Abstract
Background
The chronome (from chronos, time, and nomos, rule; time structure) of lipid peroxidation and anti-oxidant defense mechanisms may relate to the efficacy and management of preventive and curative chronotherapy.
Patients and methods
Thirty patients with liver cirrhosis, 25–45 years of age, and 60 age-matched clinically healthy volunteers were synchronized for 1 week with diurnal activity from about 06:00 to about 22:00 and nocturnal rest. Breakfast was around 08:30, lunch around 13:30 and dinner around 20:30. Drugs known to affect the free-radical system were not taken. Blood samples were collected at 6-h intervals for 24 h under standardized, presumably 24-h synchronized conditions. Determinations included plasma lipid peroxides, in the form of malondialdehyde (MDA), blood superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GR) activities, and serum total protein, albumin, ascorbic acid, and uric acid concentrations.
Results
A marked circadian variation was demonstrated for each variable in each group by population-mean cosinor (P < 0.01). In addition to anticipated differences in overall mean value (MESOR), patients differed from healthy volunteers also in terms of their circadian pattern.
Conclusion
Mapping the broader time structure (chronome) with age and multifrequency rhythm characteristics of antioxidants and pro-oxidants is needed for exploring their putative role as markers in the treatment and management of liver cirrhosis.
Keywords: Chronome, Anti-oxidant defense mechanisms, Lipid peroxidation, Circadian, Cirrhosis of liver, Chronoprevention, Marker rhythm
1. Introduction
The liver is vulnerable to a wide variety of metabolic, toxic, microbial, circulating and neoplastic insults. Cirrhosis of liver is the most common type of chronic liver disease and is one of the most important health problems responsible for high mortality and morbidity in developed as well as developing countries. Cirrhosis of liver is among the top 10 causes of death in western populations. The involvement of free radicals in the pathogenesis of liver injury has been investigated for many years in a few well-defined experimental systems [1]. Liver injury due to acute or chronic alcohol abuse has been proved dependent on its oxidative metabolism at the cytosolic, peroxisomal, and/or microsomal level [2]. According to Shaw et al. [3], lipid peroxidation may be an important mechanism in the pathogenesis of alcoholic liver disease. A statistically significant increase in plasma lipid peroxides and ascorbic acid and a statistically significant decrease in reduced glutathione and superoxide dismutase activity have been reported in cirrhotic patients in comparison to healthy controls [4]. Circadian variations of different blood and urinary variables have been reported in healthy Indians showing different circadian patterns from those found in the west [5-12]. To our knowledge, there is no mention in the available literature regarding chronomics of circulating lipid peroxides and other intracellular anti-oxidant enzymes in the hemolysate of patients with cirrhosis of liver. The present study aims to fill the gap by providing reference values for circadian changes in lipid peroxides, intracellular anti-oxidant enzymes and other molecules in clinical health and to assess any deviation from such norms in cirrhosis of liver in an attempt to understand the mechanism of oxidative stress and its involvement in cirrhosis of liver.
2. Subjects and methods
This investigation includes 30 clinically and histopathologically validated cases of cirrhosis of liver admitted in the medical wards of Gandhi Memorial and Associated Hospitals, CSM Medical University, Lucknow, and 60 clinically healthy volunteers, comprised mainly of medical students, staff members and their families. The age of the healthy participants ranged from 21 to 40 years and that of the patients from 25 to 45 years. The patients were thoroughly examined to ensure the absence of any other illness. Prior to the collection of blood samples, participants refrained from taking any drug preparation that would affect or alter the oxidative stress or its defensive mechanism. All participants were synchronized for 1 week to a schedule of diurnal activity from about 06:00 to about 22:00 and nocturnal rest. All subjects took their usual (although not identical) meals three times daily; breakfast around 08:30, lunch around 13:30 and dinner around 20:30, without any change in their fluid intake. At 06:00, 12:00, 18:00 and 00:00, 6 ml of blood was collected from each subject in plain and sterile vials containing heparin as anticoagulant. The plasma was separated and analyzed for lipid peroxidation in terms of malondialdehyde (MDA) [13]. The hemolysate was prepared from the red cells and used for the measurement of the activities of the following enzymes: superoxide dismutase (SOD) [14], catalase (CAT) [15], glutathione peroxidase (GPx) [16] and glutathione reductase (GR) [17]. Serum total protein, albumin, ascorbic acid and uric acid concentrations were measured spectrophotometrically [18-20].
