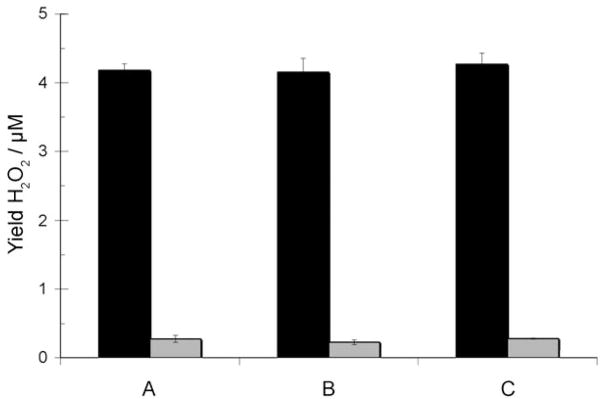
Production of H2O2 from oxygenated CuI–peptide solutions was monitored using the horseradish peroxidase (HRP)/Amplex Red assay. Hydrogen peroxide is produced from solutions of CuI–Aβ over the course of one hour, in amounts significantly greater than CuI-only or peptide-only control reactions.[8,18] Most intriguingly, all three systems, whether incorporating the third His residue (His6) or not, or incorporating the potentially redox-active Tyr10 or not, produce assayable H2O2 in similar yields and rates of formation. Mechanistically, reduction of O2 to H2O2 requires two electrons (Scheme 1). Thus, in the absence of an exogenous reductant (as in these experiments), stoichiometry requires that a second electron must be provided either by a second copper ion in the CuI–Aβ moiety or by the peptide itself, potentially by tyrosine oxidation [Eq. (2)].
Based on our results (Figure 3), the similar efficiency of CuI(FHH) in H2O2 production, compared to that of the Tyr-containing species, suggests that electrons are supplied only by the oxidation of copper. Furthermore, the similar rates and yields among all three species suggest His6 is not significantly involved in CuI–Aβ–O2 reactivity. In other words, the uniformity in results from these preliminary experiments with the three copper–peptide species suggests that they react with O2 to produce H2O2 by the same mechanism—Equation (1), wherein two separate Aβ–CuI moieties are involved and each CuI–Aβ species is a CuI(His)2 complex.[19] Together, these results suggest that the CuI(His)2 unit may not only be the predominant binding mode of CuI ions to Aβ peptides, but also the structure directly responsible for the behavior (including ROS production) of reduced CuI–Aβ species.
Figure 3.
Yields of H2O2 from reactions of O2 with 25 μM copper(I)–peptide solutions, as determined by HRP/Amplex Red assay. CuI complex: black, peptide-only: gray. A) Aβ (6–14); B) Aβ (10–14); C) FHH. Error bars represent standard errors from five trials.
In summary, our EXAFS spectroscopy and CO-binding studies have clearly demonstrated the preference of CuI ions for two-coordinate geometry in binding to fragments of the Aβ N-terminal region through a contiguous His13–His14 motif. That this structure is retained, even in the presence of three histidine residues (His6, His13, His14) and additional potential donors (Tyr10, Asp7, Glu11, Ser8, backbone carbonyl O, amide N), is striking. The two-coordinate geometry of CuI–Aβ may prove critical to understanding the redox chemistry of Cu–Aβ, and thus to understanding oxidative stress in AD. Preliminary ROS results indicate that the two-coordinate CuI(His)2 structure is significant for explaining the behavior (H2O2 production) of CuIAβ, or that a third His in the sequence (His6) may not be crucial. All the current literature suggests His6 as a ligand for the oxidized CuII ion. These studies, absent of any CuI–Aβ structural information, conclude that a three-histidine binding environment is probably important for the CuII/CuI-promoted production of ROS. We have shown herein that this may not be the case. AD oxidative stress chemistry is dependent upon: 1) the CuII/CuI redox cycle, and 2) ROS production from CuI/H2O2 and/or CuI/O2 chemistry. The energetics and kinetics of both may be highly tuned by the preferred stable copper(I)–bis(histidine) structure, which we have previously demonstrated has unique redox properties.[6] As such, the study of CuI–Aβ may hold additional information, key to the understanding of Cu–Aβ oxidative stress.