Data were evaluated by conventional statistical analysis and by the single and population-mean-cosinor procedures [21-23]. Accordingly, the MESOR (a chronome-adjusted mean), the circadian double amplitude (a measure of the extent of predictable change within a day) and the circadian acrophase (a measure of the timing of overall high values recurring each day) were determined.
3. Results
A circadian rhythm was invariably demonstrated by population-mean cosinor in healthy volunteers and in patients with cirrhosis of liver for all variables. By comparison to the healthy controls, the patients with cirrhosis of the liver had a higher MESOR of MDA (P < 0.001) and an acrophase advanced by almost 3 h (P < 0.001), Fig. 1. The MESOR of SOD activity was lower in the patients with liver cirrhosis (P < 0.001), Fig. 2. CAT activity was lower at all sampling times in cirrhotic patients by comparison with the healthy subjects. The MESOR difference is significant (P < 0.001), as is the difference in circadian amplitude (P < 0.001), Fig. 3. GPx activity was also decreased at all sampling times in cirrhotic patients by comparison with healthy subjects. As compared to healthy controls, patients with liver cirrhosis have a lower MESOR (P < 0.001) and a reduced circadian amplitude (P < 0.001) of GPx, Fig. 4. GR activity was also decreased at all sampling times in cirrhotic patients by comparison with healthy subjects. In addition to a large difference in the MESOR of GR activity (P < 0.001) and in circadian amplitude (P < 0.001), the acrophase was delayed by 19 ° or about 1 h and 16 min, Fig. 5.
Fig. 1.
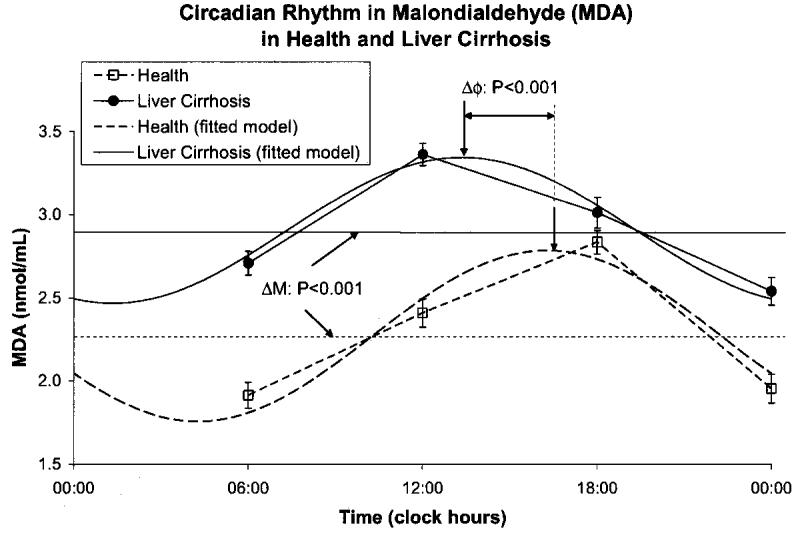
Fig. 2.
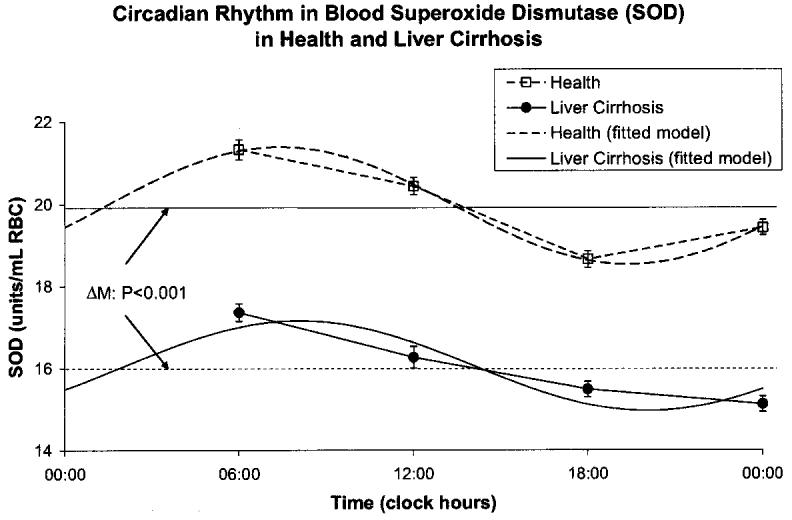
Fig. 3.
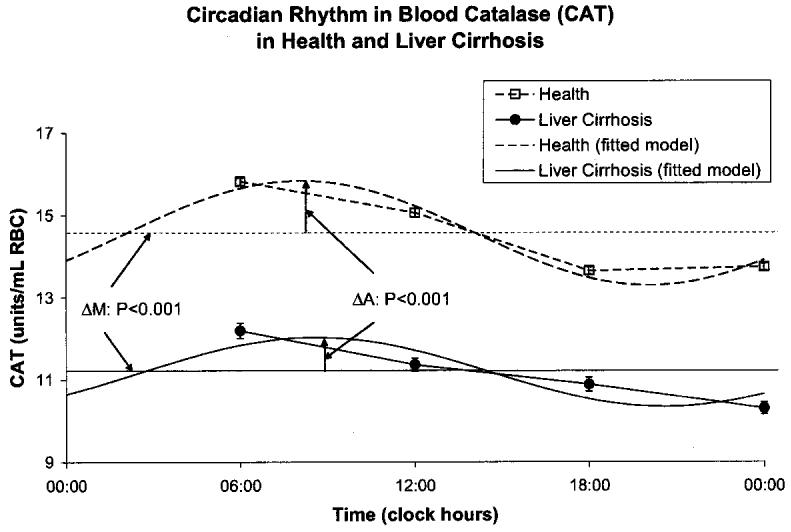
Fig. 4.
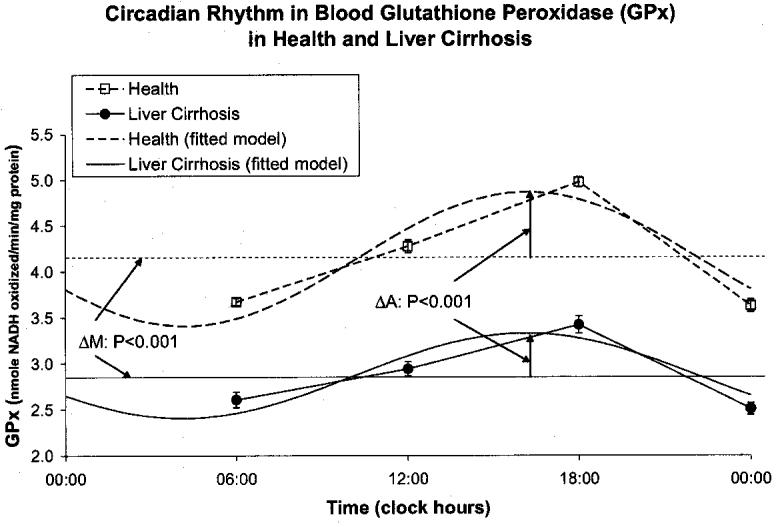
Fig. 5.