Experimental Section
Experimental procedures, including procedures for peptide synthesis and purification, preparation of solid and solution CuI–peptide samples, procedures for CO-binding and H2O2-producing experiments, and methods for EXAFS spectroscopic data collection and analysis, are available in the Supporting Information.
Supplementary Material
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200803908.
Footnotes
This work was supported by the NIH (Grants GM28962, K.D.K.; NIH Postdoctoral Fellowship, R.A.H.; NIH NS27583, N.J.B.).
References
- 1.a) Barnham KJ, Bush AI. Curr Opin Chem Biol. 2008;12:222, 228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]; b) Crichton RR, Dexter DT, Ward RJ. Coord Chem Rev. 2008;252:1189–1199. [Google Scholar]; c) Crouch PJ, Harding SME, White AR, Camakaris J, Bush AI, Masters CL. Int J Biochem Cell Biol. 2008;40:181–198. doi: 10.1016/j.biocel.2007.07.013. [DOI] [PubMed] [Google Scholar]; d) Rauk A. Dalton Trans. 2008:1273–1282. doi: 10.1039/b718601k. [DOI] [PubMed] [Google Scholar]; e) Sayre LM, Perry G, Smith MA. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]; f) Nunomura A, Castellani RJ, Zhu XW, Moreira PI, Perry G, Smith MA. J Neuropathol Exp Neurol. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]; g) Gaggelli E, Kozlowski H, Valensin D, Valensin G. Chem Rev. 2006;106:1995 – 2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 2.a) Streltsov VA, Titmuss SJJ, Epa VC, Barnham KJ, Masters CL, Varghese JN. Biophys J. 2008;95:3447–3456. doi: 10.1529/biophysj.108.134429. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Karr JW, Szalai VA. Biochemistry. 2008;47:5006–5016. doi: 10.1021/bi702423h. [DOI] [PubMed] [Google Scholar]; c) Syme CD, Nadal RC, Rigby SEJ, Viles JH. J Biol Chem. 2004;279:18169 – 18177. doi: 10.1074/jbc.M313572200. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly PS, Xiao Z, Wedd AG. Curr Opin Chem Biol. 2007;11:128 – 133. doi: 10.1016/j.cbpa.2007.01.678. [DOI] [PubMed] [Google Scholar]
- 4.Jiang DL, Men LJ, Wang JX, Zhang Y, Chickenyen S, Wang YS, Zhou FM. Biochemistry. 2007;46:9270 – 9282. doi: 10.1021/bi700508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Baruch-Suchodolsky R, Fischer B. Biochemistry. 2008;47:7796–7806. doi: 10.1021/bi800114g. [DOI] [PubMed] [Google Scholar]; b) Streltsov VA, Varghese JN. Chem Commun. 2008:3169–3171. doi: 10.1039/b803911a. [DOI] [PubMed] [Google Scholar]; c) Raffa DF, Rickard GA, Rauk A. J Biol Inorg Chem. 2007;12:147 – 164. doi: 10.1007/s00775-006-0175-9. [DOI] [PubMed] [Google Scholar]
- 6.Himes RA, Park GY, Barry AN, Blackburn NJ, Karlin KD. J Am Chem Soc. 2007;129:5352 – 5353. doi: 10.1021/ja0708013. [DOI] [PubMed] [Google Scholar]
- 7.Periodic reverse-phase HPLC confirmed that no peptide decomposition occurred when stored in this manner for weeks. No aggregation or precipitation occurred.
- 8.See Supporting Information.