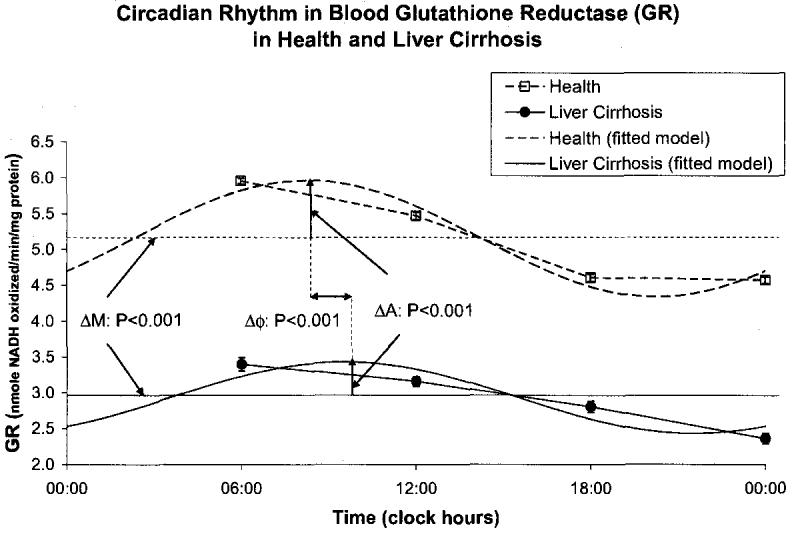
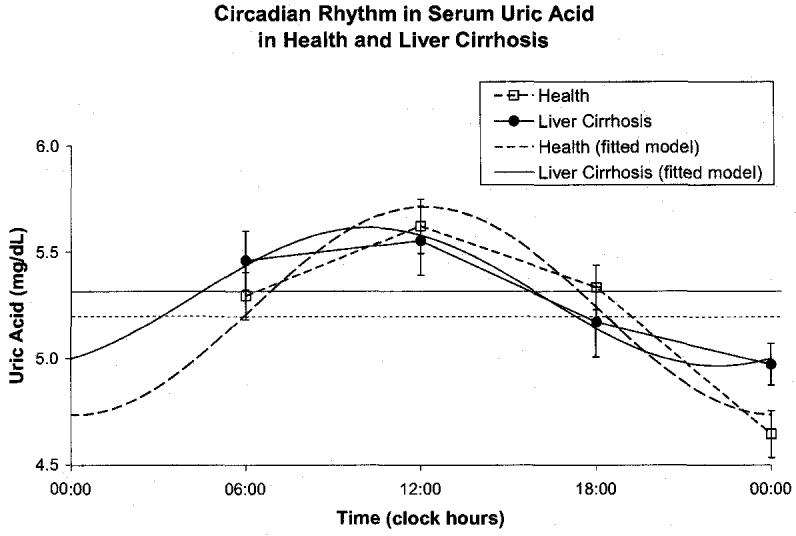
Cirrhotic patients had a decreased amplitude of total serum protein (P < 0.001) and an acrophase delayed by 66 ° or 4 h and 24 min (P < 0.001), but a similar MESOR, Fig. 6. They also had a decreased MESOR of serum albumin (P < 0.001) and an advanced acrophase (P = 0.033) with a reduced circadian amplitude (P < 0.001), Fig. 7, confirming the possible role of albumin in liver disorders. Serum ascorbic acid had a higher MESOR (P < 0.001) and an advanced acrophase (P = 0.001) in the cirrhotic patients by comparison to healthy subjects, Fig. 8. No major difference was found between the two groups for uric acid concentration, Fig. 9.
Fig. 6.
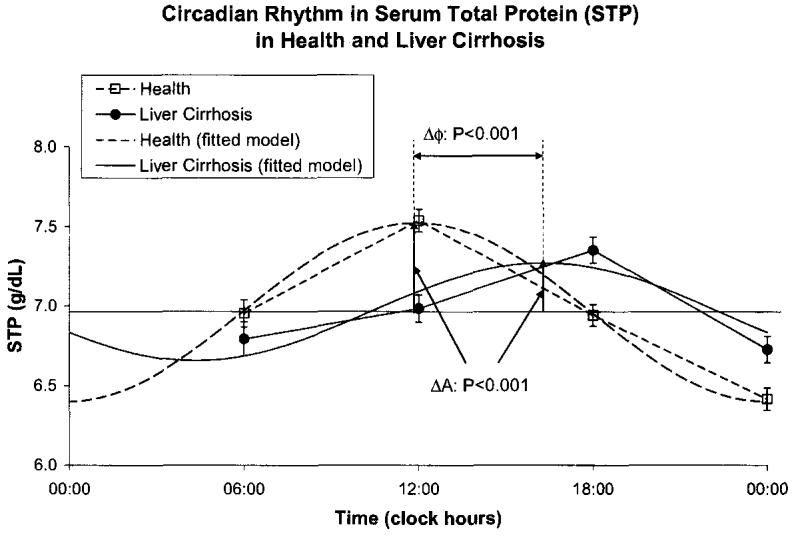
Fig. 7.
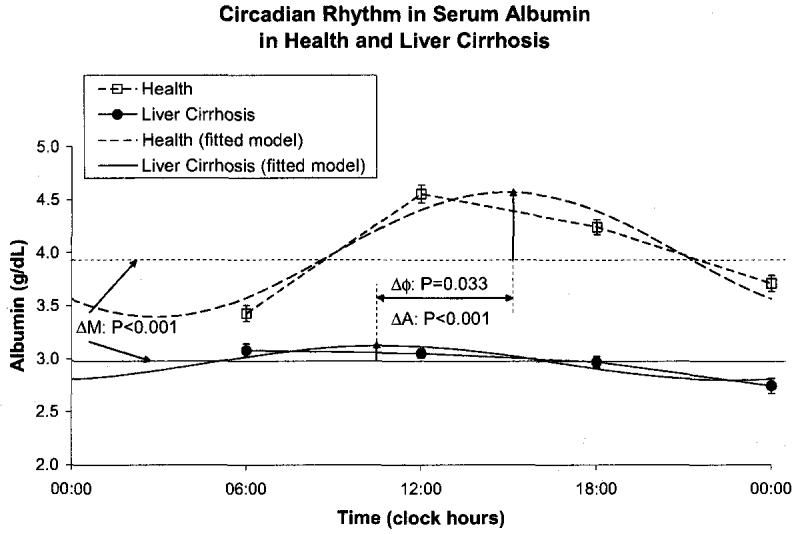
Fig. 8.
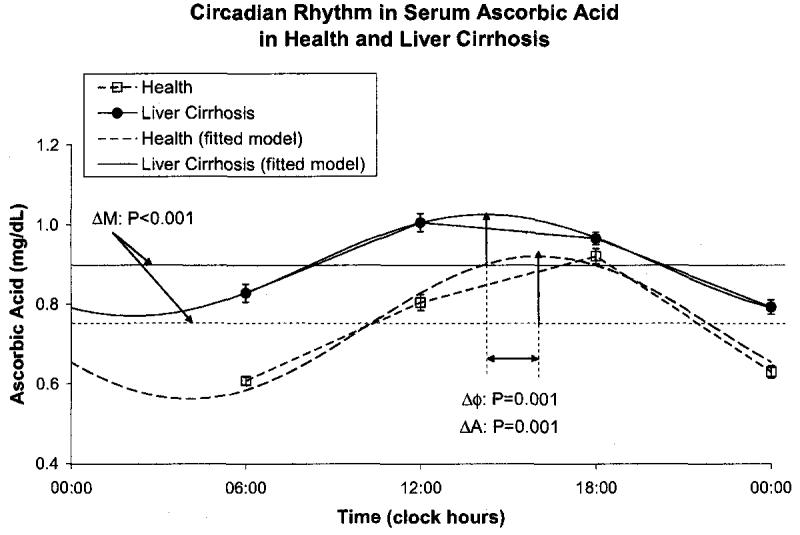
Fig. 9.