- 9.Blackburn NJ, Strange RW, Reedijk J, Volbeda A, Farooq A, Karlin KD, Zubieta J. Inorg Chem. 1989;28:1349 –1357. [Google Scholar]
- 10.Kau LS, Spira-Solomon DJ, Pennerhahn JE, Hodgson KO, Solomon EI. J Am Chem Soc. 1987;109:6433 – 6442. [Google Scholar]
- 11.a) Sanyal I, Karlin KD, Strange RW, Blackburn NJ. J Am Chem Soc. 1993;115:11259–11270. [Google Scholar]; b) Habiyakare A, Lucken EAC, Bernardinelli G. J Chem Soc Dalton Trans. 1991:2269–2273. [Google Scholar]; c) Munakata M, Kitagawa S, Shimono H, Masuda H. Inorg Chim Acta. 1989;158:217–220. [Google Scholar]; d) Engelhardt LM, Pakawatchai C, White AH, Healy PC. J Chem Soc Dalton Trans. 1985:117–123. [Google Scholar]; e) Sorrell TN, Jameson DL. J Am Chem Soc. 1983;105:6013–6018. [Google Scholar]; f) Agnus Y, Louis R, Weiss R. J Chem Soc Chem Commun. 1980:867–869. [Google Scholar]; g) Lewin AH, Cohen IA, Michl RJ. J Inorg Nucl Chem. 1974;36:1951–1957. [Google Scholar]; h) Okkersen H, Groeneve Wl, Reedijk J. Recl Trav Chim Pays-Bas. 1973;92:945 – 953. [Google Scholar]
- 12.a) Cole AP, Mahadevan V, Mirica LM, Ottenwaelder X, Stack TDP. Inorg Chem. 2005;44:7345–7364. doi: 10.1021/ic050331i. [DOI] [PubMed] [Google Scholar]; b) Chou CC, Su CC, Yeh A. Inorg Chem. 2005;44:6122–6128. doi: 10.1021/ic050568e. [DOI] [PubMed] [Google Scholar]; c) Voo JK, Lam KC, Rheingold AL, Riordan CG. J Chem Soc Dalton Trans. 2001:1803–1805. [Google Scholar]; d) Rondelez Y, Séneque O, Rager MN, Duprat AF, Reinaud O. Chem Eur J. 2000;6:4218–4226. doi: 10.1002/1521-3765(20001117)6:22<4218::aid-chem4218>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]; e) Casella L, Gullotti M, Pallanza G, Rigoni L. J Am Chem Soc. 1988;110:4221–4227. [Google Scholar]; f) Pasquali M, Floriani C, Gaetanimanfredotti A. Inorg Chem. 1980;19:1191 – 1197. [Google Scholar]
- 13.Jaron S, Blackburn NJ. Biochemistry. 1999;38:15086 – 15096. doi: 10.1021/bi991341w. [DOI] [PubMed] [Google Scholar]
- 14.Le Clainche L, Giorgi M, Reinaud O. Eur J Inorg Chem. 2000:1931 –1933. doi: 10.1021/ic000072r. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn NJ, Rhames FC, Ralle M, Jaron S. J Biol Inorg Chem. 2000;5:341 – 353. doi: 10.1007/pl00010663. [DOI] [PubMed] [Google Scholar]
- 16.a) Opazo C, Huang XD, Cherny RA, Moir RD, Roher AE, White AR, Cappai R, Masters CL, Tanzi RE, Inestrosa NC, Bush AI. J Biol Chem. 2002;277:40302–40308. doi: 10.1074/jbc.M206428200. [DOI] [PubMed] [Google Scholar]; b) Huang XD, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. Biochemistry. 1999;38:7609 – 7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 17.Huang XD, et al. J Biol Chem. 1999;274:37111 – 37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- 18.a) When carried out by the same method as the copper(I)–peptide experiments[8] copper(I)-only control reactions give a yield of H2O2 that never exceeds (and is often less than) 40% of that detected for copper(I)–peptide (that is, <1.5 μM, compared to >4 μM for copper(I)–peptide). It should be noted that there is some unexplained dependence on experimental conditions, especially the order of addition of reagents; for example, if CuI solutions are added to aerobic, buffered HRP/Amplex Red assay, strong signalling of H2O2 is indicated; b) if CuI ions are in excess (2:1) relative to the peptide, the results are unchanged, qualitatively indicating that CuI ion binds the peptide strongly. Quantitative determinations are in progress; c) assayed yields of H2O2 are, at most, approximately 30%, considering background and small amounts of Amplex oxidation by copper(II)–peptide and copper(I)–peptide/O2 (data not shown). It is unknown whether relative inefficiency of HRP/Amplex Red trapping is responsible for the sub-stoichiometric yield, or if the copper(I)/(II)–peptide itself consumes some peroxide. Catalase attenuates the signal. We are currently carrying out experiments to “trace” all copper(I) electron equivalents.
- 19.A recent publication implicates a soluble “dimer” (with two Aβ moities and, thus, possibly two Cu ions) as the minimal unit responsible for AD toxicity. See Shankar GM, Li S, Mehta TH, García-Muñoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Nature Medicine. 2008;14:837 – 842. doi: 10.1038/nm1782.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200803908.