4. Discussion
We found a marked circadian variation in MDA, total protein, albumin, ascorbic acid and uric acid concentration and SOD, CAT, GPx, and GR activities in healthy Indians and patients suffering from cirrhosis of liver. Our results on elevated plasma lipid peroxides in liver cirrhosis are in agreement with other reports [1,3,4]. An advanced circadian acrophase in liver cirrhosis has not been previously reported to our knowledge, however. Lipid peroxidation in cell membranes and subcellular organelles has been proposed as a primary mechanism for cellular membrane dysfunction and tissue injury associated with free-radical initiated processes. Elevated concentrations of lipid peroxides may disturb relations between protective and aggressive factors at the tissue and molecular level leading to hepatic damage. Although much is known about the chemistry of lipid peroxidation and cellular defense mechanisms, chronobiological studies are needed to quantify the various cellular components involved in these processes to achieve better management, prognosis and treatment. Chronomes of putative anti- and pro-oxidants have recently been mapped to explore their putative chronochemotherapeutic role as markers in cancer chronoprevention and management of ovarian cancers [12].
SOD was found to be decreased in liver cirrhosis with no noticeable alteration in circadian amplitude or acrophase. Lower activities of CAT, GPx and GR and less Prominent circadian variations (reduced amplitudes) in cirrhotic patients were also observed, pointing to an overall decrease in anti-oxidant defensive mechanisms in such patients. A decrease in SOD activity and reduced glutathione content in liver cirrhosis has been reported earlier [4]. SOD is the first among the scavenger enzyme series to ameliorate the damage caused in cells by free radicals [24]. CAT, GPx and GR are involved in the removal of hydrogen peroxides and several other toxic peroxides. These anti-oxidant enzymes form the primary enzymatic defense against toxic oxygen reductive metabolites. Such metabolites have been implicated in the damage brought about by ionizing radiations, as well as in the effects of several cytostatic compounds [25]. The reduced activity of these primary defensive enzymes could be due to a direct and greater involvement of reactive oxygen species in the pathogenesis of cirrhosis of liver disturbing the pro-oxidant vs. anti-oxidant ratio, thereby precipitating the hepatic damage. Belanger et al. [26] reported temporal variations in the hepatic concentration of glutathione (GSH), which could be responsible for time-dependent variations in GPx and GR activities, as observed herein.
A reduced circadian variation in total serum protein and albumin and an overall lowering of serum albumin was found in patients with cirrhosis of liver as compared to healthy volunteers. Whereas the acrophase of total serum protein was delayed in the patients, an acrophase advance characterized their serum albumin, contributing to an altered A/G ratio. Patients with liver cirrhosis also had elevated MESOR of ascorbic acid, but a reduced circadian amplitude and an advanced acrophase. Nalini et al. [4] reported increased ascorbic acid concentrations in cirrhotic patients, which could be due to a changed pro-oxidant vs. anti-oxidant ratio. To our knowledge, alterations of the circadian rhythm in serum ascorbate associated with cirrhosis of liver have not yet been reported. The circadian variation in uric acid concentrations demonstrated herein is in keeping with earlier reports [6,8,27]. The demonstration of a circadian rhythm in all variables investigated herein suggests that these variables could also serve as putative markers to optimize the timing of treatment administration and to assess the patients' response to treatment.
Biological rhythms are characteristic features of living organisms, showing a specific time for the peak activity of a particular variable in a specific species [28,29]. Altered rhythm characteristics of one or more variables may indicate a deviation from normal physiology. Herein, we showed an altered chronome of anti-oxidant defense mechanisms in cirrhotic patients. The increased plasma lipid peroxides and decreased enzyme activities clearly indicate the involvement of free radicals in the etiopathogenesis of the disease, while the increase in ascorbate and urate concentrations and the altered timing of serum proteins is indicative of the changed pro-oxidant vs. anti-oxidant status in cirrhotic patients.
Chronobiological studies provide the capability of therapeutic intervention at a time when this intervention is useful and best tolerated and avoidance when it is not [30]. The chronobiologic approach to treatment, by exploring the rhythmic nature of oxidants and antioxidants, is especially critical and meaningful when potentially damaging or toxic agents have to be used. But far beyond this application, the time factor has to be introduced in just about all aspects of clinical pharmacology and many “time honored” customs like “three times a day” medications will have to be replaced by more meaningful, and often more effective and less toxic chronobiologic treatment schedules. The choice of the “right time” will require chronobiologic knowledge, interpretation and experience since treatment at the “wrong time” can be potentially harmful [27,30]. Further studies are needed to correlate the lipid peroxide concentrations with free-radical scavengers, its nature, status and rhythm after administration of known dietary and therapeutic antioxidants in such pathological situations and thereby opening new chapters in understanding the pathogenesis of the disease.
References
- 1.Poli G. Liver damage due to free radicals. Br Med Bull. 1993;49:604–20. doi: 10.1093/oxfordjournals.bmb.a072634. [DOI] [PubMed] [Google Scholar]
- 2.Lieber CS. Biochemical and molecular basis of alcohol-induced injury to liver and other tissue. New Eng J Med. 1988;319:1639–50. doi: 10.1056/NEJM198812223192505. [DOI] [PubMed] [Google Scholar]
- 3.Shaw S, Jayatillele E, Ross WA, Gordon ER, Lieber CS. Ethanol-induced lipid peroxidation: Potentiation by long-term alcohol feeding and attenuation by methionine. J Lab Clin Med. 1981;98:417–24. [PubMed] [Google Scholar]
- 4.Nalini G, Hariprasad C, Narayanan VA. Oxidative stress in alcoholic liver disease. Ind J Med Res. 1999;110:200–3. [PubMed] [Google Scholar]
- 5.Singh RK, Nakra VK, Pandey HN, Arora SR. Studies on circadian periodicity of plasma, breast milk and urinary calcium in lactating Indian women. Trop Geogr Med. 1984;36:345–49. [PubMed] [Google Scholar]
- 6.Singh RK, Bansal A. Studies on circadian periodicity of serum and urinary urate in healthy Indians and renal stone formers. Prog Clin Biol Res. 1987;227B:305–13. [PubMed] [Google Scholar]
- 7.Singh RK, Wu J, Zhou S, Halherg F. Circadian rhythmic human circulating cholesterol in health, during fasting and on vegetarian vs. omnivorous diets. Chronobiologia. 1989;16:183. [Google Scholar]
- 8.Singh RK, Bansal A, Bansal SK, Rai SP. Circadian rhythms of common laboratory profiles in serum and urine of healthy Indians. Prog Clin Biol Res. 1990;341B:559–66. [PubMed] [Google Scholar]
- 9.Singh RK, Bansal A, Bansal SK, Singh AK, Mahdi AA. Circadian periodicity of urinary inhibitor of calcium oxalate crystallization in healthy Indians and renal stone formers. Eur Urol. 1993;124:387–92. doi: 10.1159/000474334. [DOI] [PubMed] [Google Scholar]
- 10.Singh R, Singh RK, Mahdi AA, Misra S, Rai SP, Singh D, et al. Studies on circadian periodicity of urinary corticoids in carcinoma of breast. In vivo. 1998;12:69–73. [PubMed] [Google Scholar]
- 11.Singh R, Singh RK, Mahdi AA, Saxena SP, Comtlissen G, Halberg F. Circadian periodicity of urinary volume, creatinine and 5-hydroxyindole acetic acid excretion in healthy Indians. Life Sci. 2000;66:209–14. doi: 10.1016/s0024-3205(99)00582-2. [DOI] [PubMed] [Google Scholar]
- 12.Singh R, Singh RK, Mahdi AA, Singh RK, Kumar A, Tripathi AK, et al. Circadian periodicity of plasma lipid peroxides and other antioxidants as putative markers in gynecological malignancies. In vivo. 2003;17:593–600. [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiabarbituric reaction. Anal Biochem. 1979;95:151–7. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.McCord JM, Fridovich I. Superoxide dismutase: an enzyme function for erythrocuprin. J Biol Chem. 1969;24:6049–55. [PubMed] [Google Scholar]
- 15.Aebi H, Suter H. Protective function of reduced glutathione against the effect of pro-oxidative substances and of irradiation in the red cell. In: Flohe L, Benhar HC, Sies H, Waller HD, Wendel A, editors. Glutathione. Georg Thieme; Stuttgart: 1974. pp. 192–9. [Google Scholar]
- 16.Pagila DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;2:158–69. [PubMed] [Google Scholar]
- 17.Hazelton GA, Lang CA. GSH content of tissue in aging mouse. Biochem J. 1985;188:25–30. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–8. [PubMed] [Google Scholar]
- 19.Natelson S. Techniques of clinical chemistry. 3rd ed Charles C Thomas; Springfield, IL: 1971. p. 286. [Google Scholar]
- 20.Eichhorn E, Zalmanwaki S, Rotenburg EA, Fanias B. Uric acid estimation in serum and urine. J Clin Pathol. 1961;14:450–3. doi: 10.1136/jcp.14.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halberg F, Johnson EA, Nelson W, Runge W, Sothern R. Autorhythmometry-procedures for physiologic self-measurements and their analysis. Physiol Ther. 1972;1:1–11. [Google Scholar]
- 22.Bingham C, Arbogast B, Comé1issen Guillaume G, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 23.Cornélissen G, Halberg F. Chronomedicine. In: Armitage P, Colton T, editors. Encyclopedia of biostatistics. Vol. 1. John Wiley & Sons Ltd.; Chichester, UK: 1998. pp. 642–9. [Google Scholar]
- 24.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marklund SL, Westman NG, Lundgren E, Ross G. Copper and zinc containing superoxide dismutase, manganese containing superoxide dismutase, catalase and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982;42:1955–51. [PubMed] [Google Scholar]
- 26.Belanger PM, Desgagne M, Boutet M. The mechanism of the chronohepatotoxicity of chloroform in rat: correlation between covalent binding to hepatic subcellular fractions and histologic changes. Ann Rev Chronopharmacol. 1988;5:235–8. [Google Scholar]
- 27.Haus E, Touitou Y. Chronobiology in laboratory medicine. In: Touitou Y, Haus E, editors. Biologic rhythms in clinical and laboratory medicine. Springer-Verlag; Berlin, Heidelberg, New York: 1992. pp. 673–708. [Google Scholar]
- 28.Halberg F. Implications of biologic rhythms for clinical practive. Hosp Pract. 1977;12:139–49. doi: 10.1080/21548331.1977.11707067. [DOI] [PubMed] [Google Scholar]
- 29.Mills JN. Human circadian rhythms. Physiol Rev. 1966;46:128–71. doi: 10.1152/physrev.1966.46.1.128. [DOI] [PubMed] [Google Scholar]
- 30.Halberg F, Comé1issen G, Wang ZR, Wan C, Ulmer W, Katinas G, et al. Chronomics: circadian and circaseptan timing of radiotherapy, drugs, calories, perhaps nutriceuticals and beyond. J Exp Ther Oncol. 2003;3:223–60. doi: 10.1111/j.1533-869x.2003.01097.x. [DOI] [PubMed] [Google Scholar]


